Abstract
Aromatase activity has commonly been associated with male infertility characterized by testicular dysfunction with low serum testosterone and/or testosterone to estradiol ratio. In this subset of patients, and particularly in those with hypogonadism, elevated levels of circulating estradiol may establish a negative feedback on the hypothalamic–pituitary–testicular axis by suppressing follicle-stimulating hormone (FSH) and luteinizing hormone (LH) production and impaired spermatogenesis. Hormonal manipulation via different agents such as selective estrogen modulators or aromatase inhibitors to increase endogenous testosterone production and improve spermatogenesis in the setting of infertility is an off-label option for treatment. We carried out a systematic review and meta-analysis of the literature of the past 30 years in order to evaluate the benefits of the use of aromatase inhibitors in the medical management of infertile/hypoandrogenic males. Overall, eight original articles were included and critically evaluated. Either steroidal (Testolactone) or nonsteroidal (Anastrozole and Letrozole) aromatase inhibitors were found to statistically improve all the evaluated hormonal and seminal outcomes with a safe tolerability profile. While the evidence is promising, future prospective randomized placebo-controlled multicenter trials are necessary to better define the efficacy of these medications.
Keywords: aromatase inhibitor, hypogonadism, male infertility, meta-analysis, systematic review
INTRODUCTION
About 15% of couples do not achieve pregnancy within 1 year and seek medical treatment for infertility. In infertile couples, male factor infertility contributes to 50% of cases.1,2 Primary hypogonadism is characterized by impaired testicular function, which may affect spermatogenesis and/or testosterone synthesis. Oligozoospermic and azoospermic men (i.e., due to nonobstructive spermatogenic dysfunction) have been found to be hypogonadal in approximately 43%–45% of cases.3,4
Estradiol (E2) plays an important role in several functions of gonadal axis regulation and spermatogenesis. In men, most bioavailable E2 is primarily created through peripheral aromatization of circulating testosterone (T) in adipose tissue. Several studies have described treatments which block E2-mediated negative feedback on the hypothalamic–pituitary axis (HPA), which, in turn, may lead to increases in luteinizing/follicle-stimulating hormone (LH/FSH) secretion and T production.5,6,7
Aromatase inhibitors (AIs) are well established for the treatment of metastatic breast cancer, and their use has also been adapted to the treatment of hypogonadal men by altering the T/E2 ratio.8,9,10,11 Multiple AIs have been used in off-label trials in the literature, but the most commonly tested steroidal AI has been testolactone, and the most commonly tested nonsteroidal AIs have been anastrozole and letrozole.12
There is no consensus in the literature regarding the role of AIs in the medical management of hypogonadal oligo-azoospermic males, although multiple studies have examined these medications for this purpose.13 The aim of the present systematic review and meta-analysis was to critically evaluate the literature for the effects of AIs on the treatment of hypogonadism and infertility through assessment of hormonal profile, semen parameters, and drug tolerability.
MATERIALS AND METHODS
Our goal was to examine the current evidence of the use of AIs in the medical management of hypoandrogenic males and to evaluate the results of trials reporting the available off-label use of AIs (e.g., testolactone, letrozole, and anastrozole) as a treatment for male infertility. In particular, we analyzed the impact of AIs in T and T/E2 ratio modification, impact of AIs on semen parameters, drug tolerability, and side effects.
Evidence acquisition
We performed a systematic search in PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) up to December 2018, without language restriction, to identify clinical trials implementing the use of AIs as a treatment for male infertility and reporting the clinical outcomes on serum hormones and/or semen parameters. The feature of related articles in PubMed was used to identify further papers. The reference lists of the studies included were also screened. Only original articles were included and critically evaluated. We excluded case reports as well as abstracts and reports from meetings. An expert librarian was involved in the design of the search strategy and in the conduct of the literature search. Accordingly, we searched publications using the following primary and secondary fields: “male infertility” and “aromatase inhibitors” and “hypogonadism” and “oligozoospermia” and “cryptozoospermia” and “azoospermia” and “serum testosterone and/or estradiol” and “sperm concentration and total sperm count” (primary fields); “testosterone to estradiol ratio” and “Testolactone” or “Letrozole” or “Anastrozole” and “side effects” and “nonrandomized and randomized trials” (secondary fields). All search terms included in the present review are summarized in Supplementary Table 1. For all studies, we evaluated the level of evidence (LE) according to the European Association of Urology (EAU) guidelines (Table 1).14
Supplementary Table 1.
Research terms analysis (primary and secondary fields)
| Key words – primary fields | Key words – secondary fields |
|---|---|
| Male infertility | Testosterone to estradiol ratio |
| Aromatase inhibitors | Testolactone |
| Hypogonadism | Anastrozole |
| oligozoospermia | Letrozole |
| Cryptozoospermia | Aromatase inhibitors side effects |
| Azoospermia | Bone metabolism |
| Serum testosterone | Osteoarticular side effects |
| Serum estradiol | Klinefelter’s syndrome |
| Sperm concentration | Obesity |
| Total sperm count | Randomized clinical trial |
| Sperm morphology | Nonrandomized clinical trial |
| Sperm motility |
Table 1.
