Abstract
BACKGROUND:
The analgesic effects of transcranial Direct Current Stimulation (tDCS) combined with physical therapy remain unclear.
OBJECTIVE:
To systematically review available evidence comparing tDCS with any physical therapy modality (PTM) to PTM alone or PTM with sham tDCS on pain relief on common musculoskeletal (MSK) conditions, namely knee osteoarthritis (KOA), chronic low back pain (CLBP), myofascial pain syndrome (MPS) and fibromyalgia.
METHODS:
EMBASE and MEDLINE were searched from inception to April 2019 for randomized controlled trials. Reviewers independently assessed the studies quality and extracted data according to the PRISMA protocol. The GRADE approach was used to asses quality of evidence and a “Summary of Findings” table was created. The analyses used random-effects model. The primary outcome was pain reduction after treatment.
RESULTS:
Eight articles were included. Only one study had low risk of bias. Quality of evidence was considered low or very low. Significant reduction in pain scores were found for fibromyalgia and KOA (Standardized mean difference (SMD) = −1.94 [95% CI: −3.37 to −0.49; I2=76.4%] and SMD = −2.35 [95% CI: −3.63 to −1.06; I2=69.7%] respectively). Subgroup analysis considering the type of PTM despite MSK condition revealed significant reduction in pain scores for exercise, SMD = −1.20 [95% CI: −1.683 to −0.717; I2=10.8%].
CONCLUSIONS:
Large heterogeneity and low quality of evidence and limited number of studies were found. Results suggest a potential analgesic effect of tDCS in combination with a PTM for fibromyalgia and KOA. Subgroup analysis suggests a stronger effect of tDCS when combined with an exercise based PTM.
Keywords: review, tDCS, physical therapy, pain, knee osteoarthritis, chronic low back pain, myofascial pain syndrome, fibromyalgia
INTRODUCTION
Rehabilitation therapies for chronic musculoskeletal (MSK) conditions aim to re-establish physical function in daily-life activities and to alleviate pain for subjects with limiting injuries, conditions or impairments. Different approaches have been studied to achieve these aims, including combining rehabilitation modalities with other therapies1. The use of non-invasive brain stimulation is a novel therapeutic modality that has increasing evidence of effectiveness when used in combination with rehabilitation protocols.2
Non-invasive brain stimulation (NIBS) techniques are designed to act on neuronal state via the application of magnetic or electrical field through the skull. These techniques have shown to influence pain perception3 and motor performances, which may translate to clinical improvements4. It has been proposed that these types of stimulations facilitate a change in dysfunctional excitability patterns in the brain, inducing neuronal plasticity5,6 and are beneficial to minimize pain and improve physical function7,8. Research suggests that NIBS may facilitate other rehabilitation strategies, such as physical therapy techniques, to be more effective in priming the activation of areas such as the primary motor cortex to improve pain and physical function9,10,11.
Among different types of NIBS, transcranial direct current stimulation (tDCS) has been widely used as it is relatively easy to use, portable, well-tolerated, and can be combined simultaneously with other rehabilitation strategies with minimal side effects12. However, clinical trials showed variable effect on pain scores before and after its application when combined with other rehabilitation therapies in different neurological and MSK conditions. A recent systematic review13 investigated the effect of NIBS for general chronic pain conditions and the meta-analysis of tDCS studies versus sham showed a clinical but not statistically significant difference between interventions. However, to date, there are no systematic reviews that investigated the effect of tDCS when combined with any type of physical therapy intervention on chronic MSK pain conditions. The combination of tDCS and physical therapy techniques (e.g. exercises) are thought to enhance clinical effects on pain, as compared to tDCS alone, by increasing the brain responsiveness to the corticomotor benefits of exercise or other physical therapy interventions (e.g. increased cortical excitability, improved motor control, enhanced muscle activation14), and by adding effects on the pain system function. We believe that investigating if this combination of interventions is effective for MSK chronic pain conditions may direct future research and clinical work to explore this approach which ultimately may help the design of more effective rehabilitation protocols, hence optimize care for chronic MSK conditions.
We have systematically reviewed the evidence from randomized controlled trials to try to answer the research question of “what is effect of tDCS when combined with any type of physical therapy intervention modality (PTM) on pain intensity scores in common MSK conditions such as knee osteoarthritis (KOA), chronic low back pain (CLBP), fibromyalgia, and myofascial pain?”. This review will focus on the mentioned MSK conditions as these conditions are commonly present in the day to day of an MSK rehabilitation clinic. Understanding the actual benefit from combining tDCS to other physical therapy modalities is clinically important as it may assist the development of better rehabilitation protocols for chronic MSK conditions.
METHODS
Search Strategy
Medline and EMBASE databases were searched for articles in English language. Search terms included multiple variants of the terms Transcranial direct current stimulation, fibromyalgia, myofascial pain, low back pain, KOA, additional to Medical Subject Headings (MeSH). A broad search strategy was employed to ensure the detection of all potentially reported forms of PTMs (details of search strategy can be found in Appendix.1). Snowball searching was performed by screening the references of retrieved studies. Published articles included from inception until April 5th, 2019.
Selection Criteria
We included randomized controlled trials and the following inclusion criteria: 1) age above 18 years; 2) presence of low back pain or fibromyalgia or KOA or myofascial pain diagnosed for at least >3 months; 3) intervention performing tDCS combined with a form of physical therapy modality (defined as any physical agents such as electrical, light, thermal or mechanical energy used in the field of physical therapy to produce therapeutic response); 4) presence of physical therapy treatment alone or physical therapy combined with sham tDCS as one of the comparison groups; 5) assessment of pain intensity level at baseline and after the intervention. The exclusion criteria were: 1) other chronic MSK conditions; 2) intervention performing other types of NIBS; 3) interventions with tDCS without direct comparison to a PTM. This systematic review is registered at PROSPERO database manager for systematic reviews (Registration number: CRD42019127037).
