Abstract
In the current COVID-19 pandemic, there has been concern regarding the use of ibuprofen and other nonsteroidal anti-inflammatory agents by COVID-19 infected patients. Aminosalicylates (5-ASAs) are structurally similar and have anti-inflammatory functions that resemble those of nonsteroidal anti-inflammatory agents. Since 5-ASAs are a mainstay treatment for inflammatory bowel disease, the authors review the pharmacology of both classes of drugs and discuss the potential relevance of 5-ASAs in the ongoing discussion of medication use in patients infected with COVID-19.
Key Words: ibuprofen, nonsteroidal anti-inflammatory agents, aminosalicylates, COVID-19, irritable bowel disease
There has been recent controversy regarding the risk that ibuprofen (and possibly other nonsteroidal anti-inflammatory drugs, NSAIDs) may worsen the clinical course of patients that are infected with COVID-19.1 Aminosalicylates (5-aminosalicylic acids or 5-ASAs) such as mesalamine and sulfasalazine are amino-analogs of salicylate, and therefore their chemical structure and mechanisms of action are related to NSAIDs (Fig. 1). Although the primary effects of 5-ASAs occur directly in the gastrointestinal tract, 5-ASAs are absorbed systemically. It is therefore the purpose of this brief review to inform practitioners treating patients with 5-ASAs about the potential relationships between 5-ASAs and NSAIDs. At the time of this writing, both the World Health Organization (WHO) and the Food and Drug Administration (FDA) have not recommended the avoidance of ibuprofen in patients with known or suspected COVID-19 infections.2 Most recently, the International Organization for the study of Inflammatory Bowel Disease released a summary of recommendations with 76 pointed statements in regard to the risk of developing COVID-19 in the inflammatory bowel disease (IBD) patient population. Statement 14 states, “5-ASA does not increase the risk of COVID-19” however this statement is not based data collected in patients with COVID-19.3 Because these types of recommendations have the potential for changes as the pandemic progresses, it is the author’s belief that the information provided in this review can serve as a tool for discussions of potential risks of 5-ASA usage if patients with IBD become infected with COVID-19.
FIGURE 1.
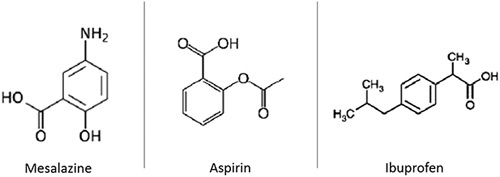
Structural similarities of mesalazine and nonsteroidal anti-inflammatory drugs, such as ibuprofen and aspirin.
ANTI-INFLAMMATORY MECHANISMS OF NSAIDs
NSAIDs reduce inflammation by reversibly and nonselectively inhibiting the cyclooxygenase (COX) enzymes COX-1 and COX-2. COX-1 and COX-2 synthesize prostanoids from arachidonic acid. In ex vivo studies, single and repeated doses of NSAIDs significantly inhibited COX-1 products more so than COX-2 products.
Prostanoids consist of prostaglandins (PG)E2, PGD2, PGF2(alpha), PGI2, and thromboxane (Tx).4 Prostanoids are responsible for many biological effects in several organ systems and have hematologic, pulmonary, renal, and cardiovascular effects.5 PGE2 and PGI2 (also known as prostacyclin) are the primary proinflammatory prostanoids that increase inflammation via increasing vascular permeability with subsequent edema formation and promotion of leukocyte infiltration. PGE2 also mediates fevers (pyresis) and is synthesized in the hypothalamus once systemic cytokines are activated by leukocytes in response to inflammation. PGD2 is produced by mast cells and contributes to inflammation in allergic responses, primarily in the lung. By inhibiting COX-1 and COX-2, NSAIDs effectively reduce regional and systemic inflammation as well as functioning as an antipyretic agent. Because of these antipyretic effects, NSAIDs are commonly prescribed in patients with upper respiratory infections (Fig. 2).
FIGURE 2.
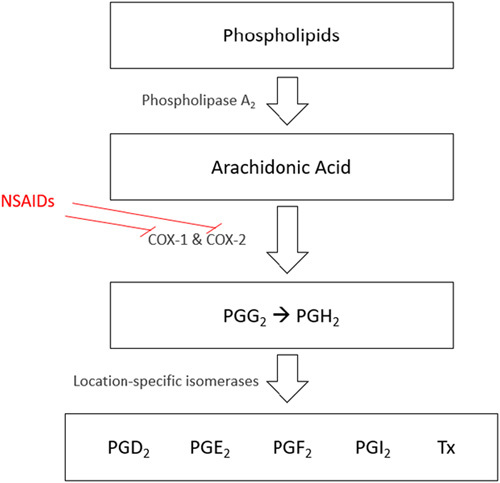
Downstream effects of NSAIDs nonselectively inhibiting COX enzymes and the downstream effects. COX indicates cyclooxygenase; NSAIDs, nonsteroidal anti-inflammatory drugs; PG, prostaglandin; Tx, thromboxane.
