Abstract
Introduction:
Pre-diabetic precedes the development of full diabetes. Studying and identification changes in pre-diabetic conditions can give the possibility to decline the development of diabetes and treat conditions associated with diabetes such as cardiovascular diseases.
Aim:
The main objectives of the present study were to investigate the potential of using Urtica pilulifera in treating the pre-diabetic rat model and to investigate its anti-oxidant impact.
Methods:
The pre-diabetic model was induced in rats through daily giving high sucrose diet (35%) for 30 days. The extraction of U. pilulifera leaves was made as described by previous studies. Thirty male Wistar rats were randomly divided into three groups, control group (n=10), pre-diabetic group (n=10), and treated group with the extract of U. pilulifera (n=10). Control group rats received standard diet; pre-diabetic group rats received standard diet and high sucrose (35%) in drinking water, treated group rats received the same conditions as a pre-diabetic group, with intra-peritoneal injection of U. pilulifera injection on daily basis. After one month experiment, blood samples were taken from all rats and tested for glucose, triglycerides, cholesterol, GSH, TAC, and MDA.
Results:
Both glucose and triglycerides levels were significantly increased in pre-diabetic groups, and significantly reduced in the treated group by the extract of U.pilulifera. The cholesterol level was not significantly changed in all groups. The levels of GSH were significantly reduced in the pre-diabetic group compared with the control group. Treatment with the extract of U. pilulifera increased the levels of GSH significantly compared with the pre-diabetic group. The levels of TAC were not significantly changed between the control group and the pre-diabetic group, but significantly increased in the treated group compared with the pre-diabetic group. The levels of MDA significantly increased in the pre-diabetic group compared with the control group, and significantly reduced in the treated group compared with the control group.
Conclusion:
High sucrose pre-diabetic model is a good model to study diabetes at early stages, and the treatment using U. pilulifera has several benefits in reducing glucose and lipid profile lipids as well as combating oxidative stress.
Keywords: pre-diabetes, oxidative stress, glucose, MDA
1. INTRODUCTION
At the global level, the prevalence of type 2 diabetes has dramatically increased (1-3). Research has shown a significant relationship between diabetes and each of obesity and insulin resistance as well as alterations in the function of β-cells in the pancreas (4, 5). The resulting influences include declining rates of insulin metabolism such as glucose, lipids, and proteins (3, 6). Diabetes can result in regulatory alterations in the hemostasis of calcium, phosphorus and magnesium ending with serious complications including cardiovascular disease and neurological disorders (7, 8). According to the American Diabetes Association (ADA), pre-diabetes can be defined as either impairment of fasting glucose (5.6–6.9 mmol/L) or impairment of oral glucose tolerance test (2-h OGTT glucose 7.8–11.0 mmol/L) (9). Other studies showed that the pre-diabetic state is characterized by a reduction of glucose tolerance and delayed insulin secretion (10).
It has been demonstrated that the state of pre-diabetes is associated with developing cardiovascular events such as myocardial abnormalities. Accordingly, as early as the cardiac changes have been determined in this state, the results are optimal in preventing the development of heart diseases (11).
Various animal models to study diabetes have been described in the literature including the high-fat diet/streptozotocin treated (HFD/STZ) rat model. In this model, there is a combination of a fatty diet and STZ (12, 13). One of the well- studied models is the male Zucker Diabetic Fatty (ZDF) rat that exhibits intolerance of glucose, insulin resistance, and hyperlipidemia. Depending on a fatty diet (6.5% fat content), rats can develop diabetes within 8 weeks of age (14).
Medical herbs have been used to treat human diseases including diabetes and other diseases since ancient times (15, 16). Dina et al (17) evaluated the extract of Urtica pilulifera in treating diabetic rat models. Researchers showed that there was a significant reduction in glucose levels.
2. AIM
The main objectives of the present study were to investigate the potential of using Urtica pilulifera in treating the pre-diabetic rat model and to investigate its anti-oxidant impact.
