Abstract
Introduction:
Immunomodulation properties of mesenchymal stem cells have attracted tremendous attention that eventually could regress liver fibrosis process.
Aim:
The study aims to demonstrate the immunomodulation activities of Umbilical cord-derived Mesenchymal stem cells (UC-MSCs) affecting interleukin-10 (IL-10) and hyaluronic acid (HA) secretion post intraperitoneal injection of CCl4, potent hepatotoxin, induced liver fibrosis among experimental rats.
Methods:
There were 18 Sprague-Dawley (SD) rats divided into three treatment groups (G1 sham group, G2 untreated liver fibrosis group, and G3 UC-MSCs treated-group) and isolated in Stem Cell and Cancer Research Facility, Semarang, Indonesia. Blood examination was conducted after 3 and 14 days of UC-MSCs transplantation using sandwich based ELISA followed by the histopathological analysis of rat liver tissue. ANOVA and posthoc LSD tests were determined the significance against all groups based on their quantitative measurement.
Results:
UC-MSCs have been successfully extracted and isolated as well as positive with osteogenic differentiation (Alizarin dye). In further analysis, there were significant mean differences among all groups through the ANOVA test, both IL-10 and HA secretion, concurrent with low-grade liver fibrosis in G3. IL-10 elevates during the early phase of UC-MSCs transplantation, and HA significantly reduced on the 14th day of transplantation, it characterizes the liver fibrosis that has been attenuated.
Conclusion:
The transplantation of UC-MSCs has given an opportunity for the treatment of a wide range of chronic liver diseases through the immunomodulation properties via its paracrine effects that regulate specific cytokine to suppress fibrosis development.
Keywords: Mesenchymal stem cells, fibrosis, chronic liver disease (CLDs), interleukin-10, hyaluronic acid
1. INTRODUCTION
Liver fibrosis disrupts normal parenchyma of liver structure producing collagenous scar that finally could lead to liver cirrhosis and hepatocellular carcinoma (HCC), end-stage of liver disease; this initiates a calamity in liver tissue and occurs as a response for the noxious stimuli causing chronic liver diseases, such as viral infection, alcoholic consumption, autoimmune disease, and fatty liver (1, 2). Liver cirrhosis and HCC have contributed to 2.5% of premature deaths worldwide, and more than 300 million people are diagnosed having one of the chronic liver diseases with viral hepatitis as the leading cause. The curative management for the conditions is liver transplantation (LT) (3); however, the shortage of resources, lifelong use of immunosuppressant drugs, and cost issues have been impeded the application of LT for the general population although liver transplantation could extend the life expectancy of the recipient (4). Besides, there are 18.0 per 100 patients who underwent negative outcomes of LT and 1.8 per 100 patients were diagnosed with graft failure. Liver transplantation has also been carried out for more than 25.000 patients worldwide and has exceeded the number of donors. Thus, dead candidates are inevitable on the waiting list (5, 6).
Mesenchymal stem-cells appears as one of the developing breakthroughs and has a potential in alleviating liver fibrosis as well as reversing the liver fibrotic progression via self-renewal, hepatocyte differentiation, immune modulation, and low immunogenicity properties (7-9). The primary sources for MSCs originate from the umbilical cord, or umbilical-cord derived mesenchymal stem cells (UC-MSCs), are more advantageous than bone marrow-derived, mainly because of abundantly and easily accessed into the umbilical cord, excellent hepatic differentiation, no ethical problems, and low viral contaminated-product (10, 11). The ability of UC-MSCs in transforming liver structure has been described as superior in several study reports, mainly through paracrine effect inducing trans-differentiation to hepatocyte-like cells and immunomodulator (12).
Inflammation generated by the exposure of harmful stimuli has emerged as the regulator of fibrotic development and played pivotal parts in the initiation phase and maintenance phase of the liver fibrosis. Injury inundates the liver environment with inflammatory mediators produced by surrounding damaged epithelial and endothelial cells, thus recruiting many types of inflammatory cells in peripheral towards the inflamed area (13). Pro-fibrotic substances, such as cytokines and enzymes, will finally induce hepatic stellate cells (HSCs) activation which triggers collagenous deposition in liver tissue (14). Interleukin- 10 (IL-10) is an anti-inflammatory cytokine and it categorically turns into suppressor for HSCs function as well as an inducer for HSC apoptosis, preventing further collagenous accumulation (15). HSCs are an essential precursor for myofibroblast differentiation, then at once producing extracellular matrix as the main component for fibrosis (16). Meanwhile, hyaluronic acid (HA) is one the composition of the extracellular matrix which synthesized by HSCs per se and it affects cell migration and proliferation; moreover, it is also degraded by liver sinusoidal endothelial cells (LSECs) (17). In recent literature, higher levels of HA represent the extent and degree of liver fibrosis. IL-10 and HA are the two-component that characterize their roles in affecting liver fibrosis development through signaling pathway activation of specific components during inflammation while UC-MSCs will modulate the immunologic response to alleviate liver fibrosis via its paracrine effect (18).