Screening and Data Extraction
Articles were exported to Covidence (web-based systematic review software) for screening and qualitative assessment process. Two authors (P.T. and L.A.), independently screened all titles and abstracts. If an abstract met inclusion criteria, the full text of the article was reviewed for confirmation. Discrepancies were resolved by the senior investigator (S.P.). After which, three authors (P.T., H.A.., L.A.) extracted the data into organized spreadsheets that included: study location; publication year; study design; MSK condition studied; PTM used; sample size; interventions; population characteristics; pain scale used; time frame of pain assessment; tDCS characteristics; duration of symptoms; pain scores and measure of variability in each intervention arm.
Our primary outcome was the mean difference in pain score between baseline and a time frame post intervention. We anticipated that studies will have varying time intervals in measuring the post intervention pain score, thus we defined a short term follow up time frame to be a pain score measurement that occurred post completion of the intervention and ≤ 8 weeks from baseline. A long-term follow-up time frame was defined as a pain score measurement that occurred post intervention and > 8 weeks from baseline. Mean pain scores and variability estimates were collected from the included studies and each estimate was assigned to be either a short-term vs. a long-term value. A web-based program (WebPlotDigitizer, version 4.2) was used to extract pain scores from articles which presented final data as figures only15,16. Finally, we calculated the mean differences and standard deviations of the mean differences between each time frame and baseline.
Risk of Bias Assessment
Three independent reviewers (P.T., L.A., H.A.) assessed the included studies using the Cochrane risk of bias tool from the Cochrane Collaboration for randomized control trials17,18. Risk was assigned to be unclear if the specific category was not clearly described in the study methods. If there were any conflicting judgments, the authors met to resolve them.
Quality of Evidence
The GRADE approach19 was used by two independent authors (P.T., L.A.) to rate the quality of the evidence for the intervention comparison. Evidence was rated according to the GRADE criteria. For comparison where there was only a single randomized study (with under 300 participants), the quality was downgraded twice as it was considered inconsistent, potentially imprecise (i.e. if wide 95%CI) and with potential publication bias, providing low quality of evidence. If there were discrepancies among authors, they met to come to an agreement. A “Summary of findings” table was created to present the result and the criteria explanation is shown in the table.
Statistical Analysis
Analysis of the analgesic effect of tDCS/PTM combination vs. PTM only was reported according to MSK condition. Due to expected clinical heterogeneity, we decided a priori to use a random-effect models with weights based on the DerSimonian and Laird method using standardized mean differences (SMD) as different pain scales were reported among the studies. We presented the results SMD with 95% CIs. For each condition, we examined between study statistical heterogeneity using the I2 and Cochran’s Q test with I2greater than 50% considered significant evidence for heterogeneity. Potential sources of heterogeneity were planned a priori to be examined with meta-regression analysis.
A subgroup analysis was performed considering the type of PTM (exercise based or electrical stimulation) despite the MSK condition. Finally, for each condition, a funnel plot to assess potential publication bias and Egger’s and Begg’s test was planned to formally test the assumption of publication bias. All tests were 2-tailed, using a p <0.05 threshold for significance, and performed using STATA 15 software (StataCorp. 2017: Release 15. College Station, TX). Clinical significance was determined according to the average baseline scores of the two intervention groups using previously reported minimal clinical important differences (MCID) and SMD values for chronic MSK pain20. For CLBP, the considered MCID was 20mm21,22 and SMD = 0.49, for fibromyalgia SMD = 0.30, Myofascial pain syndrome SMD = 0.60 and for KOA MCID = 27mm23,24 and SMD= 0.27.
RESULTS
