Abstract
Context:
Recent studies suggested that magnetic resonance imaging (MRI) followed by targeted biopsy (“MRI-stratified pathway”) detects more clinically significant prostate cancers (csPCa) than the systematic transrectal ultrasound–guided prostate biopsy (TRUS-Bx) pathway, but controversy persists. Several randomized clinical trials (RCTs) were recently published, enabling generation of higher-level evidence to evaluate this hypothesis.
Objective:
To perform a systematic review and meta-analysis of RCTs comparing the detection rates of csPCa in the MRI-stratified pathway and the systematic TRUS-Bx pathway in patients with a suspicion of prostate cancer (PCa).
Evidence acquisition:
PubMed, EMBASE, and Cochrane databases were searched up to March 18, 2019. RCTs reporting csPCa detection rates of both pathways in patients with a clinical suspicion of prostate cancer were included. Relative csPCa detection rates of the MRI-stratified pathway were pooled using random-effect model. Study quality was assessed using the Cochrane risk of bias tool for randomized trials. A comparison of detection rates of clinically insignificant PCa (cisPCa) and any PCa was also performed.
Evidence synthesis:
Nine RCTs (2908 patients) were included. The MRI-stratified pathway detected more csPCa than the TRUS-Bx pathway (relative detection rate 1.45 [95% confidence interval {CI} 1.09–1.92] for all patients, and 1.42 [95% CI 1.02–1.97] and 1.60 [95% CI 1.01–2.54] for biopsy-naïve and prior negative biopsy patients, respectively). Detection rates were not significantly different between pathways for cisPCa (0.89 [95% CI 0.49–1.62]), but higher in the MRI-stratified pathway for the detection of any PCa (1.39 [95% CI 1.05–1.84]).
Conclusions:
The MRI-stratified pathway detected more csPCa than the systematic TRUS-guided biopsy pathway in men with a clinical suspicion of PCa, for both biopsy-naïve patients and those with prior negative biopsy. The detection rate of any PCa was higher in the MRI-stratified pathway, but not significantly different from that of cisPCa.
Patient summary:
Our meta-analysis of clinical trials shows that the magnetic resonance imaging–stratified pathway detects more clinically significant prostate cancers than the transrectal ultrasound–guided prostate biopsy pathway in men with a suspicion of prostate cancer.
Keywords: Biopsy, Magnetic resonance imaging, Prostate cancer, Targeted biopsy, Meta-analysis, Systematic review
1. Introduction
Transrectal ultrasound-guided prostate biopsy (TRUS-Bx) is considered the current diagnostic standard for patients with suspected prostate cancer (PCa) based on raised serum prostatic-specific antigen (PSA) levels, abnormal digital rectal examination findings, and other risk factors. The extended sextant systematic strategy that samples 12 tissue cores from both sides of the prostate has been established as the standard procedure after a meta-analyses showed that it was superior to the prior sextant protocol, while further increase in the number of cores up to 18–24 was not shown to have a significant incremental value [1]. Although this diagnostic pathway has led to increased detection of PCa, there is concern that TRUS-Bx undersamples a significant portion of clinically significant PCa (csPCa), which potentially can progress, metastasize, and result in cancer-related mortality. A study of 7643 patients found that Gleason scores were upgraded in approximately a third of the patients from a TRUS-Bx Gleason score of ≤6 to a higher grade on radical prostatectomy [2]. On the contrary, TRUS-Bx also overdetects clinically insignificant PCa (cisPCa), leading to overtreatment and side effects of treatment such as erectile dysfunction and urinary incontinence [3]. Therefore, there is an unmet need to improve upon the current diagnostic pathway, to better identify men who would benefit from treatment without increasing unnecessary treatment-related side effects.
Recent years have seen significant advances in magnetic resonance imaging (MRI) technology. Increasing evidence suggests that MRI could noninvasively improve PCa visualization and aid in targeting prostate biopsies to abnormal areas seen on MRI [4]. In the recent PROMIS trial, multiparametric MRI (mpMRI) combining T2-weighted imaging, diffusion-weighted imaging, and dynamic contrast-enhanced (DCE) MRI, showed good sensitivity (88%) and a negative predictive value (76%), and a meta-analysis on the updated Prostate Imaging Reporting and Data System (PI-RADS) version 2 showed sensitivity of up to 90% for detecting csPCa [5,6]. Based on this improved visualization of PCa by MRI, an approach with prebiopsy MRI followed by targeted biopsy (TBx), namely, the “MRI-stratified pathway,” has become feasible, and studies have shown that MRI-guided TBx may improve the detection of csPCa while reducing that of cisPCa [7]. Nevertheless, the vast majority of studies along with systematic reviews and meta-analyses dealing with this topic may have an inherent bias as they were based on retrospective/prospective cohort studies or within-person paired comparative studies (where both systematic TRUS-Bx and MRI-guided TBx were performed for each individual) [7–9]. A meta-analysis of randomized controlled trials (RCTs) provides the highest level of evidence and thus a more conclusive answer on the value of the MRI-stratified pathway for PCa diagnosis [10]. There are several recently published RCTs comparing the MRI-stratified pathway and the systematic TRUS-Bx pathway, including the PRECISION study, which showed that the MRI-stratified pathway detected 12% more csPCa and 13% less cisPCa [11]. Therefore, we performed a systematic review and meta-analysis of RCTs comparing the detection rates of csPCa in the MRI-stratified pathway and the systematic TRUS-Bx pathway in patients with a suspicion of PCa.
2. Evidence acquisition
This study was carried out conforming to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. The aim of this systematic review and meta-analysis was to compare the cancer detection rates of the MRI-stratified pathway and those of the systematic TRUS-guided biopsy pathway with regard to csPCa (primary objective) and any PCa or cisPCa (secondary objectives) in patients with a suspicion of PCa.
2.1. Literature search
A computer database search was performed using PubMed, EMBASE, and Cochrane Database of Systematic Reviews, which were updated until March 18, 2019. The search query, shown in the Supplementary material, was constructed based on the “Population/Intervention/Comparator/Outcomes/Study design” (PICOS) criteria using keywords and their related terms of prostate cancer, MRI, targeted biopsy, systematic biopsy, and RCT. Bibliographies of the identified articles were thoroughly checked to search for other potentially includable articles. No restriction regarding language was applied.
2.2. Inclusion and exclusion criteria
Studies were included based on the PICOS criteria: (1) “patients” with a clinical suspicion of PCa who are either biopsy naïve or have had one or more prior negative biopsy results; (2) MRI-stratified pathway, in which prebiopsy mpMRI is performed followed by MRI-guided TBx, either performing TBx only without systematic biopsy (SBx) for positive scans and no biopsy for negative scans (“MRI-TBx only pathway”) or performing both SBx and TBx for positive scans and only SBx for negative scans (“combined pathway”) as “intervention”; (3) systematic TRUS-guided biopsy pathway as a “comparator”; (4) comparison of detection rates of csPCa, cisPCa, and any PCa as “outcome”; and (5) “study design” of prospective RCTs published as either a full paper or a conference abstract.
Exclusion criteria were as follows: (1) a small number of patients (<10); (2) other publication types including nonrandomized prospective/retrospective cohort studies, reviews, guidelines, and editorials; (3) papers dealing with other topics (ie, patients who have already been diagnosed with PCa undergoing active surveillance or RCTs comparing different types of MRI-guided biopsies [in bore vs MRI-TRUS fusion]); (4) insufficient information for extracting cancer detection rates; and (5) overlap in the study population (although this was not expected, as we exclusively included prospective RCTs).