2. AIM
The study aims to investigate the role of UC-MSCs in producing low-grade liver fibrosis relating to the secretion of interleukin-10 and hyaluronic acid among experimental rats. The study reports will become the resources for the evidence of UC-MSCs immunomodulation function and ultimately strengthen the use of UC-MSCs for liver fibrosis in the future.
3. MATERIALS AND METHODS
3.1. ANIMAL MODEL
There were 18 Sprague-Dawley (SD) rats which included in the study by their similar age (12-14 weeks old), weight (200-250 gram for each), and treatment in a conditioned environment of specific pathogen-free (SPF)-grade animal room which treated for 12-h light-dark cycle and adequate nutritional support during 104 days following the study. Furthermore, the animals were divided into three groups of treatment through randomized-mode, consisting of a control group or sham group (G1), liver-fibrosis induced group (G2), and UC-MSCs-treated group (G3). G2 of liver fibrosis was established via induction of carbon tetrachloride (CCl4) injection (Sigma–Aldrich, USA) intraperitoneally at dose 1 ml/kg body weight twice weekly for 12 weeks. The ethical commission for medical research, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia, has approved the experimental protocol of the study (Reference number: 542/TGL/KEPK FK USU-RSUP HAM/2019). The Guide for the Care and Use of Laboratory Animals Eighth Edition applied for the entire process of animal treatment during the study.
3.2. ISOLATION, IDENTIFICATION, AND IMMUNOPROFILING OF UC-MSCS
UC-MSCs were obtained from pregnant SD rats under deep anesthesia. Firstly, the excision was made into several smaller pieces after the blood vessels were removed and then transferred to a T25 culture flask containing complete Dulbecco’s Modified Eagle’s medium (DMEM) (Sigma-Aldrich, Louis St, MO) supplemented with 10% Fetal Bovine Serum (FBS) (GibcoTM Invitrogen, NY, USA), and 100 IU/mL penicillin/streptomycin (Sigma-Aldrich). These cells were incubated in 5% CO2, 37°C and the medium was changed every three days. An interval of the incubation period was allocated to reach an eighty-percent of confluency. The cell trypsinization then followed the cultivation of MSC-like cells. Hereafter, the immunophenotypes of UC-MSCs were analyzed at 4th passage followed by inundating the specimen with antibodies conjugated: fluorescein isothiocyanate (FITC)-conjugated CD90, Allophycocyanin (APC)-conjugated CD73, Peridinin Chlorophyll Protein Complex (PerCP)-conjugated CD105 and phycoerythrin (PE)-conjugated Lin monoclonal antibodies for 30 min at 4°C in the dark. The fluorescence intensity of cells was determined through flow cytometry (BD Bioscience, Franklin Lakes, NJ, USA) based on light transmission.
Osteogenic differentiation must be evident for all suspected- Mesenchymal stem cells based on the minimum criteria of the International Society for Cellular Therapy (ISCT) for MSCs. Therefore, UC-MSCs differentiation potential was determined to characterize the isolated cells. These cells initially were cultured in DMEM medium supplemented with 10% FBS, β-glycerophosphate (10 mM), dexamethasone (10 nM), and L-ascorbic-acid (50 μM) (all from Sigma-Aldrich, Louis St, MO), at 37°C and 5% CO2. UC-MSCs were stained with 0.2 % Alizarin Red solution (Sigma-Aldrich) to represent calcium deposition (cells used were from the 4th passage), the red-stain appearance of Alizarin dye represents osteogenic differentiation.
