Abstract
Mice deficient in the transcription factor pleomorphic adenoma gene 1 (PLAG1) exhibit reproductive issues that are characterized, in part, by decreased progressive sperm motility in the male. However, the underlying cause of this impairment is unknown. As epididymal transit is critical for sperm maturation and motility, the morphology of the epididymis of Plag1-deficient mice was investigated and the spatial expression patterns of PLAG1 protein and mRNA were identified. Using X-gal staining and in situ hybridization, PLAG1 was shown to be widely expressed in both the epithelium and stroma in all regions of the mouse epididymis. Interestingly, the X-gal staining pattern was markedly different in the cauda, where it could be suggestive of PLAG1 secretion into the epididymal lumen. At all ages investigated, the morphology of epididymides from Plag1 knockout (KO) mice was aberrant; the tubule failed to elongate and coil, particularly in the corpus and cauda, and the cauda was malformed, lacking its usual bulbous shape. Moreover, the epididymides from Plag1 KO mice were significantly reduced in size relative to body weight. In 20% of Plag1-deficient mice, the left testicle and epididymis were lacking. The impaired morphogenesis of the epididymal tubule is likely to be a major contributing factor to the fertility problems observed in male Plag1-deficient mice. These results also establish PLAG1 as an important regulator of male reproduction, not only through its involvement in testicular sperm production, but also via its role in the development and function of the epididymis.
Keywords: development, epididymis, pleomorphic adenoma gene 1, tubule coiling, Wolffian duct
INTRODUCTION
The zinc finger transcription factor pleomorphic adenoma gene 1 (PLAG1) was initially discovered as being involved in pleomorphic adenomas of the salivary gland1 and is now known to play a role in the development of a number of tumor and cancer types.1,2,3,4 The normal biological role of PLAG1 appears to be largely associated with growth and reproduction.2 Embryonic and postnatal growth analysis has shown that Plag1 knockout (KO; Plag1−/−) mice are significantly smaller than wild-type (WT; Plag1+/+) littermates.3 Many of the target genes of PLAG1, as determined by microarray analyses, are involved in growth and cell proliferation, including insulin-like growth factor 2, bone-derived growth factor, cytokine-like factor 1, and placental growth factor.4 In addition to reduced growth in mice, Hensen et al.3 also reported that fertility is reduced in Plag1 KO mice; compared with WT males, Plag1 KO males exhibited decreased conception rates when mated with WT females. Plag1 mRNA was detected in the embryonic urogenital ridge and the adult testis.3
More recently, it has been shown that PLAG1 is required for normal sperm production and sperm motility in mice.5 Mice deficient in PLAG1 exhibit decreased daily sperm production compared with age-matched WT littermates, and cauda fluid from mature KO males shows a 49% reduction in motile spermatozoa, an 80% reduction in progressively motile spermatozoa, and a decreased number of spermatozoa exhibiting rapid motility.5 This reduced sperm motility is likely to be a major reason for the decreased fertility of Plag1-deficient mice. The underlying cause of the reduced sperm motility observed in Plag1 KO mice has not yet been identified. As spermatozoa gain their motility during transit through the epididymis, it is likely that the function of the epididymis of Plag1 KO mice is defective. The rodent epididymis is generally divided into four major regions (the initial segment, caput, corpus, and cauda) and further divided into 10 transcriptionally unique segments in mice6 and 19 in rats.7 As they transit through the epididymal tubule, spermatozoa undergo a multitude of structural and biochemical modifications that are crucial for proper maturation and the production of spermatozoa capable of both motility and fertilization of an egg.8,9 In rodents, the cauda epididymidis also functions for the storage of mature spermatozoa.9
In this study, we investigated the epididymis as the underlying cause of the sperm abnormalities in Plag1-deficient mice. We show that PLAG1 is widely expressed throughout the entire epididymal tubule and stroma in mature male mice and demonstrate that the morphology of the epididymis from Plag1-deficient mice exhibits decreased tubule elongation and coiling and an overall reduction in relative size.
MATERIALS AND METHODS
Animals
All animal procedures undertaken in this study were approved by the Animal Ethics Committee of La Trobe University (AEC17-54 and AEC17-27) and the La Trobe Institutional Biosafety Committee (GMSC17-15), Bundoora, Victoria, Australia. All animal care and experimental procedures were conducted in compliance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (8th edition, 2013) of the National Health and Medical Research Council. Founder mice were a kind gift from Prof. Wim Van de Ven (Laboratory for Molecular Oncology, Center for Human Genetics, Catholic University of Leuven, Leuven, Belgium); the generation of the Plag1 KO mouse line has been previously outlined.3 Genotypes of mice were determined by PCR with genomic DNA isolated from ear clips, with the following primers: forward 5'-ATGGCCACTGTCATTCCTGGTGATTTGTCA-3' and reverse 5'-CCTGTGTGTACCACCATGTGTCTCCGGACA-3' to detect the WT Plag1 allele, and forward 5'-GCATCGAGCTGGGTAATAAGCGTTGGCAAT-3' and reverse 5'-GACACCAGACCAACTGGTAATGGTAGCGAC-3' to detect the lacZ reporter gene.
Breeding analysis
Three Plag1 KO, heterozygous (HET; Plag1+/−), and WT males aged 10–12 weeks were each housed with two 10–16-week-old WT females for a period of 5 months. Each mouse was genotyped twice, by two different researchers, to ensure accuracy of genetic status. The number of litters born and litter size were recorded. At the conclusion of the breeding analysis, the male mice were dissected to ensure both testes and epididymides were present, which was the case for all animals.