Characteristics of the trials analyzed
| Study, year, country | Study design | Treatment enclosed | Sample size (n) | Age (year), median (range); mean±s.d. | Infertility etiology, n (%) | Follow-up (month) | LE |
|---|---|---|---|---|---|---|---|
| Clark and Sherins19 1989, USA | Prospective, randomized, double-blind, placebo-controlled crossover (single center) | Testolactone 2 g daily Placebo | Total: 25 | NR | Idiopathic | Baseline, 8, 16 | 1b |
| Pavlovich et al.21 2001, USA | Prospective, nonrandomized, case–control (single center) | Testolactone 100–200 mg daily | Total: 104 Testolactone (n=74) Control (n=40) | 37 (31–43) 40 (37–40) | Idiopathic: 12 (26.6), Klinefelter’s syndrome: 6 (13.3), Chromosome Y microdeletion: 5 (11.1), cryptorchidism: 5 (11.5), varicocele: 14 (21.1) | Baseline, 3 | 2a |
| Raman and Schlegel22 2002, USA | Prospective, nonrandomized, case–control (single center) | Testolactone 100–200 mg daily Anastrozole 1 mg daily | Total: 140 Testolactone (n=74) Anastrozole (n=101) | NR | Testolactone (n=74): Klinefelter’s syndrome: 17 (22.9), varicocele repair: 18 (24.3), varicocele present: 12 (16.2), overweight (BMI >35 kg m−2): NR; Anastrozole (n=101): Klinefelter’s syndrome: NR, varicocele repair: 30 (29.7), varicocele present: 33 (32.6), overweight (BMI >35 kg m−2): 16 (15.8) | Baseline, 3 | 2a |
| Saylam et al.23 2011, Turkey | Prospective, nonrandomized (single center) | Letrozole 2.5 mg daily | Total: 27 | 34.92±6.66 | Idiopathic hypoandrogenic | Baseline, 6 | 2a |
| Gregoriou et al. 2012, Greece24 | Prospective, nonrandomized study (single center) | Letrozole 2.5 mg daily Anastrozole 1 mg daily | Total: 29 Letrozole (n=15) Anastrozole (n=14) | NR | Idiopathic hypoandrogenic | Baseline, 6 | 2a |
| Cavallini et al.20 2013, Italy | Prospective, randomized, double-blind, placebo-controlled (multicentric) | Letrozole 2.5 mg daily Placebo | Total: 45 Letrozole (n=22) Placebo (n=23) | 44 (37–52) 45 (38–53) | Idiopathic hypoandrogenic: 28 (62.2) Cryptorchidism: 17 (37.7) | Baseline, 3, 6 | 1b |
| Helo et al.26 2015, USA | Prospective, randomized, double-blind (single center) | Clomiphene citrate 25 mg daily Anastrozole 1 mg daily | Total: 26 Clomiphene citrate (n=13) Anastrozole (n=13) | 35±6.5 33±3.9 | Idiopathic hypoandrogenic | Baseline, 3 | 1b |
| Shoshany et al.25 2017, USA | Retrospective survey25 (single center) | Anastrozole 1 mg daily | Total: 86 | 37 (32–41) | Idiopathic hypoandrogenic: 71 (82.5), cryptorchidism: 11 (12.7), varicocele repair: 4 (4.6) | Baseline, 4 | 3 |
BMI: body mass index; LE: level of evidence; NR: not reported; s.d.: standard deviation
Selection of the studies and criteria of inclusion
Entry into the analysis was restricted to data collected from original studies, including data from infertile couples (defined as failure to conceive for at least 12 months). Two authors (FDG and MLE) independently screened the titles and abstracts of all articles using predefined inclusion criteria. The full-text articles were examined independently by three authors (MLE, FDG, and EDB) to determine whether or not they met the inclusion criteria. Then, two authors (FDG and GMB) extracted data from the selected articles. Final inclusion was determined by consensus of all investigators.
All the female partners of the patients enrolled in the trials underwent basic diagnostic infertility evaluation and thus couples in whom female partners had a history of gynecologic surgery or ovulatory abnormalities were excluded from the analysis. The definition of hypogonadal oligo-azoospermia retrieved from the articles was based both on hormonal profiles (FSH concentrations within the normal range of reference values, low T levels [<400 ng dl−1] and concomitant presence of a T/E2 ratio <10) and on semen analyses (one or two below the normal references values according to the World Health Organization classification).15
The presence of any of the following exclusion criteria potentially responsible for impaired semen values was noted: ejaculatory duct obstruction (obstructive azoospermia after testicular biopsy) or surgery for male factor infertility (i.e., ejaculatory tract obstruction); history of mumps orchitis, drugs, and tobacco or alcohol abuse; ongoing medical treatments including assumption of gonadotropins, anabolic steroids, exogenous T use, or other hormonal therapies; previous cancer radiochemotherapy; and positive seminal cultural analysis or positive urethral swab chlamydia test.
Therefore, we included in the analysis both idiopathic etiology and patients with known causes of testicular failure (e.g., Klinefelter's syndrome, Y chromosome microdeletion, varicocele untreated, and cryptorchidism) presenting with normo/oligo/crypto/azoospermic semen parameters and characterized by the presence of suppressed T/E2 ratio.
From each single clinical trial selected, the following data were extracted: treatment setting and regimens, total number of patients, presence/absence of randomization between active treatment/placebo, number of patients treated in each study arm, control arm including/not including placebo, cointerventions, hormonal and seminal outcomes stratified according to baseline and posttreatment mean values ± standard deviation (s.d.), standard mean difference (s.m.d.) and 95% confidence intervals (CIs), whether or not the trial noted a statistically significant difference in these outcomes between the compared arms, and side effects derived from AI administration (P < 0.05 considered as statistically significant).
Statistical analyses
To assess the risk of bias (RoB), all included reports were reviewed using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool for diagnostic accuracy studies.16 Of note, the “risk of bias” tool is designed for randomized controlled trials and should be used with caution on other types of studies. The two reviewing authors independently assessed the methodological quality based on sequence generation, allocation concealment, blinding of patients and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, ITT analysis, and additional sources of bias.
We compared treatments using s.m.d. and 95% CIs. Heterogeneity was evaluated by Chi-square Q test and I2 statistic.17 For the Q test, P < 0.05 indicated significant heterogeneity; for the I2 statistics, an I2 value >50% was considered significant. The pooled s.m.d. estimate was calculated using a random effects model.18 Our results are graphically displayed as forest plots, with s.m.d. indicating not better outcome in the experimental arm. Calculations were accomplished using the Comprehensive Meta-Analysis Software, version v.2.0 (CMA, Biostat, Englewood, NJ, USA).
RESULTS
Search results
The database searches initially yielded 116 articles (PubMed: 87; Cochrane: 9; and Embase: 20). Seventy studies were excluded because they contained overlapping data or appeared in more than one database. Of these, 48 were subsequently removed due to duplication. On more detailed review, additional 33 papers were excluded for the following reasons: AIs with other topics (9), other drugs and/or breast cancer (12), animal experiment (5), and review paper or editorials (7). Full-text articles were then reevaluated and critically analyzed for the remaining 13 journal references. Of these, five did not meet the inclusion criteria. The remaining eight studies were considered for our systematic review (Figure 1 and Table 1). RoB assessment according to QUADAS-2 tool for each of the individual studies is illustrated in Supplementary Figure 1 (1.5MB, tif) .
