Abstract
Background
Obstructive sleep apnea (OSA) is associated with cardiovascular co-morbidities and mortality. Arterial stiffness is an independent predictor of cardiovascular risk and mortality, and is influenced by the presence of OSA and related comorbidities. There is a paucity of data regarding long-term evolution of arterial stiffness in CPAP-treated OSA patients. We aimed to prospectively study long term PWV variations and determinants of PWV deterioration.
Methods
In a prospective obese OSA cohort, at time of diagnosis and after several years of follow-up we collected arterial stiffness measured by carotid-femoral pulse wave velocity (PWV), clinical and metabolic parameters, and CPAP adherence. Univariate and multivariate analyses were performed in order to determine contributing factors.
Results
Seventy two OSA patients (men: 52.8%, median age: 55.8 years and median BMI of 38.5 kg/m2) with a prevalence of hypertension: 58.3%, type 2 diabetes: 20.8%, hypercholesterolemia: 33.3%, current or past smoking: 59.7%, were evaluated after a median follow-up of 7.4 [5.8; 8.3] years. Over the period of follow-up, the median increase in PWV was 1.34 [0.10; 2.37] m/s. In multivariate analysis, the increase in PWV was associated with older age (10 extra years was associated with a 5.24 [1.35; 9.12] % increase in PWV) and hypertension (a significant increase in PWV of 8.24 [1.02; 15.57] %). No impact of CPAP adherence on PWV evolution was found.
Conclusion
PWV progression in CPAP-treated OSA patients is mainly related to pre-existing cardio-metabolic comorbidities and not influenced by CPAP adherence. In this high cardiovascular risk population, it is crucial to associated weight management and exercise with CPAP treatment.
Introduction
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of partial or complete obstruction of the upper airway during sleep, resulting in chronic intermittent hypoxia and sleep fragmentation. OSA is highly prevalent in obese patients with cardio-metabolic comorbidities. [1] The association between OSA and cardiovascular diseases has been clearly demonstrated, OSA being considered as an independent risk factor for cardiovascular and metabolic co-morbidities and mortality. [2–4] Continuous positive airway pressure (CPAP), the first line therapy for OSA, was reported to reduce the incidence of late cardiovascular events in patients with severe OSA in cohort observational studies. [5] However, in the largest recent randomized controlled trials, CPAP treatment did not reduce mortality or the occurrence of late cardiovascular events in intention to treat analyses. [6,7]
Arterial stiffness is an early independent predictor of cardiovascular risk and secondary occurrence of late incident cardiovascular events. [8–10] The gold standard measure of arterial stiffness is carotid-femoral pulse wave velocity (PWV).[11–13] A 1m/s increase in pulse wave velocity is associated with a 15% increase in mortality independently of other usual cardiovascular risk factors. Arterial stiffness has been suggested as having a dose-response relationship with indices of OSA severity. [8,14–16]
Arterial stiffness increases with age and blood pressure levels (BP); [17] and is linked with chronic conditions such as metabolic syndrome, [18] diabetes, [19] or chronic obstructive pulmonary disease (COPD). [20] All these conditions, which contribute to the lifelong increase in arterial stiffness, are highly prevalent in OSA patients. [21] The deterioration in arterial stiffness over time is sustained by intermediary mechanisms such as sympathetic over-activity, endothelial dysfunction, oxidative stress and systemic inflammation that are enhanced by OSA. [12,22]
There remains a debate regarding improvement in arterial stiffness under CPAP treatment. A recent meta-analysis [11] suggested an improvement but data were obtained from non-randomized studies assessing short term CPAP interventions with small sample sizes. [11,14] To date, no study has reported long term variations of arterial stiffness in CPAP-treated OSA patients. The goal of the current study was to prospectively assess the changes in PWV and their determinants in OSA patients treated by CPAP for at least four years (median duration of follow-up 7.5 years).
Materials and methods
Design and study population
Obese patients referred for sleep apnea to the Sleep department of Grenoble Alpes University Hospital between 2007 and 2010 were included in a prospective cohort study. These patients were re-examined after at least 4 years of CPAP treatment, with cardio-metabolic assessments including arterial stiffness. Hypertension was defined following the ESC/ESH guidelines. [23] At inclusion, patients were aged from 20 to 75 years with a body mass index (BMI) > 30 kg/m2. Patients with central apnea were excluded.
The study was conducted in accordance with good clinical practice requirements in Europe, French law, ICH E6 recommendations, and the Helsinki Declaration (1996 and 2000). The protocol was approved by an independent Ethics Committee (Comité de Protection des Personnes, Grenoble, France, IRB0006705) and registered on the ClinicalTrials.gov site (NCT02623088). All patients gave their written informed consent.
