Abstract
Introduction:
Dalbavancin is a new antibiotic against multi-drug resistant Gram (+) bacteria. Dalbavancin has an extremely long half-life. Current indication is skin and soft tissue infections (ABSSSI), but researchers have successfully administered it off-label to osteomyelitis (OM) patients.
Aim:
We present a case of successful treatment of diabetic foot (DF) OM.
Case report:
A 53-year-old male presented to our DF clinic, with recently diagnosed diabetes mellitus, with very bad glycaemic control (HbA1c=12,5%). He had diabetic neuropathy, but no peripheral arteriopathy. Two months before, because of an accident with hot water, he presented left foot ulcer, followed by ABSSSI and 1st toe and 1st metatarsal OM (plain x-ray findings). A multi-drug resistant Enterococcus faecium was isolated in cultures and a targeted treatment with tigecycline and daptomycin was administered. The patient also received 1,5 gr dalbavancin upon discharge. 2 weeks later, he continued treatment at home with linezolid and tedizolid. A complete medical record with patient’s history, informed consent and relative literature was sent to Greek National Health Care Organization (EOPYY), requesting administering off-label another 1,5 gr dalbavancin. In the meanwhile, he was admitted for iv tigecyclin, and continued treatment with linezolid at home. He finally received a second dose of 1,5 g dalbavancin. Patient received totally 14 weeks’ targeted therapy, mostly off-hospital. When he completed treatment, foot was in excellent condition and x-ray had significantly improved.
Conclusion:
Dalbavancin, due to its extremely long half-life, could potentially be the drug of choice for OM caused by multi-drug resistant Gram (+) cocci, in order to avoid hospitalization, especially on non-complient patients. Further research is necessary.
Keywords: Dalbavancin, diabetic foot, osteomyelitis, antibacterial agents, antibiotics, lipoglycopeptides, acute bacterial skin and skin structure infections (ABSSSI)
1. INTRODUCTION
Dalbavancin is a new generation antibiotic, and in particular a new semi-synthetic lipoglycopeptide which can act against multi-drug resistant Gram positive bacteria, such as penicillin resistant streptococci. Its main advantage, in comparison to vancomycin and teicoplanin, is its greater effectiveness against all types of staphylococci, including coagulase negative species (1). The older antibacterial glycopeptides vancomycin and teicoplanin were introduced to the market in the 1950’s and 1960’s, respectively (2). Compared to them, dalbavancin is more effective due to its pharmacokinetics, characterized by a long elimination t1/2 in humans, which makes a once-weekly dosing regimen possible. Clinical studies have shown that treatment with dalbavancin once a week can be effective against infections caused by multiresistant Gram positive bacteria (1). Acute bacterial skin and soft tissue structure infections (ABSSSI) patients can be treated in-hospital or as outpatients (3). Gram positive bacteria are the second cause of health care-associated infections. The latter are usually caused by Staphylococcus aureus, including its methicillin resistant strains (MRSA), Enterococcus spp. and coagulase-negative staphylococci (4). Infections of the skin and its structures are most frequently caused by Gram positive bacteria, the most common of which is Staphylococcus aureus. Dalbavancin has been tested against staphylococci, enterococci, and streptococci in 33 countries around the world, with excellent results (5). A well-conducted protocol study, which will focus on the treatment of multi-drug resistant Gram (+) bacteria, in a wide sample of medical centers in different countries, could provide us with a better understanding of the role of this potent, long-lasting, antimicrobial agent (6).
2. AIM
Dalbavancin is a new antibiotic against the multi-drug resistant Gram (+) bacteria. Dalbavancin has an extremely long half-life. Its current indication is skin and soft tissue infections (ABSSSI), but researchers have successfully administered it off-label to osteomyelitis (OM) patients. We present a case of diabetic foot (DF) OM successfully treated with Dalbavancin.
