Abstract
Response assessment or prediction to checkpoint inhibitor therapy (CIT) is an unsolved problem in current routine diagnostics of patients with melanoma. Here, we evaluated very early changes of primary and secondary lymphoid organs under CIT in multiparametric [18F]-labeled fluorodeoxyglucose-positron emission tomography (18F-FDG-PET)/MRI as possible predictors of treatment response and investigated their correlation with baseline blood immune biomarkers. Between October 2014 and November 2017, 17 patients with unresectable melanoma (8 females; 65±11 years) undergoing CIT were prospectively evaluated using whole-body 18F-FDG-PET/MRI before CIT start (t0), 2 weeks (t1) and 3 months after CIT initiation (t2). At each time point, the volume, the 18F-FDG-uptake and the mean apparent diffusion coefficient (ADC) of the spleen as well as the 18F-FDG uptake of the bone marrow were assessed. Relative lymphocyte count (RLC), relative eosinophil count (REC) and neutrophil-lymphocyte ratio (NLR) were assessed at baseline. Response Evaluation Criteria in Solid Tumours modified for immune-based therapeutics (iRECIST) and decisions from an interdisciplinary tumor board were used for treatment response evaluation at t2. iRECIST was compared with PET response criteria in solid tumors for image-based response evaluation at different time points. Comparative analysis was conducted with Mann-Whitney U test with false discovery rate correction for multiple testing and correlation coefficients were computed. In lymphoid organs, significant differences (p<0.05) between responders (9/17) and non-responders were found for the 18F-FDG-uptake in the spleen at t1 and the increase of the uptake t1-t0 (responders/non-responders: standardized uptake value lean body mass 1.19/0.93; +49%/−1%). The best correlation coefficients to baseline biomarkers were found for the 18F-FDG-uptake in the spleen at t1: NLR, r=−0.46; RLC, r=0.43; REC, r=0.58 (p<0.05), respectively. Compared with the non-responder group, the responder group showed marked increases also in the volume of the spleen (+22%/+10%), the 18F-FDG-uptake of bone marrow (+31%/−9%) at t1 and the ADCmean at t2 (+46%/+15%) compared with t0, however, not reaching significance. Our findings indicate that an effective systemic immune response in patients undergoing CIT can be detected as a significantly increased spleen activity in 18F-FDG-PET as early as 2 weeks after treatment initiation.
Trial registration number
NCT03132090, DRKS00013925.
Keywords: melanoma, programmed cell death 1 receptor, CTLA-4 antigen
Introduction
The idea to provoke an antitumor immunity by modifying the patients’ immune system has been proposed over 100 years ago.1 Today, immunotherapy has become an essential part of treatment regimens for several oncologic diseases.2–5 Checkpoint inhibitor therapy (CIT), like humanized monoclonal antibodies against the programmed-death-1 receptor or against the cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), have proven to potentially improve outcome of patients with melanoma in the long term.6 7 In brief, their mechanism of biological action is based on provoking an antitumor immune response.8 Besides tremendous therapy success in individual patients, CIT comes together with high costs and dangerous side effects while overall only 20%–40% of patients with melanoma benefit from CIT.9–12 Moreover, some non-responders even show an acceleration of tumor growth rate ≥2 under CIT which is defined as hyperprogression.13 Compared with CIT, hybrid imaging is far less expensive and comes with fewer side effects. By the early identification of responders to CIT, hybrid imaging might help to avoid ineffective or even harmful treatment regimes over a time period of usually 3 months. Early response evaluation by cross-sectional imaging (CT or MRI) or [18F]-labeled fluorodeoxyglucose-positron emission tomography (18F-FDG-PET) focusing on tumor assessment remains challenging as the mechanism of action of CIT differs from conventional chemotherapy.14–16 Blood immune biomarkers may potentially predict treatment outcome, since antitumor response requires an effective systemic immunity.17 From those biomarkers, the relative eosinophil count (REC), relative lymphocyte count (RLC) and the neutrophile-lymphocyte ratio (NLR) have been evaluated in larger melanoma patient cohorts.18–21
18F-FDG-PET is a non-invasive and quantitative imaging biomarker for glucose consumption, covering the whole body. In a recently published translational study, the potential of 18F-FDG-PET to evaluate primary (bone marrow) and secondary (spleen) lymphoid organs could be demonstrated by our group.22 While in the preclinical experiments fewer T cells and a higher number of neutrophils in the spleen led to a significantly higher 18F-FDG uptake in CIT-treated mice, the observed changes in the clinical data were small, and the examination time point varied due to the retrospective study setting. Preclinical studies in mice indicated that an antitumor immune response can be observed early after therapy start using the PET tracer (1-(2-deoxy-2-[18F]fluoroarabinofuranosyl) cytosine, which is targeting the deoxyribonucleotide salvage pathway.23 Furthermore, we were able to show in clinical PET data that a complete response to CIT in patients with melanoma can be observed already after the first cycle of therapy.16 Besides morphological data, MRI provides additional functional imaging techniques such as diffusion-weighted imaging (DWI), which monitors the diffusivity of water molecules, playing a central role in quantitative oncologic MRI.24
In this study, we aimed to evaluate whether early changes of lymphoid organs 14 days after initiation of CIT by multiparametric 18F-FDG-PET/MRI might be possible predictors of treatment response. Furthermore, correlation to baseline blood immune biomarkers was performed.
Materials and methods
Study design
Our prospectively conducted study recruited 62 patients from March 2015 until March 2018. Preliminary results of this study have been published previously to demonstrate the potential of 18F-FDG-PET to detect complete response to programmed-death-1 (PD1) therapy in 10 patients with melanoma early after therapy start.16
The present study includes adult patients with unresectable metastasized melanoma scheduled for systemic treatment with BRAF/MEK inhibitors, chemotherapy, anti-CTLA-4/PD1 antibodies or a combination therapy.