Figure 1.
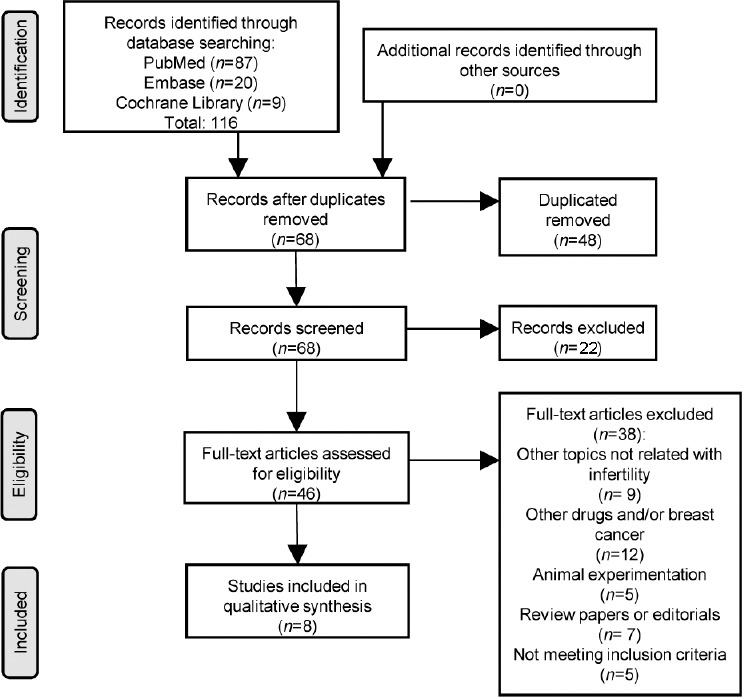
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Study locations and types
Of the 8 studies entered in the review, 5 were conducted in the US, 2 in Europe, and 1 in Turkey. Two studies were prospective, randomized, double-blind, placebo-controlled studies,19,20 with crossover between placebo and drug within the study of Clark and Sherins.19 The remaining five articles were divided into two prospective nonrandomized case–control studies,21,22 two prospective nonrandomized single-arm trials,23,24 and one retrospective survey.25 Moreover, two studies comparatively considered two different AIs (testolactone vs anastrozole and letrozole vs anastrozole) in the same study population,22,24 while one study, designed as a prospective, randomized, double-blind study, compared an AI (Anastrozole) versus a selective estrogen receptor modulator (SERM – Clomiphene Citrate).26
Study sample sizes, participant ages, and follow-up
The sample sizes varied from 25 to 140 cases analyzed in a single study. The total sample size of the eight studies was 517 patients. The total sample size of each individual treatment was 162 for testolactone, 214 for anastrozole, 64 for letrozole, and 48 for placebo. Three studies did not report participant's age.12,19,24 The range of mean age across the remaining five studies varied from 33 to 44 years. Age stratification for AIs implemented could not be performed due to available data and different statistical indices presented (mean vs median). In the studies, the follow-up after treatments ranged from 3 to 16 months (Table 1).
Impact of AIs on serum testosterone and T/E2 ratio modification
The effect of AIs on testosterone level was considered in all eight studies. Seven out of eight articles evaluated T serum modification expressed as mean ± s.d. at the baseline and at the end of the scheduled follow-up. Only the analysis of Cavallini et al.20 reported the T levels as median (range) and was not included in the following analysis. From the remaining seven studies, there were 417 men treated with Als: the overall baseline T level was 320.1 ± 98.2 ng dl−1 whereas after treatment was 475.6 ± 60.3 ng dl−1 with a mean difference of 155.5 ng dl−1 (overall mean increase 48.5%). Supplementary Table 2a summarizes the overall hormonal serum modifications and stratified for single AIs identified among the studies analyzed.
Supplementary Table 2.
(a) Overall and stratified mean hormonal modification and (b) overall and stratified mean semen parameters modification over the follow-up
| 2a | Overall mean modification | Stratification for single AIs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Testolactone (2 g/daily; 100–200 mg/daily) | Anastrozole (1 mg/daily) | Letrozole (2.5 mg/daily) | ||||||||||
| Baseline | After treatment | Δ value (%) | Baseline | After treatment | Δ value (%) | Baseline | After treatment | Δ value (%) | Baseline | After treatment | Δ value (%) | |
| Testosterone (ng dl−1) | 320.1±98.2 | 475.6±60.3 | 155.5 (48.5) | 401.2±117.1 | 479.5±85.4 | 78.2 (19.5) | 266.5±20.2 | 454±43.5 | 187.5 (70.3) | 265±14.1 | 511±22.6 | 246.2 (92.8) |
| Clark and Sherins19, 1989, USA, (n=25) | ||||||||||||
| Pavlovich et al.21 2001, USA, (n=63) | ||||||||||||
| Raman and Schlegel22 2002, USA, (n=140) | ||||||||||||
| Saylam et al.23 2011, Turkey, (n=27) | ||||||||||||
| Gregoriou et al.24 2012, Greece, (n=29) | ||||||||||||
| Helo et al.26 2015, USA, (n=26) | ||||||||||||
| Shoshany et al.25 2017, USA, (n=86) | ||||||||||||
| T/E2 ratio | 7.4±1.6 | 24.1±10.1 | 16.7 (227.2) | 5.1±0.2 | 12.5±0.2 | 7.4 (143.6) | 7.9±1.2 | 23.3±7.7 | 15.3 (193.1) | 8.5±0.7 | 37.5±2.1 | 29 (341.1) |
| Pavlovich et al.21 2001, USA, (n=63) | ||||||||||||
| Raman and Schlegel22, 2002, USA, (n=140) | ||||||||||||
