Abstract
Background:
Tranexamic acid (TXA) is an antifibrinolytic drug. Topical administration of TXA during total knee arthroplasty (TKA) is favored for certain patients because of concerns about thrombotic complications, despite a lack of supporting literature. We compared local and systemic levels of thrombogenic markers, interleukin (IL)-6, and TXA between patients who received intravenous (IV) TXA and those who received topical TXA.
Methods:
Seventy-six patients scheduled for TKA were enrolled in this randomized double-blinded study. The IV group received 1.0 g of IV TXA before tourniquet inflation and again 3 hours later; a topical placebo was administered 5 minutes before final tourniquet release. The topical group received an IV placebo before tourniquet inflation and again 3 hours later; 3.0 g of TXA was administered topically 5 minutes before final tourniquet release. Peripheral and wound blood samples were collected to measure levels of plasmin-anti-plasmin (PAP, a measure of fibrinolysis), prothrombin fragment 1.2 (PF1.2, a marker of thrombin generation), IL-6, and TXA.
Results:
At 1 hour after tourniquet release, systemic PAP levels were comparable between the IV group (after a single dose of IV TXA) and the topical group. At 4 hours after tourniquet release, the IV group had lower systemic PAP levels than the topical group (mean and standard deviation, 1,117.8 ± 478.9 µg/L versus 1,280.7 ± 646.5 µg/L; p = 0.049), indicative of higher antifibrinolytic activity after the second dose. There was no difference in PF1.2 levels between groups, indicating that there was no increase in thrombin generation. The IV group had higher TXA levels at all time points (p < 0.001). Four hours after tourniquet release, wound blood IL-6 and TXA levels were higher than systemic levels in both groups (p < 0.001). Therapeutic systemic TXA levels (mean, 7.2 ± 7.4 mg/L) were noted in the topical group. Calculated blood loss and the length of the hospital stay were lower in the IV group (p = 0.026 and p = 0.025).
Conclusions:
Given that therapeutic levels were reached with topical TXA and the lack of a major difference in the mechanism of action, coagulation, and fibrinolytic profile between topical TXA and a single dose of IV TXA, it may be a simpler protocol for institutions to adopt the use of a single dose of IV TXA when safety is a concern.
Level of Evidence:
Therapeutic Level I. See Instructions for Authors for a complete description of levels of evidence.
Total knee arthroplasty (TKA) has been associated with substantial blood loss and risk of transfusion1. Over recent years, the use of tranexamic acid (TXA), a lysine analog and antifibrinolytic agent, has become more common. Multiple studies have shown both topical and intravenous (IV) TXA to reduce blood loss after TKA, but questions still remain about the safest and most effective route of administration2-12. If the drug is administered systemically, thrombosis may be a concern in certain populations. Therefore, some practitioners advocate topical application of TXA in the surgical wound. At our institution, there are no restrictions on the use of topical TXA, whereas existing cardiovascular disease or a history of thrombosis are considered relative contraindications to IV TXA. Topical TXA is known to be absorbed to a certain extent systemically, but its effect on systemic coagulation and fibrinolysis has not been studied, to our knowledge2.
While several meta-analyses have been conducted on the safety and efficacy of TXA3-5, we are not aware of any studies of the distribution of TXA both systemically and in the wound when given IV or topically. Knowing the levels of TXA both systemically and in the wound, and its effect on thrombogenic markers according to the route of administration, may help clinicians determine the optimal care of high-risk patients. Therefore, additional research on the mechanisms of TXA and its effect on the coagulation and fibrinolytic pathways has been recommended6,7.
In this study, we examined wound and systemic levels of plasmin-anti-plasmin (PAP, a marker of fibrinolysis), prothrombin fragment 1.2 (PF1.2, a marker of thrombin generation), and the TXA itself when the drug was given through an IV route and when it was administered topically. Therapeutic TXA levels should result in lower PAP levels, implying less fibrinolysis. If TXA is thrombogenic, higher PF1.2 levels indicative of increased thrombin generation should be seen. Measuring both wound and systemic TXA levels may help elucidate the drug’s site of action when it is given either topically or through an IV route. Interleukin (IL)-6 levels were also compared between the IV and topical groups, as some studies13 have suggested that TXA is proinflammatory and others14 have indicated the opposite.
Materials and Methods
Ethics
This randomized double-blinded trial received institutional review board approval, was registered at ClinicalTrials.gov (NCT02540226), and adheres to Consolidated Standards of Reporting Trials (CONSORT) guidelines. Written informed consent was obtained from all participants prior to surgery.