The study selection process was performed by one reviewer (S.W.) and was confirmed by two additional reviewers, one of them (H.A.V.) being a faculty genitourinary radiologist with 12 yr of experience and the other (C.H.S.) a radiologist with 5 yr of experience in meta-analysis).
2.3. Data extraction and quality assessment
The following study, patient, MRI, and biopsy characteristics were extracted using a standardized form: (1) study: origin (authors, year of publication, enrollment period, institution, and country), design (multicenter vs single center), and definition of csPCa; (2) patient: clinical setting (biopsy naïve or prior negative biopsy), number of patients (total, MRI-stratified pathway, and TRUS-guided biopsy pathway), age, serum PSA level, and prostate volume; (3) MRI: vendor, scanner model, magnet strength, reporting system and threshold used for TBx indication (ie, PI-RADS version 2 score >3), number of readers and their experience, and prevalence of a positive MRI scan; and (4) biopsy: whether concurrent SBx was performed in the MRI-stratified pathway (eg, “MRI-TBx only pathway” vs “combined pathway”), number of operators performing TBx and their experience, methods for registration (cognitive fusion, MRI-TRUS software registration, or in-bore direct TBx), number of cores and lesions for TB/SB, biopsy approach (transrectal vs transperineal), and detection rates csPCa, any PCa, and cisPCa.
The quality of evidence in the included studies was evaluated using the revised Cochrane risk of bias tool for randomized trials (RoB 2 tool) [13]. This tool judges the risk of bias as “a low risk of bias,” “some concerns,” or “a high risk of bias” for each of the following five domains: (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported result.
Data extraction and quality assessment were initially performed by two reviewers (S.W. and C.H.S.), and a discussion with a third reviewer (H.A.V.) was held to reach a consensus if there was disagreement between them.
2.4. Data synthesis and analysis
The primary outcome of this meta-analysis was comparison of the detection rates of csPCa of the MRI-stratified pathway and the systematic TRUS-guided biopsy pathway, and their relative detection rate. The relative detection rate was defined as the detection rate of the MRI-stratified pathway divided by that of the systematic TRUS-guided biopsy pathway. The definition of csPCa was based on that used in each study. If not specified, Gleason score ≥7 (3 + 4) cancer was categorized as csPCa when information regarding Gleason scores was provided [9,14]. A relative detection rate of >1 signifies that the MRI-stratified pathway detects more csPCa, while a rate of <1 indicates that it detects less csPCa than the systematic TRUS-guided pathway. The rationale for using this was to adjust for differences in the prevalence of csPCa across studies, while the crude rates themselves do not. The secondary outcomes were as follows: (1) comparison of the detection rate of any PCa and cisPCa between both pathways along with their relative detection rates and (2) subgroup analysis for csPCa stratified to clinically relevant variables.
Detection rates and relative detection rates were meta-analytically pooled using a random-effect (DerSimonian and Laird) method with the “meta” package in R software (version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria) [15]. Assessment of publication bias was planned for outcomes with >10 included studies using Funnel plots and Egger’s test [16].
3. Evidence synthesis
3.1. Literature search
A total of 333 articles were initially retrieved by the systematic search. After removal of duplicates, screening of the titles and abstracts, and full-text reviews, nine articles (eight full papers and one conference abstract) were considered to be relevant for our systematic review and meta-analysis [11,17–24]. Figure 1 shows the PRISMA study selection process.
Fig. 1 −.
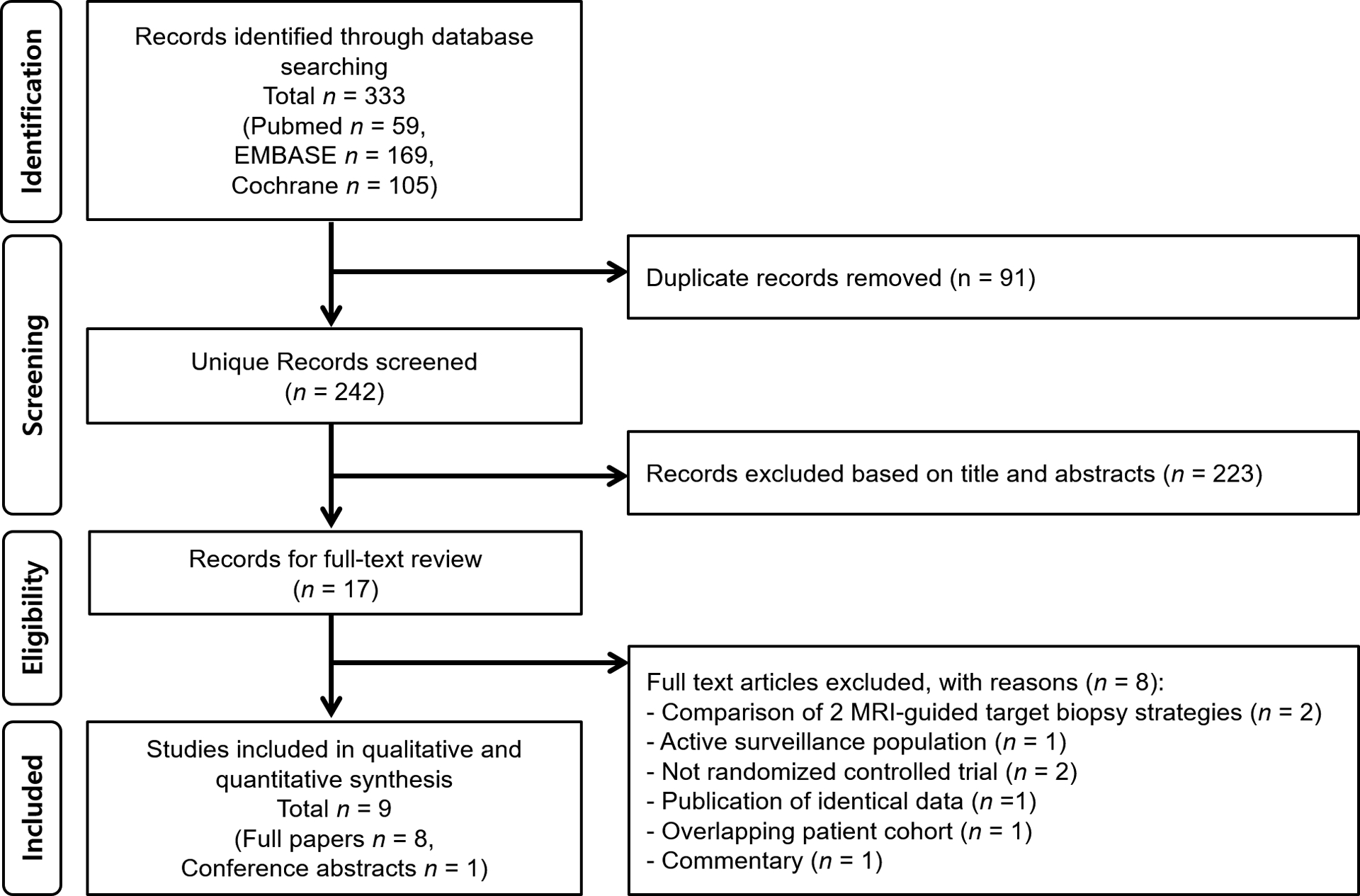
Flow diagram showing the study selection process. MRI = magnetic resonance imaging.
