Abstract
Objectives
This study aimed to characterize corrected QT (QTc) prolongation in a cohort of hospitalized patients with coronavirus disease-2019 (COVID-19) who were treated with hydroxychloroquine and azithromycin (HCQ/AZM).
Background
HCQ/AZM is being widely used to treat COVID-19 despite the known risk of QT interval prolongation and the unknown risk of arrhythmogenesis in this population.
Methods
A retrospective cohort of COVID-19 hospitalized patients treated with HCQ/AZM was reviewed. The QTc interval was calculated before drug administration and for the first 5 days following initiation. The primary endpoint was the magnitude of QTc prolongation, and factors associated with QTc prolongation. Secondary endpoints were incidences of sustained ventricular tachycardia or ventricular fibrillation and all-cause mortality.
Results
Among 415 patients who received concomitant HCQ/AZM, the mean QTc increased from 443 ± 25 ms to a maximum of 473 ± 40 ms (87 [21%] patients had a QTc ≥500 ms). Factors associated with QTc prolongation ≥500 ms were age (p < 0.001), body mass index <30 kg/m2 (p = 0.005), heart failure (p < 0.001), elevated creatinine (p = 0.005), and peak troponin (p < 0.001). The change in QTc was not associated with death over the short period of the study in a population in which mortality was already high (hazard ratio: 0.998; p = 0.607). No primary high-grade ventricular arrhythmias were observed.
Conclusions
An increase in QTc was seen in hospitalized patients with COVID-19 treated with HCQ/AZM. Several clinical factors were associated with greater QTc prolongation. Changes in QTc were not associated with increased risk of death.
Key Words: azithromycin, COVID-19, hydroxychloroquine, QT prolongation, torsades de pointes
Abbreviations and Acronyms: COVID-19, coronavirus disease-2019; ECG, electrocardiogram; HCQ/AZM, hydroxychloroquine and azithromycin; QTc, corrected QT interval
Central Illustration
In December 2019, severe acute respiratory syndrome-coronavirus-2, a novel coronavirus that causes coronavirus disease-2019 (COVID-19), was first detected in Wuhan, China (1). Since then, a global pandemic of COVID-19 has emerged, with high rates of hospital admission and respiratory failure (2). Although large multicenter clinical trials have begun evaluating potential therapies (3), several small studies have proposed that repurposed drugs, such as azithromycin and hydroxychloroquine, may offer benefit in the treatment of this disease (4,5). The U.S. Food and Drug Administration has provided emergency use authorization for health care providers to prescribe hydroxychloroquine to hospitalized patients with COVID-19, and it has been used in conjunction with azithromycin (6). However, both hydroxychloroquine and azithromycin (HCQ/AZM) are known to prolong the electrocardiographic (ECG) QT interval and could increase risk of drug-induced torsade de pointes and sudden cardiac death in these patients (7, 8, 9, 10). Because risks associated with QT prolongation in this patient population are largely unknown, the present study aimed to determine the magnitude of QT prolongation and prevalence of serious arrhythmias among patients hospitalized with COVID-19 who were treated with the combination of HCQ/AZM and to determine if QT prolongation was associated with adverse outcomes.
Methods
Patient population
This was a retrospective cohort study of consecutive patients enrolled in the Beaumont Health COVID-19 database, which was granted a waiver of consent by the institutional review board. All patients 18 years of age and older with proven COVID-19 admitted to Beaumont Hospital (Royal Oak and Beaumont Hospital, Troy, Michigan) between March 13 and April 6, 2020 were screened for study entry. Of this population, those patients who were administered concomitant HCQ/AZM underwent at least 1 day of corrected QT interval (QTc) assessment following drug initiation and who had an interpretable baseline ECG were included in the cohort. Demographics, medical history, relevant laboratory values, and clinical outcomes were recorded (Supplemental Appendix). Administration of drugs with known risk or probable risk of inducing torsade de pointes were documented (8).
Pharmacodynamics subgroup analysis
The pharmacodynamics of QTc prolongation after initiation of HCQ/AZM was assessed over the 5 days of drug administration in a subgroup of patients. Patients with QTc measurements at baseline, days 1, 2 and/or 3, and 4 and/or 5 were included in this analysis. Patients with incomplete data due to missing or technically inadequate tracings were excluded from this analysis.
Drug administration
Patients with proven or suspected COVID-19 infection were prescribed HCQ/AZM at or soon after hospital admission at the discretion of the admitting physician. The standard dose regimens prescribed were hydroxychloroquine 400 mg twice daily for 2 doses, then 200 mg twice daily for 4 days and azithromycin 500 mg once followed by 250 mg/day for 4 days. Patient care decisions were made by the hospital clinicians, and no attempt was made by study personnel to direct their care. Decisions regarding early termination of HCQ/AZM (in some cases due to excessive prolongation of the QTc interval) were at the sole discretion of the physicians supervising care.