Our search strategy identified 212 articles. After screening, 36 records required full-text review. Figure 1 shows reasons for exclusion. One placebo-controlled crossover randomized25 trial was excluded as it was unclear that the included participants had CLBP according to our definition11,25–32. Table.1 summarizes the final list of eight selected studies for the analysis. The sample size of the studies varied across conditions. A total of 355 participants were included in the analysis (216 (60.8%) with chronic back pain, 53 (14.9%) with fibromyalgia, 31 (8.7%) with myofascial pain syndrome, and 55(15.5%) with KOA. The median duration of symptoms in the combined tDCS+PTM group on all studies was 37.3 months [IQR 9.4, 98] compared to 59.7 months [IQR 6.4, 108] in the PTM only. Time between intervention and post intervention pain assessment varied among studies.
Figure 1:
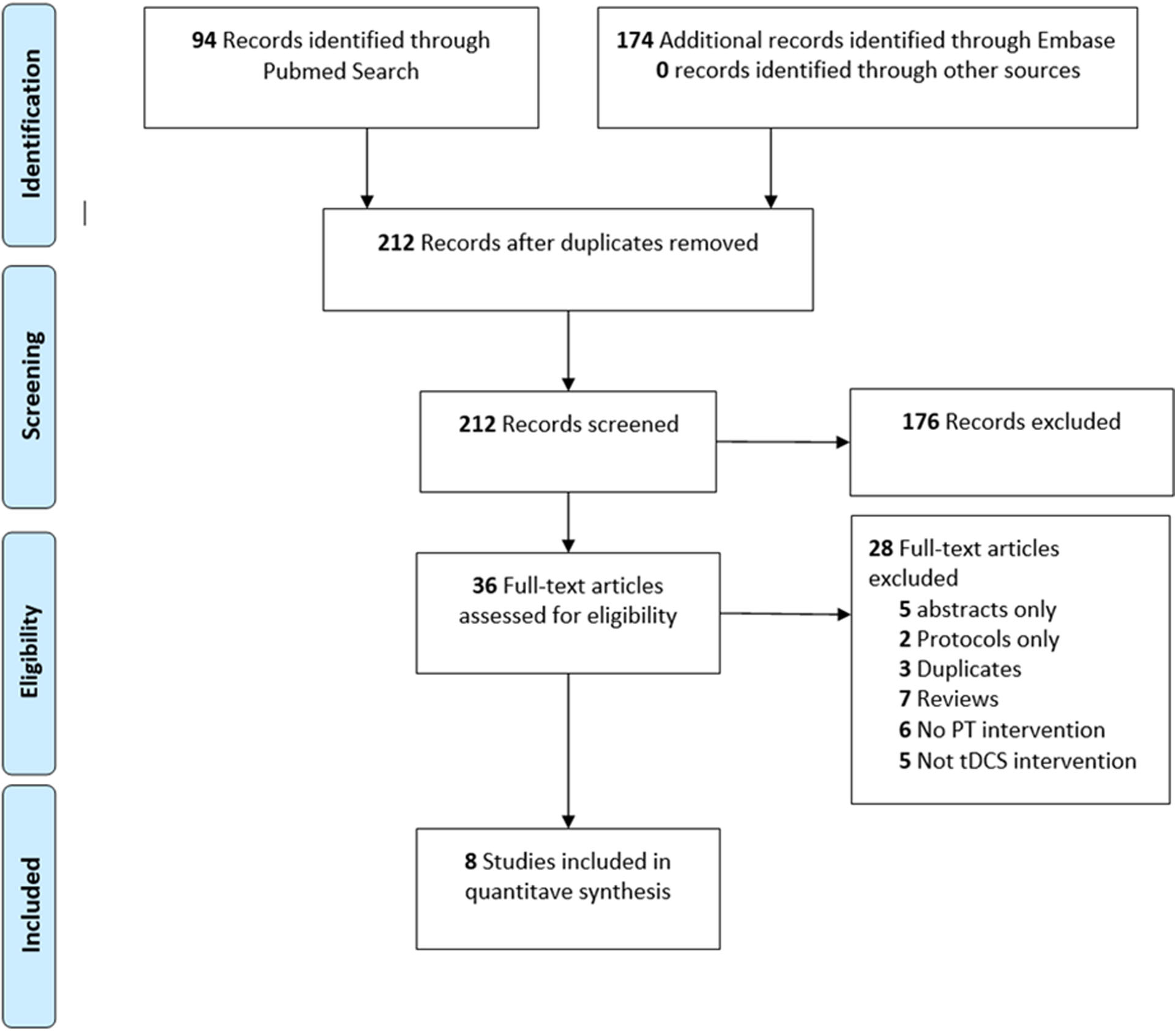
Flow diagram of study selection. PT: Physical therapy; tDCS: transcranial direct current stimulation
Table 1:
Summary of Studies That Compared the Analgesic Effect of tDCS Combined with a Physical Therapy Modality vs. Physical Therapy Alone on Common MSK Conditions.
| Author, Year | Country | Study Design | N | MSK Condition | PTM | PTM Duration | Characteristics of tDCS | Characteristics of tDCS Sessions | tDCS & PTM Sequence | Pain Scale | Pain Assessment Intervals | Intervention Groups | # of patients in each arm | Mean age ±SD (years) | % Female | Average duration of pain symptoms ± SD (months) | Timepoint Pain Assessment chosen for meta-analysis (weeks) | Mean difference in pain score between baseline and Timepoint Pain Assessment chosen for analysis ± SD | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Luedtke et al24, 2015 | Germany | RCT | 135 | Chronic Low Back Pain | CBT | daily sessions × 4 weeks | 2mA anodal on M1 left (35cm2) | Five 20-minute sessions per week × 1 week | Sequential | 0–100 VAS | Baseline, week 5, 9, 17, 29 | tDCS + CBT | 67 | 45 ± 9 | 49 | 98 ± 106 | 5 | −22 | ± | 3.8 |
| Hazime et a25l, 2017 | Brazil | RCT | 92 | Chronic Low Back Pain | PES | 3 sessions/ week × 4 weeks | 2mA anodal on M1 contralateral (35cm2) | Three 20-minute sessions per week × 4 weeks | Concurrent | 0–10 VAS | Baseline, week 4, 12, 24 | tDCS + PES | 23 | 51.3 ± 9.9 | 78.3 | 37.3 ± 39.4 | 4 | −45* | ± | 4.97 |
| Straudi et al26, 2018 | Italy | RCT | 35 | Chronic Low Back Pain | Group exercise | 10 sessions over 1 month | 2mA anodal on M1 contralateral (35cm2) | Five 20-minute sessions per week × 1 week | Sequential | 0–100 VAS | Baseline, week 1, 5, 9 | tDCS + Group Exercise | 18 | 54.3 ± 12.4 | 66.7 | 9.4 ± 9.2 | 5 | −21.2 | ± | 30.7 |
| Riberto et al27, 2011 | Brazil | RCT | 23 | Fibromyalgia | MRP | 3 sessions/week × 16 weeks | 2mA anodal on M1 contralateral (35cm2) | One 20-minute session per week × 10 weeks | Overlap | SF-36 pain domain, VAS | Baseline, week 16 | tDCS + MRP | 11 | 58.3 ± 12.1 | 100 | 9.9 ± 11.8 | 16 | −19† | ± | 5.12 |
| Mendonca et al28, 2016 | Brazil | RCT | 45 | Fibromyalgia | AE | 3 sessions/week × 4 weeks | 2mA anodal on M1 (35cm2) | Five 20-minute sessions per week × 1 week | Overlap | 0–10 VAS | Baseline, week 4, 8, 12 | tDCS + AE | 15 | 44.5 ± 14 | 93.3 | 140.6 ± 72.2 | 12 | −23* | ± | 7.67 |
| Sakrajai et al29, 2014 | Thailand | RCT | 31 | Myofascial pain syndrome | Active Stretching Exercise + Ultrasound therapy + Hot packs | daily stretching + US/hot packs 3 times/week × 2 weeks | 1mA anodal on M1 contralateral (35cm2) | Five 20-minute sessions per week × 1 week | Overlap | 0–10 VAS | Baseline, weekly × 4 weeks | tDCS + Physical Modality | 16 | 49.9 ± 8.3 | 68.8 | 5.91 ± 2.55 | 4 | −38.2* | ± | 1.98 |
| Chang et al30, 2017 | Australia | RCT | 30 | Knee Osteoarthritis | Strengthening Exercises | 2 class sessions + 2 home sessions/week × 8 weeks | 1mA anodal on M1 contralateral (35cm2) | Two 20-minute sessions per week × 8 weeks | Sequential | 0–100 VAS | Baseline, week 9 | tDCS + Exercise | 13 | 59.8 ± 9.1 | 73.3 | 86.4 ± 63.6 | 8 | −35.8 | ± | 6 |
| da Graca-Tarragó et al31, 2019 | Brazil | RCT | 60 | Knee Osteoarthritis | EIMS | 5 session/week × 1 week | 2mA anodal on M1 contralateral (35cm2) | Five 30-minute sessions per week × 1 week | Concurrent | 0–100 VAS | Baseline, day 5 | tDCS + EIMS | 15 | 66 ± 9.1 | 100 | -- | 0.71 | −64.1 | ± | 6.11 |
RCT: Randomized Controlled Trial; MSK: musculoskeletal; VAS: Visual Analog Scale; tDCS: transcranial direct current stimulation; PTM: physical therapy modality; PES: Peripheral Electrical Stimulation; CBT: Cognitive Behavioral Therapy; MRP: Multidisciplinary rehabilitation program; AE: Aerobic Exercise; EIMS: Intramuscular Electrical Stimulation.
