Abstract
Methods
Forty-four COVID-19 patients (severe/critical: N = 8, non-severe: N = 36) were examined by next generation sequencing (NGS) of nasopharyngeal test paper to observe the effect of novel coronavirus infection to the microbial composition in upper airway.
Results
In these nasopharyngeal test paper samples, 38 kinds of bacteria, 10 kinds of viruses except SARS-CoV-2, nine kinds of fungi and three kinds of atypical pathogens had been found. There was some difference in microbial composition in the upper airway between severe and non-severe cases.
Summary
These results are important for us to study the effect of SARS-CoV-2 on the local microbial composition of upper airway and prevent opportunistic infection in severe patients.
Keywords: SARS-CoV-2, microbial composition, upper airway, next generation sequencing; NGS
Introduction
By April 24, 2020, there were 2,626,321 confirmed and 181,938 deaths cases of COVID-19 globally.1 It is important that understood the effect of novel coronavirus on microbial composition on invaded mucosa for avoiding secondary infection. In recent year, next generation sequencing (NGS) technologies provide an increasingly important method for detecting microbial composition and pathogen in human.2
Methods
In our hospital, 44 novel coronavirus infection patients (severe/critical: N = 8, non-severe: N = 36) were examined by NGS of nasopharyngeal test paper to observe the effect of novel coronavirus infection on the microbial composition in upper airway. And the demographics and clinical characteristics of these 44 COVID-19 patients were collected and analyzed. The DNA libraries were sequencing using the BGISEQ-100 platform from The Beijing Genomics Institute. Species information of suspected pathogenic microorganisms can be obtained through comparison of microbial database and intelligent algorithm analysis. We can identify 12,593 pathogenic microorganisms including bacteria, fungi, viruses, parasites, mycoplasma/chlamydia accurately. Statistical analysis was performed using Statistical Package for Social Science (SPSS) Version 17.0. Measurement data was expressed as mean ± standard deviation. Continuous variables were compared using independent-sample t-test, whereas the rank sum test was used for nonparametric data. P <0.05 was considered statistically significant.
Results
Demographics and Clinical Characteristics of COVID-19 Patients
Compared with non-severe patients, older of age, higher proportion of fever and fatigue, more basic diseases, more involved lung leaves, lower white blood cells, lower lymphocytes, decreased CD3 + T cells and subsets groups, but increased CRP were shown in severe/critical patients (P<0.05, Table 1). Specially, decreased CD3 + T cells and subsets groups were risk factors for secondary infection.
Table 1.
Demographics and Clinical Characteristics of COVID-19 Patients
| Characteristic | Total (n = 44) | Severe/Critical (n = 8) | Non-Severe (n = 36) | p value |
|---|---|---|---|---|
| Age, years | 54(3–77) | 62.5(54–77) | 44(3–71) | 0.008* |
| Gender Male | 21(47.7) | 5(62.5) | 16(44.4) | 0.35 |
| Female | 23(52.3) | 3(37.5) | 20(45.6) | |
| Signs and symptoms | ||||
| Fever | 29(65.9) | 7(87.5) | 22(61.1) | 0.15 |
| Cough | 18(40.9) | 3(37.5) | 15(41.7) | 0.83 |
| Fatigue | 5(11.4) | 3(37.5) | 2(5.5) | 0.01* |
