Abstract
Characterisation of the adsorption of biomolecules, or a biocorona, on nanomaterials has proliferated in the past 10 years, as protein corona studies provide molecular level insight into mechanisms of cellular recognition, uptake, and toxicity of nanomaterials. At the crossroads of two rapidly evolving orthogonal fields, nanoscience and proteomics, the interdisciplinarity of protein corona studies creates challenges for experimental design and reporting. Here we propose a flexible checklist for experimental design and reporting guidelines to outline Minimum Information about Nanomaterial Biocorona Experiments (MINBE). The checklist for experimental design, compiled after review of reporting within the protein corona literature, provides researchers with prompts to ensure best practice experimental approaches for each stage of the workflow, collated from the nanoscience, proteomics, and bioinformatics fields. Reporting guidelines are also assembled from established sources, integrated to span the entire workflow and extended and modified to aid interdisciplinary researchers in the most challenging stages of the workflow. Where appropriate, de novo guidelines to address areas specific to protein corona studies, including exposure conditions and isolation of adsorbed proteins, were written. The MINBE guidelines provide protein corona researchers with a conduit between materials science techniques and proteomics. Implementation of these guidelines is anticipated to catalyse enhanced quality, impact, and extent of data mining and computational modelling of protein corona composition and its role in nanosafety and nanomedicine. Furthermore, high quality experimental design and reporting in the bio-nanosciences will enhance the next phase of targeted nanomedicines and sustainable nanotechnologies.
Keywords: Proteomics, LC-MS/MS, Corona, Nanoparticle, Nanomaterial, Reproducibility
Introduction
Since the emergence of the term protein corona, interdisciplinary teams have studied this spontaneously acquired coating of proteins on the surface of engineered nanomaterials (ENMs) in living systems [1]. This complex population of biomolecules includes proteins [2], but also more recently lipids [3], carbohydrates [4,5], and other small molecules [6] have been observed in the corona and represent a vital new area for bio-nano interaction research, along with natural organic matter in environmental settings [7]. The corona not only creates a new biomolecular surface for the ENMs, but can also cause ENM transformations, including altering dissolution and agglomeration [8,9]. These newly acquired chemical and physical properties, in turn, form a new biological identity for the ENM with altered uptake and interactions with cellular receptors. Ultimately, the corona modulates ENM immune response, toxicity, and distribution [1,10]. Although computational modelling has the potential to predict corona formation and organismal fate, the accuracy of models depends heavily upon the quality, depth, and breadth of collated databases of ENM corona compositions and cellular interactions.
As interest in the ENM corona has increased, the field has established a landscape of experimental approaches to characterize the corona. Although other techniques can be used to monitor patterns of change in the corona population, LC–MS/MS proteomic approaches uniquely provide both identification and quantification across the full population of proteins within the corona [8,11,12]. Initially, many studies isolated the corona with detergents and ran them through gel electrophoresis. Specific bands were then excised, digested and analysed by LC–MS/MS. This approach significantly skews the results in favour of highly abundant proteins whose concentration in the gel was sufficient to lead to visible staining, such that the resulting corona analysis may miss multiple low abundance / low staining efficiency proteins that could play important biological roles. Thus, most recent corona characterizations use in-solution/on-particle digests for the characterisation of the entire corona, with the addition of a surfactant such as Rapigest SF™ to prevent protein and peptide re-adsorption [8,11,13,14]. The typical four phase workflow for protein corona characterisation (Fig. 1 and Table 1) is highly interdisciplinary and requires expertise in ENM characterization, mass spectroscopy, and informatics. This array of specialisms introduces challenges to study design and reporting. Commonly, nanoscientists have extensive knowledge of materials characterization, but lack the background to explore informatics relevant to MS proteomics whereas the reverse is true for proteomicists. Guidance to provide depth of knowledge in these disparate techniques could help researchers ensure sound experimental design and reporting.
Fig. 1.
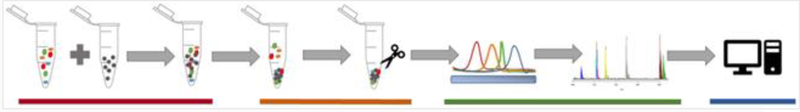
The four phases of a corona characterization include: (1) ENM exposure to biofluids and ENM characterization (red bar), (2) isolation of absorbed biomolecules and preparation for analysis (orange bar), (3) separation of biomolecules and spectroscopic characterization (green bar), and (4) informatic identification of the corona population and analysis.