3.3. HISTOPATHOLOGICAL ANALYSIS
The rats in each group were anesthetized and euthanized on days 14 after UC-MSCs injection, according to the American Veterinary Medical Association (AVMA) Guidelines. Subsequently, liver tissues were processed for paraffin embedding and were sectioned into four-μm sections using a microtome. The prepared-paraffin block was stained with hematoxylin and eosin (HE) according to standard protocols. The degree of liver fibrosis was based on a histological grading scale using the METAVIR score (19). The fibrosis was then graded on a 5-point scale, F0 no fibrosis; F1 fibrosis tissue expands to the portal area; F2 periportal fibrosis with limited septa inside the lobulus (normal architecture); F3 fibrosis septa in portal tracts and terminal hepatic vein (disrupted parenchymal structure); F4 nodular cirrhosis.
3.4. ENZYME-LINKED IMMUNOSORBENT ASSAYS (ELISA)
Blood was extracted from the periorbital venous plexus under general anesthesia on the 3rd and 14th day after administration of MSCs. The investigation of interleukin-10 and hyaluronic acid levels was measured by sandwich enzyme-linked immunosorbent assay (ELISA), both from Rat ELISA kit, Mybiosource, the United States, which perform under the manufacturer’s instructions as well as the interpretations (formulated through the analysis of the standard curve and optical density of samples spectrophotometrically at a wavelength of 450 using software). The kits included a 96-wells micro ELISA plate to accommodate the sample duplication and have been pre-coated with specific antibody for rat IL-10 and HA.
3.5. STATISTICAL ANALYSIS
The data were provided in the mean ± standard deviation formula and double-checked in Statistical Package for the Social Sciences (SPSS) version 22. A one-way ANOVA analyzed the mean difference of interleukin-10 and hyaluronic acid among groups with the least significance difference (LSD) comparison post hoc test for further analysis comparing the results in each group. Finally, it determined the significance of the result based on p-value, p < 0.05 was considered as statistically significant.
4. RESULTS
4.1. THE ISOLATION, CHARACTERISTICS, AND IMMUNOPROFILING OF UC-MSCS
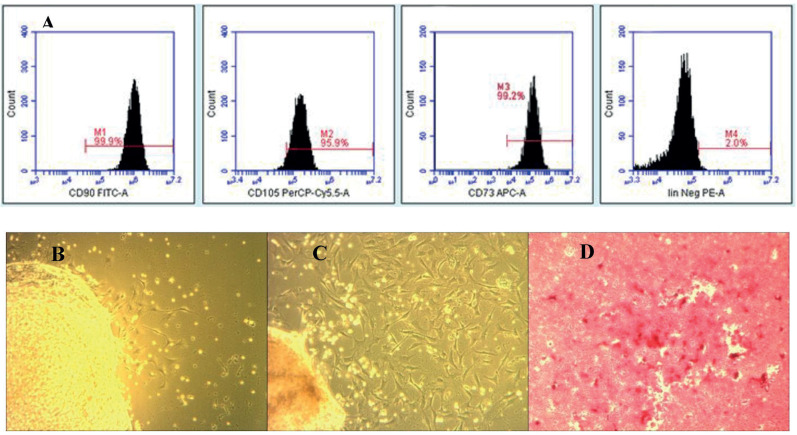
Umbilical-cord derived mesenchymal stem cells expressed CD73 (99.2%), CD90 (99.9%), and CD105 (95.9%) with no expression of the negative marker Lin and it was confirmed through phenotype surface identification of flow cytometric analysis. The morphology of UC-MSCs was similar to the long spindle-shaped cells of mesenchymal stem cells with high proliferative activity and plastic adherence under an inverted microscope. In addition, UC-MSCs had osteogenic differentiation ability concluding the cell lineage as mesenchymal stem cells based on the minimum criteria for MSCs (Figure 1).
Figure 1. UC-MSC@ had steogenic differentiation ability concluding the cell lineage as mesenchymal stem cells.