Electrocardiographic monitoring
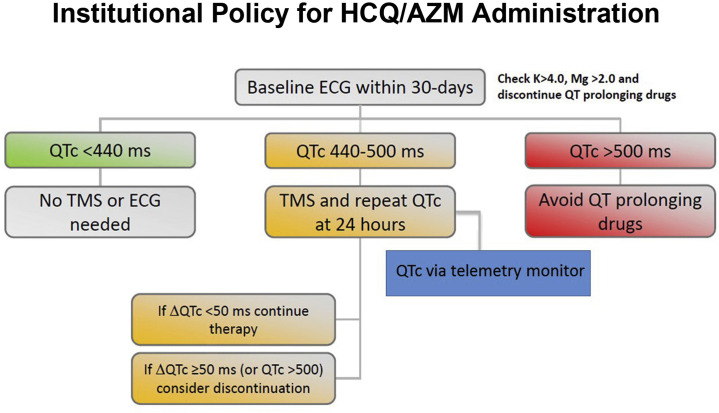
An institutional policy was created during the COVID-19 pandemic to balance safe monitoring for QT interval prolongation in patients prescribed HCQ/AZM while also limiting ECG technician exposure to infected patients and preserving personal protective equipment (Figure 1 ). Per institutional policy, a baseline QTc was measured from a 12-lead ECG before initiation of HCQ/AZM. If the patient had an ECG on file within 30 days of drug initiation, that QTc interval value could be used as the baseline measure. Due to limits on telemetry availability during the COVID-19 pandemic, institutional guidelines recommended no further QTc monitoring for patients with baseline QTc intervals of <440 ms unless there was another indication for telemetry monitoring present. The institutional policy recommended that patients with QTc intervals of ≥440 ms were to be placed on telemetry for daily QTc and arrhythmia monitoring during drug initiation. Multiple repeat 12-lead ECGs to monitor the QTc interval were discouraged. It was at the discretion of the attending physician whether to initiate or discontinue hydroxychloroquine, azithromycin, and/or telemetry.
Figure 1.
Institutional Policy for QT Interval Monitoring
Institutional policy for QT interval monitoring during inpatient administration of hydroxychloroquine and azithromycin (HCQ/AZM) in patients with COVID-19. ECG = electrocardiography; K = potassium; Mg = magnesium; QTc = corrected QT interval; TMS = telemetry monitoring system.
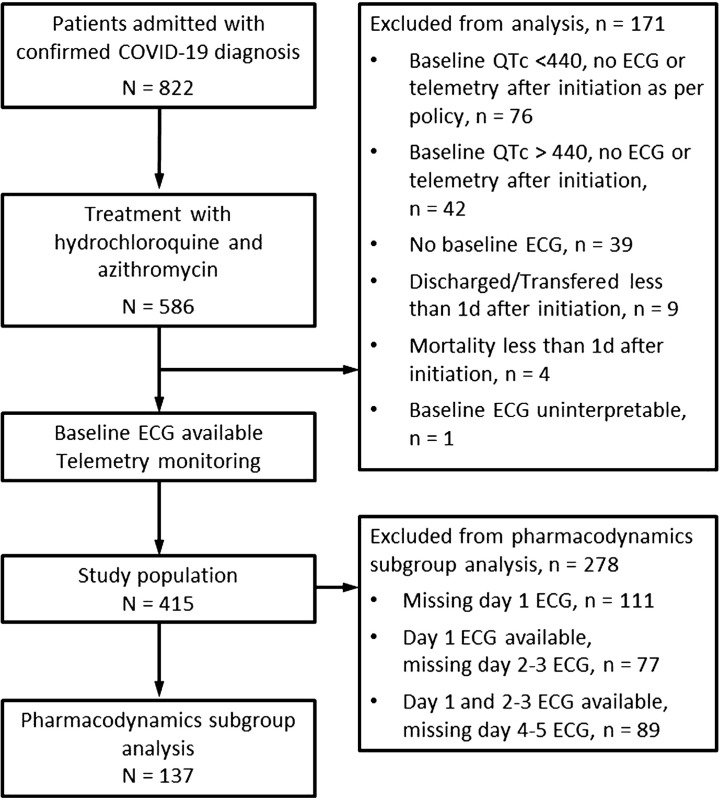
Available lead II telemetry monitor strips and ECGs were reviewed for 5 consecutive days while the patient was on combination drug therapy. The day 1 QTc measured on telemetry was defined as the first day after initiation of both HCQ/AZM. The QTc data were only recorded when patients were on combination therapy. Those without an interpretable baseline ECG or available telemetry and/or ECG monitoring for at least 1 day after drug administration were excluded from analysis (Figure 2 ). The ECGs and telemetry recordings were analyzed off-line. The QT intervals were measured manually using the tangent method and dividing by the square root of the average RR interval (Bazett’s formula) to derive the QTc (11). For patients with intraventricular conduction delays (paced rhythms or bundle branch block), a modified QTc was calculated using the following formula: modified QTc = (QT duration − [QRS duration − 120 ms]) /√RR (12).