Patient Recruitment
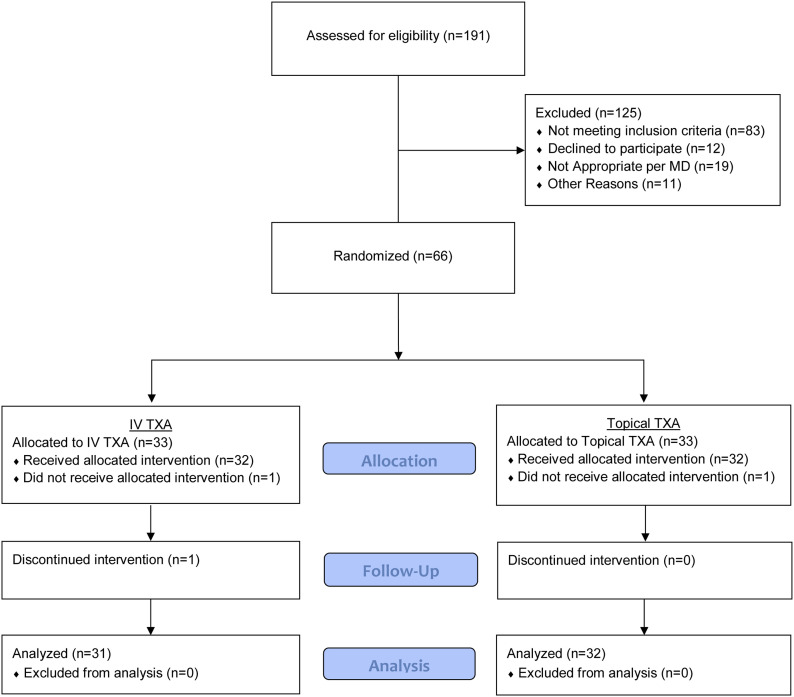
Patients 18 to 80 years of age who were being treated with a primary unilateral TKA by participating surgeon coinvestigators between December 2016 and December 2017 were eligible to participate in the study. Exclusion criteria included steroid therapy, hypersensitivity to TXA, renal and hepatic dysfunction, coronary artery disease, advanced lung disease, a history of venous thromboembolism, hypercoagulability, and cerebrovascular disease. Patient enrollment is depicted in Figure 1 and Appendix Figure 1.
Fig. 1.
CONSORT flow diagram showing progress through the study phases by the 66 patients. Sixty-three patients were included in the final analysis.
Randomization and Blinding
Patients were randomized in a 1:1 ratio in size-4 and 6 blocks, via a computer-generated randomization schedule, to receive IV or topical TXA. Unblinded pharmacy personnel implemented the randomization. Patients, surgeons, anesthesiologists, nurses, and research assistants collecting data were blinded to group allocation.
Study Interventions
All patients received combined spinal-epidural anesthesia, an adductor canal block with 0.25% bupivacaine, and an arterial line.
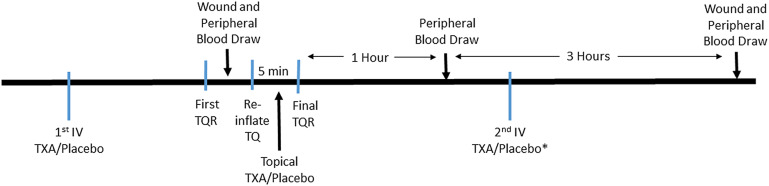
Prior to tourniquet inflation, the patients in the IV group received 1.0 g of IV TXA and the topical group received 100 mL of an IV placebo (saline solution). Three hours after the first administration of the IV TXA or placebo, an identical preparation was administered in the post-anesthesia care unit (PACU). Five minutes prior to final tourniquet release, the topical group received 3.0 g of TXA in 75 mL of saline solution and the IV group received 75 mL of saline solution directly on the wound. In all cases, a ConstaVac drain (Stryker) was inserted immediately prior to wound closure and was removed 4 hours after tourniquet release. The treatment dose for both groups was determined from previous studies2,8-11.
Both wound and peripheral blood samples were collected at several time points (Fig. 2). Peripheral blood was drawn from the arterial line and collected prior to topical administration, 1 hour after tourniquet release, and 4 hours after tourniquet release. To obtain intraoperative wound blood prior to final tourniquet release and wound closure, the knee was irrigated and the tourniquet was momentarily released to allow blood pooling. Once the blood was collected, the tourniquet was reinflated, after which the prosthesis was cemented. Then the topical TXA or placebo solution was poured into the wound, where it remained undisturbed for 5 minutes. Afterward, the tourniquet was deflated for the rest of surgery. In the PACU, 1 hour after tourniquet release, peripheral blood was collected prior to the administration of the second dose of IV TXA or placebo in all patients. Three hours later, peripheral blood was collected and wound blood was collected from the drain. All blood samples were placed on ice and centrifuged at 3,500 rpm for 5 minutes. The plasma was frozen and stored at −80°C. Preoperative and postoperative blood samples were drawn to assess hemoglobin and hematocrit levels per our institution’s standard of care for the duration of the inpatient hospital stay.