| Saylam et al.23 2011, Turkey, (n=27) | ||||||||||||
| Helo et al.26 2015, USA, (n=26) | ||||||||||||
| Shoshany et al.25 2017, USA, (n=86) | ||||||||||||
| Estradiol (pg ml−1) | 36.9±13.7 | 26.8±13.1 | −10 (−27.3) | 47.3±17.1 | 39.9±14.4 | −7.4 (−15.7) | 34.4±10.6 | 23±5.8 | −11.3 (−34.2) | 26.3±0.5 | 14.7±0.2 | −11.5 (−44.1) |
| Clark and Sherins19 1989, USA, (n=25) | ||||||||||||
| Pavlovich et al.21 2001, USA, (n=63) | ||||||||||||
| Raman and Schlegel22 2002, USA, (n=140) | ||||||||||||
| Saylam et al.23 2011, Turkey, (n=27) | ||||||||||||
| Gregoriou et al.24 2012, Greece, (n=29) | ||||||||||||
| Helo et al.26 2015, USA, (n=26) | ||||||||||||
| Shoshany et al.25 2017, USA, (n=86) | ||||||||||||
| 2b | Overall mean modification | Stratification for single AIs | ||||||||||
| Testolactone (2 g/daily; 100–200 mg/daily) | Anastrozole (1 mg/daily) | Letrozole (2.5 mg/daily) | ||||||||||
| Baseline | After treatment | Δ value (%) | Baseline | After treatment | Δ value (%) | Baseline | After treatment | Δ value (%) | Baseline | After treatment | Δ value (%) | |
| Sperm concentration (×106 ml−1) | 7.9±5.4 | 17.2±8.1 | 9.2 (116.3) | 10.8±7.4 | 20.1±12.5 | 9.3 (86.1) | 5.1±0.5 | 14.3±1.7 | 9.2 (181.3) | NE | NE | NE |
| Pavlovich et al.21 2001, USA, (n=63) | ||||||||||||
| Raman and Schlegel22, 2002, USA, (n=140) | ||||||||||||
| Shoshany et al.25 2017, USA, (n=86) | ||||||||||||
| Sperm motility (%) | 18.6±12.4 | 27.4±12.5 | 8.7 (47) | 20.8±8.7 | 33.1±17.1 | 12.2 (58.6) | 29.7±15 | 32.7±9 | 3.1 (10.2) | 9.1±2.8 | 17.5±6.4 | 8.5 (95.2) |
| Pavlovich et al.21 2001, USA, (n=63) | ||||||||||||
| Raman and Schlegel22, 2002, USA, (n=140) | ||||||||||||
| Saylam et al.23 2010, Turkey, (n=27) | ||||||||||||
| Gregoriou et al.24 2012, Greece, (n=29) | ||||||||||||
| Shoshany et al.25 2017, USA, (n=86) | ||||||||||||
| Total sperm count (×106/ejaculated Vol.) | 4.6±2.4 | 7.9±2.3 | 3.2 (69.8) | NE | NE | NE | NE | NE | NE | 3.1±0.2 | 6.2±1.3 | 2.9 (92) |
| Clark and Sherins19, 1989, USA, (n=25) | ||||||||||||
| Saylam et al.23 2011, 2010, Turkey, (n=27) | ||||||||||||
| Gregoriou et al.24 2012, Greece, (n=29) | ||||||||||||
NE: not evaluable
The study by Clark and Sherins19 using testolactone (n = 25) was the only experience demonstrating no difference in total T concentrations through the treatment period. In contrast, the double-blind, placebo-controlled study by Cavallini et al.20 (n = 45) reported a significant difference of 249 (range: 200.3–298.1) ng dl−1 versus 1198 (range: 768–1501) ng dl−1 at the baseline and after 6 months of letrozole administration (P < 0.01). Moreover, this experience demonstrated a significant increase in T concentrations in the letrozole group (n = 22) when compared with the placebo-controlled group (n = 23) at the end of the follow-up (median: 1198 [range: 768–1501] ng dl−1 vs median: 266.2 [range: 210.2–315.6] ng dl−1).
Only five out of eight articles reported the effect of treatment on T/E2 ratio.21,22,23,25,26 Eight arms of treatment (n = 374) expressed T/E2 ratio values as mean ± s.d. at the baseline and at the end of scheduled follow-up. The baseline T/E2 ratio for the eight evaluated arms of treatment was 7.4 ± 1.6, and after AI treatment, T/E2 ratio was 24.1 ± 10.1, with a mean increase of 16.7 (overall mean increase 227%) (Supplementary Table 2a).
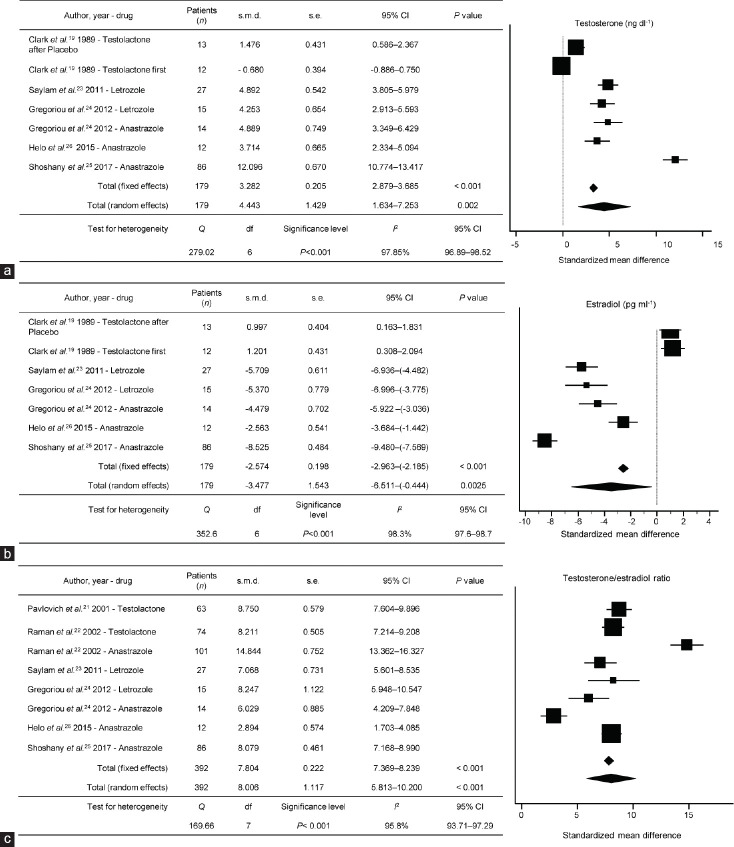
A meta-analysis was also performed to examine the effect of AIs on T levels in five eligible studies19,23,24,25,26 and T/E2 ratio in six eligible studies.21,22,23,24,25,26 AI therapy significantly increased T levels from the baseline (s.m.d: 4.443, 95% CI: 1.634–7.253; P = 0.002, I2 = 97.85%; Figure 2a and 2b) and T/E2 ratio from the baseline (s.m.d: 8.006; 95% CI: 5.813–10.200; P < 0.001 I2 = 95.8%; Figure 2c).
