Abstract
Hypoxia-induced erythropoietin signaling plays an important role in tumor growth and invasion. In the present study, we investigated the contribution of erythropoietin signaling pathway to castration-resistant prostate cancer and the development of a neuroendocrine phenotype. Immunohistochemical staining showed that the erythropoietin and erythropoietin receptor scores in castration-resistant prostate cancer and androgen-dependent prostate cancer were 7.55 versus 4.5 and 7.45 versus 5.9, respectively (P < 0.001). Furthermore, a cell proliferation assay was conducted, and the differential expression of erythropoietin and erythropoietin receptor in LNCaP cells and hypoxia-induced LNCaP cells was evaluated using western blot and quantitative real-time PCR. The proliferation capacity of hypoxia-induced LNCaP cells was similar in cultures of both fetal bovine serum and charcoal-stripped fetal bovine serum, suggesting that LNCaP cells acquired hypoxia-induced androgen-independent growth. After 2 weeks of hypoxic culture, LNCaP cells showed a neuroendocrine cell change and increased expression of neuron-specific enolase, erythropoietin, and erythropoietin receptor; knockdown of erythropoietin receptor reversed the hypoxia-induced upregulation of neuron-specific enolase in the LNCaP cells. In conclusion, the concurrent upregulation of erythropoietin and erythropoietin receptor in castration-resistant prostate cancer suggests that the erythropoietin/erythropoietin receptor autocrine loop plays an important role in the progression of castration resistance and is responsible for the development of a neuroendocrine phenotype.
Keywords: erythropoietin, erythropoietin receptor, hypoxia, prostatic neoplasm
INTRODUCTION
Prostate carcinoma (PCa) is the second-most frequent cancer and the leading cause of cancer death among males worldwide.1 Hypoxia is believed to play an important role in the progression of PCa, particularly in castration-resistant progression.2 However, the detailed mechanism remains unknown.
Erythropoietin (EPO) and erythropoietin receptor (EPOR) function as downstream targets of the hypoxia signaling pathway in PCa, as reported in our earlier studies.3,4 We found different patterns of EPO/EPOR upregulation in normal, benign, and malignant human prostatic tissues; in addition, we observed higher expression levels of EPO and EPOR in the tissues obtained from PCa and high-grade prostate intraepithelial neoplasia.3,4 Thus, in the present study, we further investigated the upregulation of EPO/EPOR as a possible mechanism to explain the progression of hypoxia-related, castration-resistant PCa.
MATERIALS AND METHODS
Clinical samples
The study protocols involving human materials were approved by the Institutional Ethics Committee of Changhai Hospital (Shanghai, China). All of the samples were obtained from specimens archived in Changhai Hospital with informed consent signed by the patients. Samples of benign prostatic hyperplasia (BPH) were obtained from 20 patients with an age range of 58–76 years (mean ± s.d.: 70.0±3.2 years) who had undergone a procedure for the transurethral enucleation of the prostate. Samples from patients with androgen-dependent prostate cancer (ADPC) were obtained from 20 patients with an age range of 59–75 years (mean ± s.d.: 71.0±4.6 years) who had undergone a prostate biopsy in the hospital but had not taken endocrine therapy before the biopsy. Samples from patients with castration-resistant prostate cancer (CRPC) were obtained from 20 patients (age: 59–77 years, mean ± s.d.: 73.0±3.4 years) who had received a transurethral resection of the prostate with previous therapy methods, including androgen deprivation therapy and castration and androgen blockade; all of these patients had received a diagnosis of CRPC before their surgeries. Hematoxylin and eosin (H & E)-stained slides of all cases were reviewed, and the diagnoses were confirmed by two senior pathologists.
Immunohistochemical staining
Immunohistochemical assays were performed on formalin-fixed, paraffin-embedded sections, as previously described.5 The following rat polyclonal EPO and EPOR primary antibodies were used: EPO (H-162, sc-7956) and EPOR (C-20, sc-695) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Then, 4- thick glass slide sections were deparaffinized in Hemo-D and rehydrated in graded alcohols, followed by an endogenous peroxidase block in 3% H2O2 and antigen retrieval in boiling 10% citrate buffer. Then, slides were incubated with the polyclonal antibodies against EPO and EPOR (1:200 dilution) overnight at 4°C and subsequently incubated with a horseradish peroxidase (HRP)-labeled dextran polymer coupled to an anti-rabbit antibody for 30 min at room temperature, after which they were washed three times with Tris-buffered saline containing Tween-20 (TBST, pH = 7.6). Finally, the slides were developed with diaminobenzidine for 10 min and counterstained with hematoxylin after washing three times with TBST.