| Nasal discharge | 2(4.5) | 0(0) | 2(5.5) | 0.49 |
| Pain (headache/sore throat/muscle aches, etc) | 9(20.4) | 2(25) | 7(19.4) | 0.72 |
| T, °C | 37.0(36.5–37.8) | 37.7(37.3–38.0) | 36.8(36.5–37.7) | 0.04* |
| Underlying diseases(N/Y) | 16(36.4) | 5(62.5) | 11(30.5) | 0.09 |
| Circulatory diseases (hypertension, coronary heart disease) | 7(15.9) | 2(25) | 5(13.9) | 0.44 |
| Respiratory basic diseases (chronic bronchitis, lung cancer) | 3(6.8) | 2(25) | 1(2.8) | 0.02* |
| Endocrine system basic diseases (diabetes) | 3(6.8) | 2(25) | 1(2.8) | 0.02* |
| Other systemic diseases (fractures, cerebral infarction, etc.) | 5(11.4) | 1(12.5) | 4(11.1) | 0.91 |
| Laboratory findings | ||||
| White blood cell count, ×109/L | 4.61(3.67–6.48) | 3.52(3.14–4.59) | 4.82(3.83–6.56) | 0.04* |
| Lymphocyte count, ×109/L | 1.60 (1.08–2.13) | 0.85(0.45–1.46) | 1.76(1.36–2.46) | 0.001* |
| Neutrophil count, ×109/L | 2.30(1.79–3.47) | 2.08(1.30–3.34) | 2.36(1.88–3.61) | 0.38 |
| Monocyte count, ×109/L | 0.47(0.37–0.66) | 0.34(0.26–0.52) | 0.49(0.40–0.71) | 0.06 |
| NLR | 1.78(0.4–9.38) | 2.16(0.74–9.38) | 1.70 (0.40–5.68) | 0.025* |
| PCT | 2(4.5) | 1(12.8) | 1(2.8) | 0.23 |
| CRP, mg/L | 2.45(0.56–7.62) | 22.0(8.68–42.5) | 1.15(0.48–5.06) | 0.001* |
| CD3(+) T lymphocytes,/ul | 1057(742.8–1561) | 565 (226.0–794.5) | 1162(891.3–1734) | 0.001* |
| CD3(+)CD4(+) T lymphocytes,/ul | 579(408.3–831.5) | 325(174.5–483.8) | 631.5(449.8–842.5) | 0.006* |
| CD3(+)CD8(+) T lymphocytes,/ul | 355(263.8–514.5) | 159(64.5–281.8) | 457.5(318.8–553.0) | 0.0002* |
| PaO2, mmHg | 99.9(86.6–105.0) | 91.1(75.7–101.2) | 101.0(89.1–105.3) | 0.12 |
| CT imaging features | ||||
| One or both lungs | 2(0–2) | 2(1–2) | 1(0–2) | 0.02* |
| Number of lung lobes (0–5) | 2next (0–5) | 5(1–5) | 1(0–5) | 0.001* |
| Ground glass lesions | 29(65.9%) | 7(87.5%) | 22(61.1%) | 0.15 |
| Consolidation | 7(15.9%) | 3(37.5%) | 4(11.1%) | 0.06 |
| Bronchial abnormalities | 4(9.1%) | 2(25%) | 2(5.5%) | 0.08 |
| Mediastinal lymph node enlargement | 1(2.3%) | 1(12.5%) | 0(0) | 0.04* |
| Pleural effusion | 1(2.3%) | 1(12.5%) | 0(0) | 0.04* |
Note: *P<0.05.
Abbreviations: T, temperature; NLR, neutrophil-to-lymphocyte ratio; PCT, procalcitonin; CRP, C-reactive protein; PaO2, arterial partial pressure of oxygen.
The Microbial Composition of Upper Airway in COVID-19 Patients
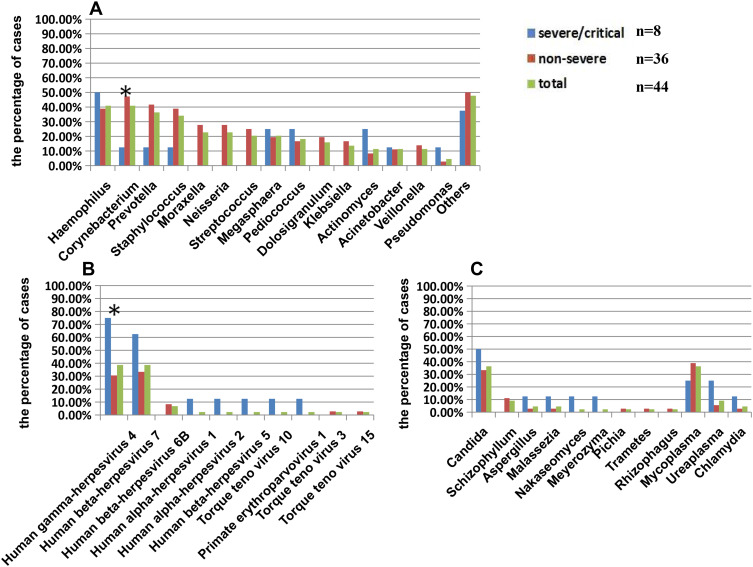
In these nasopharyngeal test paper samples, 38 kinds of bacteria, 10 kinds of viruses except severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), nine kinds of fungi and three kinds of atypical pathogens had been found. The most common bacteria detected in upper airway in these patients were Haemophilus (40.9%), Corynebacterium (40.9%), Prevotella (36.36%), Staphylococcus (34.09%), Moraxella (22.72%), Neisseria (22.72%), Streptococcus (20.45%), Megaphaera (20.45%), Pediococcus (18.18%), and Dolosigaranulum (15.91%) (Figure 1A). Human herpes virus and Torque teno virus were the main viruses detected in nasopharyngeal test paper, but no Influenza virus was found. The most common viruses were Human gamma herpesvirus 4 (38.6%) and Human beta herpesvirus 7 (38.6%), and the other viruses were less than 10% (Figure 1B). Candida (36.36%) was the main fungus found in upper airway of patients, and Aspergillus was detected in one case of severe and one case of non-severe