4.2. UC-MSCS TRANSPLANTATION GENERATES LOW-GRADE LIVER FIBROSIS
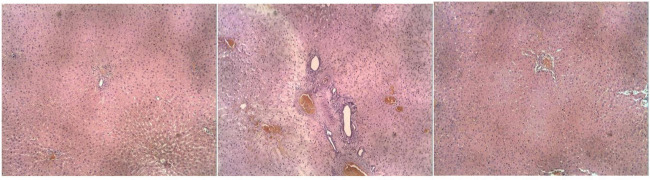
In the final session of treatment, the liver of experimental rats was extracted for the histopathological analysis using H&E. Pathologists evaluated the liver specimen, and the degree of liver fibrosis was stratified accordingly. There was a fibrous expansion of most portal areas with marked bridging (F3) in the CCl4-treated group without UC-MSCs transplantation or G2. There was also a marked vacuolar degeneration and neutrophilic infiltration in higher magnification, which indicated hepatic fibrosis was unchanged among rats in a similar group. Finally, the satisfactory results of CCl4 injection as fibrosis inducer are evident through the blind interpretation. CCl4 has been widely known to induce centrilobular necrosis and nephrotoxic properties following the intraperitoneal injection in experimental rats. In other findings, there is a reduction of liver fibrosis (F1) in the treatment group with fine structure and no bridging fibrosis after 14-days of UC-MSCs transplantation compared to coarse fibrosis, particularly in the portal area among the control group while the sham group appeared with no fibrosis (Figure 2).
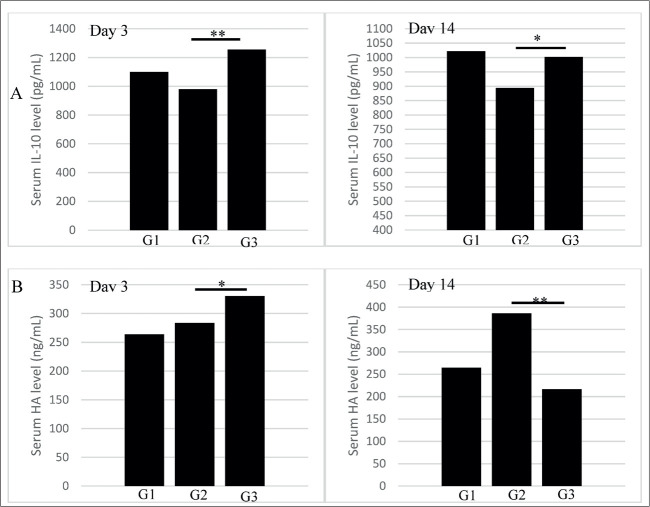
Figure 2. The effect of UC MSCs transplantation on serum IL-10 and HA level at day 3 and 14.
4.3. UC-MSCS TRANSPLANTATION MODULATES INTERLEUKIN-10 AND HYALURONIC ACID SECRETION
The administration of CCl4 in rats has induced liver fibrosis and it was confirmed through histopathological analysis concurrent with several findings of IL-10 and HA levels which were previously measured using sandwich ELISA. In the 3rd and 14th day of the blood examination, the early period of UC-MSCs treatment has successfully induced IL-10 secretion followed by a mild reduction in 14th day but if it was compared to the remaining groups, G3 induced the highest IL-10 secretion (3rd day= 1256.75 ± 123.02 pg/mL; 14th day= 1002.31 ±126.67 pg/mL) and the study demonstrated the significant result of the overall mean difference between CCl4-treated (G2) and UC-MSCs group (G3) of IL-10 secretion through posthoc analysis (937.105 pg/mL versus 1129.53 pg/mL; P<0.05, respectively). Meanwhile, HA levels surge at the beginning of the fibrosis induction but a 14-day use of UC-MSCs has declined HA secretion into the steepest levels with significant posthoc analysis with G2 in the 3rd day (330.36 ±15.21 ng/mL versus 283.76 ±13.38; P= 0.020, consecutively) and 14th day (216.55 ±11.12 versus 386.37±21.28; P=0.000, consecutively) even compared to the sham group (Figure 3). It confirmed the hypothesis that the administration of UC-MSCs affects the secretion of IL-10 and HA through its immunomodulation function.
Figure 3. Liver sections of the histopatological examination obtained from rat in three groups.
5. DISCUSSIONS
Fibrosis is a hallmark of pathological conditions in chronic liver diseases (CLDs), and the essential stage in the pathogenesis of CLDs progressing to liver cirrhosis. Inflammation takes place as a proficient inducer for liver fibrosis through the interaction between inflammatory cells, cytokines, and specific signaling pathways (20). The vicious cycle firstly occurs as a result of hepatocyte and endothelial damage that will induce more profound inflammatory surge responses to bolstering fibrosis establishment. Inflammatory responses have been associated with HSCs activation and finally turn the cells into pro-inflammatory cytokines producer that preserves fibrosis development (21, 22).
