Abstract
Multi-drug resistant (MDR) Gram-negative (GN) infections for which there are few available treatment options are increasingly common. The development of new antibiotics for these pathogens is challenging due to the inability of most small molecules to accumulate inside GN bacteria. Using recently developed predictive guidelines for compound accumulation in Escherichia coli we have converted the antibiotic Ribocil C, which targets the FMN riboswitch, from a compound lacking whole-cell activity against wild-type GN pathogens into a compound that accumulates to a high level in E. coli, is effective against Gram-negative clinical isolates, and has efficacy in mouse models of GN infection. This compound allows for the first assessment of the translational potential of FMN riboswitch binders against wild-type Gram-negative bacteria.
Keywords: antibacterial, compound accumulation
Graphical Abstract
INTRODUCTION
Deaths from drug-resistant bacterial infections in the European Union rose three-fold from 2007 to 2015, with over 27,000 deaths occurring in 2015.1 Notably, six specific drug-resistant bacterial pathogens accounted for this rise, four of which are Gram-negative (GN), with cephalosporin-resistant E. coli contributing the greatest to both incidence and deaths (285,758 infections and 8,750 deaths in 2015). Therefore, new therapeutics are needed, especially those that engage untapped cellular targets.2–11 Although there are multiple novel antibacterial targets and many promising small-molecule antibiotic leads for Gram-positive (GP) infections, whole-cell activity in GN pathogens has proven difficult to achieve.12 This inactivity is due to the impermeability of GN bacteria to most drug-like molecules, conferred through an outer membrane of densely packed lipopolysaccharides (LPS) and promiscuous efflux pumps.13–16
A creative cell-based screen conducted at Merck & Co identified Ribocil C (Figure 1A) as a synthetic compound with potent activity against GP bacteria (S. aureus) and against E. coli strains with permeability defects.17 Ribocil C targets the flavin mononucleotide (FMN) riboswitch, ultimately inducing bacterial death through riboflavin starvation. Importantly, there are no human homologues of the FMN riboswitch and indeed no riboswitches are known in mammalian systems,18–21 suggesting the potential for bacterial cell selectivity for compounds that engage this target. While Ribocil C kills S. aureus in culture, GP organisms can scavenge riboflavin from their environment22 thus tempering enthusiasm for Ribocil C specifically, and the FMN riboswitch target in general, for S. aureus. In contrast, E. coli strains with deletions within genes in the riboflavin biosynthesis pathway appear unable to scavenge exogenous riboflavin during mouse models of infection,17 and Ribocil C has efficacy in mouse models of infection using permeability-deficient strains of E. coli.17 Interestingly and contrastingly, exogenous riboflavin does squelch the antibacterial activity of Ribocil C in permeability defect E. coli grown in culture.17,22 The only means to truly assess the suitability of the FMN riboswitch as a target for GN pathogens is to identify appropriate compounds and evaluate them in vivo; unfortunately Ribocil C and its derivatives have no notable activity against wild-type GN bacteria.17, 22
Figure 1.
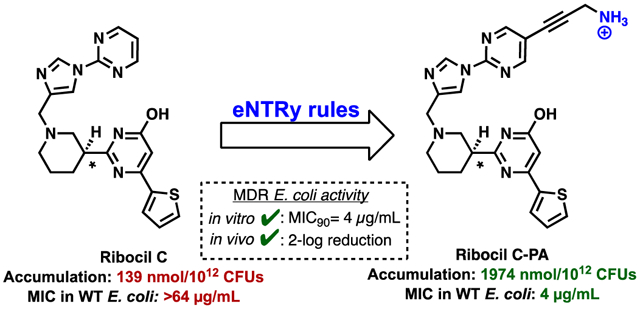
Ribocil C as a starting point to access a GN-active antimicrobial that engages the essential FMN riboswitch. A) Accumulation in E. coli MG1655 and Minimum Inhibitory Concentration (MIC) values against strains. MICs were performed in M9-MOPS media per CLSI guidelines. B) Location prioritized for amine installation, based on the co-crystal of Ribocil B bound to the FMN riboswitch aptamer of F. nucleatum (PDB 5C45). C) Target compounds designed to have enhanced accumulation in GN bacteria.
We suspected that the inactivity of Ribocil C against wild-type GN pathogens was due to low intracellular accumulation, as is the case for many antibiotics that are only active against GP organisms. Thus, if strategic modifications to Ribocil C could be made to facilitate enhanced bacterial accumulation without disrupting target engagement, this approach could provide the first wild-type GN-active antibiotic that targets the FMN riboswitch. Here we detail the application of recently described permeation rules to arrive at Ribocil C variants that accumulate in E. coli. The lead compound identified through this strategy has activity against multiple wild-type GN organisms, including dozens of multi-drug resistant (MDR) GN clinical isolates, and reduces bacterial burden and extends survival in mice infected with antibiotic-resistant E. coli.