Fig. 2.
Timeline of blood draws and interventions. *The second IV TXA or placebo dose was given 3 hours after the first. TQ = tourniquet and TQR = tourniquet release.
Outcomes
The primary outcome was the level of PAP complex in plasma of peripheral blood 4 hours after tourniquet release. Other outcomes included PF1.2, IL-6, and TXA levels in collected peripheral and wound blood. PAP-complex and PF1.2 levels were determined with an enzyme-linked immunosorbent assay (ELISA) (Human Plasmin-Antiplasmin Complex, PAP ELISA Kit [Biomatik] and Standards/Control Enzygnost F1+2 Kit [Siemens]). IL-6 levels were determined with the V-PLEX Human IL-6 Kit (Meso Scale Discovery).
Clinical outcomes included the total amount of drainage in the ConstaVac, total amount of estimated blood loss over the course of the hospital stay measured with Good’s formula, and the total hidden amount of blood loss over the course of the hospital stay12. The time to discharge from physical therapy (PT) and the length of the hospital stay were also assessed. Hemoglobin and hematocrit levels and units of blood transfused were measured until hospital discharge. The prevalance of thrombosis was also assessed.
Statistical Analysis
The study was powered for PAP levels 4 hours after tourniquet release. In a previous trial, we found the mean PAP level (and standard deviation [SD]) to be 1,087 ± 536 μg/L 4 hours after tourniquet release in primary unilateral TKA15. We determined that a sample size of 30 patients per group would provide 80% power at a 2-sided alpha level of 0.05 to detect a 400-μg/L (37%) difference in the mean PAP level 4 hours after tourniquet release between the groups. We planned to enroll a total of 66 patients to account for attrition and protocol violations.
The IV and topical TXA groups were compared for balance with regard to baseline measurements by calculating standardized differences, with imbalance defined16 as a standardized difference with an absolute value greater than 1.96 × (2/32)1/2 = 0.490.
PAP, PF1.2, IL-6, hemoglobin, hematocrit, and TXA blood levels were compared between the 2 groups and between systemic and wound blood samples (PAP only) via generalized linear modeling using the generalized estimating equation (GEE) approach. GEE was used to account for the correlation between repeated measurements for the same patient. Intraoperative and postoperative systemic blood levels of PAP, PF1.2, IL-6, and TXA were log-transformed for analysis due to skewed distributions. The log transformation was also applied to the intraoperative and postoperative wound blood PAP, IL-6, and TXA levels, whereas the PF1.2 levels were dichotomized as being more than or less than the detection limit of 3,600 pmol/L. Intraoperative and postoperative systemic PAP, PF1.2, IL-6, hemoglobin, and hematocrit blood-level comparisons were adjusted for baseline systemic blood levels via linear regression. Effect sizes are presented as ratios of geometric means with 95% confidence intervals (CIs) for log-transformed continuous outcomes, as odds ratios (ORs) with 95% CIs for dichotomous outcomes, and as difference in means with 95% CIs for continuous outcomes.
Total blood loss and time to discharge were compared between the groups using Wilcoxon rank-sum tests, with effect sizes are presented as Hodges-Lehmann estimates of location shift with 95% CIs.
All statistical hypothesis tests were 2-sided. Statistical analyses were performed with SAS version 9.4 (SAS Institute).
Results
Seventy-six patients scheduled for primary TKA were randomized to receive either IV or topical TXA. The trial was paused after randomization of 10 patients in order to perform assay validation. The protocol was refined, and the trial was resumed with a new randomization. Demographics and the preoperative hemoglobin level, hematocrit, and platelet count are presented in Table I.
TABLE I.
Baseline Patient Characteristics and Measurements
| IV TXA Group (N = 31) | Topical TXA Group (N = 32) | Standardized Difference | |
| Patient characteristics* | |||
| Age† (yr) | 65.6 ± 8.4 | 65.0 ± 6.9 | 0.077 |
| Sex (male/female) (no.) | 11/20 | 12/20 | 0.042 |
| BMI† (kg/m2) | 31.6 ± 7.1 | 31.1 ± 5.2 | 0.074 |
| ASA grade (II/III) (no.) | 28/3 | 32/0 | 0.455 |
| Preoperative CBC† | |||
| Hemoglobin (g/dL) | 135 ± 11 | 138 ± 11 | −0.196 |
| Hematocrit (%) | 40.8 ± 3.2 | 41.0 ± 2.8 | −0.061 |
| Platelet count (platelets/µL) | 241 ± 50 | 241 ± 60 | −0.002 |
BMI = body mass index, ASA = American Society of Anesthesiologists, CBC = complete blood-cell count.
The values are given as the mean and SD.