Table 1.
Checklist of questions to guide experimental design when characterizing the ENM biocorona.
| Experimental design checklist for biocorona characterization |
|---|
| Phase 1: ENM exposure to biofluids and ENM characterization |
| How can the biofluid be handled and stored to best reflect biological conditions? |
| What is the most relevant and appropriate dosage of ENMs in the chosen biofluid or hypothesis? |
| Does the study aim for analysis of the biocorona pre- or post-equilibrium? How was the timeline for equilibrium evaluated? |
| Are replicates and controls included? And, where possible, do plans include parallel processing of samples for proteomics and additional characterisation? |
| Phase 2: Isolation of absorbed biomolecules and preparation for analysis |
| Is the biocorona separation thorough enough to remove all unbound biomolecules? |
| Is the separation technique likely to alter the chemical structure of the biocorona, or the profile of biomolecules? |
| Will the biocorona clean-up steps affect the profile of your biomolecules? |
| What clean-up methods are likely to least alter the biocorona, e.g. through selective or pervasive protein loss? |
| Phase 3: Separation of biomolecules and spectroscopic characterization |
| What type of separation will be best for these samples (in gel, on-particle etc.)? |
| How can the protocol be modified to best maintain consistency across samples? |
| What instruments are best for these samples and quantification? |
| What controls can be included to check for |
| Phase 4: Informatic identification of the biocorona population and analysis. |
| What informatics database will be used for identifying the biomolecules? |
| What kind of post-processing is required for quantification? |
| What statistical analysis of the dataset can be used to assess data quality? |
| How will the full dataset of biomolecules and their characteristics be organized, analysed, and presented? |
Recent scrutiny of the preproducibility (a term introduced to describe whether a study has been reported in sufficient detail for others to undertake it) of scientific literature raises concerns about reporting adequate information for independent verification of results and reviewing methodological soundness [15]. The interdisciplinarity of nano-bio studies further complicates preproducibility, as well as experimental design and data clarity. Recently, the Minimum Information Reporting in Bio–Nano Experimental Literature (MIRIBEL) guidelines were published to improve data reusability, quantification, practicality, and quality in aspects of ENM and biologics characterisation in bio-nano interface studies [16]. These guidelines form an excellent basis for experiments up to the moment of forming a corona. Here, MIRIBEL is extended to encompass corona characterisation by creating a conduit between the fields of ENMs research and biological mass spectrometry. The reporting guidelines presented are already established in the individual fields but are integrated here and modified for corona studies. The integrated guidelines, with summaries of their scientific underpinnings, and the corona workflow and reporting checklist, have been developed to enhance interoperability, preproducibility as the requisite to reproducibility, and utility of corona characterizations. If implemented, the MINBE guidelines will ease comparisons across datasets and enable predictive modelling of corona formation and ENM impact. When applied, this will accelerate innovation of targeted bioactive ENMs and sustainable nanotechnologies.
Lessons learned for protein corona characterization
Despite publication of a suggested protocol for profiling of protein coronas roughly five years ago, [12] the field has yet to adopt a best practice approach. In part, this is due to the broad range of research questions, which demand variations in the protocol for analysis. For example, ENMs extracted from blood plasma should not be prepared and analysed with the same approach as ENMs isolated from fish guts. It also stems from the enormous variability in ENM characteristics, especially density, which makes it impossible to provide an exact specification for centrifugation time and speed to optimise the pelleting of the ENM with their associated proteins. Yet, ten years after coining the term protein corona, the field has reached a certain maturity. As corona specific characterization methods coalesce, the loose guidelines presented in Table 1, developed from the analysis of literature reporting of corona studies presented briefly below, can aid experimental reproducibility and provide reviewers with a guide for providing feedback on submitted studies.