RESULTS
Design of GN-active versions of Ribocil C.
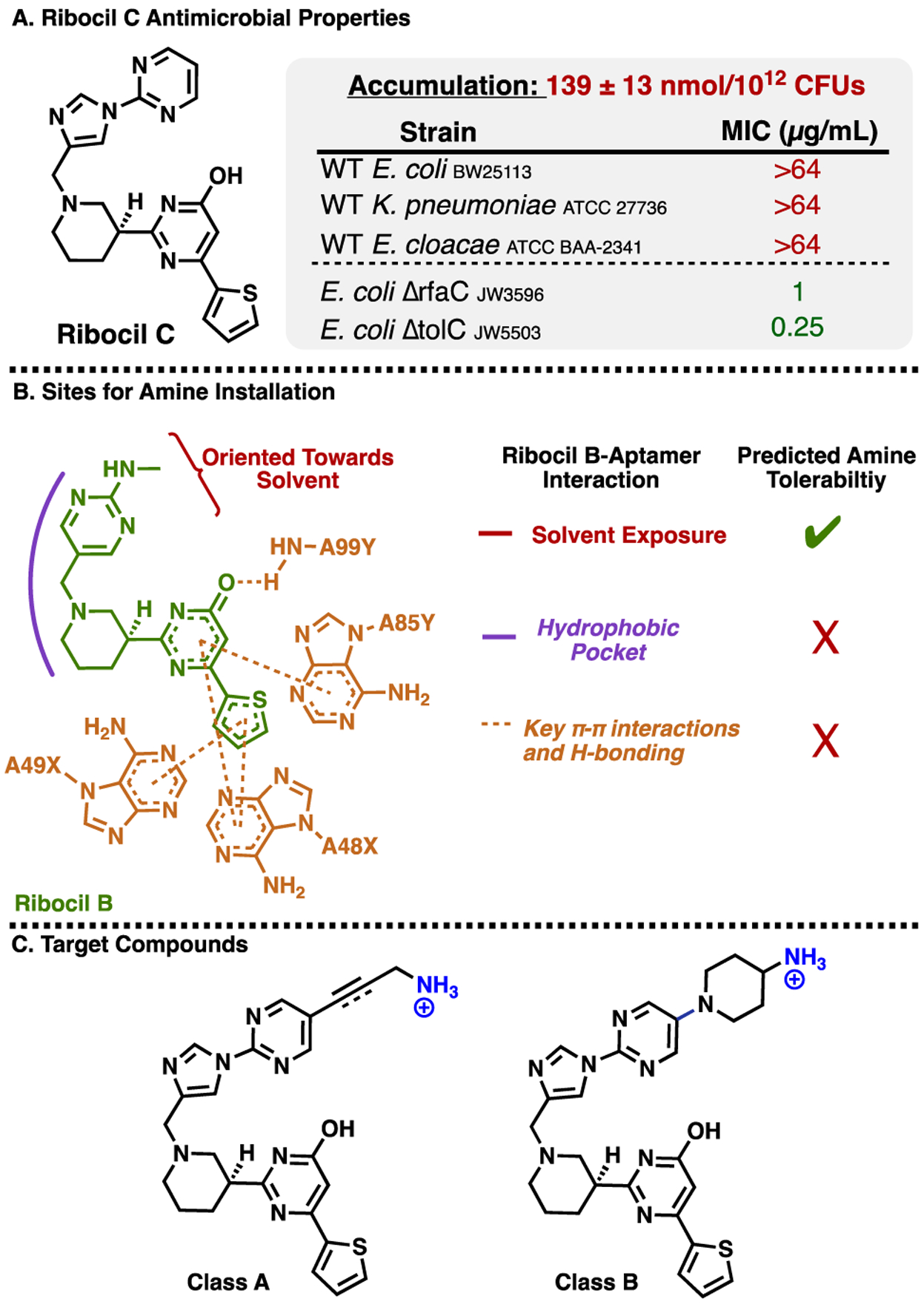
We recently reported the elucidation of physicochemical traits that bias compounds for accumulation in E. coli.23 These “eNTRy rules”24 detail that a high accumulating molecule should possess an ionizable nitrogen (with a primary amine being most favorable), low three-dimensionality, and a low number of rotatable bonds. Using a recently developed web-application for calculating eNTRy parameters (www.entry-way.org)25 it was observed that Ribocil C meets two of the three eNTRy criteria, with low three-dimensionality (globularity=0.058) and five rotatable bonds, but lacking a primary amine. The synthesis of Ribocil C (See Supporting Information) and subsequent evaluation (Figure 1A) revealed that indeed this compound accumulates very poorly in E. coli (Figure 1A, accumulation=139 nmol/1012 CFUs, accumulation relative to positive and negative controls shown in Supporting Figure 1) and demonstrates no detectable activity at its solubility limit in standard microbial susceptibility assays against reference strains of wild-type GN bacteria (Figure 1A). However, Ribocil C has marked activity against E. coli strains possessing either a truncated LPS (E. coli ΔrfaC) or efflux pump deficiency (E. coli ΔtolC) (Figure 1A), suggesting that if the compound were tailored to accumulate in wild-type E. coli, it would be a potent antibiotic.