X-gal staining
Seven-week-old KO, HET, and WT male mice (n = 5 per genotype) were killed by CO2 asphyxiation. Epididymides were dissected and immediately immersed in 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS) for 2 h at 4°C. The tissues were cryoprotected in 10% (w/v) sucrose in PBS for 2 h and then 30% (w/v) sucrose in PBS until the tissues sank. The tissues were quick-frozen in cooled isopentane and cryosectioned into 5-μm serial sections. Slides were incubated in X-gal staining solution (0.5 mg ml−1 X-gal [5-bromo-4-chloro-3-indoyl-β-D-galactopyranoside; PanReac Applichem, Darmstadt, Germany], 5 mmol l−1 K3 Fe(CN)6, 5 mmol l−1 K4 Fe(CN)6, 2 mmol l−1 MgCl2, 0.01% [w/v] sodium deoxycholate, 50 mmol l−1 EGTA, and 0.02% [v/v] IGEPAL CA-630 [Sigma-Aldrich, St. Louis, MO, USA]) in PBS in the dark at 37°C overnight. The sections were then rinsed in PBS and counterstained for 30 s with Nuclear Fast Red (Sigma-Aldrich) before coverslipping. The tissue sections were imaged with an Olympus BX41 microscope with an Olympus DP25 camera (Olympus Scientific Solutions Americas Inc., Waltham, MA, USA).
In situ hybridization
Epididymal sections from 7-week-old Plag1 WT and KO male mice (n = 5 per genotype) were made as described above for X-gal staining. In situ hybridization was performed as previously described10 with the following alterations: riboprobes were transcribed from 1 μg of linearized pCRII-TOPO plasmid (Thermo Fisher Scientific, Waltham, MA, USA) containing a 752-bp insert (503–1254 bp of the mouse Plag1 coding sequence, GenBank accession number NM_019969.3) in digoxigenin (DIG) RNA Labeling Mix and 40 U SP6 or T7 RNA polymerase (Roche Diagnostics, Risch-RotKreuz, Switzerland). The slides were incubated overnight in the hybridization buffer containing 800 ng ml−1 antisense or sense DIG-labeled probe at 66°C. Coverslips were removed and the slides were washed two times for 5 min in 0.2× saline sodium citrate at room temperature, followed by three 5-min washes in PBS with 0.1% (v/v) Triton X-100 (Sigma-Aldrich). The protocol continued as described before.10 As an additional negative control, epididymis sections from a Plag1 KO male were included to ensure there was no nonspecific probe binding. The sections were imaged as described above.
Epididymal histology and morphology
Male mice aged 3, 5, 28–33, and 50–55 weeks (n = 5 per genotype and per age) were killed by CO2 asphyxiation. Epididymides were dissected, immediately immersed in 4% paraformaldehyde in PBS, and kept overnight at 4°C. The tissues were cryoprotected in 30% sucrose in PBS until the tissues sank. The tissues were quick-frozen in cooled isopentane and cryosectioned into 5-μm serial sections and mounted onto glass slides. The sections were thawed at room temperature for 30 min and then stained with Mayer's hematoxylin (Trajan Scientific and Medical, Ringwood, Victoria, Australia) for 2 min and counterstained with eosin (Thermo Fisher Scientific) for 20 s. Tubule cross sections in serial epididymal sections from 7-week-old Plag1 KO, HET, and WT mice (n = 5 per genotype) were manually counted (Supplementary Figure 1 (382.3KB, tif) ) and compared between genotypes. A minimum of five longitudinal epididymal sections were analyzed per animal. Crosswise sections were made from the caudae epididymidis from 7-week-old Plag1 KO and WT mice (n = 5 per genotype). Three to six cross sections in the XY plane per animal were measured for luminal diameter using Olympus cellSens Standard 1.17 software (Olympus Scientific Solutions Americas Inc.), by drawing a straight line across the maximum luminal width and recording the line length (Supplementary Figure 1 (382.3KB, tif) ). The mean luminal diameter was calculated for each animal and compared between genotypes. Epididymal weight and body weight were measured in five KO and five WT males aged 3 weeks and in five KO and five WT adult males aged at least 11 weeks old. Epididymal weights relative to body weight were calculated and compared between genotypes.
Statistical analyses
Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). Means were compared using an unpaired Student's t-test or a one-way ANOVA with post hoc Tukey's HSD test for multiple comparisons, with significance set at P < 0.05. Fisher's exact test was used to assess the association between genotype and presence/absence of the left testis and epididymis.
RESULTS
Plag1-deficient male mice and infertility
There was no statistical difference between HET and WT males in litter size and number of litters born in matings with WT females, but no litters were born from matings with Plag1 KO males with WT females during the 5-month observation period (Table 1).
Table 1.