The specificity of the staining was confirmed by processing sections from the same paraffin block without the primary antibody (negative control). Cytoplasmic or membrane staining that was clearly distinguishable from the background was considered positive. At least 500 epithelial cells that showed a positive immunoreactivity within each area were evaluated for ADPC and CRPC. The percentages of cells with no staining (0) or weak (1), moderate (2), or intense staining (3) were determined by a visual inspection under ×100, and a staining score was calculated through Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) using the following formula: the weighted mean of stain intensity (intensity percentage of cells)/total percentage of cells. The scoring system not only considered the staining intensity but also considered the percentages of the cells that exhibit EPO and EPOR staining. EPO and EPOR expressions were graded semi-quantitatively according to the results of the staining score. The slides were evaluated twice at different times by two investigators who were unaware of the pathologic characteristics of the samples, and the resulting mean level was also used for the statistical analyses.
Cell culture
Androgen-dependent human prostate cancer LNCaP cells were purchased from ATCC and stored in our laboratory. LNCaP cells were grown in RPMI 1640 (with phenol red) (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 1% penicillin–streptomycin, or grown in RPMI 1640 (without phenol red) supplemented with 10% charcoal-stripped fetal bovine serum (CS-FBS; Gibco) for the assay of androgen-independent growth. Cells were cultured in HERAcell incubators (Thermo Electronic Corporation, Asheville, NC, USA) at 37°C, 95% relative humidity, and 5% CO2 with 20% oxygen for the normoxia conditions or 1% oxygen for the hypoxia conditions.
Cell proliferation assay
The proliferation rates were determined using a CCK-8 Kit (Tojindo, Shanghai, China) according to the manufacturer's instructions. LNCaP cells under and not under hypoxic conditions were cultured in the CS-FBS and in normal medium containing 10 nmol l−1 dihydrotestosterone (DHT), respectively. Cells were plated in a 96-well plate with 1 × 103 cell per well and were cultured for specific time periods. Each well of cells was then incubated in 20 μl of CCK-8 solution in addition to the 200 μl of culture medium for 1.5 h at 37°C. The absorbance was measured at 450 nm in triplicate.
Western blot analysis
Twenty-four micrograms of protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) and subsequently blotted onto a polyvinylidene fluoride (PVDF) membrane. The transferred membranes were blocked in 4% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) and then incubated with primary antibodies overnight at 4°C. Anti-EPO and anti-EPOR were obtained from Santa Cruz Biotechnology, and the anti-neuron-specific enolase (anti-NSE) antibody was purchased from Abcam (Cambridge, MA, USA). Following incubation with the primary antibody, the membranes were rinsed and incubated with the HRP-conjugated anti-rabbit secondary antibody and developed using a standard enhanced chemiluminescent (ECL) system, and the signal was captured by an Amersham Imager 600 (GE Healthcare, Madison, WI, USA). All of the procedures were performed in triplicate.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cultured cells or frozen tissues using TriZol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription of total RNA (2 μg) was performed using a reverse transcription (RT)-PCR Kit (Roche, Basel, Switzerland), according to the manufacturer's instructions. For qRT-PCR analysis, cDNA was amplified with a SYBR Green PCR Kit (Roche) on an ABI PRISM 7900 sequence detection system, with the following EPO primers (sense 5'-ACC AAC ATT GCT TGT GCC AC-3' and antisense 5'-TCT GAA TGC TTC CTG CTC TGG-3') and EPOR primers (sense 5'-ACC GTG TCA TCC ACA TCA AT-3' and antisense 5'-GCC TTC AAA CTC GCT CTC TG-3'). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified as an internal control. To ensure the reproducibility of the results, the qRT-PCR analysis was performed in triplicate.