Figure 2.
Screening, Inclusion, Exclusion, and QTc Monitoring
Screening, inclusion, exclusion, and QTc monitoring for patients enrolled to assess the effects of HCQ/AZM on QTc prolongation. Abbreviations as in Figure 1.
Study endpoints
The primary study endpoint was to quantitate the magnitude of QTc prolongation observed in a hospitalized cohort of patients with COVID-19 treated with HCQ/AZM and to identify risk factors in this patient population for significant QTc prolongation. Secondary endpoints were the incidences of sustained ventricular tachycardia or ventricular fibrillation, and the relationship of QTc prolongation to in-hospital sustained ventricular tachyarrhythmias and mortality during the study period. Cause of death was adjudicated by review of the resuscitation records from all patients with attempted resuscitation, and the reviewers of these data were blinded to the QTc data. Patients who died without monitoring because of transfer to “comfort care only” status were defined as non-arrhythmic deaths. High-grade ventricular arrhythmias were defined as ventricular tachycardia or ventricular fibrillation >30 s in duration or that required urgent medical intervention. Secondary ventricular tachyarrhythmias observed during the course of cardiac resuscitation subsequent to a bradycardic or pulseless electrical activity arrest were not designated as high-grade ventricular arrhythmic events.
Data and statistical analysis
All authors participated in multiple aspects of the trial, including data collection (T.F.O., C.J.B., A.R., A.M.T., R.G., J.S.), data compilation and analysis (all), statistical analysis (A.D.), and manuscript preparation (T.F.O., C.J.B., A.E.A., B.D.W., A.R., A.M.T., A.D., D.E.H.). Repeated measures of 10% of the QTc data were performed, and the average intraclass correlation coefficient was 0.946. Descriptive analyses were performed to assess differences between groups. Continuous variables were expressed as mean ± SD. Categorical variables were expressed as numbers and percentages. The differences between the groups were evaluated using the 2-sample Student's t-test for continuous variables and Fisher’s exact test for categorical variables. A multivariate logistic regression analysis was conducted, evaluating the impact of key demographic and clinical factors associated with QTc ≥500 ms. A second multivariate analysis was conducted to evaluate the impact of key demographic and clinical factors associated with QTc prolongation, as well as changes in the QTc interval on mortality. These analyses were performed using the Cox proportional hazards model. QTc changes from baseline to days 1 through 5 were included as a time-varying covariate in the second analysis. Hazard ratios, 95% confidence intervals, and p values were computed from this model to characterize the influence of each factor on mortality. A 2-sided p value <0.05 was considered statistically significant. No formal adjustments for multiplicity were applied. The analyses were performed using R software (version 3.6.1, R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 586 patients were admitted during the study period with a proven diagnosis of COVID-19 and treated with combination therapy of HCQ/AZM. Of those patients, 415 met the inclusion criteria, and 171 were excluded because of incomplete or technically limiting ECGs or clinical data (Figure 2). The demographics of the included and excluded patients are listed in Table 1 . The excluded group was younger, had shorter baseline QRS duration, and had shorter QTc intervals (attributable to the institutional policy that ECG telemetry monitoring was not required in patients with baseline QTc intervals <440 ms).
Table 1.