Systemic PAP levels did not differ significantly either intraoperatively or at 1 hour after tourniquet release between the topical TXA group and the IV TXA group; this indicated that the systemic levels of antifibrinolytic activity were similar in the 2 groups after the IV group received a single dose of IV TXA. However, 4 hours after tourniquet release, the systemic PAP levels in the patients who received IV TXA were significantly lower (p = 0.049) than in the topical group, indicative of higher antifibrinolytic activity after the second dose of IV TXA (Table II). PAP levels in the wound intraoperatively and at 4 hours after tourniquet release also did not differ significantly between the groups (Table II). Within the topical group, the wound PAP levels were significantly lower than the systemic PAP levels 4 hours after tourniquet release, implying less antifibrinolytic activity systemically at this point in time. This is consistent with the fact that the IV group received 2 doses of IV TXA, maintaining its antifibrinolytic activity systemically 4 hours after tourniquet release, while the topical group received only 1 dose of TXA (Table III, p = 0.021). Systemic PF1.2 levels did not differ significantly between the groups at any time point (Table IV), implying that there was no increase in thrombin generation with IV TXA.
TABLE II.
Comparison of PAP Levels Between IV and Topical TXA Groups
| IV TXA Group | Topical TXA Group | Ratio of Geometric Means (95% CI) * | P Value | |||
| No. | Mean ± SD (µg/L) | No. | Mean ± SD (µg/L) | |||
| Systemic PAP blood level* | ||||||
| Intraoperative | 31 | 1,240.3 ± 469.4 | 32 | 1,200.9 ± 430.4 | 1.02 (0.86, 1.21) | 0.808 |
| Postoperative (1 hr after tourniquet release) | 31 | 1,219.1 ± 427.1 | 32 | 1,160.6 ± 439 | 1.07 (0.91, 1.26) | 0.436 |
| Postoperative (4 hr after tourniquet release) | 31 | 1,117.8 ± 478.9 | 32 | 1,280.7 ± 646.5 | 0.85 (0.72, 1.00) | 0.049 |
| Wound PAP blood level | ||||||
| Intraoperative | 30 | 759.9 ± 316.2 | 29 | 797.5 ± 470 | 1.04 (0.78, 1.40) | 0.766 |
| Postoperative (4 hr after tourniquet release) | 26 | 1,032 ± 354.5 | 31 | 1,041.2 ± 365.8 | 1.00 (0.83, 1.21) | 0.967 |
Comparisons adjusted for baseline systemic PAP blood levels.
TABLE III.
Comparison of Systemic and Wound PAP Levels 4 Hours After Tourniquet Release by Treatment Group
| Systemic PAP Blood Level | Wound PAP Blood Level | Ratio of Geometric Means (95% CI) | P Value | |||
| No. | Mean ± SD (µg/L) | No. | Mean ± SD (µg/L) | |||
| IV TXA group | 26 | 1,078.2 ± 483.4 | 26 | 1,032 ± 354.5 | 0.99 (0.83, 1.18) | 0.943 |
| Topical TXA group | 31 | 1,290.6 ± 654.8 | 31 | 1,041.2 ± 365.8 | 1.22 (1.03, 1.45) | 0.021 |
TABLE IV.
Comparison of PF1.2 Levels Between IV and Topical TXA Groups
| IV TXA Group | Topical TXA Group | Ratio of Geometric Means or Odds Ratio (95% CI) | P Value | |||
| No. | Value* | No. | Value* | |||
| Systemic PF1.2 blood level† | ||||||
| Intraoperative | 31 | 400.2 ± 235.6 | 32 | 377.3 ± 218.4 | 1.04 (0.83, 1.30) | 0.736 |
| Postoperative (1 hr after tourniquet release) | 31 | 661 ± 243.5 | 32 | 636.2 ± 241.6 | 1.03 (0.86, 1.23) | 0.774 |
| Postoperative (4 hr after tourniquet release) | 31 | 812.9 ± 349.9 | 32 | 868.8 ± 336.2 | 0.92 (0.74, 1.13) | 0.412 |
| Wound PF1.2 blood level >3,600 pmol/L | ||||||
| Intraoperative | 31 | 16 (52%) | 32 | 14 (44%) | 1.37§ (0.51, 3.70) | 0.533 |
| Postoperative (4 hr after tourniquet release) | 28 | 13 (46%) | 32 | 11 (34%) | 1.75§ (0.63, 4.86) | 0.286 |
The values for the systemic blood levels are given as the mean and SD in pmol/L. The values for the wound blood levels are given as the number and percentage of patients who had a level above the threshold.
Comparisons adjusted for baseline systemic PF1.2 blood levels.
Odds ratio.
There was an upregulation of IL-6 levels in all patients, regardless of group, 4 hours after tourniquet release (Table V). IL-6 wound levels were significantly higher than systemic levels 4 hours after tourniquet release (p < 0.001) (Table V), with no significant difference between the topical and IV groups.
TABLE V.