To assess the current state of reporting, we reviewed the most cited protein corona papers using LC-MS/MS from the last few years. Although ENM exposure methods and characterization were often clearly defined and well reported, later stages in the workflow (shown in Fig. 1) were less clearly described. Key reporting recommendations based on gap analysis include:
Demonstrate reproducibility (workflow phases 1–4): A hallmark of reproducibility is replication. Yet, authors often overlook inclusion of a statement on replicates, technical or biological. Similarly, few articles discussed quality control (QC) and evaluation of data quality. By incorporating QC samples such as a stock tryptic digest of HeLa cells or of universal protein standards, LC–MS/MS performance can be monitored over batches to assess instrument sensitivity and consistency [17,18]. It is also vital to incorporate both positive and negative controls into the experimental set up. Negative controls would follow the entire workflow with incubation of the ENM in pure water to assess sources of contamination and any existing protein present. A positive control represents the workflow without the ENMs and is thus a digest of the biological matrix.
Streamline purification methods (workflow phase 2): Protein precipitation methods can alter the protein profile dramatically. For example, a common solvent (trichloroacetic acid in acetone) precipitation method in corona preparations selectively eliminates human serum albumin from blood plasma [19], a protein that is normally present in high concentrations [20]. Instead, we recommend direct digestion of proteins on the ENM to minimize steps where sample can be altered or lost – a protocol for this is included in Faserl et al. [14].
Pay attention to quality and efficiency of separation and detection (workflow phase 3): The quality and efficiency of the separation and detection are critical for reliable identification and quantification of protein content of the ENM corona. Because label-free quantification is predominant to date, researchers must pay attention to difficulties in sample to sample reproducibility for precursor selection and the daughter ion intensities used for this quantification. If protein intensity is reported, as opposed to fold changes or ratios between samples, it is important to state how the label free quantification was performed, which includes the number of peptides used to quantify the protein and if it is the average intensity or summed intensity of these peptides that is reported.
Take care with informatics (workflow phase 4): Often overlooked, informatics methods are as essential as wet-lab details to further reproducibility and confidence in protein identification and quantification. Rarely were details such as false discovery rates parameters for protein identification reported, yet, these specifics are essential for accuracy and confidence in protein identification and quantification.
These inconsistencies suggest that the field could benefit from a broad checklist for experimental design in addition to reporting guidelines. This checklist, shown in Table 1, will help researchers to address important considerations at each step of the workflow before stepping into the lab.
Minimum information about nanomaterial corona experiments (MINBE) reporting guidelines
Once a well-planned corona experiment has been carried out, the preproducibility of the work depends upon the quality of reporting. Precedent for reporting guidelines is well-established in the biosciences and includes over forty standards for various experimental approaches [MIBBI] [21]. Implementation of standards provides the platform for increased quality of reporting without disproportionate increase in cost, requiring only a marginal amount of additional time from users. Aligned with previous minimum information reporting guidelines for proteomics experiments [MIAPE] [22], proteomics analysis with LC–MS/MS (MIAPE-MS) [23], and proteomics data reporting [MIAPE-MSI] [24], we aimed to balance the importance of detail in reporting with a flexibility that allows for growth in new experimental analyses, techniques, and instrumentation. The MINBE reporting guidelines for ENM corona studies are presented in Table 2.
Table 2.
Reporting standards for corona characterization. Colours within the subheadings correspond to those in the experimental steps outlined in Fig. 1.