Sleep study and sleepiness assessment
Overnight polysomnography (PSG) was used to diagnose OSA and characterize severity. [24–26] The apnea-hypopnea index (AHI) was calculated as the number of apnea and hypopnea events per hour of sleep. Daytime sleepiness was evaluated using the Epworth Sleepiness Scale (ESS). [27] Mean nocturnal oxygen saturation (SaO2) and time spent under 90% of SaO2 were also collected in order to characterize sleep apnea severity. Overnight sleep studies were scored according to international guidelines. [28]
Arterial stiffness assessement
Carotid-femoral PWV, a validated measure of arterial stiffness, was assessed for each patient [12,13] using a Complior device (Alam Medical®, France). [29] Carotid-femoral PWV is the ratio on distance to transit time between two pressure waves recorded transcutaneously at carotid and femoral arterial sites. The distance travelled by the pulse wave was measured with an external tape-measure across the body surface. For the 30 min-long PWV measurements the subject was fasted and rested and in an elongated supine position. Two electrodes were placed one on the carotid artery and the other on the femoral artery until a quality signal was obtained, characterized by a clear rise of the systolic curve and a smooth diastolic curve for at least 10 seconds. At least two PWV measurements were systematically done. The mean value between the two measurements was retained if the difference between measurements was less than 0.5 m/s. When the difference was above 0.5 m/s, a third measurement was made and the median value of the three measurements was used.
Metabolic and inflammatory biomarkers
On waking, after 10 hours fasting, a peripheral blood sample was drawn. Fasting glucose, HbA1c, serum insulin, lipids, and high-sensitivity C-reactive protein (hsCRP) levels were measured using standard procedures.
Respiratory function
Arterial blood gas measurements and pulmonary function tests (measured using Medisoft® devices) were performed. Significant airway obstruction was defined as FEV1/FVC<70%, according to standard definitions. [30]
CPAP treatment
According to French and international recommendations, [31] patients with moderate or severe OSA were treated with CPAP. [2,32] Adherence was defined as a mean CPAP use of at least 4 hours per night. [33] CPAP adherence used for data analysis was corresponding to objective compliance measured in the 3 to 6 months preceding follow-up visit.
Follow-up
After 4 to 9 years of follow-up, new measurements of the same parameters as at baseline were done, except for PSG.
Statistical analysis
Statistical analyses were performed with SAS v9.4 software (SAS Institute Inc., Cary, NC, United States). A p-value < 0.05 was considered as significant. Continuous data are presented as median and interquartile range (IQR) and categorical data as frequency and percentage. A comparison of the main quantitative variables at baseline and at follow-up was performed using a non-parametric Mann-Whitney test. A non-parametric Wilcoxon signed-rank test was used to compare the PWV before and after CPAP use. Due to the non-normality of PWV values, a log-transformation was performed and a log-linear mixed effect model with a patient random effect adjusted for the delay between the two measurements was used to analyze the evolution in arterial stiffness. A univariate analysis between PWV and potentially contributing factors was performed to select variables for the multivariate model. Variables with a p-value less or equal to 0.20 were retained and introduced into the multivariate analysis in association with predefined clinically relevant variables. Adjustment for age, sex and CPAP treatment. Due to the log transformation of the PWV, the final estimate presented in the multivariate analysis corresponded to 100*Beta (where beta was a parameter of the log-linear model and can be directly interpreted as the percent of increase or decrease in the PWV at follow-up). Due to the low number of missing values, a simple imputation method was used to impute missing data: quantitative variables were imputed using the median and qualitative variables were imputed using the most frequent value.
Results
Patient characteristics
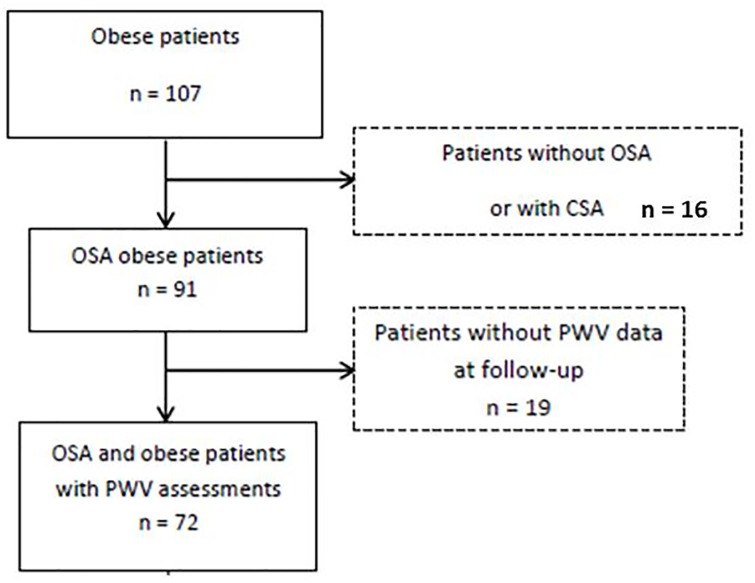
As shown in the study flowchart (Fig 1), 107 obese patients were initially included in this prospective cohort. Among them, 91 were followed and treated for OSA and for 72 patients PWV was reassessed at long-term.