3.2. Characteristics of included studies
Tables 1–3 show the study, patient, MRI, and biopsy characteristics of each study. In brief, all but one study were single-center studies. Seven studies were based on biopsy-naïve patients and two on patients with prior negative biopsy results. The clinical definition of csPCa was Gleason score ≥7 (3 + 4) in three, ≥7 (4 + 3) in one, and ≥7 (3 + 4) with additional criteria involving core information in five. The number of total patients ranged from 85 to 1140. In five studies 3-Tesla scanners were used, and 1.5-Telsa scanners were used in three studies. The multicenter study used both, but was analyzed in the group using 3-Tesla scanners as these were used predominantly (184/246) [11]. MRI was interpreted using either PI-RADS (version 1 in three, version 2 in two, and unknown version in one) or an institutional scale (n = 3) mostly by experienced radiologists. The prevalence of a positive MRI scan in the MRI-stratified pathway ranged from 50% to 100%. TBx was done by cognitive fusion in five studies and by MRI-TRUS software registration in two studies. The multicenter study used either of these methods, but was analyzed as part of the latter method as this was used in the majority of patients (219/252) [11]. In-bore direct TBx was not used in any study. Concurrent SBx was performed in the MRI-stratified pathway in all but two studies [11,21]. Systematic biopsies were performed using a median of 10–13 cores via the transrectal route.
Table 1 –
Study and patient characteristics
| Author | Year | Institution | Country | Enrollment period | CsPCa definition | Clinical setting | Patient no. | Median age, yr (range)a | Median PSA, ng/ml (range)a | Median prostate volume, ml (range)a | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | MRI-stratified pathway | SBx pathway | ||||||||||
| Baco et al [17] | 2016 | Oslo University Hospital | Norway | September 2011–June 2013 | GS ≥7 or MCCL ≥5 mm | Biopsy naïve | 175 | 86 | 89 | 64 (58–69) 65 (59–69) |
6.9 (5.2–9.2) 7.6 (5.9–10.4) |
45 (33–60) 40 (29–52) |
| Bello et al [18] | 2018 | Hospital Universitario de Canarias | Spain | February 2015–October 2017 | GS ≥7 or MCCL ≥5 mm | Biopsy naïve | 303 | 182 | 121 | NR | NR | NR |
| Kasivisvanathan et al [11] | 2018 | 23 centersb | 11 countriesb | February 2016–August 2017 | GS ≥7 | Biopsy naïve | 500 | 252 | 248 | 64.4c 64.5 |
6.75 (5.16–9.35) 6.50 (5.14–8.65) |
40.5 (32.0–54.8) 43.7 (33.3–60.0) |
| Panebianco et al [19] | 2015 | Sapienza University | Italy | October 2011–March 2014 | GS ≥7 or percentage of CL involved with PCa ≥50% | Biopsy naïve | 1140 | 570 | 570 | 64 (51–82) | NR | NR |
| Park et al [20] | 2011 | Samsung Medical Center | South Korea | July 2008–December 2009 | GS ≥7 | Biopsy naïve | 85 | 44 | 41 | 63 (40–82) 61 (37–92) |
6.1 (4.0–9.7) 5.6 (2.9–9.9) |
37 (17–94) 38 (15–87) |
| Porpiglia et al [21] | 2017 | San Luigi Gonzaga Hospital | Italy | November 2014–April 2016 | GS ≥7 or MCCL ≥5 mm | Biopsy naïve | 212 | 107 | 105 | 64 (58–70) 66 (60–70) |
5.9 (4.8–7.5) 6.7 (5.5–8.5) |
46.2 (34.5–71.6) 45.7 (34.6–65.0) |
| Sciarra et al [22] | 2010 | Sapienza University | Italy | January 2007–January 2009 | GS ≥7 (4 + 3) | Prior negative biopsy | 180 | 90 | 90 | 63.5 (49–74)c | 6.2 (4.0–9.3) 6.0 (4.0–9.0) |
45.5 (30.0–63.0) 45.0 (30.0–60.0) |
| Taverna et al [23] | 2016 | Humanitas Mater Domini | Italy | January 2013–June 2014 | GS ≥7 | Prior negative biopsy | 200 | 100 | 100 | 65 ± 19c 63 ± 15 |
12.63c 13.04 |
NR |
| Tonttila et al [24] | 2016 | Oulu University Hospital | Finland | April 2011–December 2014 | GS ≥7, >2+ cores, or MCCL ≥3 mm | Biopsy naïve | 113 | 53 | 60 | 63 (60–66) 62 (56–67) |
6.1 (4.2–9.9) 6.2 (4.0–10.7) |
27.8 (23.5–36.6) 31.8 (26.1–44.3) |
CL = core length; CsPCa = clinically significant prostate cancer; GS = Gleason score; MCCL = maximum cancer core length; MRI = magnetic resonance imaging; NR = not reported; PCa = prostate cancer; PSA = prostate-specific antigen; SBx =systematic transrectal ultrasound-guided biopsy; TRUS = transrectal ultrasound.
Data were provided separately for the MRI-stratified pathway (upper) and the systematic TRUS-guided biopsy pathway (lower).
Helsinki University Hospital, Finland; Centro de Urologia CDU, Argentina; Sapienza University, Italy; Mayo Clinic, Rochester, MN, USA; MRC Oulu, University of Oulu and Oulu University Hospital, Oulu, Finland; San Raffaele Hospital, Italy; University College London and University College London Hospital, UK; Martini Klinik, Hamburg, Germany; London North West Healthcare NHS Trust, UK; Hampshire Hospitals NHS Foundation Trust, UK; Erasmus University Medical Center, Rotterdam, the Netherlands; University of Chicago, USA; Whittington Health NHS Trust, UK; CHU Lille, France; Jewish General Hospital, Montreal, Canada; Ghent University Hospital, Belgium; Princess Alexandra Hospital NHS Trust, UK; University Hospital Bern, Switzerland; Bordeaux Pellegrin University Hospital, France; Royal Free London NHS Foundation Trust, UK; Radboudumc, the Netherlands; University Hospital Heidelberg and German Cancer Research Center, Heidelberg, Germany; and Hospices Civils de Lyon, Centre Hospitalier Lyon Sud, France.
Median.
Table 3 –
Biopsy characteristics
| Author | TBx | SBx | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Navigation | Route | Concurrent SBx | Total no. of cores | No. of lesions per patient | No. of cores per lesion |
Operators | Route | No. of cores | ||
| No. | Experience | |||||||||
| Baco et al [17] | MRI/TRUS fusion | TR | Yes | Median (range): 2 (1–4) | ≤2 | ≤2 | 1 | No. of previous biopsies: >200 | TR | 12a |
| Bello et al [18] | NR | NR | Yes | NR | NR | NR | NR | NR | TR | NR (“standard”) |
| Kasivisvanathan et al [11] | Cognitive (33), MRI/TRUS fusion (219) | TR (227), TP (25) | No | Median (range): 4 (3–7) | Median (range): 1 (1–3) | ≤4 | 33 | No. of previous biopsies: 100 (IQR 28–250) | TR | Median (range): 12 (12–12) |
| Panebianco et al [19] | Cognitive | TR | Yes | 2 | 1 | 2 | 2 | >3 yr | TR | 14 |
| Park et al [20] | Cognitive | TR | Yes | Mean (range): 0.84 (0–3) | NR | 0–3 | 1 | >2 yr | TR | Mean (range): 11.1 (10–14)b |
| Porpiglia et al [21] | MRI/TRUS fusion | TR (55), TP (26) | Noc | Mean: 6.0 | 1 (54 pts), 2 (27 pts) | NR 3–6 |
2 | >1 yr | TR | Median (range): 12 (12–12) |
| Sciarra et al [22] | Cognitive | TR | Yes | 0–6 | 0–3 | 2 | 1 | Experienced | TR | 10 |
| Taverna et al [23] | Cognitive | TR | Yes | NR | No limit | 4 | 1 | NR | TR | 13 |
| Tonttila et al [24] | Cognitive | TR | Yes | 2.0 | 1 (26 pts), 2 (14 pts) | 1.8 (1st lesion), 1.1 (2nd lesion) | 3 | None | TR | Median (range): 12 (10–12) |
DRE = digital rectal examination; IQR = interquartile range; MRI = magnetic resonance imaging; NR = not reported; pts = patients; SBx = systematic TRUS-guided biopsy; TBx = targeted biopsy; TP = transperineal; TR = transrectal; TRUS = transrectal ultrasound.