PET/MRI scans were performed just before planned treatment initiation (t0, baseline scan), 2 weeks (t1, study evaluation) and 3 months (t2, reference standard) after therapy start. Exclusion criteria were contraindications for MRI (metal implants, claustrophobia, etc), for gadolinium-based contrast agent, acute infections or other acute diseases, pregnant or breastfeeding women or a disability for informed consent.
Patient cohort
For the purpose of this study, we included patients who fulfilled the following criteria:
Treatment solely with CIT (anti-CTLA-4 and/or PD1 antibodies and no additional therapy of any kind);
Examination at all three time points;
Spleen and/or bone free of metastases at all time points.
A metastatic involvement might lead to an increase of metabolic activity, a change of organ volume or diffusivity either due to cancer cells or due to a local immune activation under therapy. A flow diagram of cohort selection is given in figure 1.
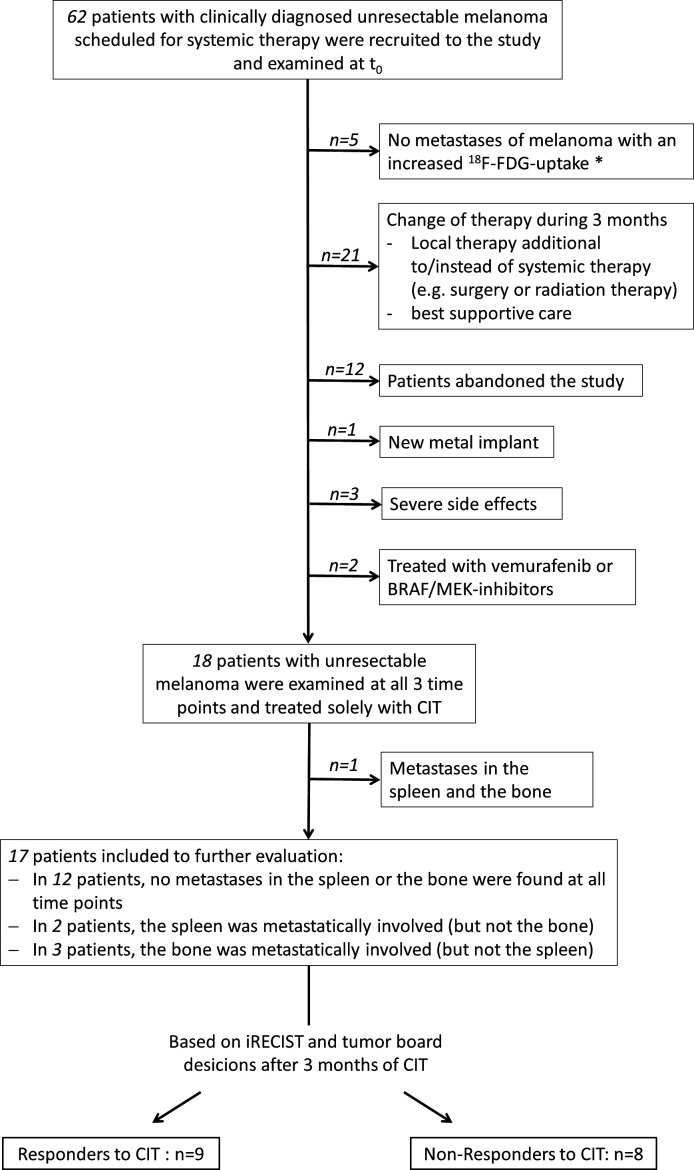
Figure 1.
Overview of cohort selection. A high number of patients quit the study and changes of therapy during the 3 months of observation resulted in a high drop-out rate. In five patients, no metastases with an increased uptake of [18F]-labeled fluorodeoxyglucose (18F-FDG) were found: in two patients, lesions turned out to be inflammatory (lung and liver), in one patient, a spinal meningeal melanocytoma was finally diagnosed and in two patients, suspicious lesions did not show an increased glucose consumption and were therefore not rated as metastases. CIT, checkpoint inhibitor therapy.
During CIT, no bone marrow stimulants such as Granulocyte-Colony Stimulating Factor were given to the patients. One week before the therapy initiation, RLC, REC and the NLR of each patient were acquired in clinical routine. Biomarkers were obtained from blood draws and analyzed using the Sysmex XN-9000 hematology analyzer. According to the literature, high RLC (>17.5%) and REC (>1.5%) and a low NLR (<4) are individually correlated with an improved treatment outcome.18–21
PET/MRI examination parameters
All patients were examined with the Biograph mMR (Siemens Healthineers, Erlangen, Germany), a fully integrated 3T-PET/MRI system. Patients fasted for at least 6 hours prior injection of 300 MBq 18F-FDG. Uptake times for PET/MRI at all time points was adherent to 120 min. Each patient received a whole-body PET/MRI scan with PET scan times of 4 min per bed position (four bed positions, cranial vertex to the thighs). PET images were reconstructed using the vendor’s software with three-dimensional (3D) ordered subset expectation maximization algorithm, 21 subsets, 2 iterations, 256×256 matrix size (voxel size: 2.8×2.8×2.0 mm3) and 4 mm Gaussian filter. A 3D T1-weighted spoiled gradient-echo sequence with Dixon-based fat-water separation in end-expiratory breath-hold was acquired to create an attenuation map. All attenuation maps were checked carefully for erroneous tissue identification. A fat saturated postcontrast T1 volumetric interpolated breath-hold examination (VIBE) was acquired in axial view with multiple breath-holds and following parameters: voxel size 1.7×1.7×3 mm3, slice thickness 3 mm, TR/TE 3.97/1.26 ms. Gadobutrol (Gadovist 1.0 mmol/mL, Bayer Vital, Leverkusen, Germany) was used as contrast agent. The DWI sequence was acquired with following parameters: image matrix 192×168, slice thickness 5 mm, no. of averages 3, b values 50 and 800 s/mm2. The apparent diffusion coefficient (ADC) maps were calculated by the vendors software.