Fig 1. Study flow chart.
CSA, central sleep apnea; OSA, obstructive sleep apnea; PWV, pulse wave velocity.
At inclusion, patients had a median age of 55.8 [47.4; 62.0] years, 52.8% were men, with a median (IQR) BMI of 38.5 [35.4; 43.1] kg/m2. Median (IQR) AHI at diagnosis was 36.1 [23.3; 75.2] events/hour. Patients with hypertension (58.3%), had type 2 diabetes (20.8%) and were current or former smokers (59.7%). Baseline data concerning medical history, comorbidities, arterial blood gases, biological parameters, sleep studies and pulmonary function tests are shown in Table 1. The comparison between imputed and non-imputed datasets is available in S1 Table of the online supplement.
Table 1. Study population characteristics at baseline.
| Anthropometric and biological characteristics | |
| Age, (years) | 55.8 [47.4–62] |
| Men | 38 (52.8) |
| BMI, (kg/m2) | 38.5 [35.4–43.1] |
| Hypertension, n (%) | 42 (58.3) |
| Stroke, n (%) | 3 (4.2) |
| Diabetes mellitus, n (%) | 15 (20.8) |
| Hypercholesterolemia, n (%) | 24 (33.3) |
| Smoking, n (%) | 40 (59.7) |
| SBP, (mmHg) | 132 [122–140] |
| DBP, (mmHg) | 79.5 [70–85] |
| HbA1c, (%) | 5.8 [5.5–6.3] |
| Fasting blood Glucose, (mmol/l) | 5.7 [5.3–6.2] |
| Insulinemia, (μUl/ml) | 8.7 [6.4–13.3] |
| hsCRP, (mg/l) | 4.2 [2.1–8.9] |
| Respiratory function | |
| FVC, (% of predicted value) | 99 [84–106] |
| FEV1, (% of predicted value) | 92 [82–103] |
| FEV1/ FVC, (%) | 80.6 [75.5–84.1] |
| FEV1/ FVC < 70%, n (%) | 7 (10.1) |
| TLC, (% of predicted value) | 103.5 [96.5–114] |
| PaCO2, (kPa) | 5.3 [5–5.6] |
| PaO2, (kPa) | 10.2 [9.6–11.2] |
| Sleep disordered breathing | |
| Epworth Sleepiness Scale | 12 [8–16] |
| AHI, (/hour) | 36.1 [23.3–75.2] |
| Mean nocturnal SpO2, (%) | 92 [89–94] |
| Sleep time spent with SpO2 < 90%, (% of total sleep time) | 11 [2–43] |
| PWV (m/s) | 9.7 [8.5–10.7] |
Categorical variables are expressed as a percentage and quantitative variables as the median (IQR). AHI, apnea hypopnea index; BMI, body mass index; DBP, diastolic blood pressure; FEV1, Forced Expiratory Volume of the first second of forced expiration; FVC, Forced Vital Capacity; HbA1c, Glycated hemoglobin; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; PWV, Pulse Wave Velocity; SBP, systolic blood pressure; SpO2, oxygen saturation; TLC, Total lung capacity.
Follow-up
The median duration of follow-up was 7.5 years. At baseline, the median value of PWV was 9.7 m/s. At the follow-up PWV assessment, the median value was 10.5 m/s corresponding to a median increase of 1.34 m/s over the follow-up period. There was a significant difference of PWV between and after CPAP use (p<0.01).
CPAP adherence of at least 4 hours/night was recorded for 72% of the patients and the median adherence to CPAP was 6.4 [5.1; 7.5] hours per night (Tables 2 & 3). The medications being used at the time of the follow-up visit are shown in S2 Table of the online supplemental material.
Table 2. Data at the follow-up PWV assessment.
| Follow-up time, (years) | 7.5 [5.8–8.3] |
| PWV, (m/s) | 10.5 [9.6–12.7] |
| Patients adherent to CPAP, n (%) | 52 (72.2) |
| Adherence to CPAP, (hours per night) | 6.4 [5.1–7.5] |
| PWV increase during the complete follow-up period, (m/s) | 1.34 [0.10–2.37] |
| PWV increase per year, (m/s per year) | 0.19 [0.01–0.36] |
Categorical variables are expressed as percentage and quantitative variables as median (IQR). PWV, Pulse Wave Velocity.