To convert Ribocil C into a GN-active antibiotic, the eNTRy rules were used to guide the design of a small compound collection. Ribocil C already meets two eNTRy rules criteria (globularity and rotatable bonds), therefore the installation of an unhindered primary amine was the chief objective. For placement of the amine, the x-ray crystal structure17 of a similar compound, Ribocil B, in complex with the FMN riboswitch aptamer of Fusobacterium nucleatum was analyzed and priority was given to solvent exposed sites (red in Figure 1B), such as in proximity to the northern pyrimidine. As such, two general classes of Ribocil C-amine variants were envisioned, possessing amines at this priority location (Figure 1C). Class A variants would be designed with amine tethers containing varying levels of saturation, so as to assess the effects of geometric/rotational freedom on target engagement, whole cell accumulation, and GN antimicrobial activity. Class B analogues were also considered as these possess low rotatable bonds, but slightly larger globularity scores.
Synthesis and antimicrobial evaluation of Ribocil C variants predicted to accumulate in GN pathogens.
The construction and preliminary assessment of target compounds proceeded through the process depicted in Figure 2A. Specific compounds with varying carbon and carbon-heteroatom linkers were synthesized, with the objective of positioning an amine in a non-intrusive region of the FMN riboswitch aptamer. To construct the target molecules, a two fragment cou- pling was adopted, harnessing a late stage reductive amination between aldehydes (represented by 1) and cyclic amine 2. Using this focused workflow, next-generation Ribocil C derivatives were rapidly constructed and then evaluated for antimicrobial activity (MIC assessments in wild-type E. coli and outer-membrane compromised E. coli), with any active compounds being subjected to enantiomer resolution and further biological evaluation (Figure 2A); multiple novel Ribocil C derivatives with amine extensions were constructed and assessed for antimicrobial activity (the full list is shown in Supporting Figure 2). Using this protocol, enantiopure compounds (-)3, (-)4, and (-)5 emerged as next-generation leads. All three of these compounds were found to have notably elevated accumulation in E. coli relative to Ribocil C while maintaining antibacterial activity against membrane compromised GN strains (Figure 2B). Additionally, compounds (-)3, (-)4, and (-)5 also have marked antibacterial activity in Enterobacteriaceae strains with intact outer membranes and functional efflux pumps, including strains of E. coli, E. cloacae, and Klebsiella pneumoniae (Figure 2B). These compounds were inactive against the other GN ESKAPE26 pathogens, Acinetobacter baumannii and Pseudomonas aeruginosa, unsurprising based on the low homology of the FMN riboswitch target of these organisms with that of Enterobacteriaceae, although these compounds do accumulate to some extent in A. baumannii and P. aeruginosa (Supp Table 1). As compound (-)3 was the most active antibiotic (Figure 2B) and also displayed little toxicity against normal human cells in culture (Supp Table 2), it was prioritized and named as Ribocil C-PA; (+)3 is devoid of antibiotic activity (Supporting Figure 2).
Figure 2.

eNTRy rule guided design of amine analogues of Ribocil C that accumulate within and kill GN pathogens. A) Workflow utilized to develop new Ribocil C variants that possess activity against wild-type GN pathogens. B) Lead compounds identified with the ability to accumulate in wild-type E. coli and inhibit growth. Antimicrobial assessment of lead derivatives against various GN pathogens. MC E. coli: Membrane Compromised E. coli. Accumulation in E. coli MG1655, units are nmol/1012 CFUs. MICs were performed in M9-MOPS media per CLSI guidelines.
Systematic construction of Ribocil variants to probe influence of functional groups on activity.
With antibiotics active against wild-type GN bacteria in hand, the next objective was to construct compounds that could illuminate the influence of various functional groups on antibiotic activity and whole-cell accumulation. These synthetic efforts were focused on modifications to derivative 3. Towards this end and as shown in Figure 3, Boc-protected 6 was converted to free amine 3, which was then transformed to dimethyl amine (compound 7) and acetamide (compound 8) variants. Late stage reduction of 6, followed by protecting group removal, provided saturated analogue 9. Finally, amine analogues with alternative linker lengths were accessed via reductive amination between 10a/10b/10c and 2 to provide, after deprotection, amines 11a-c (Figure 3B).
Figure 3.