PROINFLAMMATORY MECHANISMS OF NSAIDs
Interestingly, NSAIDs contribute to nonspecific inflammation, particularly within the gastrointestinal tract. This inflammation plays a role in the loss of protein and blood and there is endoscopic evidence of sharply demarcated ulcers in the colon that resolve after discontinuation of NSAIDs.6 The proinflammatory mechanisms are enhanced in patients with IBD as compared with the general population. NSAIDs also increase mucosal permeability, induce intracellular ATP deficiency, and increase enterohepatic circulation.7 Prostaglandins affect the colonic immune system and they help to maintain the integrity of mucosal barriers. Therefore, by reducing prostaglandin levels with inhibition of COX-1 and COX-2, colonic mucosal wall integrity weakens, and patients can consequently develop IBD flares. COX-1 is constitutively active, but COX-2 is only induced upon interaction with proinflammatory cytokines. In response to inflammatory stimuli, nuclear factor kappa-light-chain-enhancer of activated B cells produces cytokines such as IL-1 and tumor necrosis factor-alpha. Increases in these proinflammatory cytokines at local sites of inflammation lead to induction of COX-2. In both animal and human models, COX-2 expression was found to be higher in affected intestinal and colonic mucosa, which correlated with IBD activity and played a role in mucosal healing.7 Therefore, inhibiting COX-2 specifically can have detrimental effects on the healing of inflamed intestinal and colonic mucosa, which can be seen at both a pathologic level and an endoscopic visualizable level. It is standard practice for patients with IBD to avoid intake of NSAIDs, primarily because NSAIDs may worsen the natural disease course.
ANTI-INFLAMMATORY MECHANISMS OF 5-ASA
5-ASA has anti-inflammatory and immunosuppressive properties via multifactorial mechanisms. One well understood anti-inflammatory mechanism of 5-ASA is similar to NSAIDs. Both classes of drugs inhibit the synthesis of prostaglandins and leukotrienes. High levels of PGE2 are found in the stools of patients with ulcerative colitis and prostaglandins are known entities in the pathogenesis of IBD.8 Furthermore, PGI2 and Tx have been found to increase in the serum of patients with IBD resulting in stimulation of bowel motility and mucous production. By inhibiting the COX enzymes, 5-ASAs reduce the production of inflammatory prostaglandins in the gastrointestinal tract and this inhibition results in remission of clinical symptoms in patients with IBD. Interestingly, laboratory studies demonstrate that NSAIDs reduce inflammation by the same mechanism, but rather than having a role in treating IBD, they are known to exacerbate ulcerative colitis and Crohn’s disease.8 In addition to inhibition of COX-2, 5-ASAs block the aggregation of leukotrienes, such as LTB4 (which stimulates neutrophil accumulation and degranulation), in the colonic mucosa. Altering neutrophil chemotaxis and macrophage activity reduces cell damage and subsequent inflammation.8
SYSTEMIC ABSORPTION OF 5-ASA
Because 5-ASAs work primarily as topical gastrointestinal agents, orally administered forms of 5-ASA are designed for the release of active drug release at their site(s) of action in the small and large intestine. The amount of ingested 5-ASA, N‐Ac‐5ASA and prodrugs that appear in the urine and feces is the most common method of determining how much of the drug is absorbed. A systematic review of the pharmacokinetic profiles of a variety of 5-ASA preparations included data for urinary 5-ASA excretion for a variety of 5-ASA preparations including sulfasalazine, olsalazine, balasalazide, asacol, and pentasa. Mean 5-ASA excretion ranged from 11% to 53%, indicating that significant absorption of these drugs occurs.9 It may therefore be assumed that there is enough absorption of 5-ASA to produce systemic anti-inflammatory effects. This is borne out by their use in the treatment of rheumatologic diseases.10
NSAIDs AND COVID-19 INFECTION
On March 19, 2020, the WHO sent a message in a Tweet that “Based on currently available information, WHO does not recommend against the use of ibuprofen.”11 The original concern about the risk of NSAIDs use worsening the severity of COVID-19 infections arose in a short review in the Lancet published on March 11.1 Fang noted that coronaviruses that cause human disease such as the severe acute respiratory syndrome coronaviruses (SARS-CoV and SARS-CoV-2) bind to their target cells through angiotensin-converting enzyme 2 (ACE-2). Since ACE-2 expression is increased in patients treated with ACE inhibitors and angiotensin II type-I receptor blockers, and potentially ibuprofen, the authors speculated that these drugs may predispose to more severe manifestations of COVID-19 infection. Figure 3 shows a schematic of the hypothetical mechanism by which NSAIDs could promote COVID-19 infection. On March 14, the French Health Minister Olivier Véran, tweeted that “taking anti-inflammatory drugs [ibuprofen (or) cortisone] could be an aggravating factor for the infection. If you have a fever, take paracetamol.”12
FIGURE 3.