Comparison of IL-6 Levels Between IV and Topical TXA Groups
| IV TXA Group | Topical TXA Group | Ratio of Geometric Means (95% CI) | P Value | |||
| No. | Mean ± SD (pg/mL) | No. | Mean ± SD (pg/mL) | |||
| Systemic IL-6 blood level* | ||||||
| Intraoperative | 31 | 1.1 ± 0.9 | 32 | 3.9 ± 11.5 | 0.95 (0.78, 1.16) | 0.618 |
| Postoperative (1 hr after tourniquet release) | 31 | 1.8 ± 1.2 | 32 | 5 ± 12.7 | 0.96 (0.77, 1.21) | 0.738 |
| Postoperative (4 hr after tourniquet release) | 31 | 17.2 ± 14.9 | 32 | 25.6 ± 33.9 | 0.92 (0.67, 1.28) | 0.640 |
| Wound IL-6 blood level | ||||||
| Intraoperative | 31 | 5.2 ± 7.6 | 32 | 6.7 ± 12.1 | 0.84 (0.49, 1.44) | 0.528 |
| Postoperative (4 hr after tourniquet release) | 28 | 5,744.8 ± 4,360.7 | 32 | 4,497.6 ± 3,325.5 | 1.23 (0.89, 1.71) | 0.205 |
Comparisons adjusted for baseline systemic IL-6 blood levels.
Systemic and wound TXA levels were higher in the IV group than in the topical group at all time points (p < 0.001) (Table VI). The mean systemic TXA levels (and SD) in the topical group were 7.2 ± 7.4 mg/L and 5.2 ± 8.8 mg/L at 1 and 4 hours after tourniquet release, respectively. At 4 hours after tourniquet release, both groups had higher wound than systemic blood levels of TXA. The calculated total blood loss was higher in the topical group than in the IV group (p = 0.026). There was no difference between groups in the amount of ConstaVac drainage blood at 4 hours after tourniquet release (p = 0.111). The mean time to discharge from PT did not differ significantly between groups (p = 0.053), whereas the mean hospital length of stay was shorter in the IV group (p = 0.025) (Table VII).
TABLE VI.
Comparison of Blood TXA Levels Between IV and Topical TXA Groups
| IV TXA Group | Topical TXA Group | Ratio of Geometric Means (95% CI) | P Value | |||
| No. | Mean ± SD (mg/L) | No. | Mean ± SD (mg/L) | |||
| Systemic TXA blood level | ||||||
| Intraoperative | 31 | 31.0 ± 15.2 | 32 | 3.9 ± 13.1 | 349.2 (90.3, 1,349.5) | <0.001 |
| Postoperative (1 hr after tourniquet release) | 31 | 19.9 ± 8.3 | 32 | 7.2 ± 7.4 | 3.7 (2.6, 5.4) | <0.001 |
| Postoperative (4 hr after tourniquet release) | 31 | 27.4 ± 13.7 | 32 | 5.2 ± 8.8 | 8.5 (5.3, 13.6) | <0.001 |
| Wound TXA blood level | ||||||
| Intraoperative | 31 | 21.1 ± 11.2 | 32 | 2.2 ± 9.2 | 339.1 (111.0, 1,036.2) | <0.001 |
| Postoperative (4 hr after tourniquet release) | 26 | 37.9 ± 16.1 | 31 | 31.4 ± 47.0 | 2.4 (1.5, 3.7) | <0.001 |
TABLE VII.
Comparison of Blood Loss and Discharge Times Between IV and Topical TXA Groups
| IV TXA Group | Topical TXA Group | Hodges-Lehmann Estimate of Location Shift (95% CI) | P Value | |||
| No. | Median (Q1, Q3)* | No. | Median (Q1, Q3)* | |||
| Calculated total blood loss (mL) | 31 | 1,237 (780, 1,456) | 32 | 1,405 (1,208, 1,700) | −259 (−500, −33) | 0.026 |
| ConstaVac drainage total blood (mL) | 28 | 100 (90, 185) | 32 | 170 (118, 240) | −40 (−80, 10) | 0.111 |
| Time to PT discharge (days) | 31 | 1.9 (1.8, 2.8) | 32 | 2.9 (2.0, 3.0) | −0.3 (−0.9, 0) | 0.053 |
| Length of hospital stay (days) | 31 | 2.3 (2.0, 2.9) | 32 | 3.0 (2.5, 3.2) | −0.2 (−0.9, −0.45) | 0.025 |
Q1 = first quartile, and Q3 = third quartile.
Hemoglobin levels were higher in the IV group than in the topical group at postoperative day 1 (POD1) (p < 0.001) and POD2 (p = 0.005). Hematocrit levels were higher in the IV group than in the topical group at POD2 (p = 0.006) (Table VIII). There were no thromboses in either the IV or the topical group. Two patients, 1 in the IV group and 1 in the topical group, received 1 unit of transfused blood during the hospital stay.