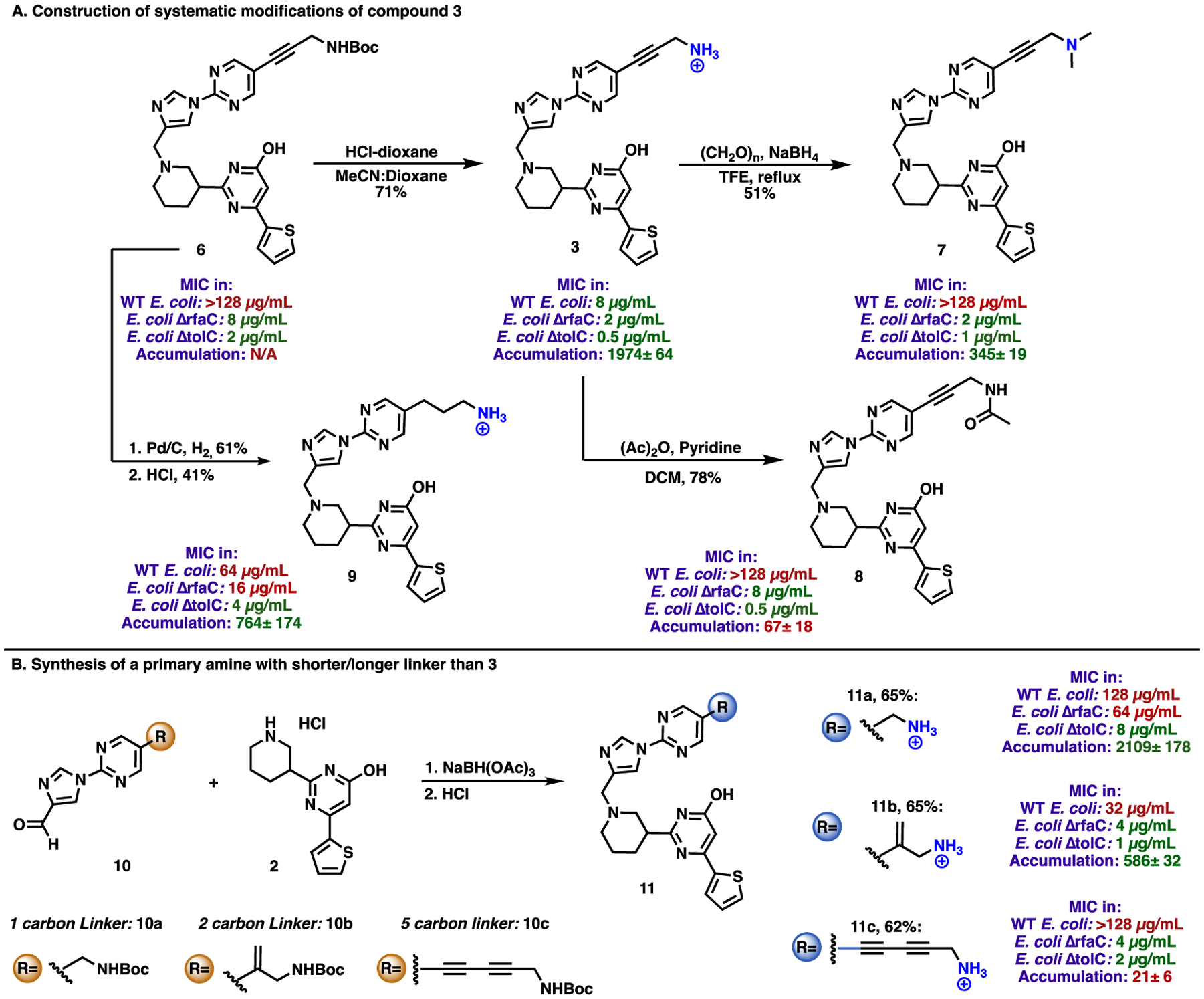
Synthetic modifications of compound 3 to probe the effect of functional groups on antimicrobial activity. Accumulation was performed in E. coli MG1655 and is represented in nmol/1012 CFUs. The accumulation of compound 6 could not be determined due to limitations of aqueous solubility. The MIC values for all compounds are reported for the racemates. The accumulation values for all compounds are reported for the racemates, with the exception of compound 3, which is reported for (-)3. MIC values were determined for E. coli BW25113 (WT E. coli), E. coli JW3596 (E. coli ΔrfaC), and E. coli JW5503 (E. coli ΔtolC). MICs were performed in M9-MOPS media per CLSI guidelines.
Antibacterial activity of novel Ribocil variants.
Compounds were assessed for their ability to accumulate in E. coli, in addition to their antibacterial activity against both wild-type E. coli and permeabilized strains. The activity of compounds against these latter strains provides some indication of their ability to engage the FMN riboswitch target, without the complication of cell permeability. As shown by the data in Figure 3, compound 3 had the best antibacterial activity against wild-type E. coli, with all modifications (6–9, acylation, dimethylation, or saturation of the linker) resulting in inactive variants against wild-type E. coli; however, all four of these compounds retained some activity against the membrane-compromised strains, suggesting a retention of some degree of target engagement. Linker length appeared important as compounds with shorter (e.g. 11a) or longer (e.g. 11c) linkers lost either target engagement or accumulation.