Treatment response assessment
All image evaluations were performed with the software syngo.via (Siemens Healthineers). Treatment evaluation of patients with melanoma was assessed with PET response criteria in solid tumors (PERCIST), which is aimed to evaluate oncologic follow-up PET examinations in clinical trials as proposed by Wahl et al 25 and with iRECIST which is based on morphological criteria of metastases adapted to immunotherapy (assessed with MRI, T1 VIBE with fat saturation, contrast-enhanced26). Treatment response evaluation at t1 and t2 were each related to the baseline scan t0. Patients showing a stable metabolic disease (SMD)/immune stable disease (iSD), a partial metabolic response (PMR)/immune partial response (iPR) or a complete metabolic response (CMR)/immune complete response (iCR) at t2 were categorized as treatment responders; progressive metabolic disease (PMD)/immune progressive disease (iPD) was categorized as non-responders. Results were correlated with the decisions of the melanoma tumor board which is routinely performed after 3 months of treatment in our hospital.
Multiparametric evaluation of lymphoid organs
The volume of the entire spleen was assessed by drawing a free-hand volume of interest (VOI) in the postcontrast T1 VIBE MRI. In a fully integrated PET/MRI, PET and MRI are acquired simultaneously and this VOI can be copied to the PET images to assess the mean standardized uptake value lean body mass (SULmean) and to the ADC map to assess the ADC mean of the spleen. More detailed, the syngo.via software generates a mask of the VOI which is than copied and interpolated to the PET or ADC images, respectively. In individual cases, the VOI had to be slightly repositioned or changed in size to ensure a sufficient alignment of VOI and PET and avoid spillover effects of the left kidney or the colon. If ADC images were affected by slight artifacts in the upper abdomen, the ADCmean values were assessed by drawing at least three regions of interest covering the spleen in an axial plane (representative areas, avoiding organ borders and artifacts) and calculating the mean value. In one patient, the attenuation map at t1 was erroneous in the upper abdomen, so no reliable SULmean of the spleen could be measured. In another patient, the ADCmean value of the spleen could not be reliably acquired due to severe artifacts. All other parameter were successfully evaluated. For the evaluation of the SULmean in the bone, a bone mask of the spine was computed using the syngo.via software Frontier MR bone scan prototype application (Siemens Healthineers). This software computes a bone mask with an atlas-based segmentation using anatomical landmarks in DIXON images (figure 2). The shape and the size of the bone mask are optimized via non-rigid registration. The bone mask was aligned to the PET data and subsequently resampled by a nearest-neighbor interpolation to circumvent additional thresholding of the interpolated mask in case of applying a linear interpolation procedure. These steps were processed in MATLAB (The MathWorks, Natick, Massachusetts, USA).
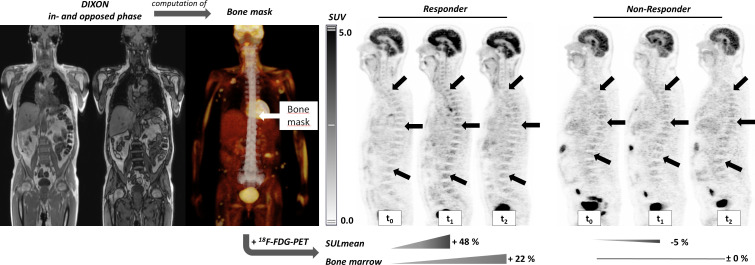
Figure 2.
Left hand side: DIXON MRI (in and opposed phase) were used to compute a bone mask of the spine (highlighted in the positron emission tomography (PET) maximum intensity projection image), which was copied to the PET images to assess the standardized uptake value lean body mass (SULmean) of the bone marrow. Right hand side: example of a responder (patient no. 10, also shown in online supplementary figure S2) and a non-responder (patient no. 11) to checkpoint inhibitor therapy. Note the changes of [18F]-labeled fluorodeoxyglucose (18F-FDG)-uptake in the bone marrow in the responder especially at t1 while there was no significant change observed in the non-responder.
jitc-2020-000656supp001.pdf (1.6MB, pdf)
The relative differences of 18F-FDG-uptake/ADCmean/volume at t1 or t2, respectively, compared with t0 were computed as follows: (value t1 or 2–value t0)/value t0*100.
Statistical analysis
MedCalc software V.18.10 (MedCalc Software, Ostend, Belgium; http://www.medcalc.org; 2018) and SPSS V.25 (SPSS Statistics, IBM, Armonk, New York, USA) were used for statistical analysis. Parameters (acquires values and relative differences) between the responder and non-responder group were compared with a Mann-Whitney U test. False discovery rate (FDR) correction was performed to account for multiple testing (rate 5%). P values of <0.05 were considered to be statistically significant. Correlation coefficients between acquired imaging parameters and baseline immune blood biomarkers were calculated.
Results
An overview of the finally included 17 patients, the results of the treatment response assessment and the baseline biomarkers are shown in table 1.
Table 1.