Table 3. Comparison between baseline and follow-up values.
| Variable | Baseline | Follow-up | P |
|---|---|---|---|
| BMI (kg/m2) | 38.5 [35.4; 43.1] | 38.4 [34.3; 41.9] | 0.19 |
| SBP (mmHg) | 132 [122; 140] | 132 [122; 138] | 0.44 |
| DBP (mmHg) | 79.5 [70; 85] | 74 [68; 81] | <.01 |
| hsCRP (mg/l) | 5.8 [5.5; 6.3] | 5.9 [5.7; 6.5] | 0.61 |
| Fasting Glucose (mmol/l) | 5.7 [5.3; 6.2] | 6.1 [5.4; 7] | 0.02 |
| Insulinemia (μUl/ml) | 8.7 [6.4; 13.3] | 12.2 [7.9; 16.7] | 0.08 |
| hsCRP (mg/l) | 4.2 [2.1; 8.9] | 4.1 [1.7; 5.8] | 0.22 |
| PaCO2 (kPa) | 5.3 [5; 5.6] | 4.9 [4.6; 5.2] | <.01 |
| PaO2 (kPa) | 10.2 [9.6; 11.2] | 11 [10.1; 11.8] | <.01 |
| Epworth Sleepiness Scale | 12 [8; 16] | 7 [4; 10] | <.01 |
BMI, body mass index; hs-CRP, high sensitivity C-reactive protein; DBP, diastolic blood pressure; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; SBP, systolic blood pressure.
P: p value for the non-parametric Mann-Whitney test.
Determinants of arterial stiffness deterioration
Univariate analysis
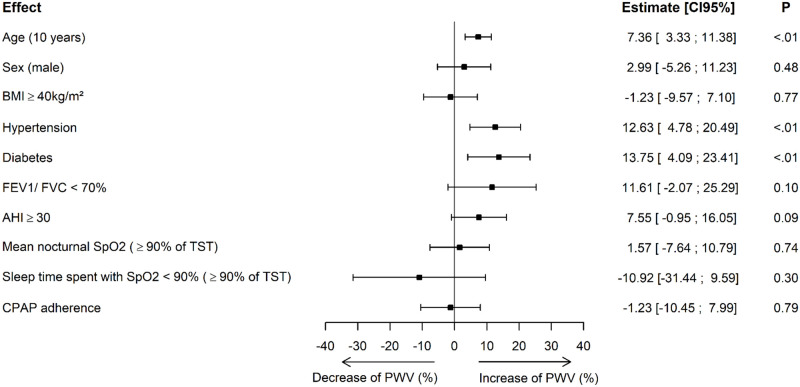
A ten year increase in age was associated with a 7.36% increase in PWV (p<0.01). High blood pressure at baseline was associated with a 12.63% increase in PWV compared to normotensive patients (p<0.01) and having diabetes was associated with a 13.75% increase in PWV compared to patients without diabetes (p<0.01). There was no association between changes in PWV results over the years and BMI or indices of OSA severity at baseline. CPAP adherence was not linked to change in PWV. (Fig 2).
Fig 2. Univariate analysis.
BMI, body mass index; FEV1, Forced Expiratory Volume in the first second of forced expiration; FVC, Forced Vital Capacity; AHI, apnea hypopnea index; TST, total sleep time, CPAP, continuous positive airway pressure. Interpretation: An increase of ten years in age is associated with a 7.36% increase in PWV. Having high blood pressure at baseline was associated with a 12.63% increase in PWV compared to normotensive patients. Having diabetes was associated with a 13.75% increase in PWV compared to patients without diabetes.
Multivariate analysis
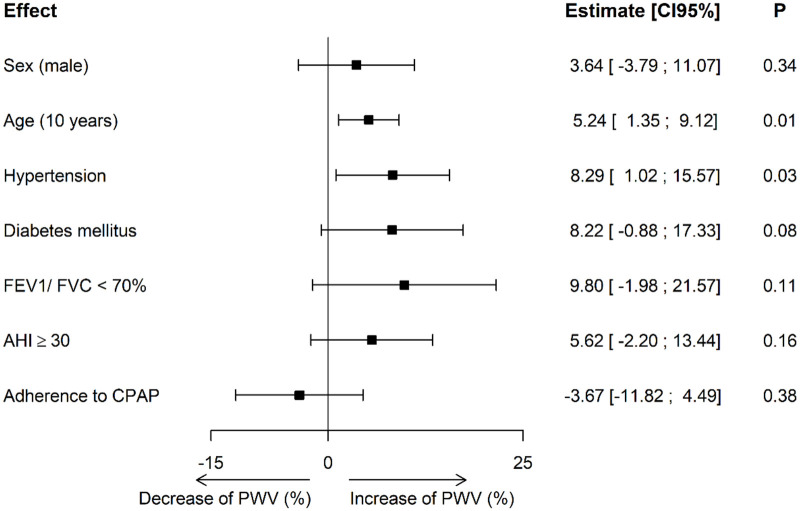
After adjustment for follow-up duration, age, gender, hypertension, diabetes, COPD, and CPAP adherence PWV was shown to increase significantly more in CPAP-treated OSA patients with hypertension (p = 0.03). A trend close to significance was apparent for type 2 diabetic patients (p = 0.08) and with airway obstruction (p = 0.11). The multivariate analysis did not demonstrate a long-term impact of CPAP adherence on PWV evolution (p = 0.54). (Fig 3).
Fig 3. Multivariate analysis.