RNA interference
Expression of EPOR was decreased in LNCaP cells with the following siRNAs: siRNA#1: 5'-CCC TTA TGA GAA CAG CCT TAT-3'; siRNA#2: 5'-CCU ACC UGG UAU UGG AUG A-3'; and siRNA#3: 5'-GGA UGA AGG UUC AGA AAC A-3'. Transfection was performed using RNAiMax (Invitrogen) according to the manufacturer's instructions. Cells that did not receive any treatment were used as a negative control.
Statistical analyses
The Kruskal–Wallis test was used to compare differences in the EPO and EPOR staining scores among groups. Unpaired Student's t-test was used to compare mRNA expression between the normoxia and hypoxia groups. Calculations were performed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA) software. Figure 1–3 are generated using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). A two-sided P < 0.05 was considered statistically significant (*P > 0.05; **P < 0.01; and ***P < 0.001).
Figure 1.
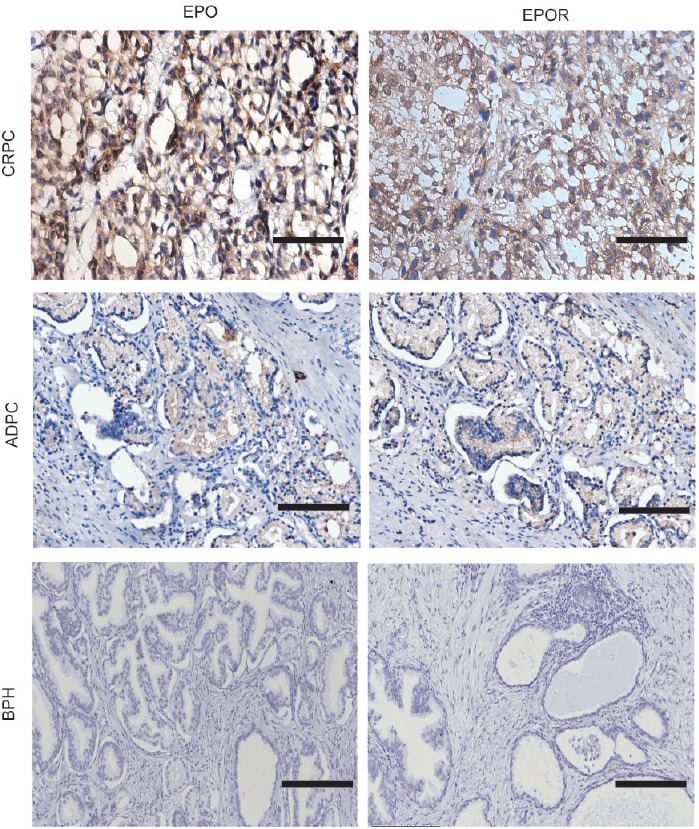
Representative immunohistochemical staining of EPO and EPOR in BPH, ADPC, and CRPC samples (Gleason score: 4 + 4; ×100; scale bars = 100 μm). EPO: erythropoietin; EPOR: erythropoietin receptor; BPH: benign prostatic hyperplasia; ADPC: androgen-dependent prostate cancer; CRPC: castration-resistant prostate cancer.
Figure 3.