Overview of patients finally included to our evaluation
| Patient no. | Age | AJCC | Therapeutic agent | PERCIST / iRECIST | Overall response |
NLR | RLC | REC | Spleen SUV t1-t0 (%) | |
| t1 | t2 | |||||||||
| 1* | 59 | IV | CTLA-4 | SMD/iSD | PMD/iPD | NR | 4.7 | 15.6 | 0.9 | +6.8 |
| 2 | 74 | IV | PD1 | PMD/iSD | PMD/iSD | R | 3.0 | 21.0 | 2.2 | +32.8 |
| 3* | 53 | IV | PD1 | PMD/iSD | PMD/iPD | NR | 4.4 | 15.7 | 1.6 | +16.9 |
| 4† | 51 | IV | CTLA-4 | SMD/iSD | CMR/iCR | R | 2.9 | 22.5 | 0.8 | N/A |
| 5 | 49 | IV | PD1 | PMR/iPR | CMR/iCR | R | 1.5 | 34.6 | 3.1 | +9.5 |
| 6* | 59 | IV | CTLA-4 | PMD/iPD | PMD/iPD | NR | 6.2 | 12.3 | 2.2 | +4.2 |
| 7 | 84 | IV | PD1 | PMR/iPR | CMR/iPR | R | 2.3 | 26.5 | 3.8 | N/A |
| 8 | 64 | IV | PD1 | SMD/iSD | PMD/iPD | NR | 3.6 | 18.9 | 2.1 | −20.5 |
| 9 | 75 | IV | PD1 | SMD/iSD | PMD/iPD | NR | 4.2 | 17.2 | 0.8 | −14.3 |
| 10 | 66 | IV | PD1 | SMD/iSD | SMD/iSD | R | 3.5 | 19.6 | 0.5 | +60.0 |
| 11 | 64 | IV | PD1 | PMD/iPD | PMD/iPD | NR | 4.4 | 16.2 | 1.9 | −2.3 |
| 12 | 60 | IV | PD1 | PMR/iPR | CMR/iCR | R | 4.1 | 16.6 | 2.3 | +25.8 |
| 13 | 82 | IV | PD1+CTLA-4 | SMD/iSD | PMR/iSD | R | 3.1 | 22.2 | 1.4 | +64.8 |
| 14 | 53 | IV | PD1+CTLA-4 | PMR/iSD | CMR/iPR | R | 1.5 | 30.8 | 4.8 | +129.9 |
| 15 | 76 | IV | PD1+CTLA-4 | PMR/iSD | PMR/iPR | R | 1.7 | 31.7 | 3.0 | +20.4 |
| 16† | 57 | IV | PD1+CTLA-4 | SMD/iSD | PMD/iPD | NR | 1.8 | 29.3 | 4.7 | N/A |
| 17 | 74 | IV | PD1 | SMD/iSD | PMD/iPD | NR | 4.2 | 17.0 | 0.7 | +2.2 |
†Staging of melanoma according to the AJCC eighth edition. Treatment response evaluation at t1 and t2 were each related to the baseline scan t0. Overall response by taking imaging and clinical decisions into account, categorized as (Non-)Responders, (N)R. Patients with a metastatic involvement of the bone are marked with (*), of the spleen with (†). In those patients, the bone or the spleen, respectively, was excluded from further evaluation. In patient no. 7, the attenuation map at t1 was erroneous in the upper abdomen, so no reliable SULmean of the spleen could be measured. Spleen SUV t1-t0 (%): the relative change of 18F-FDG-uptake of the spleen between t1 and t0.
*Patients with a metastatic involvement of the bone.
†Patients with a metastatic involvement of the spleen.
AJCC, American Joint Committee on Cancer; CMR, complete metabolic response; CTLA-4, cytotoxic T-lymphocyte-associated protein-4; 18F-FDG, [18F]-labeled fluorodeoxyglucose; iCR, immune complete response; iPD, immune progressive disease; iPR, immune partial response; iSD, immune stable disease; N/A, not available; NR, non-responders; PD1, programmed-death-1; PERCIST, PET response criteria in solid tumors; PMD, progressive metabolic disease; PMR, partial metabolic response; SMD, stable metabolic disease; SULmean, standardized uptake value lean body mass; SUV, standardized uptake value.
After 3 months of treatment, 9/17 patients were categorized as non-responders (PMD/iPD). Examples of metabolic and morphological treatment response are shown in figure 3.
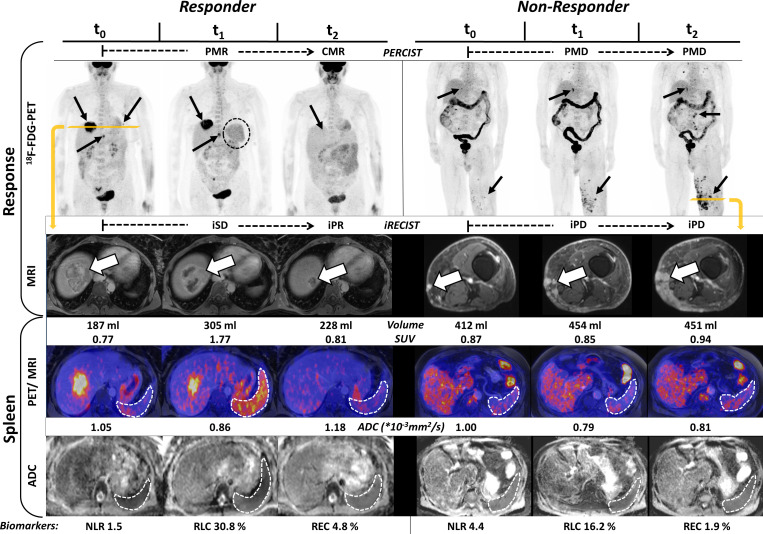
Figure 3.