3. CASE REPORT
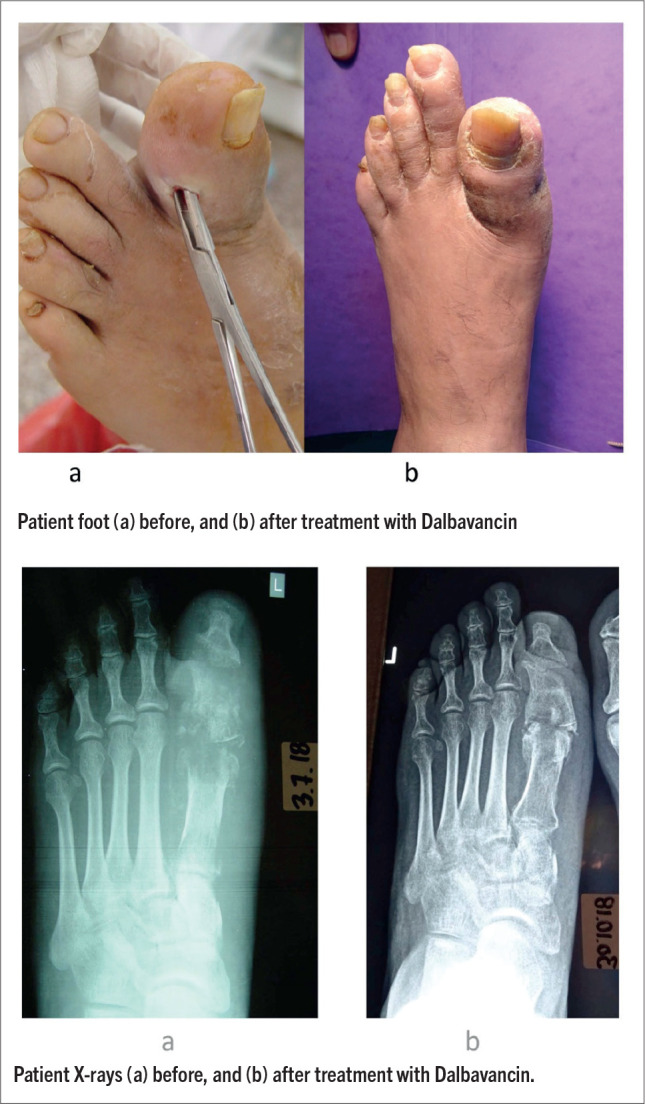
A 53-year-old male photographer was referred to our diabetic foot clinic from another hospital. The patient presented with type 2 Diabetes mellitus (DM), diagnosed one month ago. He was non-complient to diabetes treatment (he was on insulin and GLP-1 analogue), and had a very poor glycaemic control (HbA1c=12,5%). He had diabetic neuropathy (abnormal 10g monofilament, biothesiometer and Neuropad® test), but no peripheral arteriopathy (Doppler ultrasonography). Two months earlier, without knowing that he had diabetes, the patient presented left foot ulcer after an accident with hot water, followed by soft tissue infection (acute bacterial skin and skin structure infection, ABSSSI), and 1st toe and 1st metatarsal osteomyelitis (OM), with plain x-ray findings (Figure 1a, 2a). He was admitted to hospital, and DM diagnosis was made upon that time. A multi-drug resistant Enterococcus faecium was isolated in repeated cultures. Inflammation markers (WBC, ESR, c-reactive protein) were minimally affected. In-hospital targeted treatment with tigecycline (50mgx2, loading dose 100mg) and daptomycin (500mgx1) was administered iv for 10 days. The patient also received 1,5 gr dalbavancin upon discharge. After a 2-weeks’ interval, he continued treatment at home with linezolid (600mgx2, 2 weeks), followed by tedizolid (200mgx1, one week), per os. At the same time, a complete medical record with patient’s history, informed consent and relative literature was sent to Greek National Health Care Organization (EOPYY), requesting administering off-label another 1,5 gr dalbavancin. In the meanwhile, he was re-admitted to hospital for iv tigecycline (same dose, 7 days), and continued treatment afterwards with per os linezolid (same dose) at home for another two weeks. He finally received a second dose of 1,5 g dalbavancin. In total, patient received 14 weeks’ targeted antibiotic therapy, mostly off-hospital. By the end of treatment, foot was in excellent condition and there was a significant improvement in patient’s plain X-rays (Figure 1b, 2b).