FEV1, Forced Expiratory Volume of the first second of forced expiration; FVC, Forced Vital Capacity; AHI, apnea hypopnea index; CPAP, continuous positive airway pressure. Interpretation: A 10-year increase in age was associated with a 5.24% increase in PWV. Compared to the baseline PWV value of the multivariate model, this is associated to a significant increase of 0.35m/s of PWV for 10-year age increase. Having hypertension at baseline was associated to a significant increase in PWV of 8.24%.
Discussion
To our knowledge, this is the first study assessing long-term variations (median follow-up 7.5 years) of arterial stiffness in obese OSA CPAP-treated patients. During this period, the median PWV increase under CPAP was 1.34 m/s. In multivariate analysis, PWV progression was significantly dependent of age and hypertensive status. Neither indices of OSA severity at diagnosis nor CPAP adherence contributed significantly to the long-term trajectory of arterial stiffness.
Sleep apnea is known to impact vascular age. [14,34,35] We should compare our obese OSA population (median age 55 years) to the same age group in the general population, they probably show greater arterial stiffness at baseline, as assessed by PWV. The Arterial Stiffness Collaboration [36] reported a median (± 2 SD) PWV of 8.1 (6.3–10.0) m/s for the 50–59 year age group in the healthy population compared to 9.7 [8.5; 10.7] in our study population. A 1 m/s increase in aortic PWV corresponds to a 15% increase in all-cause mortality after adjustment for confounders. [8] This association between OSA and elevated measurements of arterial stiffness had been previously described independently of BP [15,37] or metabolic syndrome. [38] However, in a recent individual patient meta-analysis, [39] we showed that cross-sectional elevated arterial stiffness in patients with OSA is mainly driven by the conventional cardiovascular risk factors; age, BP and the presence of diabetes, while apnea severity indices had limited influence. The current data extend these results by demonstrating that long-term OSA treatment by CPAP does not check the progression in arterial stiffness.
A PWV decrease after CPAP initiation had been reported in several mostly small sample size, uncontrolled and short-term studies. [11] The largest study with a long-term follow-up showed that PWV decreased significantly over the first 6 months of treatment and then gradually increased between 6 and 24 months. [40] As in our study this late increase in PWV might be explained not only by age-related progression in arterial stiffness but also by the long-term burden of uncontrolled co-morbidities.
Hypertension is the main condition associated with PWV progression, with a reciprocal relationship between the two. [36,41–43] Severe OSA and hypertension are both associated with an increase in arterial stiffness, with cumulative effects when the two diseases coexist [14,34,44,45] In morbidly obese OSA patients CPAP has been shown to produce a small but significant reduction in blood pressure in relatively short term randomized controlled trials. [46] The SAVE study showed a non-significant systolic blood pressure difference between CPAP-treated and usual care groups of <1.0 mmHg over a mean follow-up of 3.7 years. [47] Further data on mean BP and visit-to-visit BP variability (BPV) over the first 24-months of the SAVE study have recently been reported. [48] The initial reduction in visit-to-visit BPV and mean BP was lost after 12 months and was associated with a decrease in CPAP adherence. These results are in accordance with our findings, suggesting that non-sustained reductions in mean BP and the relatively small potential effect size of CPAP are not enough to counteract the development of comorbidities and limit arterial stiffness progression. CPAP adherence was relatively high in our study population but no reduction in PWV values was observed. The follow-up long term assessment did not include a PSG as CPAP efficiency was evaluated by the index of residual events downloaded from the CPAP software’s. It is unexpected that the severity of OSA changed dramatically as there was no significant change in BMI (38.5 [35.4; 43.1] versus 38.4 [34.3; 41.9] for baseline and follow-up respectively; Table 3).
Other acknowledged contributors to arterial stiffness progression are type 2 diabetes or glucose intolerance in pre-diabetic states, [19,48] and metabolic syndrome. [49] Again, OSA and metabolic syndrome synergistically act to increase PWV [38] and type 2 diabetes has a major impact toward increasing arterial stiffness in patients with metabolic syndrome. [49,50] In the present study, the association with type 2 diabetes did not reach significance in multivariate analysis, but this can certainly be explained by an insufficient sample size resulting in lack of statistical power.
The combination of COPD and OSA is called “overlap syndrome” [51] and is associated with a worse prognosis compared to that of patients with only one of the two diseases. [52–55] Our data failed to show an independent association between COPD and high arterial stiffness [20,56–58] and additive effects of COPD on the cardiovascular damage seen in patients with OSA. [59]
Conclusion and perspectives
There is an increase in PWV over the study period. In multivariate analysis, determinants of PWV progression are old age and hypertension. Optimal management of OSA-associated comorbidities is needed for patients on CPAP treatment [60,61] in order to slow deterioration in arterial stiffness, reduce the occurrence of late cardiovascular events and to improve survival.
Supporting information
AHI, apnea hypopnea index; HbA1c, Glycated hemoglobin; hs-CRP, high sensitivity C-reactive protein; DBP, diastolic blood pressure; FEV1, Forced Expiratory Volume of the first second of forced expiration; FVC, Forced Vital Capacity; SBP, systolic blood pressure; SpO2, oxygen saturation; TLC, total lung capacity.