Breeding analysis of Plag1 wild-type, heterozygous, and knockout males mated with wild-type females
| Breeding parameter | Genotype | Statistical analysis (t-test) | ||
|---|---|---|---|---|
| WT | HET | KO | ||
| Litter size (mean±s.d.) | 5.87±1.99 | 5.37±2.12 | 0 | WT versus HET, P=0.8960 |
| Number of litters (mean±s.d.) | 3.33±0.58 | 2.83±0.29 | 0 | WT versus HET, P=0.4000 |
WT: wild-type; HET: heterozygous; KO: knockout; s.d.: standard deviation
PLAG1 expression in the mouse epididymis
In Plag1 KO mice, the Plag1 gene sequence is replaced with a lacZ reporter gene, which encodes β-galactosidase. This allows the use of X-gal staining to identify the spatial expression of PLAG1 in KO and HET animals. X-gal staining was widely found in the epididymis of KO animals; staining was observed in the epithelium, stromal cells, and the smooth muscle layer. In the caput and proximal corpus, the X-gal signal was concentrated and in close association with the nuclei of the basal, principal, apical, and smooth muscle cells (Figure 1); however, in the distal corpus and the cauda, the signal in the epithelium became diffuse and localized to the apical cytoplasm (Figure 1). Concentrated nuclear expression of PLAG1 was still observed in the basal cells, stromal cells, and smooth muscle layer in the distal corpus and cauda. This expression pattern was also seen in HETs, although expression was, as expected, not as strong as that in KOs (Supplementary Figure 2 (1.1MB, tif) ). There was no X-gal signal in the epididymal sections of most WT animals, although a very faint and hazy signal, clearly distinct from that in the HETs and KOs, was seen in some sections of some animals (Supplementary Figure 3 (1.1MB, tif) ).
Figure 1.
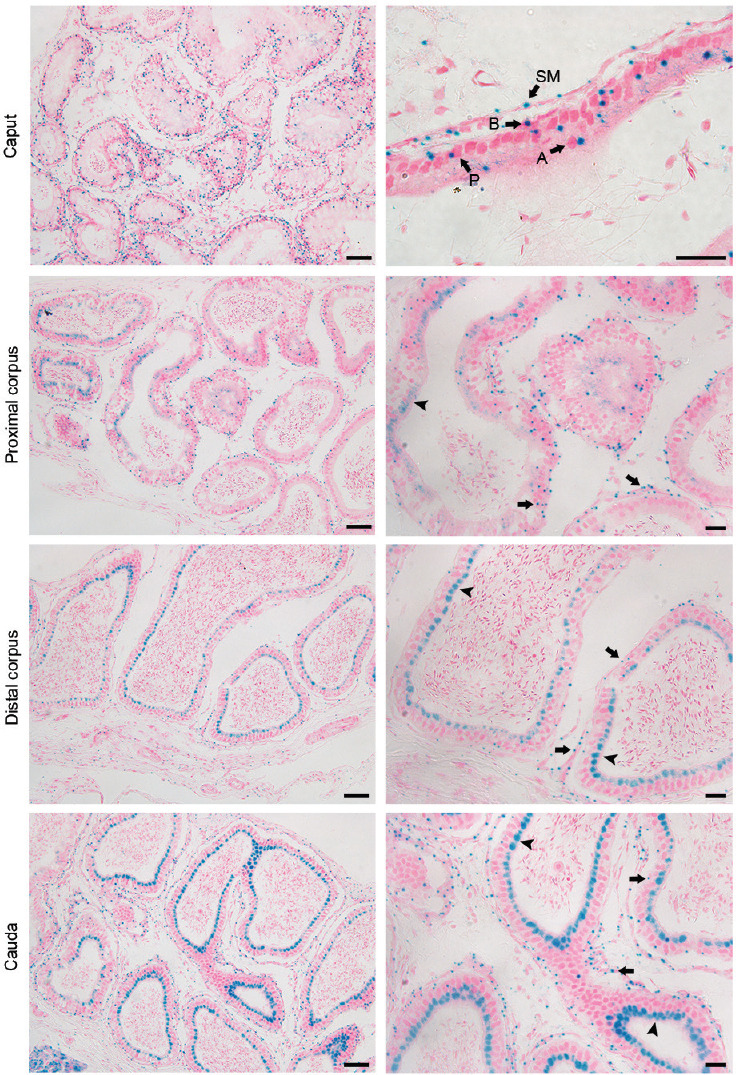
Localization of PLAG1 expression by X-gal staining in the epididymis of 7-week-old Plag1 knockout mice. Scale bars represent 50 μm in low-magnification images (left column) and 20 μm in high-magnification images (right column). Arrows indicate nucleus-associated PLAG1 signal; arrowheads indicate PLAG1 signal in the apical cytoplasm. PLAG1: pleomorphic adenoma gene 1; P: principal cell; B: basal cell; A: apical cell; SM: smooth muscle cell.
Regional expression of Plag1 mRNA in the mouse epididymis
Plag1 mRNA was detected by in situ hybridization in the principal, basal, and apical cells of the epithelium and the smooth muscle cells and stromal cells, in all regions of the epididymis (Figure 2). Less-abundant expression was observed in the cauda compared with the initial segment, caput, and corpus. There was no signal in sections stained with the sense probe and no nonspecific staining in the KO control (Supplementary Figure 4 (1.1MB, tif) ).
Figure 2.