Assessing the activity of Ribocil C-PA against a large panel of MDR clinical isolates of E. coli and K. pneumoniae.
Extensive panels of MDR clinical isolates of E. coli (n=42) and K. pneumoniae (n=54) were assessed for their susceptibility to (-)3, also called Ribocil C-PA. Within these collections of clinical isolates there are strains with resistance to a wide variety of antibiotics, including carbapenems, tetracyclines, aminoglycosides, sulfonamides, trimethoprim, and colistin (see Supporting Table 3 for full list of resistance profiles). Ribocil C-PA markedly out performs Ribocil C in these susceptibility studies (Figure 4).
Figure 4.

Assessment of Ribocil C-PA and Ribocil C against MDR clinical isolates of A) E. coli (n=42) and B) K. pneumoniae (n=54). MICs were performed in M9-MOPS media per CLSI guidelines. See Supporting Table 3 for list of resistance genes in clinical isolate panels. A full listing of this MIC data is in Supporting Tables 4–5.
Mode-of-action and resistance frequency of Ribocil C-PA.
To assess the mode-of-action of Ribocil C-PA, two key experiments were conducted. The first was to evaluate E. coli production of flavins in the presence of Ribocil C-PA. As detailed in previous reports, non-native FMN riboswitch ligands inhibit the downstream transcription and translation of key enzymes involved in riboflavin biosynthesis.17 Riboflavin serves as a precursor to FMN and flavin adenosine dinucleotide (FAD), therefore depletion of these intracellular flavins would be expected for an antibiotic that acts through targeting this riboswitch. Using an LC-MS/MS assay to monitor flavin levels in E. coli cultures treated with Ribocil C-PA (at 0.5x the MIC), reduction in flavins was observed (Figure 5), indicating that Ribocil C-PA selectively and competitively engages the FMN riboswitch. A control experiment showed the inactive enantiomer, compound (+)3, had no effect on flavin production in E. coli (Figure 5).
Figure 5.

Flavin reduction in E. coli BW25113 at sub-inhibitory concentration (2 μg/mL) of Ribocil C-PA. The inactive enantiomer, (+)3, was also assessed at 2 μg/mL and demonstrates no inhibition of flavin production. Flavin monitoring was performed after cultures were treated with either vehicle or compound for 20 hours. Data are shown as means ± s.d. and statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons. NS, not significant (P> 0.05). *P< 0.015).
As a second experiment, spontaneous E. coli mutants resistant to Ribocil C-PA were generated, and the FMN riboswitch was sequenced; this experiment was conducted in E. coli BW25113, K. pneumoniae ATCC 27736, and E. coli ΔtolC (JW5503). Significant target-based resistance was observed in all of these strains (Supporting Figure 3, at 8X MIC E. coli FOR= 2.8 ×10−6; at 8X MIC K. pneumoniae FOR= 2.4 ×10−6). Ribocil C and Ribocil C-PA yield similar frequencies of resistance against E. coli ΔtolC (Supporting Figure 4, at 8X MIC Ribocil C FOR=3.2 ×10−6; Ribocil C-PA FOR=2.1 × 10−6). After generating and sequencing 20 different E. coli BW25113 clones resistant to Ribocil C-PA, 16 different mutations were observed, all of which mapped back to either the FMN riboswitch aptamer or expression platform (Figure 6). Ribocil C-PA resistant bacteria were assessed for changes in fitness by comparing their growth rates to those of wild-type E. coli. As shown in Supporting Figure 5, growth rates for resistant mutants and wild-type E. coli were nearly identical, suggesting limited changes in cell culture fitness and viability between these distinct genotypes.
Figure 6:

Mutations observed after sequencing the FMN riboswitch in Ribocil C-PA resistant clones of E. coli BW25113 generated at 8X the MIC (32 μg/mL) of Ribocil C-PA.
Efficacy in mouse models of GN infection.
Both Ribocil C and Ribocil C-PA were tolerated when administered to mice at 100 mg/kg (IP), twice-a-day for three days, and this dosing regimen was thus used in the infection models. Ribocil C-PA was evaluated head-to-head versus Ribocil C in two E. coli infection models, an acute pneumonia infection (bacterial burden model) and septicemia (bacterial survival model). In these experiments, a nearly 2-log reduction in bacterial burden was achieved for Ribocil C-PA in mice infected with two different pathogenic strains of E. coli (Figure 7A), while 80% (12/15) of mice burdened by septic infection were successfully rescued (Figure 7B); Ribocil C showed no statistically significant reductions in bacterial burden in vivo against either E. coli strain tested, and was ineffective at rescuing mice in a model of E. coli septicemia (Figure 7).