IL-10 is a potent anti-inflammatory cytokine triggering immune tolerance that could halt fibrosis development through its suppressing effect and HSCs modulation properties (23, 24); moreover, it inhibits HSCs activation and finally prevents further damage. In the study, it is evident that UC-MSCs have modulated the IL-10 secretion following the 3rd and 14th day of UC-MSCs transplantation compared to the untreated-group. The pattern of secretion has been linked with the immunomodulation properties of UC-MSCs. Furthermore, it is proven that the establishment of liver fibrosis has successfully decreased IL-10 levels, which statistically significant based on posthoc analysis between G2 and G3 as well as G1 and G2. Likewise, the consistent results of the histopathological study have established the fact that the higher IL-10 levels were associated with low-grade fibrosis. In recent reports, there has been evidence that IL-10 reduces the secretion of pro-inflammatory cytokines thus delaying hepatic necrosis and further development of liver fibrosis, mainly via the activation of tyrosine kinase 2 and Janus tyrosine kinase 1 (JAK1) pathway that drives into one of the IL-10 responsive genes, suppressor of cytokine signaling 3 (SOCS3), which correlated with decreased expression of TNFα and IL-1β (25).
In some cases, the ineffective mechanism of anti-inflammatory pathways in IL-10 promoter region polymorphism individuals with liver fibrosis appears to reduce IL-10 expression that leads to the vigorous activity of pro-fibrosis cytokines (26). In this stage, UC-MSCs emerges as the controller for the development of liver fibrosis as IL-10 was secreted slightly higher in the early period of injection rather than in 14th days of the treatment that has attenuated the inflammation. Some studies have reported the lower levels of IL-10 among liver fibrosis population while elevates after UC-MSCs transplantation for a particular period, and it is applied for other underlying pathological conditions, such as acute ischemic stroke, arthritis, and other CNS degenerative diseases (27-29). In a study, the activation of macrophage and dendritic cells has also increased IL-10 secretion induced by mesenchymal stem cells (30).
Chronic liver inflammation is responsible for the construction of liver fibrosis through matrix extracellular deposition in which hyaluronic acid (HA) predominantly composed this essential substance, subsequently producing firm and sturdy liver fibrosis (31, 32). Based on the study results, HA has reached the peak levels exceeding 300 ng/mL among rats in G3 during the early development of liver fibrosis and UC-MSCs transplantation but significantly reduced at last. Liver fibrosis has also generated a considerable amount of HA among rats in the remaining group relating to the fibrosis degree, for instance, it increased the HA production among untreated groups (G2) in the 14th day of blood examination with high-grade fibrosis; in contrast, HA levels still unchanged in G1 without fibrosis induction. Therefore, UC-MSCs act as an inflammatory controller at this stage, so there was a shift from inflammatory to proliferative phase during the period of fibrosis inducing more HA production representing the plausible response for liver fibrosis and the accumulation of extracellular matrix. Consequently, UC-MSCs alleviates liver fibrosis through its regeneration properties inducing endogenic stem cell activation as well as impeding extracellular matrix deposition that ultimately reduces HA secretion (33, 34); it also signs the satisfactory results of the transplantation. There has been only one study comparing HA levels following UC-MSCs transplantation; Zhang et al reported the lower HA secretion as well as lower levels of some liver markers, such as serum laminin, type IV collagen, and type III procollagen peptide (PIIINP) after 24 and 48 weeks of stem cell treatment in patients with decompensated liver cirrhosis (35, 36).
6. CONCLUSION
The transplantation of UC-MSCs can ameliorate liver fibrosis through its immunomodulation properties via regulating IL-10 and hyaluronic acid secretion in the animal model of liver fibrosis. MSCs have several abilities to regulate liver inflammation, inhibit the activation and induce apoptosis of hepatic stellate cells (HSCs); this circumstance will also grant the regeneration process of UC-MSCs function to hepatocyte, and it did not exist among untreated experimental rat or G2.
Author’s contribution:
All authors have similar substantial contributions to conception and design, acquisition, analysis and interpretation of data, drafting and article publication.
Conflicts of Interest:
There were no conflicts of interest
Financial support and sponsorship:
Nil.