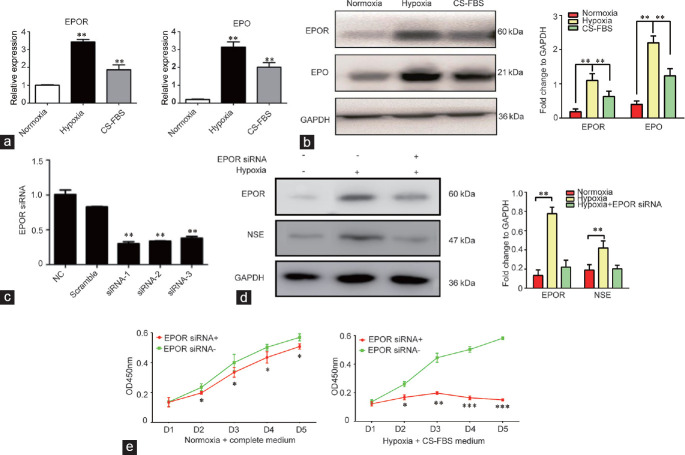
(a) Expression levels of EPO and EPOR in an LNCaP cell culture under hypoxic conditions and normoxic conditions with CS-FBS, as determined by qRT-PCR. (b) Expression levels of EPO and EPOR in hypoxia-induced LNCaP cells and LNCaP cells under normoxic condition or CS-FBS, as determined by western blot. The quantification analysis is presented in the right panel. (c) EPOR knockdown using siRNA, as determined by qRT-PCR. (d) The expression of NSE in LNCaP cells cultured under hypoxic conditions after EPOR knockdown, determined by western blot. The quantification analysis is presented in the right panel. (e) Cell proliferation results of LNCap cells cultured under hypoxic conditions in a CS-FBS culture and normoxic conditions with a complete medium after EPOR knockdown, as determined by a CCK8 assay. The experiments were performed in triplicate *P > 0.05, **P < 0.01 and ***P < 0.001. qRT-PCR: quantitative real-time polymerase chain reaction; EPO: erythropoietin; EPOR: erythropoietin receptor; NSE: neuron-specific enolase; CS-FBS: charcoal-stripped fetal bovine serum; GAPDH: glyceraldehyde-phosphate dehydrogenase; CCK8: cell counting kit 8.
RESULTS
Upregulation of EPO and EPOR in CRPC
EPO and EPOR expressions were upregulated under both ADPC and CRPC conditions (Figure 1). A predominantly cytoplasmic pattern of staining was observed for EPO and EPOR, but membrane immunoreactivity was also noted for EPOR. The mean EPO scores under CRPC and ADPC conditions were 7.55 and 5.4, respectively; the mean EPOR scores under CRPC and ADPC conditions were 7.45 and 5.9, respectively (P < 0.001; Table 1).
Table 1.
Erythropoietin and erythropoietin receptor scores of androgen-dependent prostate cancer and castration-resistant prostate cancer
| ADPC | CRPC | P | |
|---|---|---|---|
| EPO, mean±s.d. | 5.40±0.35 | 7.55±0.14 | <0.001 |
| EPOR, mean±s.d. | 5.90±0.32 | 7.45±0.15 | <0.001 |
Quantitative histologic evaluation of EPO and EPOR was analyzed by Image-Pro Plus 6.0. EPO: erythropoietin; EPOR: erythropoietin receptor; ADPC: androgen-dependent prostate cancer; CRPC: castration-resistant prostate cancer; s.d.: standard deviation
Hypoxia-induced, neuroendocrine-like, and castration-resistant growth of LNCaP cells
LNCaP cells showed a neuroendocrine-like change after a 2-week hypoxia culture that was accompanied by cell body shrinkage and multiple neurite extensions (Figure 2a), and the western blot results revealed an increase in the neuroendocrine-phenotype marker neuron-specific enolase (NSE) (Figure 2b). Using the CCK-8 assay, we further measured the cell proliferation change of hypoxic LNCap cells with/without culture in the CS-FBS medium. The results showed that the cell proliferation of LNCap cells cultured in the CS-FBS medium was dramatically inhibited. However, the hypoxic culture could rescue the inhibitory role of CS-FBS with respect to proliferation. Statistical analysis showed a significant difference between the CS-FBS and hypoxia + CS-FBS culture groups (Figure 2c). These results suggested that hypoxia occurred in the castration-resistant neuroendocrine differentiation of LNCaP cells.
Figure 2.