Estimates changed to mm scale;
Change from baseline – T Long term (on SF-36 scale) .
Risk of Bias Assessment
Studies varied on bias levels. In studies which were deemed with moderate to high risk of bias, the most common potential causes of bias were the inadequate description of randomization and concealment procedures. In many of the included studies the sample size in each intervention arm of interest is considered small. Quality assessment of included RCT studies is provided in Appendix.2.
Quality of evidence
Table 2 summarizes the findings for the quality of evidence based on the GRADE approach for the outcome of pain intensity for each condition. Inconsistency due to heterogeneity and imprecision due to small sample size was present in all conditions. Overall, the quality of evidence varied from very low to low for all the conditions for both short- and long-term follow-ups.
Table 2.
The GRADE “Summary of Findings” table for quality of evidence, magnitude of effect of the interventions at short- and long-term follow-up.
| The Analgesic Effect of Transcranial Direct Current Stimulation (tDCS) combined with Physical Therapy on Common Musculoskeletal Conditions | |||||
|---|---|---|---|---|---|
| Patient or population: patients with low back pain or fibromyalgia or KOA or myofascial pain diagnosed for at least >3 months | |||||
| Outcomes | Effect size | Relative and absolute effect (average % improvement (reduction) in pain (95% CIs) in relation to post-treatment score from sham group) * * Where 95%CIs do not cross the line of no effect. | No of participants (studies) | Quality of the evidence (GRADE) | |
| Fibromyalgia | |||||
| Pain intensity (< 8 weeks from baseline) measured using a visual analog scale or numerical rating scale | MD −12.0; 95%CI (−17.17, −6.83) SMD −1.66; 95%CI (−2.49, −0.82) | This equates to a 12%, 95% CI (17% to 6%) reduction in pain intensity, on a 0 to 100 pain intensity scale | 45 (1) | ⊕⊕◯◯ low1 | |
| Pain intensity (> 8 weeks from baseline) measured using a visual analog scale or numerical rating scale | SMD −1.94; 95%CI (−3.38, −0.49) | This equates to a 13%, 95% CI (16% to 8%) reduction in pain intensity, on a 0 to 100 pain intensity scale | 68 (2) | ⊕⊕◯◯ low2, 4 | |
| Low Back Pain | |||||
| Pain intensity (< 8 weeks from baseline) measured using a visual analog scale or numerical rating scale | SMD −0.19; 95%CI (−1.13, 0.74) | - | 278 (4) | ⊕◯◯◯ very low2, 3, 4 | |
| Pain intensity (> 8 weeks from baseline) measured using a visual analog scale or numerical rating scale | SMD −0.02; 95%CI (−0.92, 0.95) | - | 262 (3) | ⊕◯◯◯ very low2, 3, 4 | |
| Knee Osteoarthritis | |||||
| Pain intensity (0 to 8 weeks from baseline) measured using a 0–100 visual analog scale | MD −17.59; 95%CI (−26.31, −8.87) | This equates to a 17%, 95% CI (26% to 8%) reduction in pain intensity, on a 0 to 100 pain intensity scale | 90 (2) | ⊕⊕◯◯ low2, 4 | |
| Myofascial Pain | |||||
| Pain intensity (< 8 weeks from baseline) measured using a 0–10 visual analog scale | SMD −0.253; 95%CI (−0.96, 0.45) | - | 31 (1) | ⊕◯◯◯ very low2, 3, 4 | |
|
GRADE Working Group grades of evidence High quality: we are very conf ident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | |||||
MD: Mean Difference; SMD: Standardized Mean Difference; CI: confidence Interval; KOA: Knee Osteoarthritis
Downgraded once for imprecision due to small sample size
Downgraded once for study limitation due to high or unclear risk of bias.
Downgraded once for inconsistency due to heterogeneity.
Downgraded twice. Single randomized study (with under 300 participants) were considered inconsistent, imprecise (i.e. wide 95%CI) and with potential publication bias, providing low quality of evidence.
Analysis by Musculoskeletal Condition
To explore the pooled results according to each MSK condition, we examined the standardized mean differences in pain scales separately. Figure 2 shows the forest plots for each condition using SMD for CLBP and fibromyalgia and the weighted mean difference (WMD) for KOA. For CLBP, the pooled standardized estimate did not achieve either clinically or statistically significance. For the one study on myofascial pain syndrome, the mean difference on pain VAS (0–100) at the 4-week timepoint assessment was −38.2 ± 1.98 for the tDCS + physical modality group and −37.7 ± 1.98 physical modality only group, (p-value > 0.05). Given that the two studies on KOA used the same outcome measure (0–100 mm VAS pain scale), the weighted mean difference was also calculated (Figure 2).