(DOCX)
(DOCX)
(CSV)
(CSV)
(CSV)
Acknowledgments
Guarantor statement: Jean-Louis Pépin takes responsibility for the content of the manuscript, including the data and analysis. Other contributions: We thank Dr Alison Foote (Grenoble Alpes University Hospital) for critically editing the manuscript.
Abbreviations
- AHI
apnea-hypopnea index
- BP
Blood pressure
- BPV
BP variability
- COPD
chronic obstructive pulmonary disease
- CPAP
Continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- FEV1
Forced Expiratory Volume in the first second of forced expiration
- FVC
Forced Vital Capacity
- hsCRP
high-sensitivity C-reactive protein
- OSA
Obstructive sleep apnea
- PWV
Pulse wave velocity
- SaO2
Mean nocturnal oxygen saturation
- TLC
Total lung capacity
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded by an unrestricted grant from the French National Research Agency (ANR-12-TECS-0010) in the framework of the “Investissements d’avenir” program (ANR-15-IDEX-02), the “e-health and integrated care” Chair of excellence of the University Grenoble Alpes Foundation and the endowment fund “Agir pour les maladies chroniques”. This study was funded in part by ORKYN Society and Périmètre Association.
References
- 1.Pépin JL, Timsit JF, Tamisier R, Borel JC, Lévy P, Jaber S. Prevention and care of respiratory failure in obese patients. Lancet Respir Med. 2016;4: 407–418. 10.1016/S2213-2600(16)00054-0 [DOI] [PubMed] [Google Scholar]
- 2.Lévy P, Kohler M, McNicholas WT, Barbé F, McEvoy RD, Somers VK, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primer. 2015; 15015 10.1038/nrdp.2015.15 [DOI] [PubMed] [Google Scholar]
- 3.Murphy AM, Thomas A, Crinion SJ, Kent BD, Tambuwala MM, Fabre A, et al. Intermittent hypoxia in obstructive sleep apnoea mediates insulin resistance through adipose tissue inflammation. Eur Respir J. 2017;49. [DOI] [PubMed] [Google Scholar]
- 4.Aron-Wisnewsky J, Clement K, Pépin J-L. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65: 1124–1135. 10.1016/j.metabol.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 5.Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365: 1046–1053. 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- 6.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med. 2016;375: 919–931. 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 7.Drager LF, McEvoy RD, Barbe F, Lorenzi-Filho G, Redline S, INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists). Sleep Apnea and Cardiovascular Disease: Lessons From Recent Trials and Need for Team Science. Circulation. 2017;136: 1840–1850. 10.1161/CIRCULATIONAHA.117.029400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55: 1318–1327. 10.1016/j.jacc.2009.10.061 [DOI] [PubMed] [Google Scholar]
- 9.Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121: 505–511. 10.1161/CIRCULATIONAHA.109.886655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertens Dallas Tex 1979. 2001;37: 1236–1241. [DOI] [PubMed] [Google Scholar]
- 11.Vlachantoni I-T, Dikaiakou E, Antonopoulos CN, Daskalopoulou SS, Petridou ET. Effects of continuous positive airway pressure (CPAP) treatment for obstructive sleep apnea in arterial stiffness: a meta-analysis. Sleep Med Rev. 2013;17: 19–28. 10.1016/j.smrv.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 12.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27: 2588–2605. 10.1093/eurheartj/ehl254 [DOI] [PubMed] [Google Scholar]
- 13.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30: 445–448. 10.1097/HJH.0b013e32834fa8b0 [DOI] [PubMed] [Google Scholar]
- 14.Phillips CL, Butlin M, Wong KK, Avolio AP. Is obstructive sleep apnoea causally related to arterial stiffness? A critical review of the experimental evidence. Sleep Med Rev. 2013;17: 7–18. 10.1016/j.smrv.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 15.Doonan RJ, Scheffler P, Lalli M, Kimoff RJ, Petridou ET, Daskalopoulos ME, et al. Increased arterial stiffness in obstructive sleep apnea: a systematic review. Hypertens Res Off J Jpn Soc Hypertens. 2011;34: 23–32. 10.1038/hr.2010.200 [DOI] [PubMed] [Google Scholar]
- 16.Chung S, Yoon I-Y, Lee CH, Kim J-W. The association of nocturnal hypoxemia with arterial stiffness and endothelial dysfunction in male patients with obstructive sleep apnea syndrome. Respir Int Rev Thorac Dis. 2010;79: 363–369. [DOI] [PubMed] [Google Scholar]
- 17.McEniery CM, Yasmin null, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR, et al. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46: 1753–1760. 10.1016/j.jacc.2005.07.037 [DOI] [PubMed] [Google Scholar]