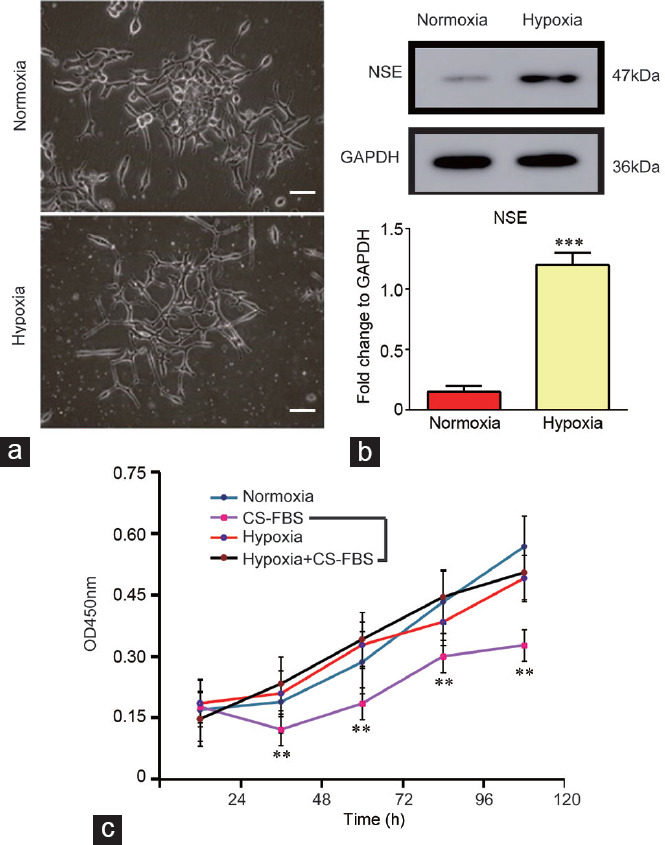
(a) Representative images of LNCaP cells cultured under hypoxic and normoxic conditions for 14 days (×100, scale bars=10 μm). (b) Western blot assay showing the expression of NSE in LNCaP cells cultured under hypoxic conditions. The quantification analysis is presented in the lower panel. (c) Cell proliferation results of LNCaP cells and 14 days hypoxia-cultured LNCaP cells cultured in medium with or without DHT, as determined by a CCK8 assay. The experiments were performed in triplicate **P < 0.01 and ***P < 0.001. DHT: dihydrotestosterone; CS-FBS: charcoal-stripped fetal bovine serum; NSE: neuron-specific enolase; GAPDH: glyceraldehyde-phosphate dehydrogenase; OD: optical density; CCK8: cell counting kit 8.
Upregulation of EPO and EPOR is involved in the progression of castration resistance in LNCaP cells
The expression levels of EPO and EPOR in 14-day hypoxia-cultured LNCaP cells, LNCaP cells cultured in CS-FBS, and controls are shown in Figure 3a and 3b. Analysis by qRT-PCR and western blot showed upregulation of EPO and EPOR in hypoxia-cultured LNCaP cells, similar to that of LNCaP cells cultured in CS-FBS, but both of these groups had higher expression levels than the controls. Furthermore, we designed three siRNAs for EPOR, and their silencing efficiency was analyzed by qRT-PCR and was determined to be 70.2%, 66.4%, and 62.2% (Figure 3c). siRNA-1, whose silencing efficiency was the highest, was chosen for the following experiments. We found that knockdown of EPOR reversed the hypoxia-induced upregulation of NSE in the LNCaP cells (Figure 3d). In addition, we measured the impact of EPOR knockdown on cell proliferation in LNCap cells. The proliferation curves show that EPOR knockdown has no obvious inhibitory effect on cell proliferation in the LNCaP cells under normoxic conditions with complete medium (Figure 3e, left). However, EPOR knockdown could dramatically inhibit the proliferation of LNCaP cells under hypoxic conditions with CS-FBS medium (Figure 3e, right).
DISCUSSION
EPO is well known for its role in erythroid differentiation but is now also widely acknowledged to have a direct effect on multiple targets, such as immune cells, bone marrow stromal cells, endothelial cells, and central nervous system cells.6,7,8 Notably, the expression of EPOR and EPO in different cancer cell lines, as well as primary cancers, has been reported,9,10,11,12 which suggests the potential for an autocrine or paracrine growth-stimulatory EPO signaling pathway in cancer cells. In 2003, Brower et al.13 published an article in the Lancet that confirmed that treatment with EPO could promote the growth of tumor cells. Acs et al.5 further proved the relationship between EPO/EPOR and hypoxia and found that the highest expression of EPO and EPOR was in the hypoxic area of the tumor, suggesting a role for the EPO signaling pathway in promoting tumor growth.