Patient Characteristics of the Screened Population, and Comparison of Patients Included Versus Patients Excluded From the Analysis
| All Patients (N = 586) | Included (n = 415) | Excluded (n = 171) | p Value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (yrs) | 64 ± 15 | 65 ± 15 | 62 ± 15 | 0.022 |
| Female | 261 (45) | 178 (43) | 83 (49) | 0.235 |
| Caucasian | 205 (35) | 147 (35) | 58 (34) | 0.775 |
| African American | 305 (52) | 211 (51) | 94 (55) | 0.413 |
| BMI (kg/m2) | 32 ± 9 | 32 ± 9 | 32 ± 8 | 0.631 |
| Comorbidities | ||||
| Hypertension | 303 (52) | 219 (53) | 84 (49) | 0.467 |
| Coronary artery disease | 64 (11) | 49 (12) | 15 (9) | 0.311 |
| Heart failure | 37 (6) | 30 (7) | 7 (4) | 0.192 |
| Atrial fibrillation | 40 (7) | 31 (8) | 9 (5) | 0.374 |
| Chronic kidney disease | 39 (7) | 27 (7) | 12 (7) | 0.856 |
| Diabetes mellitus | 176 (30) | 132 (32) | 44 (26) | 0.165 |
| Chronic lung disease | 43 (7) | 33 (8) | 10 (6) | 0.486 |
| Cigarette smoker | 14 (2) | 9 (2) | 5 (3) | 0.563 |
| Cancer | 80 (14) | 58 (14) | 22 (13) | 0.792 |
| Laboratory values | ||||
| Creatinine (mg/dl)∗ | 1.8 ± 2.1 | 1.8 ± 2.2 | 1.6 ± 1.8 | 0.294 |
| Aspartate aminotransferase (U/l)∗ | 66 ± 165 | 69 ± 194 | 60 ± 45 | 0.417 |
| Alanine aminotransferase (U/l)∗ | 48 ± 163 | 51 ± 193 | 44 ± 38 | 0.544 |
| Lactic acid (mmol/l)∗ | 1.8 ± 1.9 | 1.8 ± 1.9 | 1.7 ± 2.0 | 0.565 |
| Potassium (mmol/l)† | — | 4.2 ± 0.6 | N/A | — |
| Magnesium (mg/dl) | — | 2.0 ± 0.4 | N/A | — |
| Maximum troponin I (ng/ml) | — | 0.4 ± 2.3 | N/A | — |
| Baseline electrocardiographic parameters | ||||
| QRS duration (ms) | 90 ± 18 | 91 ± 20 | 87 ± 14 | 0.018 |
| QTc (ms) | 440 ± 26 | 443 ± 25 | 436 ± 27 | 0.009 |
| Heart rate | 91 ± 17 | 91 ± 18 | 90 ± 16 | 0.729 |
| Baseline rhythm | ||||
| Normal sinus | 505 (86) | 379 (91) | 126 (95) | 0.136 |
| Atrial fibrillation | 34 (6) | 28 (7) | 6 (5) | 0.416 |
| Paced | 5 (1) | 5 (1) | 0 (0) | — |
| Junctional | 3 (0) | 3 (1) | 0 (0) | — |
Values are mean ± SD or n (%).
SI units for creatinine (μmol/l) = mg/dl × 88.4.
SI units for magnesium (μmol/l) = mg/dl × 0.4114.
SI units for troponin I (μg/l) = ng/ml × 1.
BMI = body mass index.
Admission laboratory values.
Laboratory values at the time of initiation of hydroxychloroquine and azithromycin (HCQ/AZM)
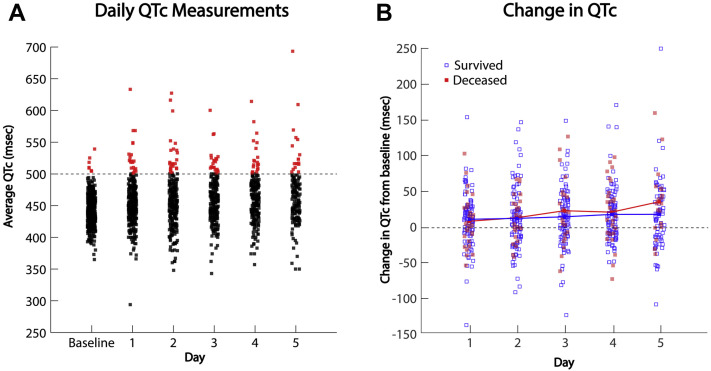
In the included study population, the baseline QTc interval was 443 ± 25 ms (range 365 to 539 ms). The QTc progressively increased coincidently with administration of HCQ/AZM (Figure 3A, Central Illustration ). The maximum QTc observed over the course of monitoring for all patients who received the drugs was 473 ± 40 ms (range 372 to 693 ms), the magnitude of QTc prolongation compared with the baseline tracing was 30 ± 39 ms (range 69 to 249 ms). The QTc was ≥500 ms after drug administration in 87 patients (21%). Notably, during their hospitalization, 255 patients (61%) received additional medications with known risk of torsade de pointes (Supplemental Table 1). Factors associated with QTc prolongation ≥500 ms are presented in Table 2 . Changes in the QTc over the 5-day prescription duration were assessed in a subset of 137 patients with QTc measurements at baseline before medication administration and on days 1 through 5. In this subgroup, the average time to maximum QTc was 2.9 ± 1.4 days. The change in the QTc from baseline increased progressively with time (Figure 3B).
Figure 3.