- 18.Koivistoinen T, Hutri-Kähönen N, Juonala M, Aatola H, Kööbi T, Lehtimäki T, et al. Metabolic syndrome in childhood and increased arterial stiffness in adulthood: the Cardiovascular Risk In Young Finns Study. Ann Med. 2011;43: 312–319. 10.3109/07853890.2010.549145 [DOI] [PubMed] [Google Scholar]
- 19.Prenner SB, Chirinos JA. Arterial stiffness in diabetes mellitus. Atherosclerosis. 2015;238: 370–379. 10.1016/j.atherosclerosis.2014.12.023 [DOI] [PubMed] [Google Scholar]
- 20.Vivodtzev I, Tamisier R, Baguet J-P, Borel JC, Levy P, Pépin J-L. Arterial stiffness in COPD. Chest. 2014;145: 861–875. 10.1378/chest.13-1809 [DOI] [PubMed] [Google Scholar]
- 21.Lévy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J. 2009;34: 243–260. 10.1183/09031936.00166808 [DOI] [PubMed] [Google Scholar]
- 22.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117: 2270–2278. 10.1161/CIRCULATIONAHA.107.741512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36: 1953–2041. 10.1097/HJH.0000000000001940 [DOI] [PubMed] [Google Scholar]
- 24.International Classification of Sleep Disorders 3rd ed. American Academy of Sleep Medicine. 2014. [DOI] [PMC free article] [PubMed]
- 25.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146: 1387–1394. 10.1378/chest.14-0970 [DOI] [PubMed] [Google Scholar]
- 26.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2012;8: 597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14: 540–545. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 28.Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, et al. AASM Scoring Manual Updates for 2017 (Version 2.4). J Clin Sleep Med JCSM Off Publ Am Acad Sleep Med. 2017;13: 665–666. 10.5664/jcsm.6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26: 485–490. 10.1161/01.hyp.26.3.485 [DOI] [PubMed] [Google Scholar]
- 30.Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD Science Committee Report 2019. Eur Respir J. 2019. [DOI] [PubMed] [Google Scholar]
- 31.Société de Pneumologie de Langue Française, Société Française d’Anesthésie Réanimation, Société Française de Cardiologie, Société Française de Médecine du Travail, Société Française d’ORL, Société de Physiologie, et al. [Recommendations for clinical practice. Obstructive sleep apnea hypopnea syndrome in adults]. Rev Mal Respir. 2010;27: 806–833. 10.1016/j.rmr.2010.05.011 [DOI] [PubMed] [Google Scholar]
- 32.Gay P, Weaver T, Loube D, Iber C, Positive Airway Pressure Task Force, Standards of Practice Committee, et al. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29: 381–401. 10.1093/sleep/29.3.381 [DOI] [PubMed] [Google Scholar]
- 33.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5: 173–178. 10.1513/pats.200708-119MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131: 1379–1386. 10.1378/chest.06-2703 [DOI] [PubMed] [Google Scholar]
- 35.Pépin J-L, Tamisier R, Baguet J-P, Lévy P. Arterial health is related to obstructive sleep apnea severity and improves with CPAP treatment. Sleep Med Rev. 2013;17: 3–5. 10.1016/j.smrv.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 36.Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values”. Eur Heart J. 2010;31: 2338–2350. 10.1093/eurheartj/ehq165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest. 2011;140: 534–542. 10.1378/chest.10-2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drager LF, Bortolotto LA, Maki-Nunes C, Trombetta IC, Alves MJNN, Fraga RF, et al. The incremental role of obstructive sleep apnoea on markers of atherosclerosis in patients with metabolic syndrome. Atherosclerosis. 2010;208: 490–495. 10.1016/j.atherosclerosis.2009.08.016 [DOI] [PubMed] [Google Scholar]
- 39.Joyeux-Faure M, Tamisier R, Borel J-C, Millasseau S, Galerneau L-M, Destors M, et al. Contribution of obstructive sleep apnoea to arterial stiffness: a meta-analysis using individual patient data. Thorax. 2018. 10.1136/thoraxjnl-2018-211513 [DOI] [PubMed] [Google Scholar]
- 40.Saito T, Saito T, Sugiyama S, Asai K, Yasutake M, Mizuno K. Effects of long-term treatment for obstructive sleep apnea on pulse wave velocity. Hypertens Res Off J Jpn Soc Hypertens. 2010;33: 844–849. 10.1038/hr.2010.77 [DOI] [PubMed] [Google Scholar]
- 41.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31: 1281–1357. 10.1097/01.hjh.0000431740.32696.cc [DOI] [PubMed] [Google Scholar]
- 42.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308: 875–881. 10.1001/2012.jama.10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin X, Chen G, Qi J, Chen X, Zhao J, Lin Q. Effect of continuous positive airway pressure on arterial stiffness in patients with obstructive sleep apnea and hypertension: a meta-analysis. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol—Head Neck Surg. 2016;273: 4081–4088. 10.1007/s00405-016-3914-8 [DOI] [PubMed] [Google Scholar]