Feldman et al.14 was the first to demonstrate the expression of functional EPOR in human prostatic epithelial cells and prostate cancer cells. Since then, an increasing number of studies have investigated the role of EPO/EPOR pathway, which is upregulated by hypoxia-inducible factor 1α, in the development and pathobiology of human prostate cancer.3,4,15,16 Recently, EPO has even been reported to be involved in the niche formation that leads to bone metastasis.17 In this study, we further found the upregulation of both EPO and EPOR in the ADPC and CRPC samples, but notably higher EPO and EPOR scores were found in the CRPC samples compared to the ADPC samples, suggesting the important functions of EPO signaling in the transition of ADPC to CRPC. These results provide strong evidence for the role of hypoxia in the progression of PCa and the development of CRPC.
The neuroendocrine (NE) phenotype of PCa and neuroendocrine prostate cancer (NEPC) tumors has been implicated in aggressive prostate cancer and contributes to the development of CRPC. We also found the hypoxia-induced neuroendocrine differentiation of LNCaP cells in vitro. After a long period of hypoxic culture, androgen-dependent LNCaP cells can even survive in an androgen deprivation environment and have an enhanced expression of both EPO and EPOR. Previously, the EPO/EPOR axis was demonstrated to modulate the growth and survival of human prostate cancer.18 Our study further confirmed the role of the EPO/EPOR autocrine loop in the castration-resistant progression of PCa.
Although the specific mechanism that is involved in hypoxia-induced castration-resistant progression of PCa remains unclear, the concurrent upregulation of EPO and EPOR should be considered as an important molecular event. Specifically, EPO can regulate cell apoptosis and the cellular response to hypoxic stress by binding the functional EPOR through Janus kinase 2 (JAK2), thus activating multiple downstream pathways, including mitogen-activated protein kinase (MAPK), nuclear factor kappa-B (NF-κB), phosphatidylinositol 3 kinase (PI3K/Akt), and signal transducer and activator of transcription 5 (STAT5).3 To test the function of EPO signaling in the transformation to the neuroendocrine phenotype of PCa, an EPOR mRNA-interfering experiment was also performed in the present study. As expected, EPOR knockdown reversed the upregulation of NSE in hypoxia-induced, androgen-independent LNCaP cells. Elevated serum NSE is currently considered to be a strong marker of progressive and metastatic CRPC.19 In addition, a high level of serum NSE strongly predicts a worsened prognosis of CRPC patients.20 Thus, the downregulation of NSE by EPOR knockdown suggests that the EPO signaling pathway promotes the neuroendocrine transmission of PCa by interacting with functional EPOR.
CONCLUSIONS
Concurrent upregulation of EPO and EPOR in CRPC and androgen-independent LNCaP cells suggests that the EPO-EPOR autocrine loop plays an important role in the tumor development and the progression to castration resistance. Hypoxia-induced EPO-EPOR signaling is also responsible for the neuroendocrine phenotype transformation, which can further demonstrate the role of hypoxia in the progression of CRPC.
AUTHOR CONTRIBUTIONS
CY performed the RT-PCR, western blots, and the RNA interference studies and also drafted the manuscript. GHC performed the cell culturing and cell proliferation assay. XC participated in the cell culturing and immunohistochemical staining and drafted the manuscript. SFQ collected clinical samples and participated in immunohistochemical staining. MFS participated in the design of the study and performed statistical analyses. TZ conceived of the study, participated in its design and coordination, and helped draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 81070602), China Postdoctoral Science Foundation (No. 43655), and Changhai Hospital GCP platform (No. 2017ZX09304030). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Geng H, Xue C, Mendonca J, Sun XX, Liu Q, et al. Interplay between hypoxia and androgen controls a metabolic switch conferring resistance to androgen/AR-targeted therapy. Nat Commun. 2018;26:4972. doi: 10.1038/s41467-018-07411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou T, Xu C, He M, Sun Y. Upregulation of erythropoietin receptor in human prostate carcinoma and high-grade prostatic intraepithelial neoplasia. Prostate Cancer Prostatic Dis. 2008;11:143–7. doi: 10.1038/sj.pcan.4500995. [DOI] [PubMed] [Google Scholar]