Figure 2.
Standardized mean difference results for hormonal serum concentrations: (a) testosterone; (b) estradiol; and (c) T/E2 ratio, during aromatase inhibitor treatment for each study over follow-up. s.m.d: standardized mean difference; s.e.: standard error; CI: confidence interval: T: testosterone; E2: estradiol; df: degree of freedom.
Impact of AIs on sperm concentration and sperm motility
Of the variables routinely evaluated in a semen analysis, the available data could be extrapolated only with regard to sperm concentration and sperm motility from those groups of patients presenting with oligo-, crypto-, and azoospermia at the baseline.
Three articles21,22,25 evaluated sperm concentration modification expressed as mean ± s.d. at the baseline and at the end of the scheduled follow-up. The analysis by Cavallini et al.20 reported this outcome as median (range) and was separately analyzed. The overall baseline total sperm concentration for the four evaluable arms of treatment was 7.9 ± 5.4 × 106 ml−1 and after treatment was 17.2 ± 8.1 × 106 ml−1, achieving a mean increase of 9.2 × 106 ml−1 (overall mean increase 116.3%). Within these three articles21,22,25 examining azoospermia (n = 66), no sperm recovery from the ejaculated semen was found in the group of azoospermic patients at the end of follow-up.
Evaluation of the different AIs was specifically performed with regard to testolactone (n = 24) and anastrozole (n = 46) due to available data. Supplementary Table 2b summarizes the overall semen profile modifications and stratified for single AI implemented in the available trials. The study by Raman and Schlegel22 was the only experience to directly compare the two drugs and showed no significant differences in any sperm parameters and in particular sperm concentration (P = 0.47). Interesting findings in sperm concentration modification were reported by Cavallini et al.20 in their cohort of crypto-azoospermic patients. In the letrozole arm, a significant increase in sperm retrieval was recorded from the baseline to the end of treatment (median: 450 [range: 0–900] ml−1 vs median: 1.387 [range: 632–1.904] × 106 ml−1; P < 0.01). Moreover, this study reported a significant difference in sperm concentration when comparing patients treated with letrozole versus placebo (median: 1.387 [range: 632–1.904] × 106 ml−1 vs median: 450 [range: 0–900] ml−1; P < 0.01). In contrast to Raman and Schlegel, Pavlovich et al., and Shoshany et al. who did not report sperm in the ejaculate of AI-treated azoospermic men,21,22,25 the experience of Cavallini et al.20 showed some spermatozoa retrieval in the ejaculate of all the azoospermic patients (n = 6) after AI treatment (i.e., letrozole) in contrast to those in the placebo arm (n = 5) that remained azoospermic. In the study of Saylam et al.23 (Letrozole 2.5mg daily), 17 (62.9%) patients presented with azoospermia, 4 (23.5%) had spermatozoa retrived in their ejaculate after treatment with sperm count increased from 0 to (1.1 ± 0.69) × 106 ml−1. However, this did not reach statistical significance (P = 0.125).
Of the five studies examining sperm motility,21,22,23,24,25 AI treatment demonstrated an increase from the baseline to the end of treatment. The overall baseline sperm motility was 18.6% ± 12.4%, whereas after treatment, it was 27.4% ± 12.5%, achieving a mean increase of 8.7% (overall mean increase of 47%). Among the studies that directly compared two AIs, Raman and Schlegel22 showed no significant difference between testolactone and anastrozole with regard to sperm motility (P = 0.63). Similarly, Gregoriou et al.24 reported a similar increase of 10.5% and 11% for anastrozole and letrozole, respectively (Supplementary Table 2b).
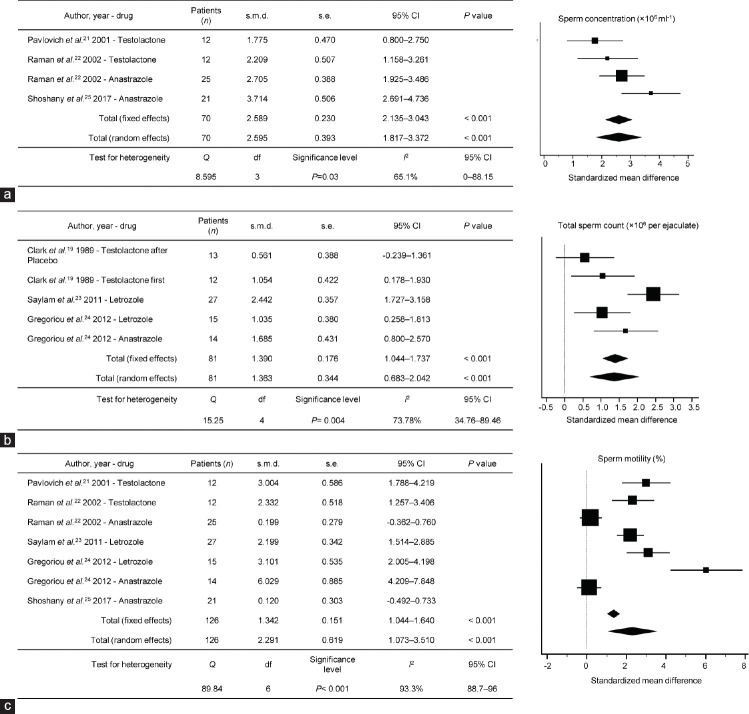
A meta-analysis was also performed to examine the effect of AIs on sperm concentration in three studies22,23,25 and sperm motility in five studies.21,22,23,24,25 AI therapy significantly increased sperm concentrations from the baseline (s.m.d: 2.595; 95% CI: 1.817–3.372; P < 0.001, I2 = 65.1%; Figure 3a and 3b) and sperm motility (s.m.d: 2.291; 95% CI: 1.073–3.510; P < 0.001, I2 = 93.3%; Figure 3c).