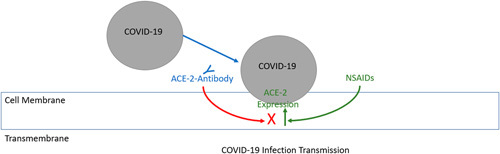
Schematic of hypothetical mechanism and interaction between NSAIDs, ACE-2 antibody expression, and the COVID-19 infection. NSAIDs are shown here to amplify the effect of ACE-2 expression, which has been proposed to facilitate acquiring the infection. To be clear, this is not a well-defined pathway and has not been demonstrated in any known physiological models. ACE-2 indicates angiotensin-converting enzyme 2; NSAIDs, nonsteroidal anti-inflammatory drugs.
His comments may stem from an account of a French infectious disease specialist that described 4 otherwise healthy young patients severely infected with COVID-19 infections. The severity of their disease course was attributed to their use of NSAIDs during the early stage of the infection.13 The argument that ibuprofen might increase the severity of COVID-19 infections was bolstered by a statement by Paul Little, a professor of primary care research at the University of Southampton. On March 16th, Dr Little commented in an article published online by the Science Media Centre that “The finding in 2 randomized trials that advised to use ibuprofen results in more severe illness or complications, helps confirm that the association seen in observational studies is indeed likely to be causal. Advice to use paracetamol is also less likely to result in complications.”14
An analysis of the 2 quoted prospective studies suggests that these studies provide little evidence that NSAIDs increase the severity of upper respiratory infection.
The first, by Little and colleagues involved 889 patients that were randomized to use several forms of advice administered to them in a sealed envelope. Advice consisted of the type of analgesic/antipyretic to take (eg, 1, take paracetamol; 2, ibuprofen; or 3, both paracetamol and ibuprofen), the regimen of analgesia use (eg, use drugs regularly 4 times a day for at least 3 days then ad lib or them as required), and the use or avoidance of steam inhalation. Results of symptom diaries on the second to the fourth day of illness were compared between the groups. The study actually demonstrated no difference in symptom severity between the analgesia groups, although the subgroup of patients with lower respiratory infection had close to 50% reduction of symptoms when advised to use ibuprofen alone (reduction of 0.40; 95% confidence interval −0.78 to −0.01) and those that used ibuprofen and paracetamol (reduction of 0.47; 95% confidence interval −0.84 to −0.10). The conclusion that patients were harmed by taking ibuprofen was based on a secondary outcome analysis of the study data, where consultation for the combination of recurring symptoms or complications was statistically higher in the groups receiving ibuprofen alone or in combination with paracetamol compared with paracetamol alone.15
The other trial by Little and colleagues was designed to assess the use of an interactive website with tailored advice for managing patients with symptoms of upper respiratory infections. Patients were randomized to either use the website or receive standard care. Patients in the website group completed a checklist of symptoms and could request personalized advice or care. If patients wanted to use medications, they were advised to optimize their use of over-the-counter medications which included paracetamol and ibuprofen. The finding of their study was that patients in the website intervention group had increased contact with National Health Services personnel (intervention 37/1574, or 2.4%; vs. control 20/1661, or 1.2%), but deceased direct physician contact (239/1574, or 15.2%; vs. 304/1664, or 18.3%), the apparent goals of the study. There were 2 paradoxical findings of the study. The first of these was that patients in the website intervention group reported a nonstatistically significant increase in illness duration (11.3 vs. 10.7 d, respectively); multivariate estimate 0.60 days longer (−0.15 to 1.36, P=0.118). They also had a nonclinically significant increase in illness severity, defined as more days of illness rated moderately bad or worse illness (0.52 d; 0.06 to 0.97, P=0.026).16
In an attempt to explain why the intervention group appeared to have a longer duration of moderately bad or worse illness compared with the control group, the study authors performed a post hoc analysis of the data. They found that by controlling for whether individuals used ibuprofen from the pages on the website, they found a reduced difference in the length of illness (0.22, −0.51 to 0.95, P=0.551) and the occurrence of moderately bad or worse symptoms (0.36, −0.08 to 0.80, P=0.105). They then concluded that it was the encouragement of the use of ibuprofen was the cause of the reported difference in duration of symptoms.