TABLE VIII.
Comparison of Hemoglobin and Hematocrit Levels Between IV and Topical TXA Groups
| IV TXA Group | Topical TXA Group | Difference in Means (95% CI)* | P Value | |||
| No. | Mean ± SD | No. | Mean ± SD | |||
| Hemoglobin (g/dL) | ||||||
| Preop. | 31 | 13.53 ± 1.13 | 32 | 13.75 ± 1.13 | ||
| 1 hr after tourniquet release (PACU) | 31 | 11.29 ± 1.21 | 32 | 11.41 ± 1.02 | 0.05 (−0.25, 0.35) | 0.741 |
| POD1 | 30 | 11.12 ± 1.04 | 31 | 10.53 ± 0.13 | 0.72 (0.35, 1.10) | <0.001 |
| POD2 | 30 | 10.28 ± 1.19 | 32 | 9.94 ± 1.03 | 0.53 (0.16, 0 .90) | 0.005 |
| Hematocrit (%) | ||||||
| Preop. | 31 | 40.8 ± 3.2 | 32 | 41 ± 2.8 | ||
| 1 hr after tourniquet release (PACU) | 31 | 33.6 ± 3.6 | 32 | 33.8 ± 2.6 | −0.01 (−1, 0.8) | 0.847 |
| POD1 | 30 | 35.6 ± 12 | 31 | 31.5 ± 4 | 4.2 (−0.1, 8.5) | 0.058 |
| POD2 | 30 | 31 ± 3.5 | 32 | 29.8 ± 2.7 | 1.4 (0.4, 2.5) | 0.006 |
Comparisons adjusted for baseline systemic hemoglobin and hematocrit blood levels.
Discussion
Two doses of IV TXA were associated with lower PAP levels in plasma, showing greater antifibrinolytic activity at the systemic level, compared with either topical TXA or a single dose of IV TKA. When topical use was compared with a single dose of IV TXA, similar coagulation and fibrinolytic profiles were noted. PF1.2 levels, a marker of thrombin generation, did not differ between groups and hence suggest that TXA does not increase the risk of thrombosis. This is consistent with the theory that TXA acts only on the fibrinolytic pathway without increasing thrombus formation. There was no difference in the inflammatory response between the groups. Wound levels of IL-6 were >100-fold higher than systemic levels, suggesting that the wound is the principal site of inflammation.
High wound levels of TXA were attained in the IV group, and they were even higher than the levels in the topical group at 4 hours after tourniquet release, most likely reflecting the second dose of IV TXA. At all time points, systemic levels of TXA were higher in the IV group. The systemic levels in the topical group (mean, 7.2 ± 7.4 mg/L at 1 hour) were in the therapeutically active range for most patients17. In another study, topical use of TXA at a dose of 3 g led to a mean serum level of 8.5 mg/L (95% CI = 7.2 to 9.9 mg/mL)2. This demonstrates that topical TXA results in therapeutic systemic levels, and its systemic effects may be similar to those of IV TXA. In our study, we used 3 g of topical TXA, which is routine at our institution. Lower doses of topical TXA, which may actually lead to lower systemic absorption, have been used3,18.
High levels of TXA in the wound, regardless of the route of administration, suggest that the wound is the major site of action for TXA. We found that the mean wound level of TXA in the IV group was higher than the systemic level at 4 hours (37.9 versus 27.4 mg/L). Previously, in a population of hemophilic patients, rapid diffusion of TXA was observed in joint fluid 1 hour after IV administration19. Wound levels of TXA in the normal patient population have not been reported, to our knowledge. Benoni et al. demonstrated more profound activation of both the coagulation and the fibrinolytic system in the wound as opposed to systemically in patients undergoing TKA20. In patients who received IV TXA, levels of D-dimers were much lower in the wound compared with the systemic circulation, leading to the conclusion that TXA inhibits fibrinolysis primarily through a local mechanism.
Concerns over the safety of IV TXA have been raised, although it has been reported to be safe even in patients with a history of thrombosis2. A recent meta-analysis showed that TXA, regardless of route, was not associated with an increased risk of venous thromboembolic events5. Despite this, many physicians continue to express concern. According to recent guidelines published by the American Association of Hip and Knee Surgeons, the recommendation for use of TXA in high-risk patients is limited because of a lack of strong supporting evidence18.
Perioperative blood loss with both regimens has been explored previously21. Several meta-analyses have concluded that the dose, dosing regimen, and route of administration of TXA have no effects on its blood-conserving properties4,5. In a study of 640 patients treated with either IV or topical TXA, greater blood loss was seen in the topical group but without any difference in the rate of transfusion3. In our study, although the amount of ConstaVac drainage did not differ between groups, total calculated blood loss was significantly higher in the topical group. This may be attributable to sustained levels of TXA in the wound from the second dose of IV TXA in the IV group. However, no difference in transfusion was noted between groups. Of note, in patients undergoing joint arthroplasty, the fibrinolytic response shows increased activity during the first 3 hours, peaking at 6 hours postoperatively, and is maintained for about 18 hours22.