Figures 1 and 2. Patient with soft tissue infection (acute bacterial skin and skin structure infection, ABSSSI), and 1st toe and 1st metatarsal osteomyelitis (OM). a: before, and b: after treatment with dalbavancin.
During treatment, patient was wearing aircast, after a three-phase scintigraphy revealed Charcot arthropathy. Patient’s last visit took place three weeks after the end of his antibiotic treatment. He was advised to continue aircast off-loading. Since then, he was lost-to-follow-up. Recently, after 1 ½ year, we finally got in touch with the patient, who informed us that his foot was in good shape, and denied a follow-up visit for personal reasons.
4. DISCUSSION
Dalbavancin is an antibacterial lipoglycopeptide agent which has received approval in the US for treating skin and skin structure infections (5). Due to its very long elimination t1/2, dalbavancin is administered once a week and is very effective against skin infections and many drug-resistant Gram positive bacteria. Through clinical trials, dalbavancin proved to be a very effective treatment for skin infections (5, 7). As mentioned above, dalbavancin has extremely long half-life and is unique in its category. It can be administered intravenously and has proved to be comparable to two-week linezolid treatment. Dalbavancin is more effective that vancomycin and teicoplanin against staphylococci and streptococci strains (5). However, little is known regarding lipoglycopeptides side-effects, because the clinical trials that have been published involved a few hundred patients, and adverse reactions may have escaped. Consequently, some patients may experience some rare side-effects, such as hypersensitivity and possible allergic reaction to dalbavancin. Therefore, infusion should last at least an hour, to avoid allergic reaction and should be performed in a hospital. In some cases, dalbavancin has caused a reversible rise of liver enzymes, mostly in patients with underlying diseases such as chronic hepatitis of alcohol abuse (2). According to Boucher et al. who conducted a study in patients who were treated with dalbavancin, these patients experienced less side-effects than patients who were treated with vancomycin or linezolid during the same treatment period (8). In a study conducted by Bouza et al., no side-effects were recorded after treatment with dalbavancin, although side-effects were difficult to establish since patients studied suffered from underlying diseases and were on concomitant medications. Dalbavancin proved to be effective when used to treat infections that demanded a long-term treatment (4). A similar study to determine the effectiveness of dalbavancin was conducted by Dunne et al. and concluded that a single iv infusion of 1500mg of the drug is noninferior and has a similar safety profile to a 2-dose regimen or 1000 mg iv on day 1 followed 1 week later by 500 mg iv (3).
It has to be noted that, in another study, Dunne et al. (7) demonstrated very good concentration of dalbavancin in bone cortex that lasted 2 weeks, which is very important for osteomyelitis, which is a disease difficult to treat (9).
Per os antibiotics eliminate the risk of complications and are more affordable, but they should be cautiously administered as there is always a risk of reduced compliance to treatment (10). So dalbavancin therapy has the unique advantage that, since the drug is given intravenously before patient’s discharge and/or in daily clinic, compliance is guaranteed and he or she avoids long-time hospitalization, thus decreasing the cost and avoiding nosocomial infections. Healthcare systems in Europe are facing a financial strain while the need for healthcare services increases. Because of population aging, patients are becoming older and hospital facilities fall behind. It is obvious that avoiding or reducing hospitalization saves money to healthcare systems. In clinical studies in 12 European countries, including Greece, the collected data regarded patients with MRSA ABSSSI, whose treatment plan switched from intravenous administration to oral formulations. Potential cost savings per patient ranged from €414 (Slovakia) to €2703 (France) (9). Bouza et al showed that in Spain, there was a notable estimated cost reduction of €3064 per patient, much more than that of the previous study, because of reduced hospitalization due to dalbavancin use (4).
It is essential to introduce programs that allow patients to continue their treatment at home, with as little adverse reactions as possible (9).