Morphological (iRECIST) and metabolic (positron emission tomography (PET) response criteria in solid tumors (PERCIST)) response assessment (upper two rows, ‘Response’) as well as multiparametric changes in the spleen (‘Spleen’) and baseline immune biomarkers (bottom line) in a responder (left hand side, patient no. 14) and a non-responder (right hand side, patient no. 11) to checkpoint inhibitor therapy. Left hand side: metastases in the liver and the lung at t0 (black arrows) with an excellent treatment response already visible at t1 in PET: the metastases in the lung disappears in PET and the metabolic activity of the liver metastases decreases significantly, resulting in partial metabolic response (PMR); diameters of metastases did not show significant changes, leading to immune stable disease (iSD) in iRECIST at t1. Avital tumor residue in the liver at t2 (immune partial response (iPR)) without specific tracer uptake (complete metabolic response (CMR)). Note the significant increase of metabolic activity and the volume of the spleen (dotted ring in the PET/MRI), especially at t1 as compared with t0, considerably less pronounced at t2. Right hand side: metastases in the liver, the lung and the soft tissue of the left leg (black arrows). New metastases are visible in both MRI and PET already at t1, resulting in PMD/immune progressive disease (iPD). The volume of the spleen slightly increases under therapy, but the metabolic activity remains stable. The apparent diffusion coefficient (ADC)mean of the spleen did not show clear trends under therapy. The dotted regions of interes (ROIs) in the images aim to highlight the spleen and are not the volume of interest (VOI) used for image evaluation.
Multiparametric evaluation of lymphoid organs
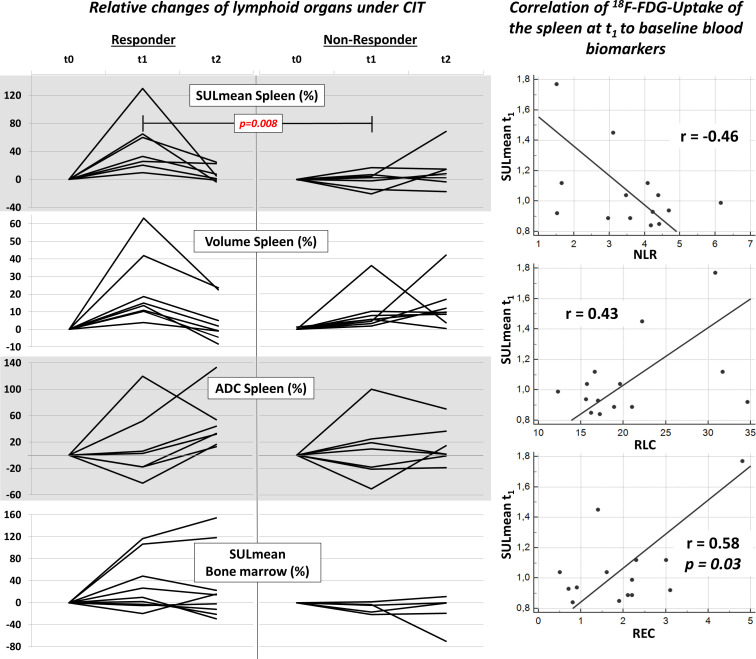
Examples for changes of the spleen and the bone marrow in responders and non-responders are shown in figures 2 and 3. Results of relative changes are demonstrated in figure 4. Significant differences between the responder and non-responder group were found in the SULmean of the spleen at t1 (mean value responder 1.19, non-responder 0.93, FDR-corrected p value 0.008) and the relative difference of SULmean t0-t1 (mean value responder +49 %, non-responder −1 %, FDR-corrected p value 0.008). In patients treated with PD1 therapy only, we also found a significant difference of the relative changes of SULmean t0-t1 of the spleen (mean value responder +32 %, non-responder −4 %, p value 0.032). Box plot diagrams of the SULmean of the spleen at t1 as well the relative changes are given in online supplementary figure S1. In the responder group, higher SULmean values were also found in the bone marrow and with regard to the volume of the spleen at t1 as compared with t0 or t2 (mean value SULmean t0/t1/t2 0.64/0.76/0.71, volume (mL) t0/t1/t2 223/270/232). Moreover, the relative changes of SULmean in the bone marrow and the volume at t1 were considerably higher in the responder group: SULmean bone marrow, +31% vs −9%; volume spleen +22% vs +10%. However, these differences were not significant (p>0.05). The ADC values did not show significant differences at t0 or t1 between the responder group and the non-responder group (ADCmean×10−3mm2/s t0/t1/t1-t0: responder, 855/899/+15%; non-responder: 824/856/+9%). At t2, the ADCmean values in the responder group were higher as compared with the non-responder group, not reaching significance: (ADCmean×10−3mm2/s t2/t2-t0: responder, 1199/+46%; non-responder: 897/+15%).
Figure 4.
Left hand side: results of the evaluation of lymphoid organs demonstrated as relative changes in % to the baseline scan t0 in the responder and non-responder group. Significant differences between the time points were found for SULmean at t1 and for relative differences between t0 and t1. Right hand side: correlation of metabolic activity of the spleen at t1 (SULmean t1) to the baseline immune biomarkers NLR, RLC and REC of all patients. NLR, neutrophile-lymphocyte ratio; REC, relative eosinophil count; RLC, relative lymphocyte count; SULmean, standardized uptake value lean body mass.
jitc-2020-000656supp002.pdf (1,002.9KB, pdf)
Correlation to baseline immune biomarkers
Significant differences between the responder and non-responder group were found for the NLR (mean value responder 2.62, non-responder 4.18, p=0.004) and the RLC (mean value responder 25.06, non-responder 17.78, p=0.008). Correlation coefficients of the evaluated imaging parameters of all patients were found highest for the SULmean at t1 with the baseline immune biomarkers, results are given in figure 4. The correlation coefficients of SULmean of the spleen with the biomarkers at all time points t0/t1/t2 were: NLR:0.30/–0.46/0.59; REC: −0.07/0.58/0.05 RLC: –0.23/0.43/–0.44. The volume or the ADC values did not show notable correlations to the baseline immune biomarkers.