Figure 7.

A) Bacterial burden model of acute pneumonia in E. coli. Acute pneumonia infections initiated in seven-week-old male CD-1 mice with E. coli AR0493 (1.7×109 CFU mouse−1 intranasally) or E. coli ELZ4081 (3.1×109 CFU mouse−1 intranasally). Mice were treated with vehicle (n=8) or compound (Ribocil C or Ribocil C-PA, 100 mg/kg IP, n=8 for each group) 8, 16, 30 and 48h post-infection, and the bacterial burden was evaluated 48h post-infection. MIC=8 μg/mL and 4 μg/mL for Ribocil C-PA against E. coli AR0493 and E. coli ELZ4081 respectively. B) Kaplan-Meier survival curve for the mouse efficacy model of E. coli sepsis. Seven-week-old male CD-1 mice were infected with E. coli AR0493 (7.91×107 CFU mouse−1; 15 mice per group) via intravenous injection. Mice were treated with compound (Ribocil C or Ribocil C-PA, 100 mg/kg IP, n=15 for each group) twice daily post-infection for 5 days. In A, data are shown as means ± s.d. and statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons. NS, not significant (P> 0.05), ****P< 0.0001. In B, statistical significance was determined by two-tailed log-rank (Mantel–Cox) test, ***P<0.001.
DISCUSSION
The bacterial-specific and essential nature of the FMN riboswitch suggests it as a promising antibiotic target. However, GP bacteria are able to scavenge riboflavin from the environment, likely making compounds that engage this target unsuitable for GP infections. With conflicting evidence about its suitability for GN infections,17,22 we set out to answer this question through the creation and assessment of an antibiotic active versus wild-type GN organisms through inhibition of the FMN riboswitch. The discovery herein of Ribocil C-PA, active in cell culture and in vivo against wild-type GN bacteria through inhibition of the FMN riboswitch, demonstrates that indeed this riboswitch could be an outstanding antibacterial target for wild-type GN pathogens. In addition, Ribocil C-PA exhibits 16-fold more potent activity against efflux deficient E. coli compared to wild-type organisms (Figure 2B), suggesting a next-generation derivative that evades efflux could have even better activity against wild-type GN pathogens.
An intriguing opportunity afforded when targeting this bacterial riboswitch is the possibility for narrow-spectrum (pathogen specific) antibiotics. Unlike many macromolecular targets, the FMN riboswitch does not have high conservation across GN organisms, with marked sequence differences between the Enterobacteriaceae, A. baumannii, and P. aeruginosa versions of this target (sequence identity of aptamer relative to E. coli): K. pneumoniae (90%); E. cloacae (87%); P. aeruginosa (72%); A. baumannii (61%)). Ribocil C-PA demonstrates activity against constituents of the Enterobacteriaceae, including in large panels of clinical isolates, but not A. baumannii and P. aeruginosa. Given the sequence divergence of the FMN riboswitch, complementary compounds that have specificity for A. baumannii or P. aeruginosa can be envisioned. Of course as infections from Enterobacteriaceae are of particular concern – drug-resistant E.coli1 but also carbapenem-resistant K. pneumoniae, which has a 30–50% mortality rate27–28 – the GN-active Ribocil C analogues described herein hold considerable promise.
With its successful conversion into a GN active antibacterial, Ribocil C joins a growing list of compounds where GN activity and/or high accumulation has been imbued via derivative synthesis guided by the eNTRy rules.23, 25, 29–31 In this context it is interesting to note that compounds (-)3, (-)4, and (-)5, all of which accumulate in E. coli, exist just outside the five rotatable bonds recommended by the eNTRy rules. As shown in Supporting Figure 6, energy minimization of these structures provides evidence of a preferable conformation that may reduce rotational freedom in this compound class. Analysis of activity data of derivatives (Figure 3) once again suggests that primary amines are most proficient at facilitating compound accumulation when compared to other ionizable nitrogens (such as the dimethyl amine), consistent with previous observations from accumulation of non-antibiotics.23 And, the comparison of amine 3 with amide 8 (Figure 3) starkly reveals the influence of the primary amine on accumulation; both these compounds are potent against membrane-compromised E. coli, but only primary amine 3 accumulates in and has antibiotic activity against wild-type GN strains. As reinforced with this conversion of Ribocil C to Ribocil C-PA, the existence of structural data (ideally an x-ray structure with the parent antibiotic bound to its target) and/or significant structure-activity relationship data facilitates the design of new compounds that meet the eNTRy rule criteria and maintain target engagement.