| Reporting component | Description |
|---|---|
| Biofluid processing [Compare to MIRIBEL guidelines [16] | |
| Collection organisms | Note sex, age, growth condition, organism part, and any known genetic variations for the source of the biofluids. If the samples were from a cell line, note passage number, cell line, source, and type. |
| Collection approach | Outline methods to collect cells and biofluids, including organism / biofluid processing. |
| Processing | Detail the purification methods, including solution conditions for biofluids before particle exposure. |
| Storage | Note method of storage, including temperature, solution conditions, and time. |
| Reaction conditions [Compare to MIRIBEL guidelines [16] | |
| Dosimetrics | Concentration of particle and biomolecules. Often reported as the ratio of particles to protein using units of mass, surface area, or particle number. |
| Sample conditions | Type and concentration of buffers, salts, other solutes, as well as pH and temperature. |
| Replicates | How many biological and technical replicates were run? |
| Nanomaterial characterization | |
| Provenance | Synthesis method, storage history, sample processing |
| Synthesized properties | Core composition, surface coating, size, and shape |
| Agglomeration state | Hydrodynamic radius, polydispersity index |
| Surface chemistry | Zeta-potential, surface ligand characterization |
| Separation techniques and processing | |
| Corona separation | Solutes, detergents, and centrifugation speed / time (or other method of separation detailed) |
| Sample clean-up | Gel size and type; types of filters used to remove particulates; solvents, amounts, and timing of any precipitation steps. |
| Protein digestion | Details of chemicals or enzymes used to alkylate and digest the protein into peptides for MS. |
| LC-MS/MS analysis of protein (Compare to MIAPE-MS [25]) | |
| LC platform | Make and model of platform, type of solvent delivery system, software, and version. |
| MS/MS platform | Make and model of platform, type of mass analyser, software, and version. |
| LC column | Make, model, length, internal diameter, porosity, column chemistry. |
| LC gradient | Time course, flow rate, temperature and solvent compositions. |
| LC solvents | Solvent manufacturer, purity, additive details. |
| Injection volume | Volume of sample injected on LC. |
| MS source settings | Electrospray ionisation voltages, gas flows, ionisation mode. |
| MS mass analyser settings | m/z range, mass resolution, calibration solution and calibration m/z range, accumulation time/AGC setting and scan rate. |
| Fragmentation method | Type of fragmentation, precursor selection, fragmentation energy, collision molecule, level of fragmentation |
| Protein identification and quantification (Compare to MIAPE-MSI [24]) | |
| Database | List the database used for protein identification, including version and any restrictions applied in the search |
| Accession number | A unique identifier for each protein |
| Confidence in protein identification | % Coverage: The percentage of the protein sequence covered. |
| Number of peptides: Total number of peptides detected for each protein, ideally 2 or more peptides. | |
| Number of unique peptides: Number of peptide sequences that are unique to the identified protein. | |
| Missed cleavages: Number of missed cleavages in the protein or peptide sequence. | |
| Protein probabilities and scores: Calculated probabilities or scores to give confidence in a protein identification. | |
| Validation | Statistical analysis or comparison of replicates should be performed to assess data quality. |
| Quantification | Details on both the normalisation and quantification method required to enable accurate reproducibility between experiments. |
MS: Mass spectrometer, LC: Liquid Chromatography, ESI: Electrospray Ionisation, m/z: mass to charge ratio, AGC: Automatic Gain Control.
Outlook
MINBE aims to provide clarity of experimental design and optimal reporting guidelines to characterize the composition ENM protein coronas. To encourage reuse by researchers, granting agencies, and publishers, the experimental checklist and reporting guidelines are available via a publicly accessible repository < https://doi.org/10.17605/OSF.IO/3JB47>. Thus far, the field has coalesced upon LC-–MS/MS approaches for protein identification and quantification. As the interdisciplinary, rapidly growing field is constantly evolving, we also encourage edits and updates.
Careful molecular level characterization of the biological surface of ENMs promises more accurate models of cellular and organismal responses to ENM at the systems level. With compilation of strong biocorona datasets, computational approaches can model individual biomolecules binding to ENMs [1,26], or predict a population of biomolecules within a corona [27]. Importantly, computational tools have predictive power across an array of samples and conditions, saving experimental time and funds. The utility of biocorona characterization extends across size-scales, from molecular level insights to cellular, organismal, and systems level predictions of ENM fate, transport, and toxicity [1,26,28,29]. Multivariate [2] and machine learning models [27,30] can mine protein corona databases and improve downstream modelling of ENM bioactivity including cellular attachment, uptake, and response. Thus far, corona modelling typically uses data collected in-house. Solid experimental design and clear, detailed reporting, will facilitate collation and mining of published datasets, enabling development of improved quantitative and qualitative structure activity relationship (QSAR) and artificial intelligence models.
With strong datasets and coordinated modelling, nanomedicine and sustainable nanotechnologies can expand. Nanomedicine will see increased selectivity for nano-based drug targeting, more adaptable diagnostics devices, and decreased clinical translation timescales. Enhanced personalisation of nanomedicines may be achievable by comparing coronas across age, gender, health status, and disease stages. Environmental applications may benefit the most, because full experimental characterization is near impossible under the broad diversity of conditions, biomolecules, and organisms in the environment. Biocorona datasets and modelling can increase accuracy of ecotoxicity, food chain interactions, and ENM transport predictions. With strong experimental design, clear reporting, and cross-study modelling in the second decade of biocorona studies, we anticipate catalysis of the design of smart, sustainable ENMs with maximized efficacy.