REFERENCES
- 1.De Luna-Saldivar MM, Marino-Martinez IA, Franco-Molina MA, Rivera-Morales LG, Alarcon-Galvan G, Cordero-Perez P, Rojas-Martinez A, Rodriguez-Padilla C, Munoz-Espinosa LE. Advantages of adipose tissue stem cells over CD34+ mobilization to decrease hepatic fibrosis in Wistar rats. Annals of hepatology. 2019;18:620–626. doi: 10.1016/j.aohep.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Cheng JY, Wong GL. Advances in the diagnosis and treatment of liver fibrosis. Hepatoma Research. 2017;3:156–169. [Google Scholar]
- 3.Byass P. The global burden of liver disease: a challenge for methods and for public health. BMC medicine. 2014;12(1):159. doi: 10.1186/s12916-014-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forbes SJ, Gupta S, Dhawan A. Cell therapy for liver disease: From liver transplantation to cell factory. Journal of hepatology. 2015;62(1):S157–169. doi: 10.1016/j.jhep.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 5.Martin P, DiMartini A, Feng S, Brown R, Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 6.Haddad L, Cassenote AJ, Andraus W, de Martino RB, de Siqueira Ortega NR, Abe JM, D’Albuquerque LA. Factors associated with mortality and graft failure in liver transplants: a hierarchical approach. PloS one. 2015;10(8):e0134874. doi: 10.1371/journal.pone.0134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Idriss NK, Sayyed HG, Osama A, Sabry D. Treatment Efficiency of Different Routes of Bone Marrow-Derived Mesenchymal Stem Cell Injection in Rat Liver Fibrosis Model. Cellular Physiology and Biochemistry. 2018;48(5):2161–2171. doi: 10.1159/000492558. [DOI] [PubMed] [Google Scholar]
- 8.Chai NL, Zhang XB, Chen SW, Fan KX, Linghu EQ. Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats. World journal of gastroenterology. 2016;22(26):6036. doi: 10.3748/wjg.v22.i26.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H, Sun J, Li Y, Duan WM, Bi J, Qu T. Human umbilical cord-derived mesenchymal stem cells suppress proliferation of PHA-activated lymphocytes in vitro by inducing CD4+CD25highCD45RA+ regulatory T cell production and modulating cytokine secretion. Cellular immunology. 2016;302:26–31. doi: 10.1016/j.cellimm.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Deng Y, Zhang Y, Ye L, Zhang T, Cheng J, Chen G, Zhang Q, Yang Y. Umbilical cord-derived mesenchymal stem cells instruct monocytes towards an IL10-producing phenotype by secreting IL6 and HGF. Scientific reports. 2016;6:37566. doi: 10.1038/srep37566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Q, Ren H, Li X, Chen Z, Zhang X, Gong W, Liu Y, Pang T, Han ZC. Differentiation of human umbilical cord mesenchymal stromal cells into low immunogenic hepatocyte-like cells. Cytotherapy. 2009;11(4):414–426. doi: 10.1080/14653240902849754. [DOI] [PubMed] [Google Scholar]
- 12.Yin F, Wang WY, Jiang WH. Human umbilical cord mesenchymal stem cells ameliorate liver fibrosis in vitro and in vivo: From biological characteristics to therapeutic mechanisms. World journal of stem cells. 2019;11(8):548. doi: 10.4252/wjsc.v11.i8.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. The Journal of clinical investigation. 2007;117(3):539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeves HL, Friedman SL. Activation of hepatic stellate cells - a key issue in liver fibrosis. Front Biosci. 2002;7(4):808–826. doi: 10.2741/reeves. [DOI] [PubMed] [Google Scholar]
- 15.Zhang LJ, Wang XZ. Interleukin-10 and chronic liver disease. World journal of gastroenterology. WJG. 2006;12(11):1681. doi: 10.3748/wjg.v12.i11.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuchiya A, Takeuchi S, Watanabe T, Yoshida T, Nojiri S, Ogawa M, Terai S. Mesenchymal stem cell therapies for liver cirrhosis: MSCs as “conducting cells” for improvement of liver fibrosis and regeneration. Inflammation and regeneration. 2019;39(1):1–6. doi: 10.1186/s41232-019-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrone G, Shah VH, Gracia-Sancho J. Sinusoidal communication in liver fibrosis and regeneration. Journal of hepatology. 2016;65(3):608–617. doi: 10.1016/j.jhep.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Chen L, Liu T, Zhang B, Xiang D, Wang Z, Wang Y. Human umbilical cord matrix stem cells efficiently rescue acute liver failure through paracrine effects rather than hepatic differentiation. Tissue engineering part A. 2012;18(13-14):1352–1364. doi: 10.1089/ten.tea.2011.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. Journal of hepatology. 2007 Oct 1;47(4):598–607. doi: 10.1016/j.jhep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Friedman SL. Liver fibrosis–from bench to bedside. Journal of hepatology. 2003;38:38–53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 21.Lee CW, Chen YF, Wu HH, Lee OK. Historical perspectives and advances in mesenchymal stem cell research for the treatment of liver diseases. Gastroenterology. 2018;154(1):46–56. doi: 10.1053/j.gastro.2017.09.049. [DOI] [PubMed] [Google Scholar]