The development of Ribocil C-PA indicates that the inhibition of the FMN riboswitch in the Enterobacteriaceae (and likely in other GN bacteria) is an effective antibacterial strategy. While the elevated frequency of resistance that is apparently associated with this target is not ideal, this issue has been surmounted for other antibiotics, for example through a large front-loading dose (e.g. fusidic acid32) or through combination regimens such as commonly employed for rifampicin, trimethoprim, and sulfamethoxazole6 and as is common when treating Mycobacterium tuberculosis.11 The validation of novel antibiotic targets will be critical in the continued battle against drug-resistant bacteria.
Supplementary Material
ACKNOWLEDGMENT
We thank L. Li (Metabolomics Center, Roy J. Carver Biotechnology Center, UIUC) for LC-MS/MS analysis. We thank Gyumin Lee for assistance with the mouse efficacy studies.
Funding Sources
We are grateful to the NIH (AI136773) and the University of Illinois for supporting this work. S.E.M was partially supported by F32AI143207 and R.J.U. is an NSF predoctoral fellow.
ABBREVIATIONS
- CLSI
Clinical and Laboratory Standards Institute
- FAD
flavin adenosine dinucleotide
- FMN
flavin mononucleotide
- FOR
Frequency of Resistance
- GN
Gram-negative
- GP
Gram-positive
- LPS
lipopolysaccharides
- MDR
Multi-drug resistant
- MIC
Minimum Inhibitory Concentration
- MTD
Maximal tolerated dose
Footnotes
Supporting Information
Supporting Figures and Tables, materials and methods, figures detailing syntheses of compounds, experimental procedures and characterization of chemical products including spectra. This material is available free of charge via the internet at http://pubs.acs.org.
REFERENCES
- 1.Gasser M; Zingg W; Cassini A; Kronenberg A; Swiss Centre for Antibiotic, R., Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in Switzerland. Lancet Infect. Dis 2019, 19 (1), 17–18. [DOI] [PubMed] [Google Scholar]
- 2.Coates ARM; Hu Y, Novel approaches to developing new antibiotics for bacterial infections. Br J Pharmacol 2007, 152 (8), 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livermore DM, The need for new antibiotics. Clin. Microbiol. Infect 2004, 10 Suppl 4, 1–9. [DOI] [PubMed] [Google Scholar]
- 4.Monserrat-Martinez A; Gambin Y; Sierecki E, Thinking Outside the Bug: Molecular Targets and Strategies to Overcome Antibiotic Resistance. Int. J. Mol. Sci 2019, 20 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry C; Hall C, Antibiotic resistance: how it arises, the current position and strategies for the future. Nurs. Times 2009, 105 (36), 20–3. [PubMed] [Google Scholar]
- 6.Silver LL, Appropriate Targets for Antibacterial Drugs. Cold Spring Harb. Perspect. Med 2016, 6 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutterlin HA; Malinverni JC; Lee SH; Balibar CJ; Roemer T, Antibacterial New Target Discovery: Sentinel Examples, Strategies, and Surveying Success In Antibacterials: Vol. I, Fisher JF; Mobashery S; Miller MJ, Eds. Springer International Publishing: Cham, 2018; pp 1–29. [Google Scholar]
- 8.Tommasi R; Iyer R; Miller AA, Antibacterial Drug Discovery: Some Assembly Required. ACS Infect. Dis 2018, 4 (5), 686–695. [DOI] [PubMed] [Google Scholar]
- 9.Zinner SH, The search for new antimicrobials: why we need new options. Expert Rev. Anti. Infect. Ther 2005, 3 (6), 907–13. [DOI] [PubMed] [Google Scholar]
- 10.Lewis K, Platforms for antibiotic discovery. Nat. Rev. Drug Discov 2013, 12 (5), 371–387. [DOI] [PubMed] [Google Scholar]
- 11.Lewis K, The Science of Antibiotic Discovery. Cell. 2020, 181 (1), 29–45. [DOI] [PubMed] [Google Scholar]
- 12.Tommasi R; Brown DG; Walkup GK; Manchester JI; Miller AA, ESKAPEing the labyrinth of antibacterial discovery. Nat Rev Drug Discov 2015, 14 (8), 529–42. [DOI] [PubMed] [Google Scholar]
- 13.Amaral L; Martins A; Spengler G; Molnar J, Efflux pumps of Gram-negative bacteria: what they do, how they do it, with what and how to deal with them. Front. Pharmacol 2014, 4 (168). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X-Z; Plésiat P; Nikaido H, The Challenge of Efflux-Mediated Antibiotic Resistance in Gram-Negative Bacteria. Clin. Microbiol. Rev 2015, 28 (2), 337–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masi M; Refregiers M; Pos KM; Pages JM, Mechanisms of envelope permeability and antibiotic influx and efflux in Gram-negative bacteria. Nat. Microbiol 2017, 2, 17001. [DOI] [PubMed] [Google Scholar]