- 4.Xu C, Zhou T, He M, Sun Y. Differential up-regulation of erythropoietin and its receptor in benign and malignant prostatic tissue. Urol Oncol. 2010;28:314–9. doi: 10.1016/j.urolonc.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Acs G, Zhang PJ, Rebbeck TR, Acs P, Verma A. Immunohistochemical expression of erythropoietin and erythropoietin receptor in breast carcinoma. Cancer. 2002;95:969–81. doi: 10.1002/cncr.10787. [DOI] [PubMed] [Google Scholar]
- 6.Hand CC, Brines M. Promises and pitfalls in erythopoietin-mediated tissue protection: are nonerythropoietic derivatives a way forward? J Investig Med. 2011;59:1073–82. doi: 10.231/JIM.0b013e3181ed30bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGee SJ, Havens AM, Shiozawa Y, Jung Y, Taichman RS. Effects of erythropoietin on the bone microenvironment. Growth Factors. 2012;30:22–8. doi: 10.3109/08977194.2011.637034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masuda S, Chikuma M, Sasaki R. Insulin-like growth factors and insulin stimulate erythropoietin production in primary cultured astrocytes. Brain Res. 1997;746:63–70. doi: 10.1016/s0006-8993(96)01186-9. [DOI] [PubMed] [Google Scholar]
- 9.Debeljak N, Solar P, Sytkowski AJ. Erythropoietin and cancer: the unintended consequences of anemia correction. Front Immunol. 2014;5:563. doi: 10.3389/fimmu.2014.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelkmann W, Bohlius J, Hallek M, Sytkowski AJ. The erythropoietin receptor in normal and cancer tissues. Crit Rev Oncol Hematol. 2008;67:39–61. doi: 10.1016/j.critrevonc.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Udupa KB. Functional significance of erythropoietin receptor on tumor cells. World J Gastroenterol. 2006;12:7460–2. doi: 10.3748/wjg.v12.i46.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedley BD, Allan AL, Xenocostas A. The role of erythropoietin and erythropoiesis-stimulating agents in tumor progression. J Oncol Pharm Pract. 2011;17:6373–80. doi: 10.1158/1078-0432.CCR-10-2577. [DOI] [PubMed] [Google Scholar]
- 13.Brower V. Normal and neoplastic prostate cells have EPO receptors. Lancet Oncol. 2003;4:69. doi: 10.1016/s1470-2045(03)00997-5. [DOI] [PubMed] [Google Scholar]
- 14.Feldman L, Wang Y, Rhim JS, Bhattacharya N, Loda M, et al. Erythropoietin stimulates growth and STAT5 phosphorylation in human prostate epithelial and prostate cancer cells. Prostate. 2006;66:135–45. doi: 10.1002/pros.20310. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Zhou J, Liu J, Tang B, Zhao F, et al. Biological characteristics of prostate cancer cells are regulated by hypoxia-inducible factor 1α. Oncol Lett. 2014;8:1217–21. doi: 10.3892/ol.2014.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranasinghe WK, Sengupta S, Williams S, Chang M, Shulkes A, et al. The effects of nonspecific HIF1α inhibitors on development of castrate resistance and metastases in prostate cancer. Cancer Med. 2014;3:245–51. doi: 10.1002/cam4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decker AM, Jung Y, Cackowski F, Taichman RS. The role of hematopoietic stem cell niche in prostate cancer bone metastasis. J Bone Oncol. 2016;5:117–20. doi: 10.1016/j.jbo.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong JY, Hoxhaj G, Socha AL, Sytkowski AJ, Feldman L. An erythropoietin autocrine/paracrine axis modulates the growth and survival of human prostate cancer cells. Mol Cancer Res. 2009;7:1150–7. doi: 10.1158/1541-7786.MCR-08-0243. [DOI] [PubMed] [Google Scholar]
- 19.Muoio B, Pascale M, Roggero E. The role of serum neuron-specific enolase in patients with prostate cancer: a systematic review of the recent literature. Int J Biol Markers. 2018;33:10–21. doi: 10.5301/ijbm.5000286. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Zhao S, Wang J, Zhu Z, Luo L, et al. Serum neuroendocrine markers predict therapy outcome of patients with metastatic castration resistant prostate cancer: a meta-analysis. Urol Int. 2019;102:373–84. doi: 10.1159/000495512. [DOI] [PubMed] [Google Scholar]