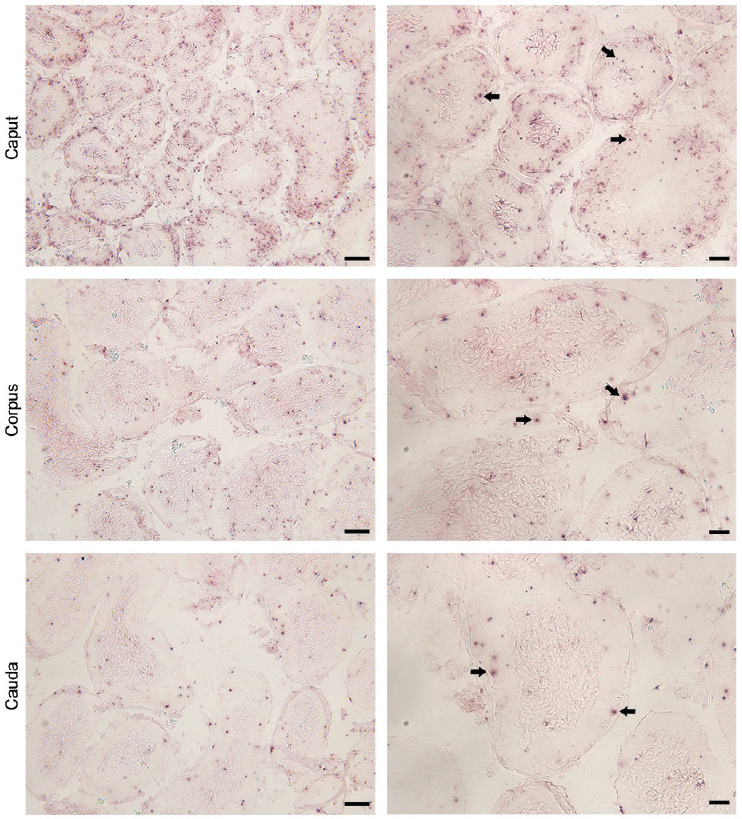
Localization of Plag1 mRNA in 7-week-old wild-type mice by in situ hybridization. Arrows indicate positive in situ hybridization signal. Scale bars represent 50 μm in low-magnification images (left column) and 20 μm in high-magnification images (right column). Plag1: pleomorphic adenoma gene 1.
Plag1-deficient mice have reduced epididymis weight and aberrant epididymis morphology
Epididymides from 3-, 5-, 28–33-, and 50–55-week-old Plag1 KO and WT mice were examined macroscopically and microscopically. In all mice, testes were descended normally. However, out of 65 male Plag1 KOs dissected, 13 (20.0%) were observed with absence of the left testis and epididymis. None of the 49 WTs dissected were lacking the left testis and epididymis. The association of the Plag1 KO genotype and absence of the left testis and epididymis was analyzed using Fisher's exact test and found to be statistically significant (P = 0.0017). At all ages, the cauda in KOs lacked the typical large bulbous shape (Figures 3, 4 and Supplementary Figures 5 (828.4KB, tif) –7 (835.4KB, tif) ). Epididymal weight relative to body weight was compared between Plag1 KO and WT mice aged 3 weeks and in adults. Epididymides from KO mice had a significantly reduced mass relative to body weight, weighing on average (s.d.) 0.025% (0.006%) compared with 0.060% (0.005%) in WTs (t-test, P < 0.0001) in 3-week-old mice, and weighing on average (s.d.) 0.062% (0.019%) compared with 0.111% (0.019%) in WTs in adult mice aged 11 weeks or older (t-test, P = 0.0035).
Figure 3.
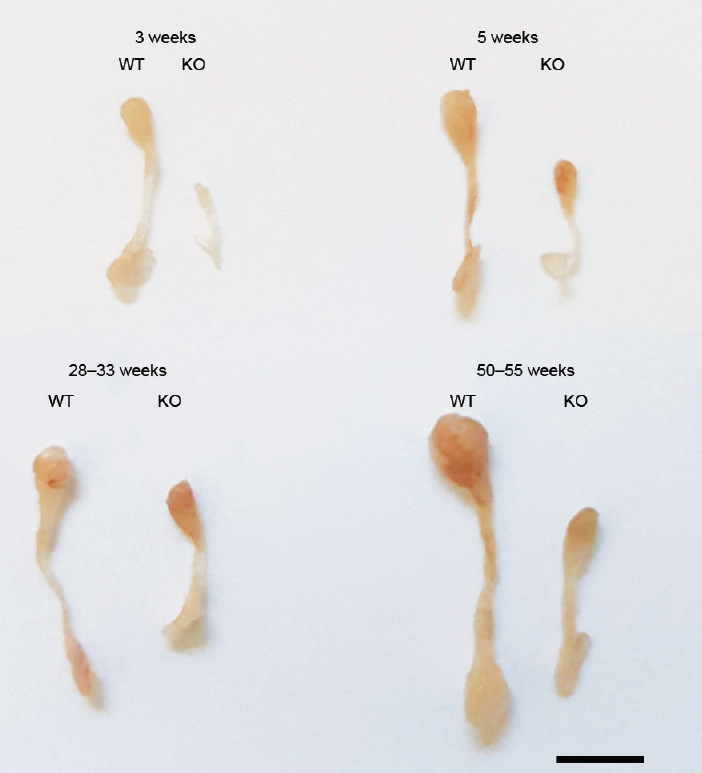
Macroscopic images of the epididymides from Plag1 WT and KO mice aged 3, 5, 28–33, and 50–55 weeks. Scale bar = 5 mm. Plag1: pleomorphic adenoma gene 1; WT: wild-type; KO: knockout.
Figure 4.
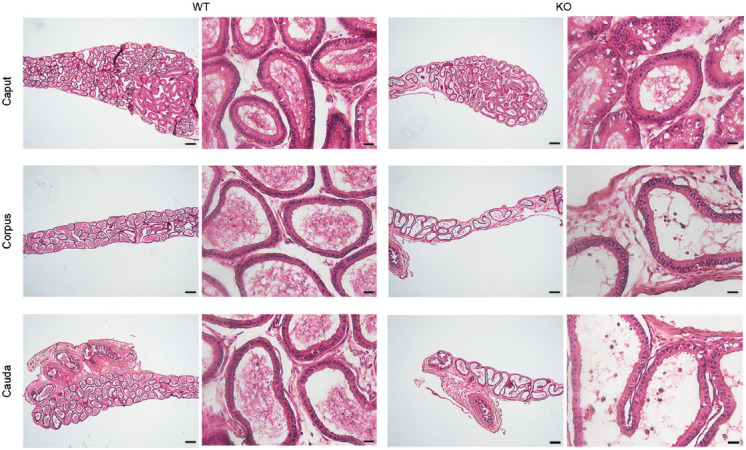
Hematoxylin-and-eosin staining of the epididymal sections from 5-week-old Plag1 WT and KO mice. Scale bars represent 200 μm in low-magnification images (left column) and 20 μm in high-magnification images (right column). Plag1: pleomorphic adenoma gene 1; WT: wild-type; KO: knockout.