Interestingly, the IV group had a shorter length of stay than the topical group. In a study of 40 patients treated with TKA who received either 1 g of TXA or no TXA, the TXA group had a shorter length of stay (4.5 versus 5.4 days)23. In a retrospective study, patients who had received TXA walked farther during their first PT session but there was no association with length of stay24. In a prospective randomized controlled trial, no difference in perioperative blood loss was found between IV and topical TXA25. The patients who received IV TXA reported less pain during the initial 24 hours after the operation, with the potential for faster rehabilitation and a shorter length of stay. The reason for the shorter length of stay in the IV group in the present study is not clear. It may have been due to higher hemoglobin levels or diminished knee pain, but we did not look at pain scores.
There are several limitations to this study. We did not include a group of patients who did not receive TXA. From an ethical standpoint, it is reasonable to assert that the literature at this point would not support TXA versus no-TXA groups. Another limitation is that IL-6 levels were collected only up to 4 hours postoperatively whereas, in other studies13,14, these levels were followed up to 48 hours postoperatively. In addition, although no thrombotic events were observed, our study was not powered for such clinical outcomes.
In conclusion, this study raises questions about whether topical TXA offers any advantages over IV TXA, and yet the use of topical TXA has no clinical contraindications. IV TXA leads to higher wound concentrations, raising the possibility that the wound is the major site of action regardless of the mode of administration. Lower systemic TXA levels were seen in the topical group, but they were still considered to be in the therapeutic range for most patients. There was no difference in systemic PAP levels 1 hour after tourniquet release, suggesting similar antifibrinolytic activity in the topical and IV TXA groups at a point at which the IV group had received only 1 dose. Increased antifibrinolytic activity was seen after the second dose of IV TXA, but without an increase in thrombin generation as shown by the PF1.2 levels, suggesting a lack of increased risk of thrombosis with such a dose of IV TXA. The effect of the inflammatory response was also similar. Consequently, the clinical rationale invoking the safety of topical over IV TXA may not be sound. In addition, IV administration allows an immediate desired effect, especially in the absence of a tourniquet. Given that therapeutic levels were reached with topical TXA and the lack of a major difference in the mechanism of action, coagulation, and fibrinolytic profile between topical TXA and a single dose of IV TXA, it may be a simpler protocol for institutions to adopt the use of a single dose of IV TXA when safety is a concern.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJS/F506).
Footnotes
Investigation performed at the Hospital for Special Surgery, New York, NY
Disclosure: REDCap use was supported by the National Center for Advancing Translational Science of the National Institutes of Health (UL1TR000457). On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJS/F505).
Data Sharing
A data-sharing statement is provided with the online version of the article (http://links.lww.com/JBJS/F507).
References
- 1.Moráis S, Ortega-Andreu M, Rodríguez-Merchán EC, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R, Gómez-Barrena E. Blood transfusion after primary total knee arthroplasty can be significantly minimised through a multimodal blood-loss prevention approach. Int Orthop. 2014. February;38(2):347-54. Epub 2013 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong J, Abrishami A, El Beheiry H, Mahomed NN, Roderick Davey J, Gandhi R, Syed KA, Muhammad Ovais Hasan S, De Silva Y, Chung F. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010. November 3;92(15):2503-13. [DOI] [PubMed] [Google Scholar]
- 3.Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Shores P, Mullen K, Bini SA, Clarke HD, Schemitsch E, Johnson RL, Memtsoudis SG, Sayeed SA, Sah AP, Della Valle CJ. The efficacy of tranexamic acid in total hip arthroplasty: a network meta-analysis. J Arthroplasty. 2018. October;33(10):3083-3089.e4. Epub 2018 Jun 27. [DOI] [PubMed] [Google Scholar]
- 4.Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Shores P, Mullen K, Bini SA, Clarke HD, Schemitsch E, Johnson RL, Memtsoudis SG, Sayeed SA, Sah AP, Della Valle CJ. The efficacy of tranexamic acid in total knee arthroplasty: a network meta-analysis. J Arthroplasty. 2018. October;33(10):3090-3098.e1. Epub 2018 May 5. [DOI] [PubMed] [Google Scholar]