- 44.Tsioufis C, Thomopoulos K, Dimitriadis K, Amfilochiou A, Tousoulis D, Alchanatis M, et al. The incremental effect of obstructive sleep apnoea syndrome on arterial stiffness in newly diagnosed essential hypertensive subjects. J Hypertens. 2007;25: 141–146. 10.1097/HJH.0b013e32801092c1 [DOI] [PubMed] [Google Scholar]
- 45.Tavil Y, Kanbay A, Sen N, Ulukavak Ciftçi T, Abaci A, Yalçin MR, et al. The relationship between aortic stiffness and cardiac function in patients with obstructive sleep apnea, independently from systemic hypertension. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2007;20: 366–372. 10.1016/j.echo.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 46.Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs Mandibular Advancement Devices and Blood Pressure in Patients With Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. JAMA. 2015;314: 2280–2293. 10.1001/jama.2015.16303 [DOI] [PubMed] [Google Scholar]
- 47.Van Ryswyk E, Anderson CS, Barbe F, Loffler KA, Lorenzi-Filho G, Luo Y, et al. Effect of CPAP on Blood Pressure in Obstructive Sleep Apnea with Cardiovascular Disease. Am J Respir Crit Care Med. 2019. 10.1164/rccm.201811-2200LE [DOI] [PubMed] [Google Scholar]
- 48.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54: 1328–1336. 10.1161/HYPERTENSIONAHA.109.137653 [DOI] [PubMed] [Google Scholar]
- 49.Pietri P, Vlachopoulos C, Vyssoulis G, Ioakeimidis N, Stefanadis C. Macro- and microvascular alterations in patients with metabolic syndrome: sugar makes the difference. Hypertens Res Off J Jpn Soc Hypertens. 2014;37: 452–456. 10.1038/hr.2013.148 [DOI] [PubMed] [Google Scholar]
- 50.Safar ME, Balkau B, Lange C, Protogerou AD, Czernichow S, Blacher J, et al. Hypertension and vascular dynamics in men and women with metabolic syndrome. J Am Coll Cardiol. 2013;61: 12–19. 10.1016/j.jacc.2012.01.088 [DOI] [PubMed] [Google Scholar]
- 51.Malhotra A, Schwartz AR, Schneider H, Owens RL, DeYoung P, Han MK, et al. Research Priorities in Pathophysiology for Sleep-disordered Breathing in Patients with Chronic Obstructive Pulmonary Disease. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2018;197: 289–299. 10.1164/rccm.201712-2510ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182: 325–331. 10.1164/rccm.200912-1869OC [DOI] [PubMed] [Google Scholar]
- 53.Zamarrón C, García Paz V, Morete E, del Campo Matías F. Association of chronic obstructive pulmonary disease and obstructive sleep apnea consequences. Int J Chron Obstruct Pulmon Dis. 2008;3: 671–682. 10.2147/copd.s4950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stone IS, Barnes NC, Petersen SE. Chronic obstructive pulmonary disease: a modifiable risk factor for cardiovascular disease? Heart Br Card Soc. 2012;98: 1055–1062. 10.1136/heartjnl-2012-301759 [DOI] [PubMed] [Google Scholar]
- 55.Lee HM, Lee J, Lee K, Luo Y, Sin DD, Wong ND. Relation between COPD severity and global cardiovascular risk in US adults. Chest. 2012;142: 1118–1125. 10.1378/chest.11-2421 [DOI] [PubMed] [Google Scholar]
- 56.McAllister DA, Maclay JD, Mills NL, Mair G, Miller J, Anderson D, et al. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176: 1208–1214. 10.1164/rccm.200707-1080OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weir-McCall JR, Struthers AD, Lipworth BJ, Houston JG. The role of pulmonary arterial stiffness in COPD. Respir Med. 2015;109: 1381–1390. 10.1016/j.rmed.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vanfleteren LEGW Spruit MA, Groenen MTJ Bruijnzeel PLB, Taib Z Rutten EPA, et al. Arterial stiffness in patients with COPD: the role of systemic inflammation and the effects of pulmonary rehabilitation. Eur Respir J. 2014;43: 1306–1315. 10.1183/09031936.00169313 [DOI] [PubMed] [Google Scholar]
- 59.Shiina K, Tomiyama H, Takata Y, Yoshida M, Kato K, Nishihata Y, et al. Overlap syndrome: additive effects of COPD on the cardiovascular damages in patients with OSA. Respir Med. 2012;106: 1335–1341. 10.1016/j.rmed.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 60.Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370: 2265–2275. 10.1056/NEJMoa1306187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vivodtzev I, Tamisier R, Croteau M, Borel J-C, Grangier A, Wuyam B, et al. Ventilatory support or respiratory muscle training as adjuncts to exercise in obese CPAP-treated patients with obstructive sleep apnoea: a randomised controlled trial. Thorax. 2018. 10.1136/thoraxjnl-2017-211152 [DOI] [PubMed] [Google Scholar]