- 16.Zgurskaya HI; Löpez CA; Gnanakaran S, Permeability Barrier of Gram-Negative Cell Envelopes and Approaches To Bypass It. ACS Infect. Dis 2015, 1 (11), 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howe JA; Wang H; Fischmann TO; Balibar CJ; Xiao L; Galgoci AM; Malinverni JC; Mayhood T; Villafania A; Nahvi A; Murgolo N; Barbieri CM; Mann PA; Carr D; Xia E; Zuck P; Riley D; Painter RE; Walker SS; Sherborne B; de Jesus R; Pan W; Plotkin MA; Wu J; Rindgen D; Cummings J; Garlisi CG; Zhang R; Sheth PR; Gill CJ; Tang H; Roemer T, Selective small-molecule inhibition of an RNA structural element. Nature 2015, 526 (7575), 672–677. [DOI] [PubMed] [Google Scholar]
- 18.Breaker RR, Riboswitches and Translation Control. Cold Spring Harb. Perspect. Biol 2018, 10 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breaker RR, Riboswitches and the RNA world. Cold Spring Harb. Perspect. Biol 2012, 4 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breaker RR, Prospects for riboswitch discovery and analysis. Mol. Cell 2011, 43 (6), 867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deigan KE; Ferre-D’Amare AR, Riboswitches: discovery of drugs that target bacterial gene-regulatory RNAs. Acc. Chem. Res 2011, 44 (12), 1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H; Mann PA; Xiao L; Gill C; Galgoci AM; Howe JA; Villafania A; Barbieri CM; Malinverni JC; Sher X; Mayhood T; McCurry MD; Murgolo N; Flattery A; Mack M; Roemer T, Dual-Targeting Small-Molecule Inhibitors of the Staphylococcus aureus FMN Riboswitch Disrupt Riboflavin Homeostasis in an Infectious Setting. Cell Chem. Biol 2017, 24 (5), 576–588.e6. [DOI] [PubMed] [Google Scholar]
- 23.Richter MF; Drown BS; Riley AP; Garcia A; Shirai T; Svec RL; Hergenrother PJ, Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 2017, 545 (7654), 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter MF; Hergenrother PJ, The challenge of converting Gram-positive-only compounds into broad-spectrum antibiotics. Ann N Y Acad Sci 2019, 1435 (1), 18–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker EN; Drown BS; Geddes EJ; Lee HY; Ismail N; Lau GW; Hergenrother PJ, Implementation of permeation rules leads to a FabI inhibitor with activity against Gram-negative pathogens. Nat. Microbiol 2020, 5 (1), 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice LB, Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis 2008, 197 (8), 1079–81. [DOI] [PubMed] [Google Scholar]
- 27.Ramos-Castaneda JA; Ruano-Ravina A; Barbosa-Lorenzo R; Paillier-Gonzalez JE; Saldana-Campos JC; Salinas DF; Lemos-Luengas EV, Mortality due to KPC carbapenemase-producing Klebsiella pneumoniae infections: Systematic review and meta-analysis: Mortality due to KPC Klebsiella pneumoniae infections. J. Infect 2018, 76 (5), 438–448. [DOI] [PubMed] [Google Scholar]
- 28.Xu L; Sun X; Ma X, Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann. Clin. Microbiol. Antimicrob 2017, 16 (1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masci D; Hind C; Islam MK; Toscani A; Clifford M; Coluccia A; Conforti I; Touitou M; Memdouh S; Wei X; La Regina G; Silvestri R; Sutton JM; Castagnolo D, Switching on the activity of 1,5-diaryl-pyrrole derivatives against drug-resistant ESKAPE bacteria: Structure-activity relationships and mode of action studies. Eur. J. Med. Chem 2019, 178, 500–514. [DOI] [PubMed] [Google Scholar]
- 30.Lukežič T; Fayad AA; Bader C; Harmrolfs K; Bartuli J; Groß S; Lešnik U; Hennessen F; Herrmann J; Pikl Š; Petković H; Müller R, Engineering Atypical Tetracycline Formation in Amycolatopsis sulphurea for the Production of Modified Chelocardin Antibiotics. ACS Chem. Biol 2019, 14 (3), 468–477. [DOI] [PubMed] [Google Scholar]
- 31.Li Y; Gardner JJ; Fortney KR; Leus IV; Bonifay V; Zgurskaya HI; Pletnev AA; Zhang S; Zhang ZY; Gribble GW; Spinola SM; Duerfeldt AS, First-generation structure-activity relationship studies of 2,3,4,9-tetrahydro-1H-carbazol-1-amines as CpxA phosphatase inhibitors. Bioorg. Med. Chem. Lett 2019, 29 (14), 1836–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandes P, Fusidic Acid: A Bacterial Elongation Factor Inhibitor for the Oral Treatment of Acute and Chronic Staphylococcal Infections. Cold Spring Harb. Perspect. Med 2016, 6 (1), a025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