QTc Measurements and Intervals
(A) QTc measurements at baseline and days 1 through 5 of HCQ/AZM therapy in the study population of 415 patients. The data points representing QTc intervals ≥500 ms are highlighted red. (B) Change in QTc interval for days 1 through 5 of HCQ/AZM therapy compared with the baseline measurements in the subgroup of 137 patients with complete data from baseline, and days 1, 2 and/or 3, and 4 and/or 5. Patients who survived to hospital discharge or the termination of the study are depicted by blue squares, and patients who died in the hospital are depicted by red squares. The mean changes in QTc from baseline are depicted by the red and the blue lines, respectively. Compared with baseline, the mean QTc intervals increased on all days: p = 0.005 for days 1 and 2, and p < 0.001 for days 3 to 5. Abbreviations as in Figure 1.
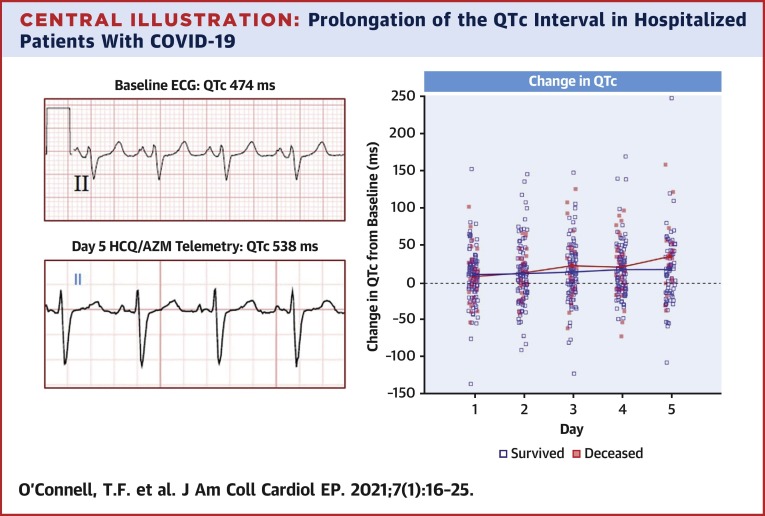
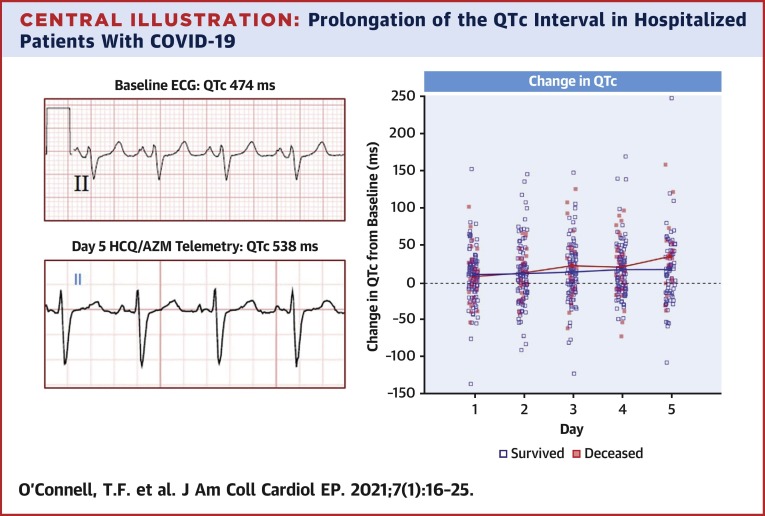
Central Illustration.
Prolongation of the QTc Interval in Hospitalized Patients With COVID-19
Examples of lead II ECG recordings from a patient admitted with severe COVID-19 infection at baseline (top left) and after 5 days of treatment with HCQ/AZM (bottom left) demonstrating significant prolongation of the QTc interval. Changes in the QTc interval after administration of HCQ/AZM compared to baseline values are shown (right panel). Patients that did not survive hospitalization are designated by solid red markers. COVID-19 = coronavirus disease-2019; ECG = electrocardiography; HCQ/AZM = hydroxychloroquine and azithromycin; QTc = corrected QT interval.
Table 2.
Clinical Factors Independently Associated With QTc Prolongation ≥500 ms
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age >65 yrs | 2.82 | 1.67–4.75 | <0.001 | 3.00 | 1.62–5.54 | <0.001 |
| History of congestive heart failure | 3.74 | 1.75–8.01 | 0.001 | 4.65 | 2.01–10.74 | <0.001 |
| Body mass index ≥30 kg/m2 | 0.48 | 0.30–0.78 | 0.003 | 0.45 | 0.26–0.78 | 0.005 |
| Peak troponin >0.04 mg/ml | 3.10 | 1.85–5.19 | <0.001 | 3.89 | 2.22–6.83 | <0.001 |
| Admission creatinine ≥1.5 mg/dl | 2.42 | 1.45–4.06 | <0.001 | 2.22 | 1.28–3.84 | 0.005 |
| Concomitant use of drugs with known risk of torsade de pointes | 1.73 | 1.04–2.90 | 0.036 | 1.51 | 0.86–2.66 | 0.154 |
SI units for creatinine (μmol/l) = mg/dl × 88.4.