The histology of the epithelium was not obviously different between WTs and KOs. At all ages, a normal pseudostratified epithelium was observed, but the epididymal tubule was less coiled and elongated in Plag1 KOs compared with WTs, particularly in the cauda, as judged by the number of tubule cross sections and luminal diameter (Figures 4, 5 and Supplementary Figures 5 (828.4KB, tif) –7 (835.4KB, tif) ). A decreased number of tubule cross sections was observed in the caput, corpus, and cauda of KOs compared with WTs and HETs (ANOVA, P < 0.01; Figure 5a), and the diameter of the epididymal tubule lumen in the cauda was increased in Plag1 KO mice in comparison with WTs (t- test, P = 0.0003; Figure 5b).
Figure 5.
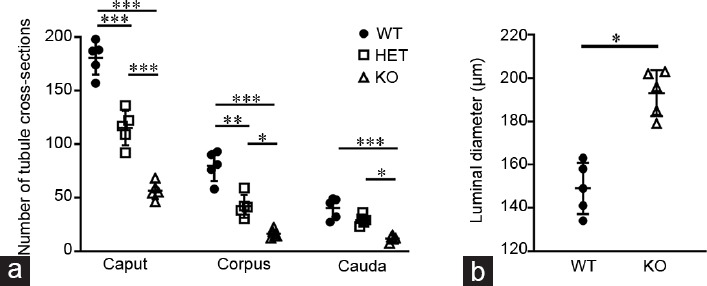
(a) Number of XY-plane epididymal tubule cross sections in the caput, corpus, and cauda of 7-week-old Plag1 WT (circles), HET (squares), and KO (triangles) mice (n = 5). Data are presented as mean ± s.d. *P < 0.01; **P < 0.001; ***P < 0.0001 (one-way ANOVA with Tukey's honest significant difference test). (b) Diameter of the tubule lumen in the cauda epididymidis of 7-week-old Plag1 WT and KO mice (n = 5). Data are presented as mean ± s.d. *P < 0.01 (t-test). s.d.: standard deviation; Plag1: pleomorphic adenoma gene 1; WT: wild-type; HET: heterozygous; KO: knockout.
DISCUSSION
Plag1-deficient mice are known to have fertility problems.3,5 Our breeding analysis indicated that Plag1 HETs are not subfertile, as there was no significant difference in the number of litters born or litter size when compared with WTs, suggesting that one copy of the Plag1 gene is sufficient for normal fertility. The Plag1 KO males in our colony were completely infertile, with no litters being born from these matings in the 5-month period of the analysis. These results are contrary to previous findings, in which Plag1 KO males were observed to successfully impregnate 7% of female WTs they were housed with.3 However, the number of males tested and the length of the mating period was not specified in that study. Plugging behavior was normal in male KOs, indicating that sexual behavior was not affected and that the infertility exhibited by Plag1-deficient males has other underlying causes.
Apart from reduced sperm production, Plag1 KO males also show reduced sperm motility,5 suggesting a role of PLAG1 in epididymal development or function. The results of this study show for the first time that PLAG1 is widely expressed in the mouse epididymis and that PLAG1 is required for normal epididymal development and structure. In the caput and proximal corpus, PLAG1 expression was localized to the nucleus of the principal, basal, and apical cells. Here, the signal was concentrated and in association with the nuclei of the cells, as is expected of transcription factors, and is the same type of PLAG1 signal observed previously in X-gal staining of the testes,5 the pituitary gland,11 and the hypothalamus.11 However, X-gal staining signal in the distal corpus and cauda epididymis was distinctly different. In the distal segments of the epididymis, X-gal signal became diffuse and localized to the apical cytoplasm. The lacZ reporter gene replacing the Plag1 gene in Plag1 KO mice is still flanked by the 5' and 3' untranslated regions of the Plag1 gene,3 which may contain mRNA localization signals that facilitate the translation of proteins close to the cytoplasmic site of their function.12,13 Therefore, we speculate that X-gal signal localization is representative of the subcellular localization of PLAG1. The relocation of the transcription factor PLAG1 to the apical cytoplasm could be indicative of it being secreted in the lumen. If PLAG1 were indeed secreted into the epididymal tubule lumen, it may interact with spermatozoa or be involved in maintaining the microenvironment of the luminal fluid. Another possible explanation for the diffuse signal in the apical cytoplasm is the transport of excessive PLAG1 protein to the cytoplasm for degradation; transcription factor levels within a cell can be regulated by proteasome-mediated protein degradation.14 Further confirmation of PLAG1 protein secretion with an anti-mouse PLAG1 antibody would be beneficial; however, several commercial antibodies and a custom-made antibody have been tested and have not produced specific staining. Other techniques such as mass spectrometry would require a large amount of epididymal luminal fluid in order to detect low levels of secreted PLAG1.