Figure 2:
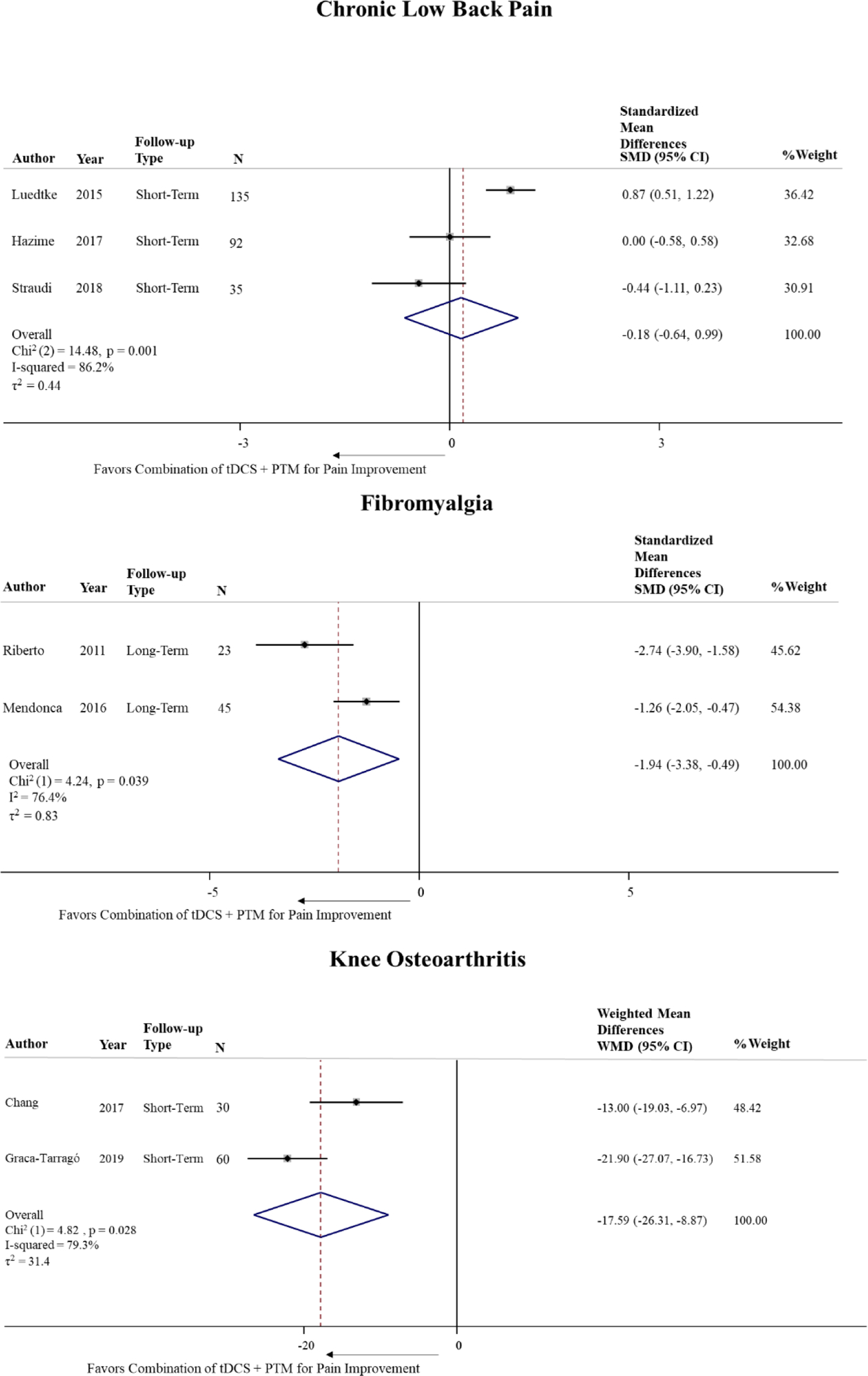
Meta-analysis of the standardized/weighed mean difference in pain score between studies including post tDCS/PTM combined intervention compared to PTM alone by MSK condition.
Meta-regression
Due to the number of studies in each condition, there was no adequate statistical power to run meta-regression analysis. Such analysis should be considered only when there are ten or more studies, according to the Cochrane Handbook for Systematic Reviews of Interventions18.
Subgroup Analysis
Considering the type of PTM despite the condition, three studies (one on CLBP, one in KOA and one in fibromyalgia) used an exercise-based intervention as a PTM, and two studies used an electrical stimulation type of intervention (one CLBP and one in KOA). The pooled standardized estimate for the combination of tDCS with an exercise based PTM was significant, SMD = −1.20 [95% CI: −1.683 to −0.717, p-value = 0.000; I2=10.8%, p-value = 0.326], and not significant for the combination with electrical stimulation, SMD = −1.48 [95% CI: −4.45 to 1.49, p-value = 0.328; I2=95.8%, p-value = 0.000] (Figure 3).
Figure 3.