Plus up to two cores for DRE or TRUS suspicious lesions.
Including up to two cores for hypoechoic lesions on TRUS.
Only SBx was performed in MRI-negative patients.
3.3. Detection rate of csPCa
The pooled estimates for the detection rates of csPCa in the MRI-stratified pathway and the systematic TRUS-guided biopsy pathway were 0.36 (95% confidence interval [CI] 0.23–0.53) and 0.25 (95% CI 0.18–0.33), respectively (Table 4). There was substantial heterogeneity for both pathways based on the Q test (p < 0.01 for both) and I2 statistics (I2 = 97% and 90%, respectively). The relative detection rate of csPCa ranged from 0.89 to 5.13, and the pooled estimated was 1.45 (95% CI 1.09–1.92), indicating that the MRI-stratified pathway detected significantly more csPCa than the systematic TRUS-guided biopsy pathway (Fig. 2). There was substantial heterogeneity (p < 0.01 for Q test and I2 = 82%).
Table 4 −.
Pooled estimate detection rates of the MRI-stratified pathway and the systematic TRUS-guided pathway, and corresponding relative detection rates for clinically significant, clinically insignificant, and any prostate cancer
| csPCa | cisPCa | Any PCa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Detection rate (95% CI) | p valuea | I2 (%) | Detection rate (95% CI) | p valuea | I2 (%) | Detection rate (95% CI) | p valuea | I2 (%) | |
| MRI-stratified pathway | 0.36 (0.23–0.53) | <0.01 | 97 | 0.10 (0.04–0.19) | 0.09 | 94 | 0.50 (0.40–0.62) | <0.01 | 94 |
| Systematic TRUS-guided biopsy pathway | 0.25 (0.18–0.33) | <0.01 | 90 | 0.10 (0.05–0.17) | <0.01 | 91 | 0.38 (0.30–0.46) | <0.01 | 87 |
| Relative detection rate | 1.45 (1.09–1.92) | <0.01 | 82 | 0.99 (0.61–1.60) | <0.01 | 72 | 1.35 (1.05–1.73) | <0.01 | 88 |
CI = confidence interval; cisPCa = clinically insignificant prostate cancer; csPCa = clinically significant prostate cancer; MRI = magnetic resonance imaging; PCa = prostate cancer; TRUS = transrectal ultrasound.
p value for the Q test.
Fig. 2 −.
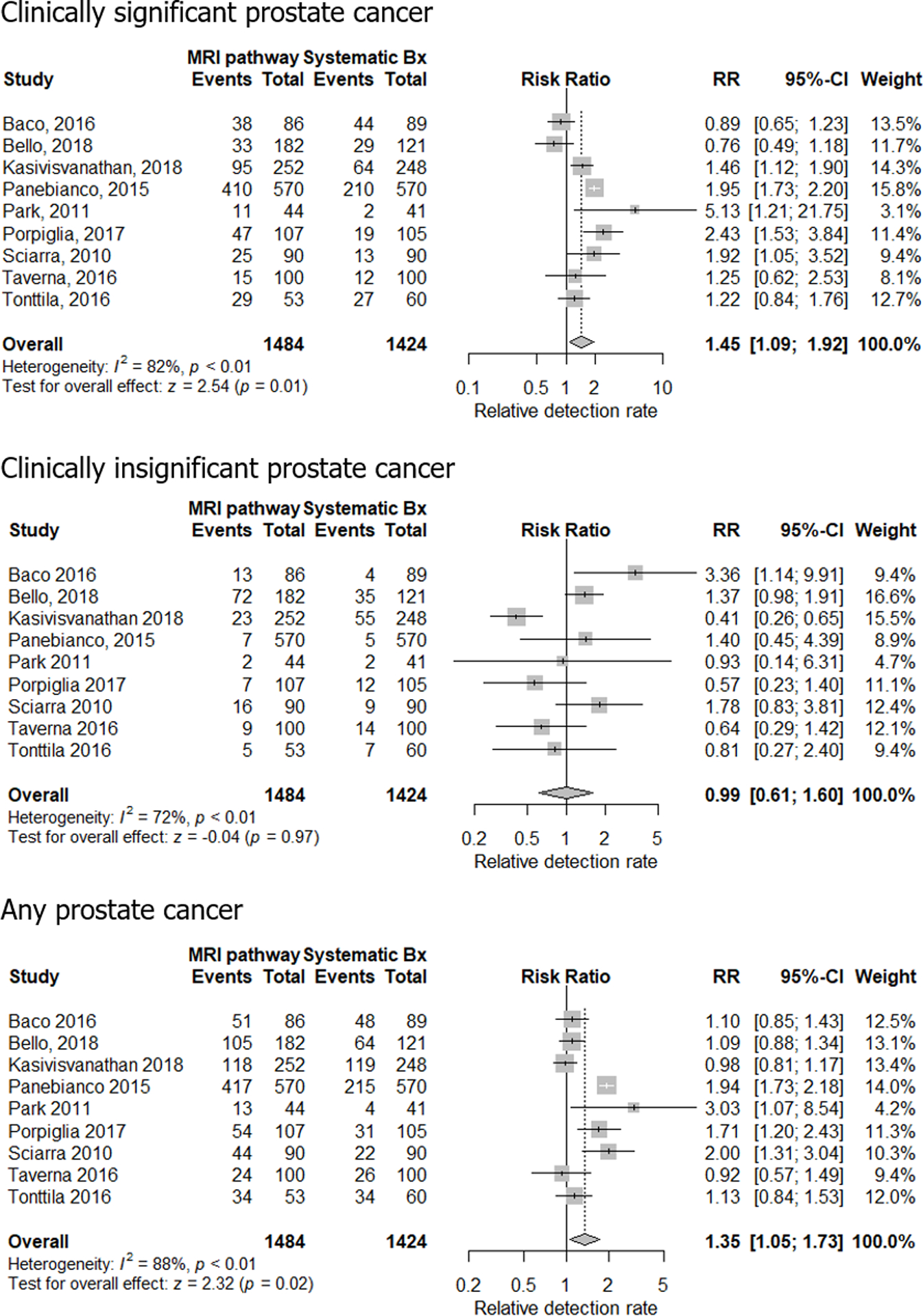
Forest plots of relative detection rate of clinically significant, clinically insignificant, and any prostate cancer in the MRI-stratified pathway and the systematic TRUS-guided biopsy pathway. A relative detection rate of >1 indicates that the MRI-stratified pathway detects more cancers, while a rate of <1 means that the MRI-stratified pathway detects fewer cancers than the systematic TRUS-guided biopsy pathway. Bx = biopsy; CI = confidence interval; MRI = magnetic resonance imaging; RR = risk ratio; TRUS = transrectal ultrasound.