3. MATERIAL AND METHODS
Induction of pre-diabetic rat model
Male adult (16 weeks-old) Wistar rats (Yarmouk University, Animal house Unit, Jordan) were chosen for this study. The average of rat weight was (196±7.25 g). For the acclimatization process, animals were kept under optimal conditions including environment with controlled temperature (22–23°C) and light-dark cycle for 1week. Then, animals were randomly divided into three groups (n=10). The first group, the control group (n=10), the second group, the pre-diabetic group (n=10) and the third group, treated group with extract of U. pilulifera (n=10). The control group received tap water and a standard diet. Pre-diabetic received tap water for drinking in addition to high sucrose diet (HSu) (35% sucrose). We followed the methodology in preparing the pre-diabetic model as described by the study of Nunes et al (11). The treated group received the same treatment as the pre-diabetic group, but treated with an intraperitoneal injection of 1.25 mg/kg of body weight daily. The extraction of U. pilulifera leaves was made as described by previous studies (18). After the end of the study, blood samples were withdrawn into blood tubes (red tubes) to collect serum.
Assessment of the following tests was made in private clinical laboratories: glucose level, lipid profile (cholesterol and triglyceride), and antioxidants (GSH, TAC, and MDA).
4. RESULTS
Glucose and lipid profile levels in study groups
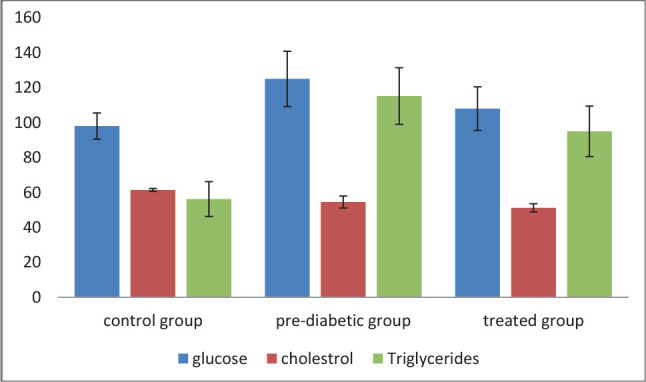
As seen in (Table 1 and Figure 1), the mean level of glucose in the control group is 98±7.5 mg/dl. Pre-diabetic conditions significantly increased the mean level of glucose to 125±15.8 mg/dl (p<0.001). Treatment with the extract of U. pilulifera significantly decreased the level of glucose from 125.15.8 mg/dl to 108±12.45 mg/dl (p<0.001). The mean levels of cholesterol were not significantly changed between study groups. On the other hand, the mean levels of triglycerides were significantly increased from 65.23±9.96 mg/dl in the control group to 115.19±16.18 in the pre-diabetic group (p<0.001). Treatment with the extract of U. pilulifera significantly decreased the mean level of cholesterol from 115.19±16.18 mg/dl in the pre-diabetic group to 95±14.43 mg/dl in the treated group (p<0.001).
Table 1. Glucose and lipid levels in study groups. Values are expressed as means± SD. *pre-diabetic group is compared with control group, **treated group is compared with pre-diabetic group.
| Variable | Control group | Pre-diabetic group | Treated group |
|---|---|---|---|
| Glucose (mg/dl) | 98±7.5 | 125±15.8* | 108±12.45** |
| Cholesterol (mg/dl) | 61.45 ± 0.85 | 54.53 ± 3.44 | 51.21±2.32 |
| Triglycerides (mg/dl) | 65.23 ± 9.96 | 115.19 ± 16.18* | 95 ±14.43** |
Figure 1. The levels of glucose and lipid profile in study groups.
The mean levels of antioxidants in study groups
As demonstrated in (Table 2, and Figure 2), the mean level of GSH was 0.32±0.052 nmol/ml. pre-diabetic conditions significantly decreased the levels of GSH into 0.15±0.026 (p<0.001). The treatment using the extract of U. pilulifera significantly increased the levels of GSH (0.22±0.031) (p<0.001). For TAC, no significant changes were observed between the control group and the pre-diabetic group. There were significantly increased levels of TAC in the treated group compared with the pre-diabetic group (p<0.001). The levels of MDA were significantly increased in the pre-diabetic group compared with the control group (p<0.001). The treatment with the extract of U. pilulifera significantly reduced the level of MDA in the treated group compared with the pre-diabetic group (p<0.001).
Table 2. The mean levels of antioxidants in study groups. Values are expressed as means± SD. *pre-diabetic group is compared with control group, **treated group is compared with pre-diabetic group.
| Variable | Control group | Pre-diabetic group | Treated group |
|---|---|---|---|
| GSH (nmol/ ml) | 0.32±0.052 | 0.15±0.026* | 0.22±0.031** |
| TAC (nmol/μl) | 1.9 ± 0.15 | 1.6±0.18 | 2.8±0.19** |
| MDA (nmol/μl) | 0.07 ± 0.1 | 0.25±0.12* | 0.13±0.10** |
Figure 2. Levels of anti-oxidants in study groups.