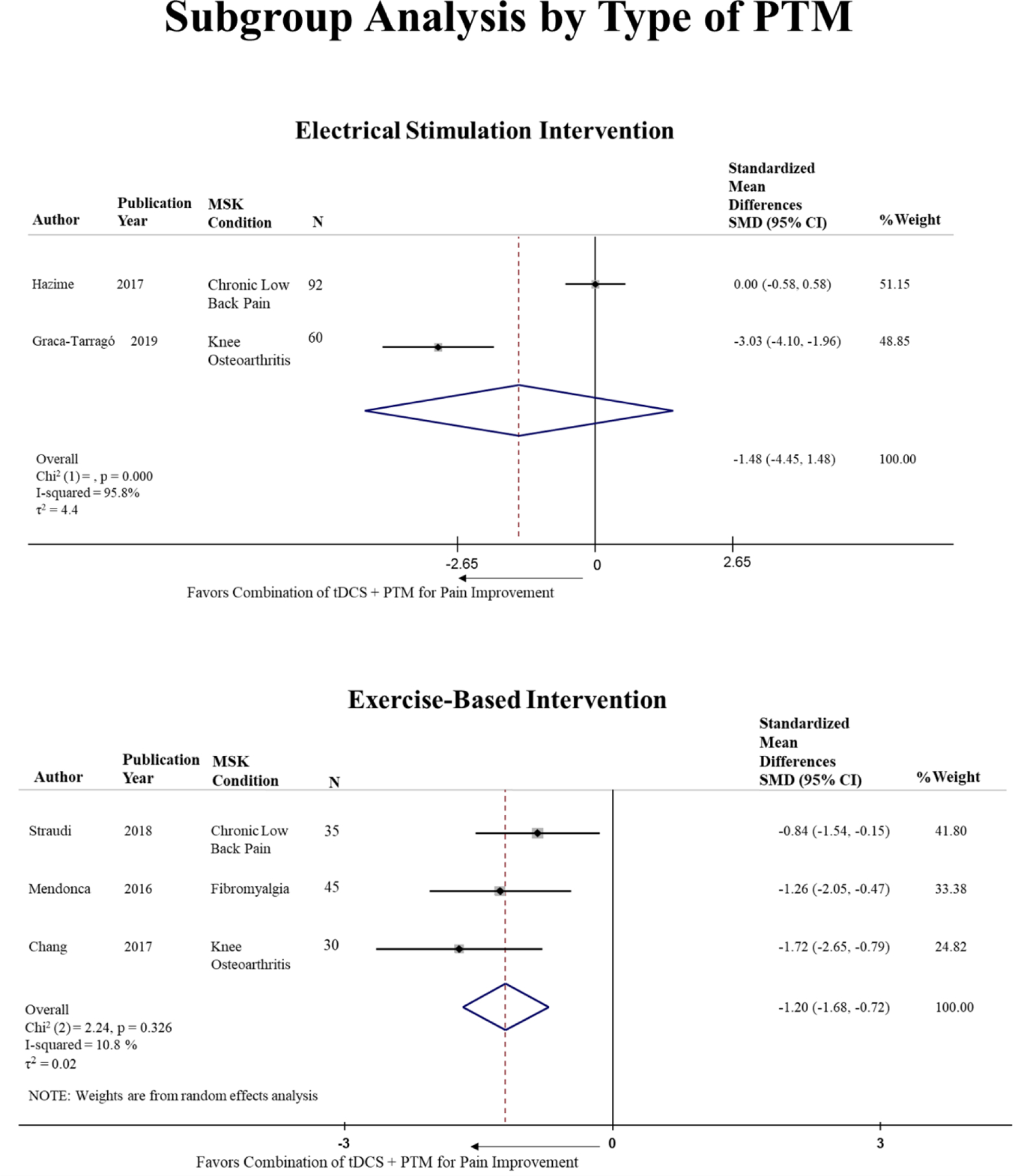
Subgroup analysis by type of CPM despite the MSK condition.
Publication Bias Assessment
The low number of studies did not allow us to perform publication bias analysis as planned as it is only adequate when there are ten or more observations/studies. However, the lack of enough studies to run this analysis is likely to suggest the presence of publication bias.
Evidence on Myofascial Pain Syndrome
Our search has found only one randomized clinical trial that investigated the analgesic effect of tDCS alone versus tDCS plus PTM on myofascial pain syndrome. The PTM was based on active stretching exercise, ultrasound therapy and the use of hot packs (3 times/week for 2 weeks) and the method of combination of the interventions was overlapping. Sample size was relatively small (tDCS + PTM group =16, PTM only group= 15). The tDCS parameters were 1mA anodal at contralateral M1 using 35cm2 electrode surface area for 20 minutes, once a day for 5 consecutive days. The average age on the tDCS + PTM and PTM only group were respectively 49.9 ± 8.2 and 45.9 ± 10.2. Symptom duration on both groups were approximately 6 months. The study used the pain VAS (0–10) as outcome and the assessments were performed at baseline and weekly for 4 weeks. The pain mean difference at the 4th week was −3.82 ± 0.2 for the tDCS + PTM group and −3.77 ± 0.2 PTM group only. The risk of bias assessment suggested a moderate to high risk of bias as important aspects of the methodology were not clearly reported.
DISCUSSION
The current study systematically reviewed all available evidence from RCTs to summarize the effect of tDCS when combined with any type of PTM versus PTM alone on pain scores in common MSK conditions namely KOA, low back pain, fibromyalgia, and myofascial pain. To our knowledge, this is the first systematic review that examined this research question. Overall, our results show that the available evidence is inconclusive due to the large heterogeneity among the studies and methodological biases.
For CLBP, the standardized estimate did not support the use of tDCS in combination with PTM with the objective of pain relief on a short-term period (<8 weeks). Our random-effects meta-analysis revealed a high level of heterogeneity among the studies. Our findings cannot confirm but factors such as number and duration of tDCS sessions, mean age and symptom duration in the intervention’s groups could potentially explain the high heterogeneity observed for the CLBP condition studies. However, our review did not have enough observations to allow a more comprehensive investigation of the sources of heterogeneity. In addition, other unknown sources of heterogeneity, such as presence of comorbidities or co-treatments (i.e. pain medication), may also be contributing to the overall result and were not possible to be analyzed with the collected data. Pooled estimates should be interpreted with caution when large heterogeneity estimates exist in meta-analysis.
The standardized estimates for fibromyalgia and for KOA suggest that these effects of are above the clinically considered standardized mean difference of the visual analog scale previously reported20,24,33. Although the non-standardized estimate calculated for the KOA studies show a result below previously reported MCID (27mm)23,24, the standardized estimate supports a significant clinical analgesic effect of the combination of tDCS with PTM when compared to PTM alone. The analgesic effect of tDCS when combined with any type of PTM seems to be beneficial to these conditions as compared to CLBP and myofascial pain syndrome. However, the quality of evidence assessment reveals very low to low levels of quality, meaning that the effect estimate is limited and there is very little confidence in it. It is likely that the true effect differs from the estimate which consequently makes the results limited. Our analysis also showed higher levels of heterogeneity for the subgroup analysis of CLBP and myofascial pain syndrome as compared with KOA and Fibromyalgia. This could be explained by the nature of the clinical conditions as CLBP is a condition with no decisive diagnosis and with varying underlying etiology. As for myofascial pain syndrome, there was only one study about this condition which makes the generalization of the findings limited, inconsistent and inconclusive.
All studies included in the analysis had a relatively small sample size. Given the randomization is effective when the sample size of the study is large enough, confounding could still produce bias in these studies. In addition, the low number of studies in this review highlights the lack of published trials in the field which may reflect a relevant publication bias.