Acknowledgements
K.E.W. acknowledges support from the National Institute of Environmental Health Sciences of the National Institutes of Health (R15ES025929) and extends a warm thanks to Kris McNeill and group at ETH Zurich, who provided a sabbatical home while much of this manuscript was written. A.J.C and I.L. acknowledge funding from the European Union Horizon 2020 project ACEnano (Grant Agreement No. 720952).
References
- [1].Ke PC, Lin S, Parak WJ, Davis TP and Caruso F, A decade of the protein corona, ACS Nano 11, 2017, 11773–11776, 10.1021/acsnano.7b08008. [DOI] [PubMed] [Google Scholar]
- [2].Walkey CD, Olsen JB, Song F, Liu R, Guo H, Olsen DWH, Cohen Y, Emili A and Chan WCW, Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles, ACS Nano.8, 2014, 3439–3455, 10.1021/nn406018q. [DOI] [PubMed] [Google Scholar]
- [3].Lee JY, Wang H, Pyrgiotakis G, DeLoid GM, Zhang Z, Beltran-Huarac J, Demokritou P and Zhong W, Analysis of lipid adsorption on nanoparticles by nanoflow liquid chromatography-tandem mass spectrometry, Anal. Bioanal. Chem. 2018, 6155–6164, 10.1007/s00216-018-1145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Martel J, Wu CY, Hung CY, Wong TY, Cheng AJ, Cheng ML, Shiao MS and Young JD, Fatty acids and small organic compounds bind to mineralo-organic nanoparticles derived from human body fluids as revealed by metabolomic analysis, Nanoscale .8, 2016, 5537–5545, 10.1039/c5nr08116e. [DOI] [PubMed] [Google Scholar]
- [5].Grintzalis K, Lawson TN, Nasser F, Lynch I and Viant MR, Metabolomic method to detect a metabolite corona on amino functionalised polystyrene nanoparticles, Nanotoxicology 2019, 1–12, 10.1080/17435390.2019.1577510. [DOI] [PubMed] [Google Scholar]
- [6].Pink M, Verma N, Kersch C and Schmitz-Spanke S, Identification and characterization of small organic compounds within the corona formed around engineered nanoparticles, Environ. Sci. Nano .5, 2018, 1420–1427, 10.1039/C8EN00161H. [DOI] [Google Scholar]
- [7].Markiewicz M, Kumirska J, Lynch I, Matzke M, Köser J, Bemowsky S, Docter D, Stauber R, Westmeier D and Stolte S, Changing environments and biomolecule coronas: consequences and challenges for the design of environmentally acceptable engineered nanoparticles, Green Chem. 2018, 10.1039/C8GC01171K. [DOI] [Google Scholar]
- [8].Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, Schlenk F, Fischer D, Kiouptsi K, Reinhardt C, Landfester K, Schild H, Maskos M, Knauer SK and Stauber RH, Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology, Nat. Nanotechnol. 8, 2013, 772–781, 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- [9].Müller J, Prozeller D, Ghazaryan A, Kokkinopoulou M, Mailänder V, Morsbach S and Landfester K, Beyond the protein corona – lipids matter for biological response of nanocarriers, Acta Biomater. 71, 2018, 420–431, 10.1016/j.actbio.2018.02.036. [DOI] [PubMed] [Google Scholar]
- [10].Lynch I and Dawson KA, Protein-nanoparticle interactions, Nano Today .3, 2008, 40–47, 10.1016/S1748-0132(08)70014-8. [DOI] [Google Scholar]
- [11].Zhang H, Burnum KE, Luna ML, Petritis BO, Kim JS, Qian WJ, Moore RJ, Heredia-Langner A, Webb-Robertson BJM, Thrall BD, Camp DG, Smith RD, Pounds JG and Liu T, Quantitative proteomics analysis of adsorbed plasma proteins classifies nanoparticles with different surface properties and size, Proteomics .11, 2011, 4569–4577, 10.1002/pmic.201100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Docter D, Distler U, Storck W, Kuharev J, Wünsch D, Hahlbrock A, Knauer SK, Tenzer S and Stauber RH, Quantitative profiling of the protein coronas that form around nanoparticles, Nat. Protoc. 9, 2014, 2030–2044, 10.1038/nprot.2014.139. [DOI] [PubMed] [Google Scholar]