5. DISCUSSION
Diabetes has already been studied extensively in the literature, particularly in animal models including rats (3, 17, and 18). Little studies have been conducted on pre-diabetes, particularly the high sucrose model (11). Pre-diabetes is the early area preceding the development of type 2 diabetes (19).
The findings of the present study showed that pre-diabetic conditions increased significantly the levels of glucose (p<0.001). This was associated with significantly increased levels of triglycerides (p<0.001). The mean level of cholesterol was not significantly changed in study groups. It is plausible to consider that increased levels of triglycerides are associated with the development of the pre-diabetic conditions and may be involved in developing other conditions like cardiovascular diseases at this stage (11, 20). Treatment with the extraction of U. pilulifera was interestingly found to significantly decrease the levels of both glucose and triglycerides compared with pre-diabetic group. Previous studies demonstrated beneficial effects against diabetes by U. pilulifera (15), but its use in pre-diabetic conditions may not be indicated up to the best knowledge of the author.
We studied the effects of pre-diabetic conditions on some anti-oxidants. Pre-diabetic conditions increased the levels of glutathione reduced (GSH) significantly compared with the control group. The accumulation of GSH has damaging effects and participates in the formation of oxidative conditions such as reactive oxygen species and reactive nitrogen species (21). Treatment with U. pilulifera significantly decreased the levels of GSH. From the study results, it is obvious that the treatment of U. pilulifera helps in reducing glucose levels and oxidative stress (15, 18, and 22). The study findings showed that the levels of total anti-oxidant capacity (TAC) were similar in both, the control group and pre-diabetic group, but the treatment with U. pilulifera significantly increased the levels TAC. TAC acts to protect against free radicals and as a result combat the oxidative effect. Although its level was not significantly increased in the pre-diabetic group, the use of U. pilulifera significantly increased the levels of TAC. In general, the importance of TAC to reduce the effects of oxidative stress was indicated in previous studies (23).
The levels of malate dehydrogenase (MDA) were significantly increased in the pre-diabetic group compared with the control group, whereas the levels of MDA were significantly decreased because of the effect of treatment with U. pilulifera. Other studies reported that MDA to be higher in diabetic patients (24). Previous studies pointed out to MDA as one of the products resulting from lipid peroxidation (25-27).
6. CONCLUSION
The results of this study showed that pre-diabetic model based on high sucrose diet can be further used in other studies to better understand diabetes from one side, and from the other side, the use of treatment with U. pilulifera is beneficial in controlling glycemia, lowering lipid profile, and acting against oxidative stress mechanisms.
Author’s contribution:
Both authors were included in all steps of preparation this article. Final proof reading was made by the first author.
Conflict of interest:
None declared.
Financial support and sponsorship:
Nil.
REFERENCES
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Unwin N, Gan D, Whiting D. The IDF Diabetes Atlas: providing evidence, raising awareness and promoting action. Diabetes Res Clin Pract. 2010;87:2–3. doi: 10.1016/j.diabres.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Skovso Sos. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Invest. 2014;5:349–358. doi: 10.1111/jdi.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guilherme A, Virbasius J, Vishwajeet P, et al. Adipocyte dysfunction linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Lianzi, Tao Li, Liu Jiaqing, Xian Wu, Wang Huihui, Li Xuemei, et al. Association between glycosylated hemoglobin A1c and bone biochemical markers in type 2 diabetic postmenopausal women: a cross-sectional study. BMC Endocrine Disorders. 2019;19:31. doi: 10.1186/s12902-019-0357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn SE, Halban PA. Release of incompletely processed proinsulin is the cause of the disproportionate proinsulinemia of NIDDM. Diabetes. 1997;46:1725–1732. doi: 10.2337/diab.46.11.1725. [DOI] [PubMed] [Google Scholar]
- 7.Zakeri Z, Azizi Z, Mehrabifar H, Hashemi M. Evaluation of bone mineral density in premenopausal women with type-2 diabetes mellitus in Zahedan, Southeast Iran. J Pak Med Assoc. 2011;61(5):443–445. [PubMed] [Google Scholar]