Discussion
In this study of multiparametric evaluation of lymphoid organs, we found that a systemic immune response in patients responding to CIT can be observed already 14 days after treatment initiation by 18F-FDG-PET, appearing as increased metabolic activity in the spleen. Changes of the volume of the spleen and the 18F-FDG-uptake of bone marrow showed the same tendency, but less pronounced. The ADC map did not add a noticeable value to early treatment response evaluation in our patient cohort.
The prediction of treatment response to CIT especially a very early response assessment is of paramount interest for oncologic treatment regimes in several fields. The biological mechanism of action makes the response evaluation of solid tumors to immunotherapy a challenging task for imaging modalities focusing on changes in metastatic lesions. In this connection, the role of 18F-FDG-PET including the evaluation of different response criteria has been investigated in several studies with small patient cohorts and equivocal results: Kaira et al 27 found PERCIST criteria to be more predictive in patients with NSCLC treated with immunotherapy as compared with RECIST 1.1.28 Cho et al 29 proposed a combination of anatomical and functional imaging parameters as a possible future approach. Anwar et al 30 even proposed new criteria for the response evaluation to CIT in PET/CT (PERCIMT). In our study, metabolic (PERCIST) and morphological (iRECIST) response evaluation was overall in good agreement. A significant difference was only found for patient no. 2 who showed a response to CIT which was rated as PMD with PERCIST and iSD with iRECIST at t2. This patient was rated as ‘responder’ according to the decision of the interdisciplinary tumor board 3 months after treatment initiation. In patients no. 3, 14 and 15, PERCIST showed a clear trend of treatment (non-)response already at t1 while iRECIST was stable (example is given in figure 3). In patients no. 7 and 14, remnants of metastases without specific tracer uptake were seen at t2 (CMR vs iPR). Previous studies could observe changes in the 18F-FDG-uptake of the spleen 3 months or later after treatment initiation in patients responding to CIT.31 32 Moreover, a recently published report on the immunotherapy symposium held on the annual congress of the European Association of Nuclear Medicine takes the spleen for response assessment of CIT in PET into consideration.33 Nevertheless, the reported changes in FDG-uptake were small, the evaluation time points vary and although it may appear likely, the link between the metabolic activity of immune organs and an immune response has not been proven yet. The recently published translational study by Schwenck et al 22 and the correlation of the metabolic activity early after treatment initiation to baseline immune biomarkers observed in our study support the hypothesis, that 18F-FDG-PET is a reliable tool to monitor an effective systemic immune response in patients treated with CIT. A further important observation from this longitudinal patient study is that the systemic immune response seems a highly dynamic process, also in 18F-FDG-PET. This may explain the differing results to our previous retrospective study with examination time points between 50 days before and 125 days after treatment initiation.22 Invasive biopsies of the spleen or bone marrow under therapy are ethically not feasible and a detailed explanation of the observations in lymphoid organs regarding changes in cellular components of the immune system is beyond the scope of our work. In this connection, anti-CD8 immuno-PET might help gain a deeper understanding of the process of immune response under CIT in the future.34 In 8/17 patients, no clear trend could be observed at t1 with PERCIST criteria (SMD) alone. In patients thereof finally responding to therapy, the 18F-FDG-uptake of the spleen as well as the relative change of 18F-FDG-uptake at t1 was considerably higher compared with the patients finally not responding to CIT (responder/non-responder with SMD at t1: SULmean 1.25/0.90; +62%/−6%). Especially in this group of patients, the supportive role of spleen evaluation might be of value and enable a early response assessment with hybrid imaging. Nevertheless, there is an overlap of the SULmean values and the relative changes of metabolic activity in the spleen t1-t0 between the responder and non-responder group, more pronounced in the SULmean values (online supplementary figure S1). Although the patient cohort is small, an increase of +25% and a SULmean >1 after 2 weeks of CIT might be reasonable first approaches for cut-off-values. Focusing on the group of patients treated with PD1 antibodies only, we also found a significant difference between the responder and the non-responder group regarding the change t0-t1 of 18F-FDG-uptake in the spleen. The predictive value of baseline blood immune biomarkers was confirmed in our study with significant differences between the two groups. Nonetheless, they have limitations and although there is a correlation between the metabolic activity of the spleen early after treatment initiation and baseline immune biomarkers, they do not necessarily mean the same thing: patient no. 12 shows a baseline NLR of >4 and a RLC of <17.5% but a complete response to CIT after 3 months; the relative differences in 18FDG-uptake t1-t0 were as follows: spleen, +25%; bone marrow, +107%. Patient no. 8 shows a baseline NLR <4, a RLC >17.5% and REC >1.5% but progressive disease after 3 months; the relative differences in 18F-FDG-uptake t1-t0 were as follows: spleen, −20%; bone marrow, −3%. Therefore, baseline immune biomarkers indicating a good treatment outcome do not necessarily translate in an increased 18F-FDG-uptake of lymphoid organs early after treatment initiation and good treatment response and vice versa. Maybe blood immune biomarkers indicate a general immune competence of a patient, but the increase of 18F-FDG-uptake in lymphoid organs confirms the actual process of CIT-induced immune response. Therefore, the relative changes of lymphoid organs in 18F-FDG-PET might add an independent value to response evaluation in CIT. However, the results of our study do not allow a general statement regarding the predictive capacity of early changes in lymphoid organs as compared with baseline immune biomarkers. As anatomic CT images today represent the standard of care for therapy monitoring, we also evaluated the volume of the spleen to investigate if this might be of help for CIT response assessment. We observed an increase of the spleen volume under therapy in most of our