SUMMARY AND DISCUSSION
There has been much attention on the public stage regarding the possibility that ibuprofen (and potentially other NSAIDs) may worsen the clinical course in patients with COVID-19 infection. Because NSAIDs have structural and mechanistic similarities to 5-ASAs, their effect on patients that are infected with COVID-19 may be questioned.
In this review of the data, we have questioned the correctness of the suggestion that NSAIDs play a role in upper respiratory tract infections. As the beneficial role of anti-inflammatory agents in management of COVID-19 infections evolves, further data on 5-ASAs in the process may emerge. Our review of the circumstances surrounding concern about NSAIDs and COVID-19 leads us to the suggestion that patients taking 5-ASAs may continue their treatment. However, patients on immunomodulatory and immunosuppressive agents for IBD should be included in the group of patients at high risk for complications of COVID-19 infection.
Footnotes
The authors declare that they have nothing to disclose.
REFERENCES
- 1.Fang L. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet, Respiratory Medicine; 2020. Available at: www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30116-8/fulltext. Accessed March 21, 2020.
- 2.Food and Drug Administration (FDA). FDA advises patients on use of non-steroidal anti-inflammatory drugs (NSAIDs) for COVID-19. U.S. Food & Drug Administration; 2020. Available at: www.fda.gov/drugs/drug-safety-and-availability/fda-advises-patients-use-non-steroidal-anti-inflammatory-drugs-nsaids-covid-19 Accessed March 24, 2020.
- 3.The International Organization for the Study of Inflammatory Bowel Disease (IOIBD). IOIBD Update on COVID19 for Patients with Crohn’s Disease and Ulcerative Colitis; 2020. Available at:www.ioibd.org/ioibd-update-on-covid19-for-patients-with-crohns-disease-and-ulcerative-colitis/ Accessed April 27, 2020.
- 4.Mazaleuskaya LL, Theken KN, Gong L, et al. PharmGKB summary: ibuprofen pathways. Pharmacogenet Genomics. 2015;25:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smyth EM, Grosser T, Wang M, et al. Prostanoids in health and disease. J Lipid Res. 2009;50(suppl):S423–S428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurahara K, Matsumoto T, Lida M, et al. Clinical and endoscopic features of nonsteroidal anti-inflammatory drug-induced colonic ulcerations. Am J Gastroenterol. 2001;96:473–480. [DOI] [PubMed] [Google Scholar]
- 7.Klein A, Eliakim R. Non-steroidal anti-inflammatory drugs and inflammatory bowel disease. Pharmaceuticals (Basel, Switzerland). 2010;3:1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small RE, Schraa CC. Chemistry, pharmacology, pharmacokinetics, and clinical applications of mesalamine for the treatment of inflammatory bowel disease. Pharmacotherapy. 1994;14:385–398. [PubMed] [Google Scholar]
- 9.Sandborn WJ, Hanauer SB. Systematic review: the pharmacokinetic profiles of oral mesalazine formulations and mesalazine pro-drugs used in the management of ulcerative colitis. Aliment Pharmacol Ther. 2003;17:29–42. [DOI] [PubMed] [Google Scholar]
- 10.Rains CP, Noble S, Faulds D. Sulfasalazine. A review of its pharmacological properties and therapeutic efficacy in the treatment of rheumatoid arthritis. Drugs. 1995;50:137–156. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO). Q: Could #ibuprofen worsen disease for people with #COVID19? 2020. Available at: https://twitter.com/WHO/status/1240409217997189128. Accessed March 19, 2020.
- 12.Wehrwein P. French Health Minister Stirs Up Questions About Ibuprofen, COVID-19. Managed Healthcare Executive; 2020. Available at: www.managedhealthcareexecutive.com/news/french-health-minister-stirs-questions-about-ibuprofen-covid-19. Accessed March 21, 2020.
- 13.Michael D. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. [DOI] [PubMed] [Google Scholar]
- 14.Science Media Centre. Expert reaction to reports that the French Health Minister recommended use of paracetamol for fever from COVID-19 rather than ibuprofen or cortisone; 2016. Available at: www.sciencemediacentre.org/expert-reaction-to-reports-that-the-french-health-minister-recommended-use-of-paracetamol-for-fever-from-covid-19-rather-than-ibuprofen-or-cortisone/. Accessed March 21, 2020.
- 15.Paul L, Michael M, Joanne K, et al. Ibuprofen, paracetamol, and steam for patients with respiratory tract infections in primary care: pragmatic randomised factorial trial. BMJ. 2013;347:f6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Little P, Stuart B, Andreou P, et al. Primary care randomised controlled trial of a tailored interactive website for the self-management of respiratory infections (Internet Doctor). BMJ Open. 2016;6:e009769. [DOI] [PMC free article] [PubMed] [Google Scholar]