3.4. Detection rate of cisPCa
The pooled estimates for the detection rate of cisPCa in the MRI-stratified pathway and the systematic TRUS-guided biopsy pathway were 0.10 (95% CI 0.04–0.19) and 0.10 (95% CI 0.05–0.17), respectively (Table 4). There was substantial heterogeneity for the systematic TRUS-guided biopsy pathway but not the MRI-stratified pathway based on the Q test (p <0.01 and 0.09, respectively) and I2 statistics (I2 = 94% and 91%, respectively). The relative detection rate of cisPCa ranged from 0.41 to 3.36 and the pooled estimated was 0.99 (95% CI 0.61–1.60), meaning that there was no significant difference in the detection rate of cisPCa between the MRI-stratified pathway and the systematic TRUS-guided biopsy pathway (Fig. 2). There was substantial heterogeneity (p < 0.01 for Q test and I2 = 72%).
3.5. Detection rate of any PCa
Pooled estimates for the detection rate of any PCa in the MRI-stratified pathway and the systematic TRUS-guided biopsy pathway were 0.50 (95% CI 0.40–0.62) and 0.38 (95% CI 0.30–0.46), respectively (Table 4). There was substantial heterogeneity for both pathways based on the Q test (p <0.01 for both) and I2 statistics (I2 = 94% and 87%, respectively). The relative detection rate of any PCa ranged from 0.92 to 3.03 and the pooled estimated was 1.35 (95% CI 1.05–1.73), implying that the MRI-stratified pathway detected significantly more any PCa than the systematic TRUS-guided biopsy pathway (Fig. 2). There was substantial heterogeneity (p < 0.01 for Q test and I2 = 88%).
3.6. Multiple subgroup analyses for csPCa
Table 5 shows the relative detection rate of csPCa in multiple subgroup analyses. The MRI-stratified pathway detected significantly more csPCa than the systematic TRUS-guided biopsy pathway in both biopsy-naïve patients and those with prior negative biopsy (pooled relative detection rates of 1.42 [95% CI 1.02–1.98] and 1.60 [95% CI 1.01–2.54], respectively). Significantly better detection rates were also seen in both single- and multicenter studies, csPCa definition of Gleason score ≥7 (4 + 3), 3-Tesla scanners, endorectal coils, PI-RADS version 2, prevalence of MRI-positive scans <0.71, and TBx only (without concurrent SBx). There were no significant differences between the subgroups except for the use of endorectal coils—studies using them showed significantly greater relative detection rates than those that did not (1.95 [95% CI 1.75–2.19] vs 1.27 [95% CI 0.88–1.83], p = 0.03).
Table 5 −.
Multiple subgroup analyses for relative detection rate of clinically significant prostate cancer
| Variable | Stratification | No. of studies | Relative detection rate (95% CI) | p valuea |
|---|---|---|---|---|
| Study design | Multicenter | 1 | 1.4608 (1.1218, 1.9023) | 0.99 |
| Single center | 8 | 1.4548 (1.0307, 2.0535) | ||
| Clinical setting | Biopsy naïve | 7 | 1.4239 (1.0241, 1.9797) | 0.68 |
| Prior negative biopsy | 2 | 1.6038 (1.0137, 2.5374) | ||
| Definition of csPCa | GS ≥7 (3 + 4) | 3 | 1.5637 (0.9921, 2.4647) | 0.60 |
| GS ≥7 (4 + 3) | 1 | 1.9231 (1.0521, 3.5151) | ||
| GS ≥7 (3 + 4) + core-related informationb | 5 | 1.3210 (0.8608, 2.0273) | ||
| MRI magnet strength | 3 Tesla | 6 | 1.3994 (1.0022, 1.9542) | 0.7654 |
| 1.5 Tesla | 3 | 1.5756 (0.7797, 3.1839) | ||
| Endorectal coil | Used | 4 | 1.9547 (1.7479, 2.1860) | 0.03 |
| Not used | 4 | 1.2707 (0.8842, 1.8260) | ||
| MRI interpretation | Institutional scale | 3 | 1.7710 (0.9484, 3.3069) | 0.79 |
| PI-RADS version 1 | 4 | 1.3460 (0.7945, 2.2803) | ||
| PI-RADS version 2 | 2 | 1.4332 (1.1191, 1.8354) | ||
| Prevalence of MRI (+) patients | >0.71c | 4 | 1.4998 (0.9757, 2.3054) | 0.62 |
| <0.71 | 4 | 1.5904 (1.1742, 2.1542) | ||
| Concurrent SBx in MRI-stratified pathway | MRI-TBx only pathway | 2 | 1.8162 (1.1085, 2.9760) | 0.35 |
| Combined pathway | 7 | 1.3484 (0.9178, 1.9810) | ||
| Navigation | Cognitive fusion | 5 | 1.4363 (0.8668, 2.3798) | 0.59 |
| MRI-TRUS software registration | 3 | 1.6869 (1.2438, 2.2879) |
CI = confidence interval; csPCa = clinically significant prostate cancer; GS = Gleason score; MRI = magnetic resonance imaging; PI-RADS = Prostate Imaging Reporting and Data System; SBx = systematic biopsy; TBx = targeted biopsy; TRUS = transrectal ultrasound.
p value for comparing between subgroups.
Maximum cancer core length, percentage of core length involved, and/or number of positive cores.
Median of studies.
3.7. Quality of evidence and publication bias
The quality of evidence of the included studies based on the revised Cochrane risk of bias tool for randomized trials (RoB 2 tool) are shown in Figure 3. All but two of the studies had some concerns for bias mainly (1) due to nonreporting of whether randomized allocation was concealed or not, and (2) because it was not clear whether pathologic assessment of biopsy specimens was done blinded to allocation. The study by Bello et al [18] was considered to be at a high risk of overall bias, as it was a conference abstract and therefore had some concerns for bias in multiple domains. The study by Porpiglia et al [21] was considered to have a low risk of bias. Publication bias was not assessed as the number of included studies was <10.
Fig. 3 −.