SI units for troponin I (μg/l) = ng/ml × 1.
CI = confidence interval; OR = odds ratio; other abbreviation as in Table 1.
Throughout the study period, no primary high-grade ventricular arrhythmias were observed in any patient in the included or excluded groups. Of the entire treatment population, there were a total of 111 deaths in-hospital. Twenty-six of 171 (15%) excluded patients died, and 85 of 415 (21%) in the included population died. Of those 85 patients, 32 patients had pulseless electrical activity or bradycardia at the initiation of the resuscitation, and 53 patients died off monitor because “do not resuscitate” orders were placed. The change in QTc interval from baseline was greater in patients who died during hospitalization compared with those who survived to hospital discharge or study termination (Figure 3B). Data from the proportional hazards model for time-varying QTc changes with concurrent mortality for the subset of 137 patients with complete QTc data and the overall population of 415 patients are presented in Table 3 . Despite the association of QTc prolongation with mortality, the only independent predictor of mortality was age.
Table 3.
Results From Proportional Hazards Cox Analysis of Association Between Time-Dependent Changes in QTc Following Concomitant HCQ/AZM Administration, and Con-Current Mortality
| HR | 95% CI | p Value (2-sided) | |
|---|---|---|---|
| Patients with complete QTc data from baseline, and days 1, 2 or 3, 4 or 5 (n = 137) | |||
| Changes in QTc (time varying) | 1.004 | 0.996−1.012 | 0.367 |
| Baseline QTc (from ECG) | 1.014 | 0.998−1.031 | 0.094 |
| Days from admission to combo administration | 1.068 | 0.947−1.204 | 0.283 |
| Age at admission | 1.087 | 1.045−1.131 | <0.0001 |
| Male | 1.295 | 0.613−2.736 | 0.499 |
| BMI | 1.065 | 1.003−1.130 | 0.039 |
| Race (White or Caucasian) | 0.617 | 0.270−1.410 | 0.252 |
| All patients (N = 415) | |||
| Changes in QTc (time varying) | 0.998 | 0.992−1.005 | 0.607 |
| Baseline QTc (from ECG) | 1.004 | 0.994−1.014 | 0.457 |
| Days from admission to combo administration | 1.081 | 0.996−1.173 | 0.064 |
| Age at admission | 1.064 | 1.042−1.086 | <0.0001 |
| Male | 0.818 | 0.526−1.271 | 0.371 |
| BMI | 1.017 | 0.993−1.040 | 0.164 |
| Race (White or Caucasian) | 0.962 | 0.606−1.525 | 0.868 |
Discussion
In response to the poor outcomes observed in patients with COVID-19 infections and lack of proven curative therapies, clinicians have turned to unproven remedies like HCQ/AZM, although this drug combination is known to prolong the QT interval and has an unknown risk of proarrhythmia in this disease. The present study was performed to quantitate the magnitude of QT prolongation in a cohort of hospitalized patients with COVID-19 treated with HCQ/AZM and to identify QT-prolonging risk factors in this population. The QTc was found to increase over the 5-day HCQ/AZM course, with the average change in baseline to a maximum QTc that measured 30 ms. This was the first study in a COVID population to identify that older age, lower body mass index, higher admission creatinine, higher peak troponin, and a history of congestive heart failure independently predicted potentially hazardous QTc prolongation ≥500 ms. However, despite the significant increases in QTc intervals in these patients, no high-grade ventricular arrhythmias were observed, and changes in QTc were not associated with increased likelihood of dying. This suggested that the actual risk of torsade de pointes in this setting was low or that the prescribing physicians were appropriately adjusting therapies in patients who had excess QT prolongation was observed.
Hydroxychloroquine is a widely prescribed anti-malarial and anti-rheumatic medication (13,14), and azithromycin is a commonly prescribed antibiotic (15). They are both currently being prescribed, often in combination, as an off-label treatment for COVID-19 (6). Both medications increase the QTc interval by blocking the KCNH2-encoded hERG potassium channel (16, 17, 18). Before the COVID-19 pandemic, most of the clinical evidence of hydroxychloroquine’s QT prolonging properties was limited to case reports (19,20). Azithromycin’s QT-prolonging properties were better studied in a large case-control study that demonstrated that hospitalized patients treated with azithromycin were more likely to have severe QT prolongation (21). The present study confirmed evidence of the QT-prolonging properties of HCQ/AZM and extended these results to a COVID-19 population. The QTc progressively and significantly increased during HCQ/AZM administration, similar to other studies in COVID-19 populations (22, 23, 24, 25). Confirmation of QTc prolongation in this population will better inform physicians of this important side effect of HCQ/AZM that will necessitate QTc assessment before drug initiation and QTc monitoring during treatment for many patients. Identifying QTc prolonging risk factors will be important before prescribing HCQ/AZM and when deciding on a monitoring strategy.