- [13].Tenzer S, Docter D, Rosfa S, Wlodarski A, Kuharev J, Rekik A, Knauer SK, Bantz C, Nawroth T, Bier C, Sirirattanapan J, Mann W, Treuel L, Zellner R, Maskos M, Schild H and Stauber RH, Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona:a comprehensive quantitative proteomic analysis, ACS Nano .5, 2011, 7155–7167, 10.1021/nn201950e. [DOI] [PubMed] [Google Scholar]
- [14].Faserl K, Chetwynd AJ, Lynch I, Thorn JA and Lindner HH, Corona isolation method matters: capillary electrophoresis mass spectrometry based comparison of protein corona compositions following on-particle versus in-solution or in-gel digestion, Nanomaterials 9, 2019, 898, 10.3390/nano9060898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stark PB, Before reproducibility must come preproducibility world-view, Nature 557, 2018, 613, 10.1038/d41586-018-05256-0. [DOI] [PubMed] [Google Scholar]
- [16].Faria M, Björnmalm M, Thurecht KJ, Kent SJ, Parton RG, Kavallaris M, Johnston APR, Gooding JJ, Corrie SR, Boyd BJ, Thordarson P, Whittaker AK, Stevens MM, Prestidge CA, Porter CJH, Parak WJ, Davis TP, Crampin EJ and Caruso F, Minimum information reporting in bio–nano experimental literature, Nat. Nanotechnol. 13, 2018, 777–785, 10.1038/s41565-018-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tabb DL, Vega-Montoto L, Rudnick PA, Variyath AM, Ham A-JL, Bunk DM, Kilpatrick LE, Billheimer DD, Blackman RK, Cardasis HL, Carr SA, Clauser KR, Jaffe JD, Kowalski KA, Neubert TA, Regnier FE, Schilling B, Tegeler TJ, Wang M, Wang P, Whiteaker JR, Zimmerman LJ, Fisher SJ, Gibson BW, Kinsinger CR, Mesri M, Rodriguez H, Stein SE, Tempst P, Paulovich AG, Liebler DC and Spiegelman C, Repeatability and reproducibility in proteomic identifications by liquid chromatography−Tandem mass spectrometry, J. Proteome Res. 9, 2010, 761–776, 10.1021/pr9006365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bittremieux W, Tabb DL, Impens F, Staes A, Timmerman E, Martens L and Laukens K, Quality control in mass spectrometry-based proteomics, Mass Spectrom. Rev. 37, 2018, 697–711, 10.1002/mas.21544. [DOI] [PubMed] [Google Scholar]
- [19].Liu G, Zhao Y, Angeles A, Hamuro LL, Arnold ME and Shen JX, A novel and cost effective method of removing excess albumin from plasma/serum samples and its impacts on LC-MS/MS bioanalysis of therapeutic proteins, Anal. Chem. 86, 2014, 8336–8343, 10.1021/ac501837t. [DOI] [PubMed] [Google Scholar]
- [20].Walkey CD and Chan WCW, Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment, Chem. Soc. Rev. 41, 2012, 2780–2799, 10.1039/C1CS15233E. [DOI] [PubMed] [Google Scholar]