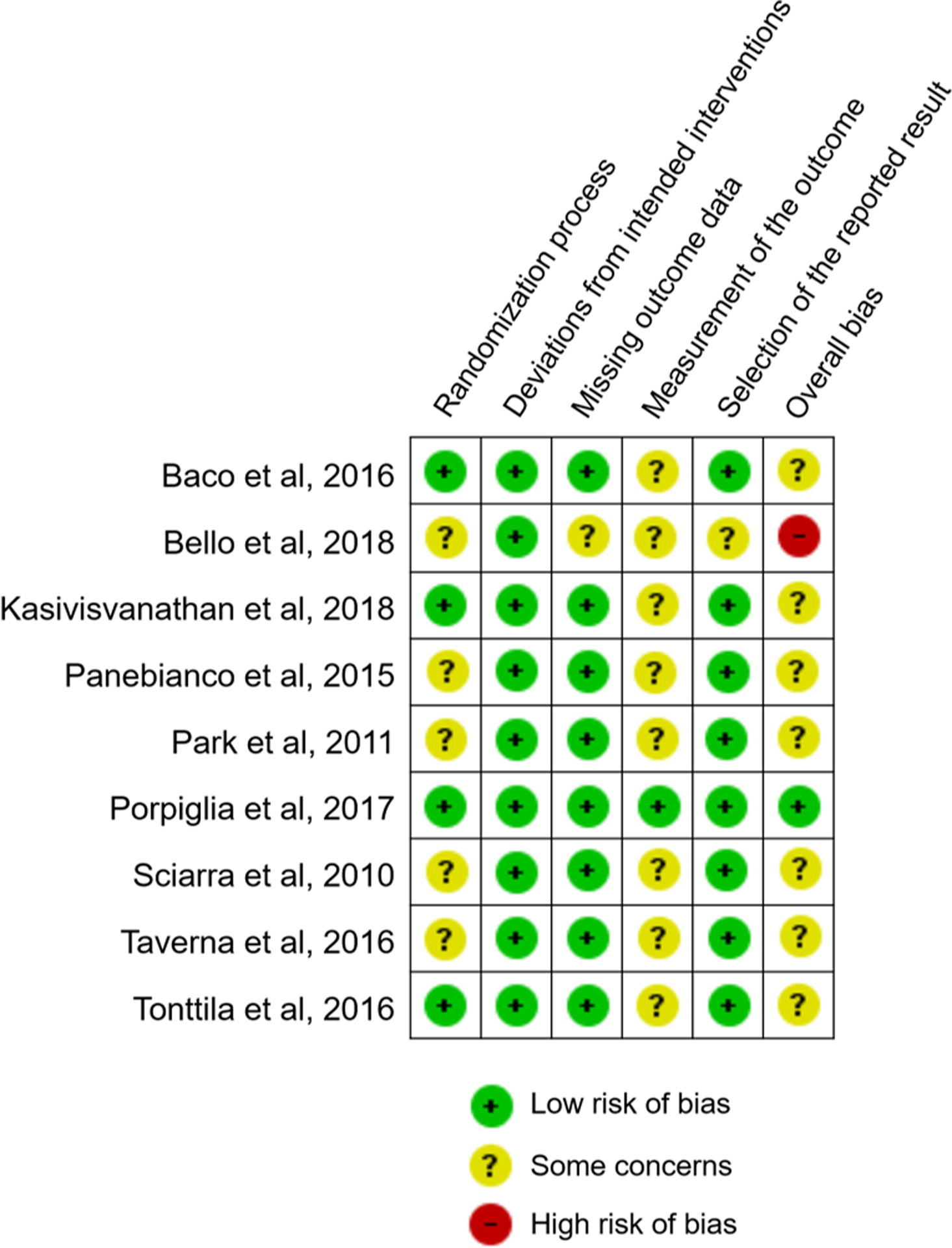
Summary chart of risk of bias assessment for included studies using the revised Cochrane risk of bias tool for randomized trials (RoB 2 tool).
3.8. Discussion
In this meta-analysis, we compared the detection rate of csPCa in the MRI-stratified pathway and the systematic TRUS-Bx pathway in patients with a suspicion of PCa in RCTs. The relative detection rate of the MRI-stratified pathway was 1.45 (95% CI 1.09–1.92), meaning that it detected approximately 50% more csPCa than the systematic TRUS-Bx pathway. Based on this, prebiopsy MRI followed by MRI-guided TBx is anticipated to improve detection and risk stratification of PCa. Our meta-analysis is in agreement with prior meta-analyses addressing the comparison between MRI-guided TBx and systematic TRUS-Bx, which have consistently shown the benefit of the MRI-stratified pathway [7–9,25]. However, this meta-analysis further substantiates the incremental value of the MRI-stratified pathway due to several of its unique characteristics. First, it was exclusively based on RCTs and therefore provides the highest level of evidence, while previous studies were predominantly or completely based on nonrandomized cohorts or within-person paired comparative studies. Second, prior studies aimed to directly compare the “sensitivity” of MRI-guided TBx and systematic TRUS-Bx, introducing an inherent bias in the prevalence of csPCa and positive MRI scans. For instance, in the meta-analysis by Schoots et al [7], which compared MRI-guided TBx and systematic TRUS-Bx, all patients had a positive MRI result. Given that our meta-analysis included only RCTs with patients having a clinical suspicion of PCa, the prevalence could be considered to more closely reflect the target population. Third, we took into consideration the whole MRI-stratified clinical pathway, which consists of the diagnostic performance of mpMRI for detecting csPCa followed by accurate TBx aimed at MRI-detected suspicious lesion. Prior meta-analyses primarily focused on only the TBx component. Fourth, all but two of our included studies were published after 2015, while most of the previous meta-analyses were based on studies published in 2014 or earlier [7,8,25]. There have been remarkable improvements in MRI technology during the recent years, and our meta-analysis possibly better reflects contemporary MRI and biopsy performance.
The detection rates of cisPCa in the MRI-stratified pathway and the systematic TRUS-Bx pathway were not significantly different with a pooled relative detection rate of 0.89 (95% CI 0.49–1.62). We speculate that this may have been primarily because the majority of the included studies performed concurrent SBx in the MRI-stratified pathway. The overall detection rate of any PCa was higher in the MRI-stratified pathway with a relative detection rate of 1.39 (95% CI 1.05–1.84), possibly attributed to the enhanced detection of csPCa and nonsignificantly different detection of cisPCa. Previous studies have shown that MRI-guided TBx without concurrent SBx shows almost twofold better performance in avoiding unwanted detection of cisPCa [7]. In keeping with this, two of the included studies in this meta-analysis that did not perform concurrent SBx for the MRI-stratified pathway (“MRI-TBx only pathway”) showed lower detection rates of cisPCa (relative rates of 0.41 and 0.57) [11,21].
At subgroup analysis, improved detection of csPCa in the MRI-stratified pathway was demonstrated in both biopsy-naïve patients and those with prior negative biopsy (1.42 [95% CI 1.02, 1.97] and 1.60 [95% CI 1.01, 2.54], respectively). In a previous meta-analysis predominantly based on nonrandomized cohorts, it was reported that systematic TRUS-Bx might be sufficient for biopsy-naïve patients due to only minimal increased sensitivity of MRI-guided TBx (pooled relative sensitivity of 1.10 [95% CI 1.00–1.22]) compared with the more evident benefit in those who had prior negative biopsy (pooled relative sensitivity of 1.54 [95% CI 1.05–2.26]) [7]. Another meta-analysis also predominantly based on more recent nonrandomized studies reported that the relative sensitivities were 1.15 [95% CI 1.07–1.31] and 1.45 [95% CI 1.08–1.69] for biopsy-naïve and prior negative biopsy populations, respectively [9]. In addition, in a recent Cochrane review dealing with the MRI-TBx only pathway in paired agreement studies, this pathway was superior only in the prior negative biopsy (1.44 [95% CI 1.19–1.75]) and not in the biopsy-naïve patients (1.05 [95% CI 0.95–1.16]) [26]. Based on these results, there seems to be a trend for a greater benefit of the MRI-stratified pathway in patients who had prior negative biopsy results. This may be due to the fact that in these patients, PCa may be located in areas such as the anterior or apical tumors where routinely performed systematic TRUS-Bx may miss these tumors, whereas prebiopsy MRI can depict lesions in these locations, potentially leading to enhanced cancer detection and more accurate Gleason scoring [27–29]. Regardless of the differences in the degree of benefit, our meta-analysis of RCTs along with prior meta-analyses of nonrandomized studies consistently demonstrate improved detection of csPCa using the MRI-stratified pathway in both clinical settings.
Studies using endorectal coils were shown to have significantly higher relative detection rates than the studies that did not. It is unclear whether this could relate to theoretical technical benefits such as potential higher spatial resolution images [30]. However, the use of endorectal coils can also cause artifacts, anatomical distortion, and patient discomfort, and therefore their use should be carefully decided based on physician, scanner, and patient-related variables. Other subgroup analyses did not show significant differences. Although not overtly manifested in our meta-analysis, possibly due to a small number of included studies, it has been shown that definitions of positive prostate MRI (including MRI protocols, interpretation schemes, and cutoff values) can affect the diagnostic performance of mpMRI for detecting csPCa [31]. For instance, the revised PI-RADS version 2 has been shown to yield higher sensitivity (0.95 vs 0.88) than, albeit similar specificity (0.73 vs 0.75) to, the original PI-RADS version 1 [6]. In addition, that one of the included studies used DCE MRI and MR spectroscopy warrants mention, as there is debate over the incremental value of DCE in mpMRI and MR spectroscopy is not currently considered necessary due to low spatial resolution and long acquisition times [4,32]. With regard to registration methods (cognitive, software registration, or in bore), there is also controversy regarding the optimal strategy. In this meta-analysis, no significant differences were found between cognitive and fusion, but no study performed direct in-bore TBx. In line with our results, a recent multicenter RCT comparing the three TBx techniques did not observe significant benefit of any technique over the other [33]. Nevertheless, there is concern that this multicenter study was underpowered, and our meta-analysis does not specifically deal with the comparison of TBx techniques; therefore, further studies will be needed to elucidate this issue.