It has previously been shown that testes from Plag1-deficient male mice have a lower relative weight, which presumably contributes to the lower daily sperm production observed in these mice.5 In this study, we have shown that the relative epididymal weight in 3-week-old and adult mice is also reduced. The decreased sperm motility and infertility phenotype of Plag1-deficient mice indicates that there is an impairment in sperm maturation, which is likely to be a consequence of the defective epididymal elongation and coiling observed in these mice, as the length of the tubule is well known to be crucial for its function in sperm maturation. As sperm transit the extensive length of the epididymal tubule, they undergo a series of structural and biochemical changes in a very specific sequence.8,9,15,16 This precise sequence of maturation events is facilitated by segmentation of the rodent epididymis. Each segment has unique gene and protein expression patterns and is further defined by the presence of connective tissue septa.16 As our data show, in Plag1-deficient mice, the epididymal tubule fails to elongate to the age-appropriate length. Hensen et al.3 previously reported that the epididymis from Plag1 KOs showed no obvious abnormalities; however, the data were not shown and no images were provided. Our results are in direct contrast to this; we observed and showed clear morphological abnormalities in the epididymis of Plag1 KO mice. Presumably, the epididymis in these mice would not be able to facilitate the required sequence of events of sperm maturation and the identity and structure of the epididymal segments may be impaired, which would in turn influence the genes and proteins that should be expressed in each segment. The transformation of the embryonic Wolffian duct into a convoluted tubule over 1 m long in mice and over 6 m long in humans17 is a puzzling process that is not yet well understood. The lack of coiling observed in the epididymis of Plag1 KO mice is indicative of impaired tubule elongation; however, it is not clear whether the intense tubule coiling of the epididymis is a result of the rapid proliferation of epithelial cells and tubule elongation exerting force on the surrounding tissue, or the mechanical pressure exerted by the mesenchyme surrounding the tubule forcing coiling, or a combination of the two mechanisms.18,19 The absence of the typical bulbous shape of the cauda epididymidis in Plag1 KO mice exemplifies this enigma. Does the cauda lack the bulbous shape because the tubule has not elongated properly, or is there a defect in the development or homeostasis of the mesenchyme resulting in a lack of mechanical forces required for tubule development? The epididymal morphology of Plag1-deficient mice implies a role for PLAG1 in these dynamics of epididymal development. Furthermore, the absence of the left testis and epididymis was found to be significantly associated with the Plag1 KO genotype. This condition was not observed in any Plag1 WT mice. In humans, absence of one testis (monorchidism or unilateral anorchidism) occurs in 3% of males and is more commonly on the left side.20 As the major cause of anorchidism is vascular accidents during gestation,21 PLAG1 deficiency may affect vascular development, resulting in an insufficient blood supply to the developing left testis and epididymis. This would ultimately result in the degeneration of the left testis and epididymis during development.
Several studies have identified a number of genes that are required for normal epididymal development and function, including Ptk7,22 Pkd1,23 Pkd2,24 and Lgr4.25,26 Knockout rodent models for each of these genes exhibit decreased coiling of the Wolffian duct and the epididymis and decreased fertility. Mesoderm-specific Ptk7 KO results in a shorter, dilated, and significantly less coiled Wolffian duct at E18.5, which testosterone supplementation cannot restore.22 Inhba is required for anterior Wolffian duct coiling and its activity appears to be dependent of testosterone signaling.27 In both Pkd1 KO and Pkd2 KO mice, coiling of the caput epididymidis failed to initiate at E16.5.23,24 Pharmacological inhibition of WNT (Wingless and INT-1) signaling in mice results in decreased Wolffian duct coiling, while epithelium-specific deletion of the β-catenin (a WNT signaling transducer) gene results in the complete absence of coiling in the epididymis from 1-day-old pups and the constitutive activation of WNT signaling also abolishes any epididymal coiling.28 Furthermore, LGR4, an R-spondin receptor that activates canonical WNT signaling, is crucial for normal postnatal epididymis elongation and coiling.25 Lgr4 KOs exhibit normal epididymides at birth; however, from postnatal day 3 onward, epididymides are dilated, the duct fails to continue to elongate, and coiling is drastically reduced compared with WT controls.25 The similarities in defective tubule coiling between these gene KO models and Plag1 KOs suggest that there may be similar pathways and processes affected in the animals, or that PLAG1, as a transcription factor, has upstream control of these genes or pathways, either directly or indirectly. Further, all of these transgenic mouse models, including the Plag1 KO mice, demonstrate that decreased or defective epididymal tubule coiling is typically associated with decreased fertility. The aforementioned studies focused mainly on the morphology of the embryonic Wolffian duct; however, it would be worthwhile to investigate whether or not the phenotype is restored later in adulthood. Interestingly, mice in which Inhba is replaced with an activin B transgene are found to have a completely normal epididymis morphology by adulthood, despite displaying reduced epididymal weight and delayed epithelial development during earlier postnatal development.29 Evidently, development may be simply delayed in some of the transgenic models and studies would benefit from investigating the adult epididymis.
In summary, PLAG1 is essential for normal epididymal morphology and the establishment of an epididymal tubule that is the appropriate size and length for normal function. Lack of PLAG1 results in reduced coiling of the epididymal tubule, which, as in other KO models, has been associated with reduced fertility. The wide expression of PLAG1 in the epididymis and its potential secretion into the epididymal lumen in adult mice indicates that PLAG1 may also play a role in the epididymal function of sperm maturation and storage. Currently, the target genes of PLAG1 in the epididymis have not been identified. Target genes may include those that have been demonstrated to be crucial for epididymal elongation and coiling, such as the genes described above.