Figure 3.
Standardized mean difference results for semen parameters: (a) sperm concentration; (b) total sperm count; and (c) sperm motility, during aromatase inhibitor treatment for each study over follow-up. s.m.d: standardized mean difference; s.e.: standard error; CI: confidence interval; df: degree of freedom.
Drug tolerability and sides effects
We searched for the complications/side effects and reasons for discontinuing treatment reported within the different articles. All these variables are summarized in Table 2. The side effects reported were not life-threatening and resolved after the suspension of the therapy. In general, while AI treatment was overall well tolerated, various side effects were reported. Out of 436 patients on AI therapy, 14 (3.2%) discontinued the therapy due to the presence of side effects. Among the different adverse events, subclinical hepatic dysfunction, decrease/loss in libido, and drug intolerance were the most represented achieving a total number of events of 24 (5.5%), 11 (2.5%), and 10 (2.3%), respectively. All three drugs implemented were associated with episodes of subclinical hepatic dysfunction described both as asymptomatic liver function test increase and/or mild increases in transaminases and bilirubin values alone. Decrease or loss of libido was documented for anastrozole and letrozole administration. Studies also reported urticarial cutaneous rash as a reported side effect.19,20,26 Of note, no significant osteoarticular events or complications related to decreased bone density (rate of osteoporosis: 6.9% [2/29], Gregoriou et al.;24 3.4% [3/86], Shoshany et al.;25 Table 2) were reported due to concerns of potential interference with bone metabolism. Moreover, no significant difference in terms of osteoporosis event rate was reported in the study of Gregoriou et al.24 when compared with the placebo group (letrozole: 6.9% vs placebo: 5.5%). However, the follow-up in these studies24,25 was limited, which is consistent with the literature regarding long-term bone density effects in men.
Table 2.
Reported side effects from trials included
| Author, year, country | Sample size (n) | Treatment enclosed | Drug intolerance, n (%) | Transient weakness, n (%) | Arthralgia and/or decreased bone density, n (%) | Nausea, n (%) | Headache, n (%) | Diarrhea, n (%) | Loss of hair, n (%) | Subclinical hepatic dysfunction, n (%) | Decrease/loss of libido, n (%) | Discontinuation due to side effects, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clark and Sherins19 1989, USA | 25 | Testolactone 2 g daily | 8 (32) | NR | NR | 3 (12) | NR | NR | NR | NR | NR | 2 (8) |
| Pavlovich et al.21 2001, USA | 45 | Testolactone 100–200 mg daily | NR | NR | NR | NR | NR | NR | NR | 8 (17.7) | NR | NR |
| Raman and Schlegel22 2002, USA | 74 | Testolactone 100–200 mg daily | NR | NR | NR | NR | NR | NR | NR | 5 (6.9) | NR | NR |
| 104 | Anastrozole 1 mg daily | NR | NR | NR | NR | NR | NR | 8 (7.4) | 5 (4.8) | NR | ||
| Saylam et al.23 2011, Turkey | 27 | Letrozole 2.5 mg daily | NR | NR | NR | NR | 2 (7.4) | NR | NR | NR | NR | NR |
| Gregoriou et al.24 2012, Greece | 15 | Letrozole 2.5 mg daily | NR | 1 (6.6) | 2 (6.9) | 1 (6.6) | 2 (13.3) | NR | NR | 1 (6.6) | NR | NR |
| 14 | Anastrozole 1 mg daily | NR | NR | NR | 2 (14.2) | 1 (7.1) | 1 (7.1) | NR | 2 (14.2) | NR | NR | |
| Cavallini et al.20 2013, Italy | 22 | Letrozole 2.5 mg daily | 2 (9) | NR | NR | NR | NR | NR | 1 (4.5) | NR | 5 (22.7) | 4 (18.1) |
| 23 | Placebo | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| Helo et al.26 2015, USA | 24 | Anastrozole 1 mg daily | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Shoshany et al.25 2017, USA | 86 | Anastrozole 1 mg daily | NR | NR | 3 (3.4) | NR | NR | NR | NR | NR | 1 (1.1) | 8 (9.3) |
| Total | 436 | 10 (2.3) | 1 (0.3) | 5 (1.1) | 6 (1.4) | 5 (1.1) | 1 (0.3) | 1 (0.3) | 24 (5.5) | 11 (2.5) | 14 (3.2) |
NR: not reported
DISCUSSION
The purpose of our analysis was to evaluate the state of evidence regarding the use of AIs for men with testicular dysfunction. Despite heterogeneity in the literature with respect to patient characteristics, study design, and measured outcomes, a systematic review and meta-analysis was carried out for multiple hormonal and semen variables.
The trend of T serum modification over the follow-up represented the main outcome analyzed among the studies included in the review. Overall, all the studies reported a significant increase in serum T after AI administration (overall mean increase 48.5%; LE:1b). Only two studies22,24 using two different AIs on the same cohort population were identified. In particular, no difference in improvement of T concentrations between individual AIs was noted. For example, in the study of Raman and Schlegel,22 no difference between steroidal (testolactone) and nonsteroidal AIs (anastrozole) could be assessed. Moreover, we were not able to compare the two available randomized placebo-controlled trials19,20 included in the review due to different study designs (randomized placebo-controlled with crossover vs randomized placebo-controlled), different drugs administered, and different statistical indices presented in the results section (mean ± s.d. vs median [range]). Of these, the study by Clark and Sherins19 (testolactone) was the only one reporting no significant differences in total T concentrations over treatment, whereas the study by Cavallini et al.20 (letrozole) demonstrated a significant improvement of serum T concentrations in the nonsteroidal AI treatment group compared to placebo administration (LE:1b). While an overall serum T improvement was demonstrated (s.m.d: 4.443, 95% CI: 1.634–7.253; P = 0.002, I2 = 97.85%; Figure 2a and 2b), we could not conclude that there were significant differences in efficacy between the multiple AIs due to lack of available data.