patients respectively. Mycoplasma was shown in 36.36% of all patients (Figure 1C). There was some difference in microbial composition in upper airway between severe and non-severe cases. In non-severe patients, the proportion of Corynebacterium was higher (47.22% vs.12.5%) (Figure 1A), while Human gamma herpesvirus 4 was lower (30.55% vs. 75%) (Figure 1B), compared with severe ones. SARS-CoV-2 declined types of bacteria but increased types of other viruses in upper airway in severe COVID-19 patients in a way.
Figure 1.
The microbial composition of upper airway in patients with SARS-CoV-2 infection.
Notes: The percentage of cases with different kinds of bacteria (A), virus (B), fungus and atypical pathogens (C) detected by next generation sequencing of nasopharyngeal test paper was shown in this figure. *P<0.05, there was significant difference between severe and non-severe groups.
Discussion
The influence of the SARS-CoV-2 on the microbial environment of airway mucosa is not clear. Due to the highly infectious characteristics of SARS-CoV-2, it is difficult to obtain the secretion samples from lower airway. There are many common normal parasitic bacteria in the upper respiratory tract. Common bacteria have aureus, coagulase negative staphylococcus, pulmonary chain, group A streptococcus, enterococcus, neisseria meningitis, diphtheria corynebacterium and so on. And the common fungus is Candida albicans.3 We studied the influence of SARS-COV-2 on the microbial environment of upper airway mucosa by analyzing the microbial composition of upper respiratory tract of COVID-19 patients. Particularly for severe/critical patients, in which lymphocyte declining and immune function inhibition are obvious, we should pay more attention to some opportunistic infections. For example, combining infection of Human gamma herpesvirus 4 had been found both in nasopharyngeal test paper and blood samples of 2 critical patients by NGS, accompanying with SARS-CoV-2 infection.
Acknowledgment
We would like to extend gratitude to BGI.
Abbreviations
COVID-19, 2019 coronavirus disease; NGS, next generation sequencing; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; T, temperature; NLR, neutrophil-to-lymphocyte ratio; PCT, procalcitonin; CRP, C-reactive protein; PaO2, arterial partial pressure of oxygen.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Ethics Approval and Consent to Participate
This retrospective observational study was approved by the Research Ethics Committee of The Fifth Affiliated Hospital of Sun Yat-sen University (approvement series number K30-1) and the need for informed consent was waived, considering the retrospective study design. We will keep all patient data confidential and strictly comply with the Declaration of Helsinki.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Novel coronavirus (2019-nCoV). Situation report-95. WHO. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed April24, 2020.
- 2.Cantalupo PG, Pipas JM. Detecting viral sequences in NGS data. Curr Opin Virol. 2019;39:41–48. doi: 10.1016/j.coviro.2019.07.010 [DOI] [PubMed] [Google Scholar]
- 3.Benninger M, Brook I, J M B, et al. Bacterial interference in upper respiratory tract infections: a systematic review. Am J Rhinol Allergy. 2011;25(2):82–88. doi: 10.2500/ajra.2011.25.3594 [DOI] [PubMed] [Google Scholar]