AUTHOR CONTRIBUTIONS
JW carried out all experimental procedures and wrote the manuscript. ARJ assisted with the collection of adult epididymal weights and in situ hybridization. SCT and JGG assisted with genotyping of animals. SVHG and BDG provided advice for experimental procedures, participated in the design of the study, and assisted with manuscript preparation. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
Representative image to show histological quantification methods. Numbers indicate how tubule cross sections were counted within a field of view. The double-headed arrow indicates a tubule cross section in the XY plane (i.e., perpendicular to the tubule's longitudinal axis, resulting in a near circular shape) and how its luminal diameter was measured. The scale bar represents 50 µm.
Localization of PLAG1 expression by X-gal staining in the epididymis of 7-week-old Plag1 heterozygous (Plag1+/−) mice. Scale bars represent 50 µm in low-magnification images (left column) and 20 µm in high-magnification images (right column). Plag1: pleomorphic adenoma gene 1.
X-gal staining in 7-week-old wild-type (Plag1+/+) mice as a negative control. Scale bars represent 50 µm in low-magnification images (left column) and 20 µm in high-magnification images (right column).
Negative controls for in situ hybridization staining of Plag1 mRNA: epididymal sections from 7-week-old Plag1 WT mice stained with the sense riboprobe (left column) and epididymal sections from 7-week-old Plag1 KO mice stained with the antisense riboprobe. Scale bars represent 50 µm. WT: wild-type; KO: knockout.
Hematoxylin-and-eosin staining of epididymal sections from 3-week-old Plag1 WT and KO mice. Scale bars represent 200 µm in low-magnification images (left column) and 20 µm in high-magnification images (right column). WT: wild-type; KO: knockout.
Hematoxylin-and-eosin staining of epididymal sections from 28–33-week-old Plag1 WT and KO mice. Scale bars represent 200 µm in low-magnification images (left column) and 20 µm in high-magnification images (right column). WT: wild-type; KO: knockout.
Hematoxylin-and-eosin staining of the epididymal sections from 50–55-week-old Plag1 WT and KO mice. Scale bars represent 200 µm in low-magnification images (left column) and 20 µm in high-magnification images (right column). WT: wild-type; KO: knockout.
ACKNOWLEDGMENTS
The authors thank Melissa Middleton for her assistance with in situ hybridization. The authors acknowledge the staff of the La Trobe Animal Research and Teaching Facility for animal care and assistance with the breeding analysis. The authors thank Prof. Moira O'Bryan (Monash University, Australia) for advice on experimental design. This work was supported by funding from the Department of Physiology, Anatomy and Microbiology, and the School of Life Sciences, La Trobe University. JW is supported by an Australian Government Research Training Program Scholarship.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Šström AK, Voz ML, Kas K, Röijer E, Wedell B, et al. Conserved mechanism of PLAG1 activation in salivary gland tumors with and without chromosome 8q12 abnormalities: identification of SII as a new fusion partner gene. Cancer Res. 1999;59:918–23. [PubMed] [Google Scholar]
- 2.Juma AR, Damdimopoulou PE, Grommen SV, Van de Ven WJ, De Groef B. Emerging role of PLAG1 as a regulator of growth and reproduction. J Endocrinol. 2016;228:R45–56. doi: 10.1530/JOE-15-0449. [DOI] [PubMed] [Google Scholar]
- 3.Hensen K, Braem C, Declercq J, Van Dyck F, Dewerchin M, et al. Targeted disruption of the murine plag1 proto-oncogene causes growth retardation and reduced fertility. Dev Growth Differ. 2004;46:459–70. doi: 10.1111/j.1440-169x.2004.00762.x. [DOI] [PubMed] [Google Scholar]
- 4.Van Dyck F, Delvaux EL, Van de Ven WJ, Chavez MV. Repression of the transactivating capacity of the oncoprotein PLAG1 by SUMOylation. J Biol Chem. 2004;279:36121–31. doi: 10.1074/jbc.M401753200. [DOI] [PubMed] [Google Scholar]
- 5.Juma AR, Grommen SV, O’Bryan MK, O’Connor AE, Merriner DJ, et al. PLAG1 deficiency impairs spermatogenesis and sperm motility in mice. Sci Rep. 2017;7:5317. doi: 10.1038/s41598-017-05676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, et al. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol Reprod. 2005;73:404–13. doi: 10.1095/biolreprod.105.039719. [DOI] [PubMed] [Google Scholar]
- 7.Jervis KM, Robaire B. Dynamic changes in gene expression along the rat epididymis. Biol Reprod. 2001;65:696–703. doi: 10.1095/biolreprod65.3.696. [DOI] [PubMed] [Google Scholar]
- 8.Dacheux JL, Dacheux F. New insights into epididymal function in relation to sperm maturation. Reproduction. 2014;147:27–42. doi: 10.1530/REP-13-0420. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan R, Mieusset R. The human epididymis: its function in sperm maturation. Hum Reprod Update. 2016;22:574–87. doi: 10.1093/humupd/dmw015. [DOI] [PubMed] [Google Scholar]
- 10.Grommen SV, Scott MK, Darras VM, De Groef B. Spatial and temporal expression profiles of urocortin 3 mRNA in the brain of the chicken (Gallus gallus) J Comp Neurol. 2017;525:2583–91. doi: 10.1002/cne.24223. [DOI] [PubMed] [Google Scholar]
- 11.Juma AR, Hall NE, Wong J, Gasperoni JG, Watanabe Y, et al. PLAG1 expression and target genes in the hypothalamo-pituitary system in male mice. Mol Cell Endocrinol. 2018;478:77–83. doi: 10.1016/j.mce.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Mignone F, Gissi C, Liuni S, Pesole G. Untranslated regions of mRNAs. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-3-reviews0004. reviews00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hervé C, Mickleburgh I, Hesketh J. Zipcodes and postage stamps: mRNA localisation signals and their trans-acting binding proteins. Brief Funct Genomics. 2004;3:240–56. doi: 10.1093/bfgp/3.3.240. [DOI] [PubMed] [Google Scholar]