Genetics, medications, underlying medical conditions, and other metabolic factors predispose patients to QT prolongation, which can lead to torsade de pointes. One of 2,000 people are genetically predisposed to have a long QT interval due to congenital long QT syndrome (10). Hospitalized patients are particularly susceptible to drug-induced QT prolongation due to concomitant proarrhythmic risk factors, and those on non-antiarrhythmic QT prolonging medications have twice the risk of an in-hospital cardiac arrest. This risk further increases with prescription of multiple QTc-prolonging medications (26,27). Although no QTc value can predict the onset of torsade de pointes with certainty, data from the congenital long QT syndrome and drug-induced torsade de pointes populations have shown patients with QTc >500 ms are at increased risk (26). The U.S. Food and Drug Administration has discouraged use of HCQ/AZM outside of clinical trials or hospitalized settings with monitoring (28), and cardiovascular leaders have issued guidance to clinicians to consider withholding and withdrawing HCQ/AZM for a QTc >500 ms (29).
More than 20% of the patients in our cohort had a QTc ≥500 ms after HCQ/AZM administration. Cohort characteristics that predicted QTc prolongation were similar to those that predicted significant QT interval prolongation in a validated risk score model in hospitalized patients (30). Elevated troponin was independently associated with QTc prolongation ≥500 ms, which did not rule out ischemia as a mechanism for QTc prolongation in this cohort (31). Although other known precipitants of QTc prolongation such as hypokalemia and hypomagnesemia were not significant in our cohort, this was likely attributable to an institutional policy that recommended careful electrolyte monitoring in these patients. Despite careful monitoring of patients on HCQ/AZM, >60% of the cohort was administered at least 1 additional medication during hospitalization that increased the risk of torsade de pointes. This speaks to the medical complexity of this patient population, but also suggests inadequate awareness of the QT-prolonging effects of many common medications.
Throughout the study period, no primary high-grade ventricular arrhythmias were observed. More than 20% of the study population died, which highlighted the high mortality of hospitalized patients with COVID-19 who experienced severe respiratory illness (2). Although positive changes in QTc from baseline were associated with increased morality in univariate analysis, this was not observed in multivariate analysis. QTc prolongation might be a marker of sicker patients, as evidenced by the comorbid conditions and laboratory abnormalities that predicted QTc prolongation ≥500 ms. Because of the magnitude of QTc prolongation on HCQ/AZM, larger studies are warranted to investigate the prevalence of arrhythmias and mortality if this remains a therapy for COVID-19. Although the world waits for the results of larger trials (3), proposed systems to safely monitor the QTc (29) will remain necessary and cannot be ruled out as an explanation for the results of this study.
Study limitations
Although a specific dosing schedule was recommended for all patients, the decisions when and how to prescribe HCQ/AZM were deferred to the prescribing physicians. Due to the retrospective nature of the study, it was not possible to definitively determine whether HCQ/AZM was discontinued by clinicians in response to QTc assessments. Retrospective collection of some data and technical limitations prevented acquisition of QTc intervals from all patients on all days, so the associations between magnitude of QT prolongation and clinical and outcome data were based upon incomplete information. The initial QTc interval was obtained from a 12-lead ECG, but subsequent QTc measurements were almost exclusively obtained from a telemetry monitoring system. This policy was instituted by the health care system in an attempt to balance monitoring for QTc interval prolongation while also protecting staff and limiting use of personal protective equipment. Although the death rate was high, the rate of high-grade ventricular arrhythmias was low. The rate of appropriate implantable cardioverter-defibrillator therapy or death in patients with congenital long QT syndrome and QTc intervals >500 ms was reported as 35% over 5 years (32), but the duration of follow-up in the present study was brief, and it was not adequately powered to assess the association of drug-induced QT prolongation and mortality. In addition, the results of QT monitoring certainly affected clinical decisions, thus confounding outcome data. Also, it was possible that the COVID-19 infection could directly prolong the QTc independent of drug effects. Unfortunately, the high use of HCQ/AZM in these hospitals precluded analysis of a drug-free control arm. Finally, the cohort studied consisted of of hospitalized patients, and the results might not apply to non-hospitalized patients or prophylactic therapy regimens. Because decisions when and how to prescribe HCQ/AZM were deferred to the prescribing physicians, it was possible that the 586 of 822 patients prescribed HCQ/AZM were the sickest patients.