- 5.Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Shores P, Mullen K, Bini SA, Clarke HD, Schemitsch E, Johnson RL, Memtsoudis SG, Sayeed SA, Sah AP, Della Valle CJ. The safety of tranexamic acid in total joint arthroplasty: a direct meta-analysis. J Arthroplasty. 2018. October;33(10):3070-3082.e1. Epub 2018 Mar 22. [DOI] [PubMed] [Google Scholar]
- 6.Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Shores P, Mullen K, Bini SA, Clarke HD, Schemitsch E, Johnson RL, Memtsoudis SG, Sayeed SA, Sah AP, Della Valle CJ. Response to letter to the editor on “The safety of tranexamic acid in total joint arthroplasty: a direct meta-analysis”. J Arthroplasty. 2018. October;33(10):3368-9. Epub 2018 May 29. [DOI] [PubMed] [Google Scholar]
- 7.Wu XD, Hu KJ, Sun YY, Chen Y, Huang W. Letter to the editor on “The safety of tranexamic acid in total joint arthroplasty: a direct meta-analysis”. J Arthroplasty. 2018. October;33(10):3365-3368.e1. Epub 2018 May 29. [DOI] [PubMed] [Google Scholar]
- 8.Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011. December;93(12):1577-85. [DOI] [PubMed] [Google Scholar]
- 9.Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009. March;123(5):687-96. Epub 2008 Nov 12. [DOI] [PubMed] [Google Scholar]
- 10.Maniar RN, Kumar G, Singhi T, Nayak RM, Maniar PR. Most effective regimen of tranexamic acid in knee arthroplasty: a prospective randomized controlled study in 240 patients. Clin Orthop Relat Res. 2012. September;470(9):2605-12. Epub 2012 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014. August;29(8):1528-31. Epub 2014 Mar 21. [DOI] [PubMed] [Google Scholar]
- 12.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003. May;90(5):596-9. [DOI] [PubMed] [Google Scholar]
- 13.Grant AL, Letson HL, Morris JL, McEwen P, Hazratwala K, Wilkinson M, Dobson GP. Tranexamic acid is associated with selective increase in inflammatory markers following total knee arthroplasty (TKA): a pilot study. J Orthop Surg Res. 2018. June 18;13(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie J, Hu Q, Ma J, Huang Q, Pei F. Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss and the inflammatory response following enhanced-recovery primary total hip arthroplasty: a randomised clinical trial. Bone Joint J. 2017. November;99-B(11):1442-9. [DOI] [PubMed] [Google Scholar]
- 15.McLawhorn AS, Beathe J, YaDeau J, Buschiazzo V, Purdue PE, Ma Y, Sculco TP, Jules-Elysée K. Effects of steroids on thrombogenic markers in patients undergoing unilateral total knee arthroplasty: a prospective, double-blind, randomized controlled trial. J Orthop Res. 2015. March;33(3):412-6. Epub 2015 Jan 6. [DOI] [PubMed] [Google Scholar]
- 16.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009. November 10;28(25):3083-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999. June;57(6):1005-32. [DOI] [PubMed] [Google Scholar]
- 18.Fillingham YA, Ramkumar DB, Jevsevar DS, Yates AJ, Bini SA, Clarke HD, Schemitsch E, Johnson RL, Memtsoudis SG, Sayeed SA, Sah AP, Della Valle CJ. Tranexamic Acid Use in Total Joint Arthroplasty: The Clinical Practice Guidelines Endorsed by the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. J Arthroplasty. 2018. October;33(10):3065-9. Epub 2018 Aug 7. [DOI] [PubMed] [Google Scholar]
- 19.Ahlberg A, Eriksson O, Kjellman H. Diffusion of tranexamic acid to the joint. Acta Orthop Scand. 1976. October;47(5):486-8. [DOI] [PubMed] [Google Scholar]
- 20.Benoni G, Lethagen S, Fredin H. The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thromb Res. 1997. February 1;85(3):195-206. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Pérez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014. December 3;96(23):1937-44. [DOI] [PubMed] [Google Scholar]
- 22.Blanié A, Bellamy L, Rhayem Y, Flaujac C, Samama CM, Fontenay M, Rosencher N. Duration of postoperative fibrinolysis after total hip or knee replacement: a laboratory follow-up study. Thromb Res. 2013. January;131(1):e6-11. Epub 2012 Nov 26. [DOI] [PubMed] [Google Scholar]
- 23.Ismail K, Moll N, Koch L, Swanson K, Walsh G. Intra-operative tranexamic acid reduces blood loss and length of hospital stay after unilateral knee replacement surgery: 6AP2-5. Eur J Anaesthesiol. 2012;29:95-6.22183158 [Google Scholar]
- 24.Grosso MJ, Trofa DP, Danoff JR, Hickernell TR, Murtaugh T, Lakra A, Geller JA. Tranexamic acid increases early perioperative functional outcomes after total knee arthroplasty. Arthroplast Today. 2017. June 29;4(1):74-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei W, Dang S, Duan D, Wei L. Comparison of intravenous and topical tranexamic acid in total knee arthroplasty. BMC Musculoskelet Disord. 2018. June 13;19(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data-sharing statement is provided with the online version of the article (http://links.lww.com/JBJS/F507).