Despite the low number of studies, our meta-analysis suggests that the combination of tDCS with PTM does not show to be beneficial in relieving pain for CLBP, but it might for the conditions of fibromyalgia and KOA as compared to PTM alone. tDCS is likely to act on neuronal state to indirectly influence the central sensitization which is thought to be present in chronic MSK conditions3. Central sensitization levels can vary according to symptom chronicity and the presence of psychosocial factors such as anxiety, depression and catastrophization34. Different study samples with different MSK conditions may comprise of subjects with different levels of central sensitization which would make them more or less likely to benefit from neuromodulation interventions such as tDCS. This may explain why the combination of tDCS with PTM may work for some conditions and not others. However, the limited number of studies included in our analysis only hence the need of more studies and larger clinical trials to better estimate the pooled effect of these interventions on pain scores in common MSK conditions.
Our subgroup analysis showed that the reduction in pain scores was significant for the combination of tDCS with an exercise based PTM but not for the combination of tDCS with a type of electrical stimulation (peripheral or intramuscular). Previous research has explored the relationship between physical exercise and pain perception in MSK condition35. Greater physical activity was associated with decreased pain ratings and its association with brain areas suggests implications on pain regulation and sensory aspects of pain. One of the neural areas that may lead to this synergistic effect is the primary motor cortex that seems to be an important modulator of chronic pain36.In addition, exercise can affect other body functions that can help with its analgesic effect, such as neuroendocrine responses37. As tDCS can modulate neuronal excitability and influence brain plasticity, it is likely that an exercise intervention when combined with tDCS may add to the effect on the central nervous system areas of pain processing. Our finding supports the combination of these two intervention strategies for pain improvement in different MSK conditions.
Our review suggests that there are a low number of studies that investigated the combination of PTM with tDCS for MSK conditions and the existing literature relies on poor methodology. Such finding may be partially explained by the traditional use of NIBS only in neurological conditions. As the tDCS research developed the evidence in musculoskeletal conditions started to surge. Randomized clinical trials that are well powered and with adequate methodology to avoid bias are the level of evidence needed to clarify the effects of the combination of these interventions. Limiting factors for conducting this type of research may be the resources that such design requires, such as funding and logistics.
Our systematic review and meta-analysis have limitations that should be considered. Mainly, the high level of heterogeneity revealed by the analysis demands that the results are interpreted with caution. Our attempt to explore the sources of heterogeneity among the studies within each condition was limited by the number of studies found for each condition. We believe these results exposed the lack of studies in the field which may call for an exploration of these interventions in MSK conditions. An adequate heterogeneity investigation was again not allowed due to the number of studies. Future investigations should explore sources of heterogeneity as the ones measured in our study, thus other confounders may exist. The low sample size and risk of bias present in the selected studies are also an important limitation. Imprecise estimates are common when small sample sizes are predominant among the studies. Ultimately, our criteria, although broad, led to a small number of studies to be analyzed. The low number of studies included suggest that the current state of evidence may be limiting a definite answer to our research question. As for strengths, our extensive systematic search, the quality assessment exploration, and the detailed presentation of our results make this synthesis the best available with the current existing evidence.
CONCLUSION
Available evidence offers inconclusive results due to publication bias and large heterogeneity. The conditions that may benefit more from the combined interventions of tDCS and PTM are KOA and Fibromyalgia. The analgesic effect seems to be stronger when tDCS was combined with an exercise-based intervention, despite the MSK condition. Future research should explore the combination of interventions on these specific conditions (tDCS and exercise-based therapy). Larger clinical trials are needed to better estimate the pooled effect of these interventions on pain scores in other MSK conditions. Due to the existence of few studies in this field, our findings should not discourage any future research in this area.
Supplementary Material
Appendix.1: Systematic Review Search Strategy
Appendix.2: Risk of Bias Assessment in Included studies. Green = low risk; Red: high risk; Yellow = unclear risk / not reported.
Funding
FF is funded by NIH grant R01 AT009491-01A1
Footnotes
Conflict of Interest
The author followed the International Committee of Journal of Medical Journal Editors (ICMJE) form for disclosure of potential conflicts of interest. The listed author concurs with the submission of the manuscript. The final version has been approved by the author. The author has no financial or personal conflicts of interest.
REFERENCES
- 1.Babatunde F, MacDermid J, MacIntyre N. Characteristics of therapeutic alliance in musculoskeletal physiotherapy and occupational therapy practice: a scoping review of the literature. BMC Health Serv Res. 2017;17(1):375. doi: 10.1186/s12913-017-2311-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liew S-L, Santarnecchi E, Buch ER, Cohen LG. Non-invasive brain stimulation in neurorehabilitation: local and distant effects for motor recovery. Front Hum Neurosci. 2014;8:378. doi: 10.3389/fnhum.2014.00378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto CB, Teixeira Costa B, Duarte D, Fregni F. Transcranial Direct Current Stimulation as a Therapeutic Tool for Chronic Pain. J ECT. 2018;34(3):e36–e50. doi: 10.1097/YCT.0000000000000518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson L, Koslowsky M, Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res. 2012;216(1):1–10. doi: 10.1007/s00221-011-2891-9 [DOI] [PubMed] [Google Scholar]
- 5.Reis J, Schambra HM, Cohen LG, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106(5):1590–1595. doi: 10.1073/pnas.0805413106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Censor N, Dimyan MA, Cohen LG. Modification of Existing Human Motor Memories Is Enabled by Primary Cortical Processing during Memory Reactivation. Curr Biol CB. 2010;20(17):1545–1549. doi: 10.1016/j.cub.2010.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buch ER, Johnen VM, Nelissen N, O’Shea J, Rushworth MFS. Noninvasive associative plasticity induction in a corticocortical pathway of the human brain. J Neurosci Off J Soc Neurosci. 2011;31(48):17669–17679. doi: 10.1523/JNEUROSCI.1513-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conde V, Vollmann H, Taubert M, et al. Reversed timing-dependent associative plasticity in the human brain through interhemispheric interactions. J Neurophysiol. 2013;109(9):2260–2271. doi: 10.1152/jn.01004.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimyan MA, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of mechanisms of functional recovery after stroke. Neurorehabil Neural Repair. 2010;24(2):125–135. doi: 10.1177/1545968309345270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7(2):76–85. doi: 10.1038/nrneurol.2010.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang WJ, Bennell KL, Hodges PW, et al. Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: A pilot randomised controlled trial. 2017;12(6):e0180328. doi: 10.1371/journal.pone.0180328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortensen J, Figlewski K, Andersen H. Combined transcranial direct current stimulation and home-based occupational therapy for upper limb motor impairment following intracerebral hemorrhage: a double-blind randomized controlled trial. Disabil Rehabil. 2016;38(7):637–643. doi: 10.3109/09638288.2015.1055379 [DOI] [PubMed] [Google Scholar]
- 13.O’Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2018;3:CD008208. doi: 10.1002/14651858.CD008208.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koltyn KF, Arbogast RW. Perception of pain after resistance exercise. Br J Sports Med. 1998;32(1):20–24. doi: 10.1136/bjsm.32.1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WebPlotDigitizer - Extract data from plots, images, and maps. https://automeris.io/WebPlotDigitizer/index.html. Accessed May 12, 2019.