- 8.Asokan AG, Jaganathan J, Philip R, Soman RR, Sebastian ST, Pullishery F. Evaluation of bone mineral density among type 2 diabetes mellitus patients in South Karnataka. J Nat Sci Biol Med. 2017;8(1):94–98. doi: 10.4103/0976-9668.198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katulanda GW, Katulanda P, Dematapitiya C, Dissanayake HA, Wijeratne S, Sheriff MHR, et al. Plasma glucose in screening for diabetes and pre-diabetes: how much is too much? Analysis of fasting plasma glucose and oral glucose tolerance test in Sri Lankans. BMC Endocrine Disorders. 2019;19:11. doi: 10.1186/s12902-019-0343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renner S, Fehlings C, Herbach N, et al. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose dependent insulinotropic polypeptide function. Diabetes. 2010;59:1228–1238. doi: 10.2337/db09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nunes S, Soares E, Fernandes J, Viana S, Carvalho E, et al. Early cardiac changes in a rat model of prediabetes: brain natriuretic peptide overexpression seems to be the best marker. Cardiovascular Diabetology. 2013;12:44. doi: 10.1186/1475-2840-12-44. http://www.cardiab.com/content/12/1/44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenzen S. The mechanisms of alloxan- and streptozotocin induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 13.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 14.Ferreira L, Teixeira-de-Lemos E, Pinto F, Parada B, Mega C, et al. Effects of Sitagliptin Treatment on Dysmetabolism, Inflammation, and Oxidative Stress in an Animal Model of Type 2 Diabetes (ZDF Rat) Mediators of Inflammation. 2010 doi: 10.1155/2010/592760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AlShuwayeb Mousa H, Al-Khatib Ahed J. Molecular and chemical therapeutic features of Urtica species. European scientific journal. 2013;9(24):253–261. [Google Scholar]
- 16.Alsarhan Ali, Sultana Naznin, Al-Khatib Ahed, Kadir Mohammed Rafiq Abdul. Review on some Malaysian traditional medicinal plants with therapeutic properties. Journal of Basic and Applied Sciences. 2014;10:149–159. [Google Scholar]
- 17.Abo-elmatty Dina M, Essawy Soha S, Badr Jihan M, Sterner Olov. Antioxidant and anti-inflammatory effects of Urtica pilulifera extracts in type2 diabetic rats. Journal of Ethnopharmacology. 2013;145:269–277. doi: 10.1016/j.jep.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 18.AlKhatib AJ, Syarmine Shah M, Alkhatatbeh MA, AlKhatib IA, Maikano AB, Shuaibu SM, et al. Urtica pilulifera ameliorates diabetic impacts through upregulation the expression of hsp70 in liver of diabetic rats. European scientific journal. 2014;10(24):296–302. [Google Scholar]
- 19.Bansal N. Prediabetes diagnosis and treatment: A review. World J Diabetes. 2015;6(2):296–303. doi: 10.4239/wjd.v6.i2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredersdorf S, Thumann C, Zimmermann WH, Vetter R, Graf T, Luchner A, Riegger GA, et al. Increased myocardial SERCA expression in early type 2 diabetes mellitus is insulin dependent: In vivo and in vitro data. Cardiovasc Diabetol. 2012;11:57. doi: 10.1186/1475-2840-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human disease. Archives of physiology and biochemistry. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 22.Toldy A, Stadler K, Sasvari M, Jakus J, Jung KJ, Chung HY, et al. The effect of exercise and nettle supplementation on oxidative stress markers in the rat brain. Brain Research Bulletin. 2005;65(6):487–493. doi: 10.1016/j.brainresbull.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 23.Milajerdi A, Keshteli HA, Afshar H, Esmaillzadeh A, Adibi P. Dietary total antioxidant capacity in relation to depression and anxiety in Iranian adults. Nutrition. 2018. [DOI] [PubMed]
- 24.Fatani SH, Babakr AT, Noureldin EM, Almarzouki AA. Lipid peroxidation is associated with poor control of type-2 diabetes mellitus. Diabetes Metab. Syndr. 2016;10(2):64–67. doi: 10.1016/j.dsx.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Ghazizadeh Z, Khaloo P, Alemi P, Rabizadeh S, Mirmiranpour H, Esteghamati A, Nakhjavani M. Definition of an oxidative stress status by combined assessment of Malondialdehyde and Oxidized-LDL: A study in patients with type2 diabetes and control. Meta Gene. 2019;19:91–97. [Google Scholar]
- 26.Palinski W, Rosenfeld ME, Yla-Herttuala S, Gurtner GC, Socher SS, Butler, et al. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–1214. [PubMed] [Google Scholar]