AI therapy significantly increased T/E2 ratios (overall mean increase 227%; LE:2a). In the present review, the study by Ramanand Schlegel22 reported improvements in the T/E2 ratio. Of note, both randomized placebo-controlled trials19,20 enrolled in the present review did not report numeric information regarding the T/E2 levels modification.
The rationale behind the use of AIs as a potential tool to restore hormonal impairment and thus to treat idiopathic hypogonadal infertile males resides on the fact that more than 50% of circulating E2 is derived from peripheral aromatization of androgens.27,28 Moreover, T and E2 together with Sertoli cell-produced inhibin have found to be independently regulated, but strictly connected with the negative feedback modulating the release of pituitary gonadotropins. High E2 levels together with low circulating T exert a negative feedback on the HPA by suppressing the FSH and LH production and thus negatively regulating spermatogenesis.29,30,31 More recently, a rat model developed by Dumasia et al.32 demonstrated how exposure to agonist E2 administration may directly impact germinal epithelium by inducing germ cell apoptosis and spermiation failure by separate regulation of E2 receptors. AI administration in patients with impaired T/E2 ratio will decrease peripheral T to E2 conversion inducing a hypoestrogenic state which releases the HPA from the negative E2 effect and thus improving the hormonal profile and semen parameters.33,34
Certain limitations warrant mention. While we attempt for high scientific rigor, we are bound the existing literature which includes relatively few studies. As such, we performed a systematic review and meta-analysis only comparing available data (randomized clinical trials and prospective longitudinal and retrospective studies; Table 1) with limited ability to compare active treatments versus placebo administration. While data regarding hormonal parameters clearly improve among all the trials enrolled over the follow-up demonstrating some degree of consistency across the studies, the adoption of different seminal variables (i.e., sperm concentration or total sperm count) reduces the sample size of the evaluable population, therefore impacting on potency of the outcomes retrieved. In conclusion, we also recognize that pregnancy outcomes are not available in these studies, which limits the assessment of fertility outcomes.
Although AIs are FDA approved for the treatment of early and late-stage breast cancer, their use for male infertility is limited to off-label implementation. In our meta-analysis, the use of AIs in patients suffering from idiopathic or known causes of testicular failure resulted in a marked increase in serum T concentrations and T/E2 ratio. Moreover, a similar positive effect on semen profiles of these patients was identified, albeit in a limited number of studies. In addition, only one of four studies (Cavallini et al.20) demonstrated the efficacy of AI therapy in yielding sperm in the ejaculate of azoospermic men. Moreover, as previously observed by Schlegel,12 improvement in semen profiles appears to be more commonly achieved when selection criteria of the studies are based on lower baseline parameters. Thus, regression to the mean may result in higher values after treatment. In this setting, without future strict inclusion criteria regarding sperm concentration status, it could be difficult to identify a particular subset of patients who could expect a better prognosis from AI therapy. Moreover, lack of substantial amount of available data on patients suffering from other male factor diagnoses such as Klinefelter's syndrome and varicoceles precludes the possibility to further discuss AI use in this setting. Raman and Schlegel have demonstrated how anastrozole implementation could potentially be less efficacious in semen outcome improvement among men with Klinefelter's syndrome due to probable drug mechanism of action inability to inhibit adrenal steroidal synthesis. At the same time, in the subset of obese subfertile patients, the authors have also demonstrated how AI therapy did not show greater benefit than the rest of the population.22 With the present evidence, we were not able to establish any conclusions on such restricted category of patients. AI therapy before sperm retrieval could be a promising neoadjuvant option to increase the chances of sperm detection. Further prospective randomized studies focused on such selected category of infertile patients are therefore necessary before implementing AI in clinical practice.
CONCLUSION
Currently, the number and quality of the studies focusing on AIs for male testicular dysfunction remain low. Nevertheless, our systematic review and meta-analysis suggests the ability of AIs to improve hormone and semen profiles in a safe, well-tolerated manner. However, future randomized multicenter trials are necessary to better define the efficacy and risks of these medications in the clinical management of infertile men.
AUTHORS CONTRIBUTIONS
FDG, MLE, GMB and EDB take responsibility for the integrity of the data and accuracy of the data analysis while study concept and design of the study was developed by MLE, FDG, and MSG. All authors contributed to acquisition, analysis, and/or interpretation of data. Drafting of the manuscript was performed by FDG, AS, GMB, and MLE. Statistical analysis has been performed by MM, IS, and MF. MLE and GMB has been the study supervisor of the entire project. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
Supplementary Information in linked to the online version of the paper on Asian Journal of Andrology website.
Risk of bias assessment according to QUADAS-2. RoB: risk of bias; QUADAS-2: Quality Assessment of Diagnostic Accuracy Studies.
REFERENCES
- 1.World Health Organization. WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Couple. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 2.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–12. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 3.Dohle GR, Arver S, Bettocchi C, Jones TH, Kliesch S. European Association of Urology (EAU) Guidelines on Male Hypogonadism. 2019. [Last accessed on 2018 Mar 15]. Available from: https://uroweborg/wp-content/uploads/EAU-Guidelines-on-Male-Hypogonadism-2019v2 .