patients, especially in the first follow-up scan 2 weeks after therapy start. This peak was overall more pronounced in patients responding to CIT, however, the difference between the two groups did not reach significance, probably due to the small patient cohort. In patients with iSD at t1, the increase of spleen volume t1-t0 was higher in patients finally responding to CIT as compared with non-responders (30% vs 11%), however, less pronounced as the changes observed in 18F-FDG-PET. Besides anatomic images, MRI provides several functional imaging parameters and nowadays, DWI plays a pivotal role in oncologic whole-body imaging.24 For the evaluation of a systemic immune response, the role of ADC has not been evaluated previously. Based on our results, ADC values of the spleen do not seem to be of value for an early response assessment, but there was a trend towards higher ADC values of the spleen in the responder group at t2. The ADC map in our study was calculated based on to b values: b=50 and 800 s/mm2. This protocol is aimed for oncologic whole-body DWI. The spleen is a highly perfused organ and with a low b value of 50 s/mm2 and two acquired b values, the ADC is influenced by perfusion effects. Although there might also be true changes in the diffusivity of the spleen (eg, caused by changes in the cellularity), a main part of the increase of ADC might be caused by changes in the perfusion. In order to eliminate this effect, the acquisition of more and higher b values is necessary which is associated with longer acquisition times. We evaluated the changes in metabolic activity in the spleen and the bone marrow (and metastases) using the SULmean and did not compute a ratio to the liver as it is one of the most commonly affected organs in metastasized melanoma and might also play a role in the systemic immune response.35 Besides the spleen and the bone marrow, draining and distant lymph nodes also play a significant role in the immune system and changes under CIT were reported in the study by Tsai et al.32 An example from our patient cohort of an intensive increase of tracer uptake in the hilar lymph nodes at t1 is shown in online supplementary figure S2, known as sarcoid-like reaction.36 Although this is likely to also represent a CIT-induced systemic immune response, we did not systematically evaluate lymph nodes in this work for the following reasons: first, we cannot differentiate by imaging if an increase of tracer uptake in (draining) lymph nodes is caused by a metastatic involvement or a response to CIT. A systematic evaluation using fine needle aspirations is ethically not feasible. On the contrary, metastases in the spleen and the bone can be excluded by multiparametric PET/MRI. Second, healthy lymph nodes are (without immune activation) small and do not show a relevant tracer uptake; changes in size, ADC value or tracer accumulations are therefore susceptible to the methodology used. Future oncologic hybrid imaging will ask for quantitative and robust imaging biomarkers, which can be added to clinical routine image evaluation. By segmenting the spleen and the spine based on anatomical images, we want to propose a reproducible approach for the evaluation of primary and secondary lymphoid organs in hybrid imaging which can easily be applied. Against this background, a visual and non-quantitative evaluation of lymph nodes might not to be robust enough. The major limitation of our study is the relatively low number of patients, however, the special values of the study are the prospective design and the strict inclusion criteria. The included patients were treated solely with CIT and the lymphoid organs were monitored in a complex whole-body PET/MRI setting which is unique in the literature so far. Although the statistical power is therefore limited, the findings of our study strongly support the role of the evaluation of lymphoid organs in hybrid imaging for treatment assessment. There was a high number of patients who quit the study (figure 1), mostly due to long examination time of 1.5 hours and the resulting limited patient comfort.37 Shorter examination times can be achieved by reducing the number of MRI sequences and the use of accelerated imaging techniques38 in the future. A large proportion of non-responders can be expected in patients who have abandoned the study or who have had changes in therapy during the 3 months; this also explains the balanced proportion of responders and non-responders in the finally evaluated patient cohort.
In conclusion, our findings indicate that an effective systemic immune response in patients undergoing CIT can be observed in the spleen in 18F-FDG-PET, especially early after treatment initiation. Spleen volume and metabolic activity of bone marrow seem also be valuable in this regard but need to be confirmed in studies with larger patient cohorts in the future. These findings might help to support the evaluation of treatment response by hybrid imaging in the future.
Footnotes
Correction notice: This paper has been corrected since it was published online. The affiliation ‘Cluster of Excellence iFIT (EXC 2180) "Image-Guided and Functionally Instructed Tumor Therapies", Eberhard Karls University, 72076 Tübingen, Germany’ was missing from the paper.
Contributors: FS evaluated all imaging data, critically analyzed and interpreted the patient data and drafted the manuscript. AF interpreted clinical treatment response of patients and was responsible for patient recruitment. BW contributed to the interpretation and collection of hematological data. BG contributed to the design, the coordination and the funding of the study. MS contributed to the evaluation of imaging data regarding the bone scan prototype application and critically revised the manuscript. JS critically analyzed imaging data, supported the interpretation of hematological data and critically revised the manuscript. AO contributed to the statistical analysis and revised the manuscript. MF kindly supported the implementation of syngo.via-software Frontier MR bone scan prototype application and critically revised the manuscript. CG contributed to the design of the study, patient recruitment and the interpretation of treatment response assessment. KN critically analyzed imaging data and revised the manuscript. NS conceived the study, obtained funding and participated in its design. CLF critically analyzed imaging data and supported the coordination of the study. CP conceived the study, obtained funding and participated in its design and coordination and critically revised the manuscript. All authors read and approved the final manuscript.