- 16.Burda BU, O’Connor EA, Webber EM, Redmond N, Perdue LA. Estimating data from figures with a Web-based program: Considerations for a systematic review. Res Synth Methods. 2017;8(3):258–262. doi: 10.1002/jrsm.1232 [DOI] [PubMed] [Google Scholar]
- 17.Tool to Assess Risk of Bias in Cohort Studies.pdf. http://methods.cochrane.org/bias/sites/methods.cochrane.org.bias/files/public/uploads/Tool%20to%20Assess%20Risk%20of%20Bias%20in%20Cohort%20Studies.pdf. Accessed May 12, 2019.
- 18.Cochrane Handbook for Systematic Reviews of Interventions. /handbook. Accessed May 12, 2019.
- 19.Interpreting results and drawing conclusions - Cochrane Handbook for Systematic Reviews of Interventions - Wiley Online Library. https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119536604.ch15. Accessed January 17, 2020.
- 20.Kersten P, White PJ, Tennant A. Is the pain visual analogue scale linear and responsive to change? An exploration using Rasch analysis. PloS One. 2014;9(6):e99485. doi: 10.1371/journal.pone.0099485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hägg O, Fritzell P, Nordwall A, Swedish Lumbar Spine Study Group. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2003;12(1):12–20. doi: 10.1007/s00586-002-0464-0 [DOI] [PubMed] [Google Scholar]
- 22.Ostelo RWJG, de Vet HCW. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol. 2005;19(4):593–607. doi: 10.1016/j.berh.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 23.Katz NP, Paillard FC, Ekman E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J Orthop Surg. 2015;10:24. doi: 10.1186/s13018-014-0144-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64(1):29–33. doi: 10.1136/ard.2004.022905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schabrun SM, Jones E, Elgueta Cancino EL, Hodges PW. Targeting chronic recurrent low back pain from the top-down and the bottom-up: a combined transcranial direct current stimulation and peripheral electrical stimulation intervention. Brain Stimul. 2014;7(3):451–459. doi: 10.1016/j.brs.2014.01.058 [DOI] [PubMed] [Google Scholar]
- 26.Luedtke K, Rushton A, Wright C, et al. Effectiveness of transcranial direct current stimulation preceding cognitive behavioural management for chronic low back pain: sham controlled double blinded randomised controlled trial. Bmj. 2015;350:h1640. doi: 10.1136/bmj.h1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hazime FA, Baptista AF, de Freitas DG, et al. Treating low back pain with combined cerebral and peripheral electrical stimulation: A randomized, double-blind, factorial clinical trial. Eur J Pain. 2017;21(7):1132–1143. doi: 10.1016/j.brs.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 28.Straudi S, Buja S, Baroni A. The effects of transcranial direct current stimulation (tDCS) combined with group exercise treatment in subjects with chronic low back pain: a pilot randomized control trial. 2018;32(10):1348–1356. doi: 10.1177/0269215518777881 [DOI] [PubMed] [Google Scholar]
- 29.Riberto M, Alfieri FM, de Benedetto Pacheco KM, et al. Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. Open Rheumatol J. 2011;5(1):45–50. doi: 10.2174/1874312901105010045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial Direct Current Stimulation Combined with Aerobic Exercise to Optimize Analgesic Responses in Fibromyalgia: A Randomized Placebo-Controlled Clinical Trial. Front Hum Neurosci 2016;10:68. doi: 10.3389/fnhum.2016.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakrajai P, Janyacharoen T, Jensen MP, et al. Pain reduction in myofascial pain syndrome by anodal transcranial direct current stimulation combined with standard treatment: a randomized controlled study. Clin J Pain. 2014;30(12):1076–1083. doi: 10.1097/ajp.0000000000000069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Graca-Tarrago M, Lech M, Angoleri LDM, et al. Intramuscular electrical stimulus potentiates motor cortex modulation effects on pain and descending inhibitory systems in knee osteoarthritis: a randomized, factorial, sham-controlled study. J Pain Res 2019;12:209–221. doi: 10.2147/jpr.s181019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wandel S, Jüni P, Tendal B, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675. doi: 10.1136/bmj.c4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams LM, Turk DC. Psychosocial factors and central sensitivity syndromes. Curr Rheumatol Rev. 2015;11(2):96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLoughlin MJ, Stegner AJ, Cook DB. The relationship between physical activity and brain responses to pain in fibromyalgia. J Pain Off J Am Pain Soc. 2011;12(6):640–651. doi: 10.1016/j.jpain.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castillo Saavedra L, Mendonca M, Fregni F. Role of the primary motor cortex in the maintenance and treatment of pain in fibromyalgia. Med Hypotheses. 2014;83(3):332–336. doi: 10.1016/j.mehy.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 37.Goldfarb AH, Jamurtas AZ. Beta-endorphin response to exercise. An update. Sports Med Auckl NZ. 1997;24(1):8–16. doi: 10.2165/00007256-199724010-00002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix.1: Systematic Review Search Strategy
Appendix.2: Risk of Bias Assessment in Included studies. Green = low risk; Red: high risk; Yellow = unclear risk / not reported.