Although the MRI-TBx only pathway showed significantly higher csPCa than the systematic TRUS-guided SBx pathway, there is concern that some csPCa could still be missed. This may stem from the fact that mpMRI can detect neither all PCa nor all csPCa cases. Based on a previous study of 169 tumors (≥0.5 ml in volume or Gleason score ≥7 [4 + 3]) in 150 patients comparing mpMRI and whole mount radical prostatectomy specimens, while PI-RADS version 2 detected 94% and 95% of PCa cases with a tumor volume of ≥0.5 ml in the peripheral and transition zones, respectively, only 20% and 26% of PCa cases with Gleason score ≥7 (4 + 3) and a tumor volume of ≤0.5 ml were detected [4]. Another important reason could be the imperfect biopsy targeting of MRI-visible lesions. Studies have shown that when MRI-guided TBx does not yield csPCa, SBx cores in adjacent or “perilesional” sextants yield csPCa [34]. Furthermore, increasing the number of cores directed at the MRI-visible area [35] or just performing concurrent SBx in the ipsilateral hemiprostate could increase the detection rate of csPCa [36]. Therefore, no inferences from this meta-analysis can be made with regard to adding or omitting SBx; the most appropriate strategy should be determined individually, and tailored according to the clinician and patient’s characteristics and preferences regarding the acceptable rates of missed csPCa and cisPCa overdetection.
Our meta-analysis had some limitations. First, since it was restricted to RCTs, only a small number of studies were included. Nevertheless, a total of 2908 patients were analyzed, and even with the possibility of underpowering, we were able to derive statistically significant conclusions. Second, substantial heterogeneity was observed between the included studies. Although we observed a significant difference only between studies using endorectal coils and those that did not in the subgroup analysis, variability in MRI protocol and interpretation, threshold for TBx, registration methods for TBx, number of targets and cores for TBx, and experience of radiologists and urologists could potentially be associated with heterogeneity among the studies. Third, as with all meta-analyses, this one is subject to publication bias as studies with negative results are less likely to be published. Owing to the small number of included studies, we were unable to formally assess publication bias using funnel and Egger tests. However, we included not only full papers but also conference abstracts that were relevant to the research question in order to minimize the possibility of publication bias, as it has been recognized that even for RCTs, negative trials have a lower cumulative publication rate than those with positive results [37]. Fourth, our meta-analysis was restricted to patients with a clinical suspicion of PCa, and therefore the results of our study cannot be directly applied to those with histologically diagnosed PCa (ie, active surveillance). Although a prior meta-analysis predominantly including retrospective studies reported median relative sensitivity of 1.25 for upgrading to Gleason score ≥7 (3 + 4) on a confirmatory biopsy, a recent prospective RCT of 273 patients did not see a significant difference between the MRI-stratified pathway and the systematic TRUS-Bx pathway [9,38]. Fifth, there was heterogeneity regarding the specific type of MRI-stratified clinical pathways and the definition of csPCa among the studies. Although meta-regression analysis did not reveal significant difference between groups, caution is needed for interpretation of results.
4. Conclusions
In this meta-analysis of RCTs, the MRI-stratified pathway was shown to detect more csPCa than the systematic TRUS-guided biopsy pathway in men with a clinical suspicion of PCa. A subgroup analysis showed consistent results for both biopsy-naïve patients and those with prior negative biopsy. The detection rate of any PCa was also higher in the MRI-stratified pathway, but not significantly different from that of cisPCa. However, caution may be needed for interpretation of these results due to heterogeneity among the studies.
Supplementary Material
Table 2 –
MRI characteristics
| Author | Magnet strength (T) | Vendor (scanner) | ERC | Sequence for defining target | Reader | Reporting system and threshold for TBx | Positive scan (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| No. | Experience | ||||||||
| Baco et al [17] | 1.5 | Siemens (Avanto) | No | T2WI, DWI | 1 | >1000 prostate MRI interpretations | PI-RADS v1 ≥3 | 63/86 (73.3) | |
| Bello et al [18] | 3 | NR | NR | NR | NR | NR | PI-RADS (unknown version) ≥4 | NR | |
| Kasivisvanathan et al [11] | 1.5 (62), 3 (184) | GE (Discovery), Philips (Achieva, Ingenia), Siemens (Aera, Avanto, Prisma, Skyra, Trio) | Somea | T2WI, DWI, DCE | 37 | 5 yr (IQR 4.5–10) |
PI-RADS v2 ≥3 | 175/252 (69.4) | |
| Panebianco et al [19] | 3 | GE (Discovery MR750), Siemens (Verio) | Yes | T2WI, DWI, DCE | 2 | 13, 4 yr | PI-RADS v1 ≥3 | 440/570 (77.2) | |
| Park et al [20] | 3 | Philips (Achieva) | No | T2WI, DWI, DCE | 2 | >7 yr | NR; ≥1 positive sequence | 23/44 (52.3) | |
| Porpiglia et al [21] | 1.5 | NR | Yes | T2WI, DWI, DCE | 3 | Experienced | PI-RADS v1 ≥3 | 81/107 (75.7) | |
| Sciarra et al [22] | 1.5 | Siemens (Avanto) | Yes | DCE, MRS | 2 | Experienced | MRS (choline + creatinine/citrate >0.8 and DCE positive | 45/90 (50.0) | |
| Taverna et al [23] | 3 | NR | Yes | mpMRI (T2WI + ≥2 functional); not specified | NR | NR | PI-RADS v2; NR | 67/100 (67.0) | |
| Tonttila et al [24] | 3 | Siemens (Skyra) | No | T1WI, T2WI, DWI, DCE | 2 | Experienced | Institutional 4-point Likert scale; NR | 53/53 (100.0) | |
DCE = dynamic contrast enhanced; DWI = diffusion-weighted imaging; ERC = endorectal coil; IQR = interquartile range; mpMRI = multiparametric MRI; MRI = magnetic resonance imaging; MRS = magnetic resonance spectroscopy; NR = not reported; PI-RADS = Prostate Imaging Reporting and Data System; TBx = targeted biopsy; T1WI = T1-weighted imaging; T2WI = T2-weighted imaging; V = version.
ERC was used in only three of 23 institutions.
Financial disclosures:
Sungmin Woo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Hedvig Hricak reports that she serves on the Board of Directors of Ion Beam Applications (IBA), a role for which she receives annual compensation.
The research described in this article was supported by a National Cancer Institute (NCI) P30 Cancer Center Support Grant (P30 CA008748). The NCI had no role in the research described or the preparation of the manuscript.