- [21].Taylor CF, Field D, Sansone SA, Aerts J, Apweiler R, Ashburner M, Ball CA, Binz PA, Bogue M, Booth T, Brazma A, Brinkman RR, Clark A. Michael , Deutsch EW, Fiehn O, Fostel J, Ghazal P, Gibson F, Gray T, Grimes G, Hancock JM, Hardy NW, Hermjakob H, Julian RK, Kane M, Kettner C, Kinsinger C, Kolker E, Kuiper M, Le Novère N, Leebens-Mack J, Lewis SE, Lord P, Mallon AM, Marthandan N, Masuya H, McNally R, Mehrle A, Morrison N, Orchard S, Quackenbush J, Reecy JM, Robertson DG, Rocca-Serra P, Rodriguez H, Rosenfelder H, Santoyo-Lopez J, Scheuermann RH, Schober D, Smith B, Snape J, Stoeckert CJ, Tipton K, Sterk P, Untergasser A, Vandesompele J and Wiemann S, Promoting coherent minimum reporting guidelines for biological and biomedical investigations: the MIBBI project, Nat. Biotechnol. 26, 2008, 889–896, 10.1038/nbt.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Taylor CF, Paton NW, Lilley KS, Binz PA, Julian RK, Jones AR, Zhu W, Apweiler R, Aebersold R, Deutsch EW, Dunn MJ, Heck AJR, Leitner A, Macht M, Mann M, Martens L, Neubert TA, Patterson SD, Ping P, Seymour SL, Souda P, Tsugita A, Vandekerckhove J, Vondriska TM, Whitelegge JP, Wilkins MR, Xenarios I, Yates JR and Hermjakob H, The minimum information about a proteomics experiment (MIAPE), Nat. Biotechnol. 25, 2007, 887–893, 10.1038/nbt1329. [DOI] [PubMed] [Google Scholar]
- [23].Martínez-Bartolomé S, Deutsch EW, Binz P-A, Jones AR, Eisenacher M, Mayer G, Campos A, Bech-Serra J-J, Carrascal M, Gay M, Paradela A, Navajas R, Marcilla M, Hernáez ML, Gutiérrez-Blázquez MD, Velarde LFC, Aloria K, Beaskoetxea J, Medina-Aunon JA and Albar JP, Guidelines for reporting quantitative mass spectrometry based experiments in proteomics, J. Proteomics 95, 2013, 84–88, 10.1016/J.JPROT.2013.02.026. [DOI] [PubMed] [Google Scholar]
- [24].Binz P-A, Barkovich R, Beavis RC, Creasy D, Horn DM, Julian RK Jr., Seymour SL, Taylor CF and Vandenbrouck Y, Guidelines for reporting the use of mass spectrometry informatics in proteomics, Nat. Biotechnol. 268, 2008. [DOI] [PubMed] [Google Scholar]
- [25].Taylor CF, Binz P-A, Aebersold R, Affolter M, Barkovich R, Deutsch EW, Horn DM, Hühmer A, Kussmann M, Lilley K, Macht M, Mann M, Müller D, Neubert TA, Nickson J, Patterson SD, Raso R, Resing K, Seymour SL, Tsugita A, Xenarios I, Zeng R and Julian RK Jr., Guidelines for reporting the use of mass spectrometry in proteomics, Nat. Biotechnol. 268 (2008), 2008. [DOI] [PubMed] [Google Scholar]
- [26].Murphy CJ, Vartanian AM, Geiger FM, Hamers RJ, Pedersen J, Cui Q, Haynes CL, Carlson EE, Hernandez R, Klaper RD, Orr G and Rosenzweig Z, Biological responses to engineered nanomaterials: needs for the next decade, ACS Cent. Sci. 1, 2015, 117–123, 10.1021/acscentsci.5b00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Findlay MR, Freitas DN, Mobed-Miremadi M and Wheeler KE, Machine learning provides predictive analysis into silver nanoparticle protein corona formation from physicochemical properties, Environ. Sci. Nano 5, 2018, 64–71, 10.1039/c7en00466d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mahmoudi M, Debugging nano-bio interfaces: systematic strategies to accelerate clinical translation of nanotechnologies, Trends Biotechnol. 2018, 10.1016/j.tibtech.2018.02.014. [DOI] [PubMed] [Google Scholar]
- [29].Lin S, Mortimer M, Chen R, Kakinen A, Riviere JE, Davis TP, Ding F and Ke PC, NanoEHS beyond toxicity – focusing on biocorona, Environ. Sci. Nano 4, 2017, 1433–1454, 10.1039/C6EN00579A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Papa E, Doucet JP, Sangion A and Doucet-Panaye A, Investigation of the influence of protein corona composition on gold nanoparticle bioactivity using machine learning approaches, SAR QSAR Environ. Res. 27, 2016, 521–538, 10.1080/1062936X.2016.1197310 [DOI] [PubMed] [Google Scholar]