Funding: This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy—EXC 2180-390900677.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the local ethics committee (code: 251/2012BO1) as well as the Federal Agency for Radiation Protection (code: Z5-22463/2-2012-023). Written informed consent was given by all patients.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. with a report of ten original cases. 1893. Clin Orthop Relat Res 1991;262:3–11. [PubMed] [Google Scholar]
- 2. Lipson EJ, Forde PM, Hammers H-J, et al. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol 2015;42:587–600. 10.1053/j.seminoncol.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pflugfelder A, Kochs C, Blum A, et al. Malignant Melanoma S3-Guideline “Diagnosis, Therapy and Follow-up of Melanoma”. J Dtsch Dermatol Ges 2013;11:1–116. 10.1111/ddg.12113_suppl [DOI] [PubMed] [Google Scholar]
- 5. Xu-Monette ZY, Zhou J, Young KH. Pd-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood 2018;131:68–83. 10.1182/blood-2017-07-740993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–33. 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134–44. 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480–9. 10.1038/nature10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016;315:1600–9. 10.1001/jama.2016.4059 [DOI] [PubMed] [Google Scholar]
- 10. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 11. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–18. 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:190–209. 10.1016/j.ejca.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 13. Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920–8. 10.1158/1078-0432.CCR-16-1741 [DOI] [PubMed] [Google Scholar]
- 14. Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412–20. 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 15. Sachpekidis C, Anwar H, Winkler J, et al. The role of interim 18F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging 2018;45:1289–96. 10.1007/s00259-018-3972-9 [DOI] [PubMed] [Google Scholar]
- 16. Seith F, Forschner A, Schmidt H, et al. 18F-Fdg-Pet detects complete response to PD1-therapy in melanoma patients two weeks after therapy start. Eur J Nucl Med Mol Imaging 2018;45:95–101. 10.1007/s00259-017-3813-2 [DOI] [PubMed] [Google Scholar]
- 17. Spitzer MH, Carmi Y, Reticker-Flynn NE, et al. Systemic immunity is required for effective cancer immunotherapy. Cell 2017;168:e15:487–502. 10.1016/j.cell.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacquelot N, Roberti MP, Enot DP, et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun 2017;8:592. 10.1038/s41467-017-00608-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martens A, Wistuba-Hamprecht K, Geukes Foppen M, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res 2016;22:2908–18. 10.1158/1078-0432.CCR-15-2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer 2018;6:74. 10.1186/s40425-018-0383-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lino-Silva LS, Salcedo-Hernández RA, García-Pérez L, et al. Basal neutrophil-to-lymphocyte ratio is associated with overall survival in melanoma. Melanoma Res 2017;27:140–4. 10.1097/CMR.0000000000000333 [DOI] [PubMed] [Google Scholar]
- 22. Schwenck J, Schörg B, Fiz F, et al. Cancer immunotherapy is accompanied by distinct metabolic patterns in primary and secondary lymphoid organs observed by non-invasive in vivo18F-FDG-PET. Theranostics 2020;10:925–37. 10.7150/thno.35989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radu CG, Shu CJ, Nair-Gill E, et al. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2'-deoxycytidine analog. Nat Med 2008;14:783–8. 10.1038/nm1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Padhani AR, Koh D-M, Collins DJ. Whole-Body diffusion-weighted MR imaging in cancer: current status and research directions. Radiology 2011;261:700–18. 10.1148/radiol.11110474 [DOI] [PubMed] [Google Scholar]
- 25. Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009;50 Suppl 1:122S–50. 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143–52. 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaira K, Higuchi T, Naruse I, et al. Metabolic activity by 18F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging 2018;45:56–66. 10.1007/s00259-017-3806-1 [DOI] [PubMed] [Google Scholar]
- 28. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 29. Cho SY, Lipson EJ, Im H-J, et al. Prediction of Response to Immune Checkpoint Inhibitor Therapy Using Early-Time-Point 18F-FDG PET/CT Imaging in Patients with Advanced Melanoma. J Nucl Med 2017;58:1421–8. 10.2967/jnumed.116.188839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anwar H, Sachpekidis C, Winkler J, et al. Absolute number of new lesions on 18F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur J Nucl Med Mol Imaging 2018;45:376–83. 10.1007/s00259-017-3870-6 [DOI] [PubMed] [Google Scholar]
- 31. Dercle L, Seban R-D, Lazarovici J, et al. 18F-FDG PET and CT Scans Detect New Imaging Patterns of Response and Progression in Patients with Hodgkin Lymphoma Treated by Anti-Programmed Death 1 Immune Checkpoint Inhibitor. J Nucl Med 2018;59:15–24. 10.2967/jnumed.117.193011 [DOI] [PubMed] [Google Scholar]
- 32. Tsai KK, Pampaloni MH, Hope C, et al. Increased FDG avidity in lymphoid tissue associated with response to combined immune checkpoint blockade. J Immunother Cancer 2016;4:58. 10.1186/s40425-016-0162-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aide N, Hicks RJ, Le Tourneau C, et al. FDG PET/CT for assessing tumour response to immunotherapy : Report on the EANM symposium on immune modulation and recent review of the literature. Eur J Nucl Med Mol Imaging 2019;46:238–50. 10.1007/s00259-018-4171-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tavaré R, Escuin-Ordinas H, Mok S, et al. An effective Immuno-PET imaging method to monitor CD8-Dependent responses to immunotherapy. Cancer Res 2016;76:73–82. 10.1158/0008-5472.CAN-15-1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology 2006;43:S54–62. 10.1002/hep.21060 [DOI] [PubMed] [Google Scholar]
- 36. Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-Like reactions induced by checkpoint inhibitors. J Thorac Oncol 2018;13:1076–82. 10.1016/j.jtho.2018.04.031 [DOI] [PubMed] [Google Scholar]
- 37. Gückel B, Gatidis S, Enck P, et al. Patient comfort during positron emission tomography/magnetic resonance and positron emission tomography/computed tomography examinations: subjective assessments with visual analog scales. Invest Radiol 2015;50:726–32. 10.1097/RLI.0000000000000177 [DOI] [PubMed] [Google Scholar]
- 38. Taron J, Schraml C, Pfannenberg C, et al. Simultaneous multislice diffusion-weighted imaging in whole-body positron emission tomography/magnetic resonance imaging for multiparametric examination in oncological patients. Eur Radiol 2018;28:3372–83. 10.1007/s00330-017-5216-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-000656supp001.pdf (1.6MB, pdf)
jitc-2020-000656supp002.pdf (1,002.9KB, pdf)