- 4.Nieschlag E, Behre HM, Nieschlag S. Andrology: Male Reproductive Health and Dysfunction. Berlin: Springer Verlag; 2010. [Google Scholar]
- 5.Hussein A, Ozgok Y, Ross L, Rao P, Niederberger C. Optimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: a multicentre study. BJU Int. 2013;111:E110–4. doi: 10.1111/j.1464-410X.2012.11485.x. [DOI] [PubMed] [Google Scholar]
- 6.Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES., Jr Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012;187:973–8. doi: 10.1016/j.juro.2011.10.137. [DOI] [PubMed] [Google Scholar]
- 7.Kim ED, Crosnoe L, Bar-Chama N, Khera M, Lipshultz LI. The treatment of hypogonadism in men of reproductive age. Fertil Steril. 2013;99:718–24. doi: 10.1016/j.fertnstert.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 8.Geisler J, King N, Anker G, Ornati G, Di Salle E, et al. In vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in postmenopausal breast cancer patients. Clin Cancer Res. 1998;4:2089–93. [PubMed] [Google Scholar]
- 9.Geisler J, Haynes B, Anker G, Dowsett M, Lønning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002;20:751–7. doi: 10.1200/JCO.2002.20.3.751. [DOI] [PubMed] [Google Scholar]
- 10.de Ronde W, de Jong FH. Aromatase inhibitors in men: effects and therapeutic options. Reprod Biol Endocrinol. 2011;9:93. doi: 10.1186/1477-7827-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dias JP, Melvin D, Simonsick EM, Carlson O, Shardell MD, et al. Effects of aromatase inhibition vs. testosterone in older men with low testosterone: randomized-controlled trial. Andrology. 2016;4:33–40. doi: 10.1111/andr.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlegel PN. Aromatase inhibitors for male infertility. Fertil Steril. 2012;98:1359–62. doi: 10.1016/j.fertnstert.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Ho CC, Tan HM. Treatment of the hypogonadal infertile male – A review. Sex Med Rev. 2013;1:42–9. doi: 10.1002/smrj.4. [DOI] [PubMed] [Google Scholar]
- 14.Aus G, Chapple C, Hanûs T, Irani J, Lobel B, et al. The European Association of Urology (EAU) guidelines methodology: a critical evaluation. Eur Urol. 2009;56:859–64. doi: 10.1016/j.eururo.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Switzerland: WHO Press; 2010. [Google Scholar]
- 16.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 19.Clark RV, Sherins RJ. Treatment of men with idiopathic oligozoospermic infertility using the aromatase inhibitor, testolactone. Results of a double-blinded, randomized, placebo-controlled trial with crossover. J Androl. 1989;10:240–7. doi: 10.1002/j.1939-4640.1989.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 20.Cavallini G, Biagiotti G, Bolzon E. Multivariate analysis to predict letrozole efficacy in improving sperm count of non-obstructive azoospermic and cryptozoospermic patients: a pilot study. Asian J Androl. 2013;15:806–11. doi: 10.1038/aja.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlovich CP, King P, Goldstein M, Schlegel PN. Evidence of a treatable endocrinopathy in infertile men. J Urol. 2001;165:837–41. [PubMed] [Google Scholar]
- 22.Raman JD, Schlegel PN. Aromatase inhibitors for male infertility. J Urol. 2002;167:624–9. doi: 10.1016/S0022-5347(01)69099-2. [DOI] [PubMed] [Google Scholar]
- 23.Saylam B, Efesoy O, Cayan S. The effect of aromatase inhibitor letrozole on body mass index, serum hormones, and sperm parameters in infertile men. Fertil Steril. 2011;95:809–11. doi: 10.1016/j.fertnstert.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Gregoriou O, Bakas P, Grigoriadis C, Creatsa M, Hassiakos D, et al. Changes in hormonal profile and seminal parameters with use of aromatase inhibitors in management of infertile men with low testosterone to estradiol ratios. Fertil Steril. 2012;98:48–51. doi: 10.1016/j.fertnstert.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Shoshany O, Abhyankar N, Mufarreh N, Daniel G, Niederberger C. Outcomes of anastrozole in oligozoospermic hypoandrogenic subfertile men. Fertil Steril. 2017;107:589–94. doi: 10.1016/j.fertnstert.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Helo S, Ellen J, Mechlin C, Feustel P, Grossman M, et al. A randomized prospective double-blind comparison trial of clomiphene citrate and anastrozole in raising testosterone in hypogonadal infertile men. J Sex Med. 2015;12:1761–9. doi: 10.1111/jsm.12944. [DOI] [PubMed] [Google Scholar]
- 27.de Ronde W, Hofman A, Pols HA, de Jong FH. A direct approach to the estimation of the origin of oestrogens and androgens in elderly men by comparison with hormone levels in postmenopausal women. Eur J Endocrinol. 2005;152:261–8. doi: 10.1530/eje.1.01830. [DOI] [PubMed] [Google Scholar]
- 28.Santen RJ. Feedback control of luteinizing hormone and follicle-stimulating hormone secretion by testosterone and estradiol in men: physiological and clinical implications. Clin Biochem. 1981;14:243–51. doi: 10.1016/s0009-9120(81)90964-4. [DOI] [PubMed] [Google Scholar]
- 29.Jarow JP, Zirkin BR. The androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann N Y Acad Sci. 2005;1061:208–20. doi: 10.1196/annals.1336.023. [DOI] [PubMed] [Google Scholar]
- 30.Pitteloud N, Dwyer AA, DeCruz S, Lee H, Boepple PA, et al. Inhibition of luteinizing hormone secretion by testosterone in men requires aromatization for its pituitary but not its hypothalamic effects: evidence from the tandem study of normal and gonadotropin- releasing hormone-deficient men. J Clin Endocrinol Metab. 2008;93:784–91. doi: 10.1210/jc.2007-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raven G, de Jong FH, Kaufman JM, de Ronde W. In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab. 2006;91:3324–8. doi: 10.1210/jc.2006-0462. [DOI] [PubMed] [Google Scholar]
- 32.Dumasia K, Kumar A, Deshpande S, Sonawane S, Balasinor NH. Differential roles of estrogen receptors, ESR1 and ESR2, in adult rat spermatogenesis. Mol Cell Endocrinol. 2016;428:89–100. doi: 10.1016/j.mce.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 33.McLachlan RI, Wreford NG, O’Donnell L, de Kretser DM, Robertson DM. The endocrine regulation of spermatogenesis: independent roles for testosterone and FSH. J Endocrinol. 1996;148:1–9. doi: 10.1677/joe.0.1480001. [DOI] [PubMed] [Google Scholar]
- 34.T’Sjoen GG, Giagulli VA, Delva H, Crabbe P, De Bacquer D, et al. Comparative assessment in young and elderly men of the gonadotropin response to aromatase inhibition. J Clin Endocrinol Metab. 2005;90:5717–22. doi: 10.1210/jc.2005-0982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of bias assessment according to QUADAS-2. RoB: risk of bias; QUADAS-2: Quality Assessment of Diagnostic Accuracy Studies.