5. CONCLUSION
Dalbavancin, due to its extremely long half-life, could potentially be the drug of choice for DF OM caused by multi-drug resistant Gram (+) cocci, in order to avoid hospitalization, especially in non-complient patients. Further research is necessary. Of course, infectious disease specialist should always take into consideration drug high cost and antibiotic stewardship.
ABBREVIATIONS
- MRSA
Methicillin-resistant Staphylococcus aureus
- MSSA
Methicillin-susceptible Staphylococcus aureus
- IV
intravenous
- ABSSSI
Acute Bacterial Skin and Skin Structure Infections
- OM
osteomyelitis
- DF
diabetic foot
Patient’s Consent Form:
None non-medical interest was involved during the preparation of this article.
Author’s contribution:
Each author participated in each step of the research. Each author gave the final approval for the final version of article.
Conflicts of interest:
None declared.
Financial support and sponsorship:
The work was not sponsored nor financed.
REFERENCES
- 1.Malabarba A, Goldstein BP. Origin, structure, and activity in vitro and in vivo of dalbavancin. J Antimicrob Chemother. 2005;55(Suppl. 2):15–20. doi: 10.1093/jac/dki005. [DOI] [PubMed] [Google Scholar]
- 2.Van Bambeke F. Lipoglycopeptide Antibacterial Agents in Gram-Positive Infections: A Comparative Review. Drugs. 2015;75(18):2073–2095. doi: 10.1007/s40265-015-0505-8. [DOI] [PubMed] [Google Scholar]
- 3.Dunne MW, Puttagunta S, Giordano P, Krievins D, Zelasky M, Baldassarre J. A Randomized Clinical Trial of Single-Dose Versus Weekly Dalbavancin for Treatment of Acute Bacterial Skin and Skin Structure Infection. Clin Infect Dis. 2016;62(5):545–551. doi: 10.1093/cid/civ982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouza E, Valerio M, Soriano A, Morata L, Carus EG, Rodriguez-Gonzalez C, et al. Dalbavancin in the treatment of different gram-positive infections: a real-life experience. Int J Antimicrob Agents [Internet] 2018;51(4):571–577. doi: 10.1016/j.ijantimicag.2017.11.008. Available from: [DOI] [PubMed] [Google Scholar]
- 5.Biedenbach DJ, Bell JM, Sader HS, Turnidge JD, Jones RN. Activities of dalbavancin against a worldwide collection of 81,673 gram-positive bacterial isolates. Antimicrob Agents Chemother. 2009;53(3):1260–1263. doi: 10.1128/AAC.01453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biedenbach DJ, Jones RN. Multicenter evaluation of the in vitro activity of dalbavancin tested against staphylococci and streptococci in 5 European countries: results from the DECIDE Surveillance Program (2007) Diagn Microbiol Infect Dis [Internet] 2009;64(2):177–184. doi: 10.1016/j.diagmicrobio.2008.12.019. Available from: [DOI] [PubMed] [Google Scholar]
- 7.Dunne MW, Puttagunta S, Sprenger CR, Rubino C, Van Wart S, Baldassarre J. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother. 2015;59(4):1849–1855. doi: 10.1128/AAC.04550-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169–2179. doi: 10.1056/NEJMoa1310480. [DOI] [PubMed] [Google Scholar]
- 9.Eckmann C, Lawson W, Nathwani D, Solem CT, Stephens JM, Macahilig C, et al. Antibiotic treatment patterns across Europe in patients with complicated skin and soft-tissue infections due to meticillin-resistant Staphylococcus aureus: A plea for implementation of early switch and early discharge criteria. Int J Antimicrob Agents [Internet] 2014;44(1):56–64. doi: 10.1016/j.ijantimicag.2014.04.007. Available from: [DOI] [PubMed] [Google Scholar]
- 10.Roberts K, Pharm D. Oral Antibiotics for the Treatment of Adult Osteomyelitis: A Tough Pill to Swallow. 2014:1–20. [Google Scholar]