References
- [1].Eichler K, Hempel S, Wilby J, Myers L, Bachmann LM, Kleijnen J. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol 2006;175:1605–12. [DOI] [PubMed] [Google Scholar]
- [2].Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol 2012;61:1019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Geiger-Gritsch S, Oberaigner W, Muhlberger N, et al. Patient-reported urinary incontinence and erectile dysfunction following radical prostatectomy: results from the European Prostate Centre Innsbruck. Urol Int 2015;94:419–27. [DOI] [PubMed] [Google Scholar]
- [4].Vargas HA, Hotker AM, Goldman DA, et al. Updated Prostate Imaging Reporting and Data System (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol 2016;26:1606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815–22. [DOI] [PubMed] [Google Scholar]
- [6].Woo S, Suh CH, Kim SY, Cho JY, Kim SH. Diagnostic performance of Prostate Imaging Reporting and Data System version 2 for detection of prostate cancer: a systematic review and diagnostic meta-analysis. Eur Urol 2017;72:177–88. [DOI] [PubMed] [Google Scholar]
- [7].Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015;68:438–50. [DOI] [PubMed] [Google Scholar]
- [8].Wegelin O, van Melick HHE, Hooft L, et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique? Eur Urol 2017;71:517–31. [DOI] [PubMed] [Google Scholar]
- [9].Stabile A, Giganti F, Emberton M, Moore CM. MRI in prostate cancer diagnosis: do we need to add standard sampling? A review of the last 5 years. Prostate Cancer Prostatic Dis 2018;21:473–87. [DOI] [PubMed] [Google Scholar]
- [10].Murad MH, Asi N, Alsawas M, Alahdab F. New evidence pyramid. Evid Based Med 2016;21:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018;378:1767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- [13].Higgins JPT, Sterne JAC, Savović J, et al. A revised tool for assessing risk of bias in randomized trials In: Chandler J, McKenzie J, Boutron I, Welch V, editors. Cochrane methods. Cochrane Database Syst Rev 2016;10(Suppl 1):CD201601. [Google Scholar]
- [14].Sathianathen NJ, Butaney M, Bongiorno C, Konety BR, Bolton DM, Lawrentschuk N. Accuracy of the magnetic resonance imaging pathway in the detection of prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2019;22:39–48. [DOI] [PubMed] [Google Scholar]
- [15].Higgins JPT, Green S. https://handbook-5-1.cochrane.org/chapter_9/9_4_3_1_random_effects_dersimonian_and_laird_method_for.htm
- [16].Higgins JPT, Green S. https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm
- [17].Baco E, Rud E, Eri LM, et al. A randomized controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy. Eur Urol 2016;69:149–56. [DOI] [PubMed] [Google Scholar]
- [18].Bello A, Pérez L, Flores L, et al. MP46–09 Image-based diagnosis of prostate cancer (DICAMPRO study): randomized prospective study in biopsy-naïve population comparing diagnosis standard pathway vs an image-guided approach using mpMRI and target biopsy. J Urol 2018;199(4S):e609. [Google Scholar]
- [19].Panebianco V, Barchetti F, Sciarra A, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol 2015;33:17.e11–7. [DOI] [PubMed] [Google Scholar]
- [20].Park BK, Park JW, Park SY, et al. Prospective evaluation of 3-T MRI performed before initial transrectal ultrasound-guided prostate biopsy in patients with high prostate-specific antigen and no previous biopsy. AJR Am J Roentgenol 2011;197:W876–81. [DOI] [PubMed] [Google Scholar]
- [21].Porpiglia F, Manfredi M, Mele F, et al. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naive patients with suspected prostate cancer. Eur Urol 2017;72:282–8. [DOI] [PubMed] [Google Scholar]
- [22].Sciarra A, Panebianco V, Ciccariello M, et al. Value of magnetic resonance spectroscopy imaging and dynamic contrast-enhanced imaging for detecting prostate cancer foci in men with prior negative biopsy. Clin Cancer Res 2010;16:1875–83. [DOI] [PubMed] [Google Scholar]
- [23].Taverna G, Bozzini G, Grizzi F, et al. Endorectal multiparametric 3-Tesla magnetic resonance imaging associated with systematic cognitive biopsies does not increase prostate cancer detection rate: a randomized prospective trial. World J Urol 2016;34:797–803. [DOI] [PubMed] [Google Scholar]
- [24].Tonttila PP, Lantto J, Paakko E, et al. Prebiopsy multiparametric magnetic resonance imaging for prostate cancer diagnosis in biopsy-naive men with suspected prostate cancer based on elevated prostate-specific antigen values: results from a randomized prospective blinded controlled trial. Eur Urol 2016;69:419–25. [DOI] [PubMed] [Google Scholar]
- [25].Valerio M, Donaldson I, Emberton M, et al. Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. Eur Urol 2015;68:8–19. [DOI] [PubMed] [Google Scholar]
- [26].Drost FH, Osses DF, Nieboer D, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev 2019;4:Cd012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Komai Y, Numao N, Yoshida S, et al. High diagnostic ability of multiparametric magnetic resonance imaging to detect anterior prostate cancer missed by transrectal 12-core biopsy. J Urol 2013;190:867–73. [DOI] [PubMed] [Google Scholar]
- [28].Boesen L, Noergaard N, Chabanova E, et al. Early experience with multiparametric magnetic resonance imaging-targeted biopsies under visual transrectal ultrasound guidance in patients suspicious for prostate cancer undergoing repeated biopsy. Scand J Urol 2015;49:25–34. [DOI] [PubMed] [Google Scholar]
- [29].Seles M, Gutschi T, Mayrhofer K, et al. Sampling of the anterior apical region results in increased cancer detection and upgrading in transrectal repeat saturation biopsy of the prostate. BJU Int 2016;117:592–7. [DOI] [PubMed] [Google Scholar]
- [30].Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging – Reporting and Data System: 2015, version 2. Eur Urol 2016;69:16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moldovan PC, Van den Broeck T, Sylvester R, et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol 2017;72:250–66. [DOI] [PubMed] [Google Scholar]
- [32].Li B, Cai W, Lv D, et al. Comparison of MRS and DWI in the diagnosis of prostate cancer based on sextant analysis. J Magn Reson Imaging 2013;37:194–200. [DOI] [PubMed] [Google Scholar]
- [33].Wegelin O, Exterkate L, van der Leest M, et al. The FUTURE trial: a multicenter randomised controlled trial on target biopsy techniques based on magnetic resonance imaging in the diagnosis of prostate cancer in patients with prior negative biopsies. Eur Urol 2019;75:582–90. [DOI] [PubMed] [Google Scholar]
- [34].van der Leest M, Cornel E, Israel B, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naive men with elevated prostate-specific antigen: a large prospective multicenter clinical study. Eur Urol 2019;75:570–8. [DOI] [PubMed] [Google Scholar]
- [35].Zhang M, Milot L, Khalvati F, et al. Value of Increasing Biopsy Cores per Target with Cognitive MRI-targeted Transrectal US Prostate Biopsy. Radiology 2019;291:83–9. [DOI] [PubMed] [Google Scholar]
- [36].Bryk DJ, Llukani E, Taneja SS, Rosenkrantz AB, Huang WC, Lepor H. The role of ipsilateral and contralateral transrectal ultrasound-guided systematic prostate biopsy in men with unilateral magnetic resonance imaging lesion undergoing magnetic resonance imaging-ultrasound fusion-targeted prostate biopsy. Urology 2017;102:178–82. [DOI] [PubMed] [Google Scholar]
- [37].Leal AI, Barra WF, Saragiotto DF, Saad ED, Hoff PM. Publication bias in randomized controlled trials (RCTs) of colorectal cancer presented at ASCO Annual Meetings. J Clin Oncol 2010;28:6116. [Google Scholar]
- [38].Klotz L, Loblaw A, Sugar L, et al. Active Surveillance Magnetic Resonance Imaging Study (ASIST): results of a randomized multicenter prospective trial. Eur Urol 2019;75:300–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