- 14.Desterro J, Rodriguez M, Hay R. Regulation of transcription factors by protein degradation. Cell Mol Life Sci. 2000;57:1207–19. doi: 10.1007/PL00000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update. 2009;15:213–27. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner T, Bomgardner D, Jacobs J, Nguyen Q. Association of segmentation of the epididymal interstitium with segmented tubule function in rats and mice. Reproduction. 2003;125:871–8. doi: 10.1530/rep.0.1250871. [DOI] [PubMed] [Google Scholar]
- 17.Hinton BT, Galdamez MM, Sutherland A, Bomgardner D, Xu B, et al. How do you get six meters of epididymis inside a human scrotum? J Androl. 2011;32:558–64. doi: 10.2164/jandrol.111.013029. [DOI] [PubMed] [Google Scholar]
- 18.Joseph A, Yao H, Hinton BT. Development and morphogenesis of the Wolffian/epididymal duct, more twists and turns. Dev Biol. 2009;325:6–14. doi: 10.1016/j.ydbio.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirashima T. Pattern formation of an epithelial tubule by mechanical instability during epididymal development. Cell Rep. 2014;9:866–73. doi: 10.1016/j.celrep.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 20.Kogan S, Gill B, Bennett B, Smey P, Reda E, et al. Human monorchism: a clinicopathological study of unilateral absent testes in 65 boys. J Urol. 1986;135:758–61. doi: 10.1016/s0022-5347(17)45842-3. [DOI] [PubMed] [Google Scholar]
- 21.Wass JA, Stewart PM. Oxford Textbook of Endocrinology and Diabetes. Oxford: Oxford University Press; 2011. [Google Scholar]
- 22.Xu B, Washington AM, Domeniconi RF, Souza AC, Lu X, et al. Protein tyrosine kinase 7 is essential for tubular morphogenesis of the Wolffian duct. Dev Biol. 2016;412:219–33. doi: 10.1016/j.ydbio.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nie X, Arend LJ. Pkd1 is required for male reproductive tract development. Mech Dev. 2013;130:567–76. doi: 10.1016/j.mod.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nie X, Arend LJ. Novel roles of Pkd2 in male reproductive system development. Differentiation. 2014;87:161–71. doi: 10.1016/j.diff.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendive F, Laurent P, Van Schoore G, Skarnes W, Pochet R, et al. Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev Biol. 2006;290:421–34. doi: 10.1016/j.ydbio.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 26.Hoshii T, Takeo T, Nakagata N, Takeya M, Araki K, et al. LGR4 regulates the postnatal development and integrity of male reproductive tracts in mice. Biol Reprod. 2007;76:303–13. doi: 10.1095/biolreprod.106.054619. [DOI] [PubMed] [Google Scholar]
- 27.Tomaszewski J, Joseph A, Archambeault D, Yao HH. Essential roles of inhibin beta A in mouse epididymal coiling. Proc Natl Acad Sci U S A. 2007;104:11322–7. doi: 10.1073/pnas.0703445104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar M, Syed SM, Taketo MM, Tanwar PS. Epithelial Wnt/βcatenin signalling is essential for epididymal coiling. Dev Biol. 2016;412:234–49. doi: 10.1016/j.ydbio.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Wijayarathna R, de Kretser DM, Sreenivasan R, Ludlow H, Middendorff R, et al. Comparative analysis of activins A and B in the adult mouse epididymis and vas deferens. Reproduction. 2018;155:15–23. doi: 10.1530/REP-17-0485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative image to show histological quantification methods. Numbers indicate how tubule cross sections were counted within a field of view. The double-headed arrow indicates a tubule cross section in the XY plane (i.e., perpendicular to the tubule's longitudinal axis, resulting in a near circular shape) and how its luminal diameter was measured. The scale bar represents 50 µm.
Localization of PLAG1 expression by X-gal staining in the epididymis of 7-week-old Plag1 heterozygous (Plag1+/−) mice. Scale bars represent 50 µm in low-magnification images (left column) and 20 µm in high-magnification images (right column). Plag1: pleomorphic adenoma gene 1.
X-gal staining in 7-week-old wild-type (Plag1+/+) mice as a negative control. Scale bars represent 50 µm in low-magnification images (left column) and 20 µm in high-magnification images (right column).
Negative controls for in situ hybridization staining of Plag1 mRNA: epididymal sections from 7-week-old Plag1 WT mice stained with the sense riboprobe (left column) and epididymal sections from 7-week-old Plag1 KO mice stained with the antisense riboprobe. Scale bars represent 50 µm. WT: wild-type; KO: knockout.
Hematoxylin-and-eosin staining of epididymal sections from 3-week-old Plag1 WT and KO mice. Scale bars represent 200 µm in low-magnification images (left column) and 20 µm in high-magnification images (right column). WT: wild-type; KO: knockout.
Hematoxylin-and-eosin staining of epididymal sections from 28–33-week-old Plag1 WT and KO mice. Scale bars represent 200 µm in low-magnification images (left column) and 20 µm in high-magnification images (right column). WT: wild-type; KO: knockout.
Hematoxylin-and-eosin staining of the epididymal sections from 50–55-week-old Plag1 WT and KO mice. Scale bars represent 200 µm in low-magnification images (left column) and 20 µm in high-magnification images (right column). WT: wild-type; KO: knockout.