- 22.Zhangdi HJ, Su SB, Wang F, Liang ZY, Yan YD, Qin SY, Jiang HX. Crosstalk network among multiple inflammatory mediators in liver fibrosis. World journal of gastroenterology. 2019;25(33):4835. doi: 10.3748/wjg.v25.i33.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang YY. Can serum cytokines predict hepatic cytokine expression in liver cirrhosis? Journal of the Chinese Medical Association. 2011;74:485–486. doi: 10.1016/j.jcma.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Zhang XI, Xiong XI, Yang Z, Li P, Wang JI, Sun YU, Yang Z, Hoffman RM. Bone marrow mesenchymal stem cells reverse liver damage in a carbon tetrachloride-induced mouse model of chronic liver injury. in vivo. 2016;30(3):187–193. [PubMed] [Google Scholar]
- 25.Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World journal of stem cells. 2014;6(5):552. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grove J, Daly AK, Bassendine MF, Gilvarry E, Day CP. Interleukin 10 promoter region polymorphisms and susceptibility to advanced alcoholic liver disease. Gut. 2000;46(4):540–545. doi: 10.1136/gut.46.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JJ, Yoo SA, Park SJ, Kang YJ, Kim WU, Oh IH, Cho CS. Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clinical & experimental immunology. 2008;153(2):269–276. doi: 10.1111/j.1365-2249.2008.03683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima M, Nito C, Sowa K, Suda S, Nishiyama Y, Nakamura-Takahashi A, Nitahara-Kasahara Y, Imagawa K, Hirato T, Ueda M, Kimura K. Mesenchymal stem cells overexpressing interleukin-10 promote neuroprotection in experimental acute ischemic stroke. Molecular Therapy-Methods & Clinical Development. 2017;6:102–111. doi: 10.1016/j.omtm.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payne NL, Sun G, McDonald C, Moussa L, Emerson-Webber A, Loisel-Meyer S, Medin JA, Siatskas C, Bernard CC. Human adipose-derived mesenchymal stem cells engineered to secrete IL-10 inhibit APC function and limit CNS autoimmunity. Brain, behavior, and immunity. 2013;30:103–114. doi: 10.1016/j.bbi.2013.01.079. [DOI] [PubMed] [Google Scholar]
- 30.Saldana L, Bensiamar F, Valles G, Mancebo FJ, Garcia-Rey E, Vilaboa N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem cell research & therapy. 2019;10(1):58. doi: 10.1186/s13287-019-1156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan JA, Khan FA, Dilawar M, Ijaz A, Khan NA, Mehmood T. Serum hyaluronic acid as a marker of hepatic fibrosis. J Coll Physicians Surg Pak. 2007;17(6):323–326. [PubMed] [Google Scholar]
- 32.Rostami S, Parsian H. Hyaluronic Acid: from biochemical characteristics to its clinical translation in assessment of liver fibrosis. Hepatitis monthly. 2013;13(12) doi: 10.5812/hepatmon.13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kholodenko IV, Kurbatov LK, Kholodenko RV, Manukyan GV, Yarygin KN. Mesenchymal Stem Cells in the Adult Human Liver: Hype or Hope? Cells. 2019;8:1127. doi: 10.3390/cells8101127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meier RP, Muller YD, Morel P, Gonelle-Gispert C, Buhler LH. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem cell research. 2013;11(3):1348–1364. doi: 10.1016/j.scr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, Chen L, Lv S, Li Y, Yu S, Geng H. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. Journal of gastroenterology and hepatology. 2012;27:112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 36.Putra A, Rosdiana I, Darlan DM, Alif I, et al. Intravenous Administration is the Best Route of Mesenchymal Stem Cells Migration in Improving Liver Function Enzyme of Acute Liver Failure. Folia Medica. 2020;62:52. doi: 10.3897/folmed..e47712. [DOI] [PubMed] [Google Scholar]