Conclusions
Hospitalized patients with COVID-19 treated with HCQ/AZM had a significant and progressive increase in QTc during combination drug therapy. Several risk factors identified patients at risk of severe QTc prolongation. Despite this finding, the risk of serious arrhythmias during this brief observation period was low.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: HCQ/AZM are both known to prolong the QT interval and are associated with a risk of torsade de pointes. Addition of these drugs to other medications with known QT-prolonging effects can further increase patient risk. Patients with COVID-19 infection are susceptible to cardiac injury and significant mortality. The value of HCQ/AZN in treating this infection is unknown, but their effects on QT interval prolongation are significant. Although risk of serious arrhythmia in these patients is low, it may be increased with the use of these drugs.
TRANSLATIONAL OUTLOOK: Prospective randomized trials of HCQ/AZM treatment in patients with COVID-19 infections are needed to assess possible benefit and compare those benefits with the small but important risk of excess QT interval prolongation and drug-induced torsade de pointes.
Author Disclosures
Dr. Haines has received honoraria from Biosense Webster, Farapulse and Sagentia; and has been a consultant for Affera, Boston Scientific, Integer, Medtronic, Philips Healthcare, and Zoll. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Shirley Qu and Connie Gaines for their hard work and expertise help in assembling and organizing the database.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section and a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kupferschmidt K., Cohen J. Race to find COVID-19 treatments accelerates. Science. 2020;367:1412–1413. doi: 10.1126/science.367.6485.1412. [DOI] [PubMed] [Google Scholar]
- 4.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madrid P.B., Panchal R.G., Warren T.K. Evaluation of Ebola virus inhibitors for drug repurposing. ACS Infect Dis. 2015;1:317–326. doi: 10.1021/acsinfecdis.5b00030. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg E.S., Dufort E.M., Udo T. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020 doi: 10.1001/jama.2020.8630. 323:2493 41(24):e906-e907 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakkireddy D.R., Chung M.K., Gopinathannair R. Guidance for rebooting electrophysiology through the coronavirus (COVID-19) pandemic from the Heart Rhythm Society and the American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology: endorsed by the American College of Cardiology. J Am Coll Cardiol EP. 2020;6:1053–1066. doi: 10.1016/j.jacep.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woosley R.L., Heise C.W., Gallo T., Tate J., Woosley D., Romero K.A. QT drugs List. AZCERT, Inc. https://crediblemeds.org/new-drug-list Available at:
- 9.Ray W.A., Murray K.T., Hall K., Arbogast P.G., Stein C.M. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020;95:1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panicker G.K., Karnad D.R., Natekar M., Kothari S., Narula D., Lokhandwala Y. Intra- and interreader variability in QT interval measurement by tangent and threshold methods in a central electrocardiogram laboratory. J Electrocardiol. 2009;42:348–352. doi: 10.1016/j.jelectrocard.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Patel P., Borovskiy Y., Deo R. QTTC, a novel method for correcting QT interval for QRS duration predicts all-cause mortality. J Am Coll Cardiol. 2015;65(10 Suppl):A336. [Google Scholar]
- 13.Fanouriakis A., Kostopoulou M., Alunno A. 2019 update of the EULAR recommendations for the management of systemic lupus erythematous. Ann Rheum Dis. 2019;78:736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 14.Shippey E.A., Wagler V.D., Collamer A.N. Hydroxychloroquine: an old drug with new relevance. Cleve Clin J Med. 2018;85:459–467. doi: 10.3949/ccjm.85a.17034. [DOI] [PubMed] [Google Scholar]
- 15.Metlay J.P., Waterer G.W., Long A.C. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cubeddu L.X. Drug-induced inhibition and trafficking disruption of ion channels: Pathogenesis of QT abnormalities and drug-induced fatal arrhythmias. Curr Cardiol Rev. 2016;12:141–154. doi: 10.2174/1573403X12666160301120217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capel R.A., Herring N., Kalla M. Hydroxychloroquine reduces heart rate by modulating the hyperpolarization-activated current If: novel electrophysiologic insights and therapeutic potential. Heart Rhythm. 2015;12:2186–2194. doi: 10.1016/j.hrthm.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M., Xie M., Li S. Electrophysiologic studies on the risks and potential mechanism underlying the proarrhythmic nature of azithromycin. Cardiovasc Toxicol. 2017;17:434–440. doi: 10.1007/s12012-017-9401-7. [DOI] [PubMed] [Google Scholar]
- 19.Chen C.Y., Wang F.L., Lin C.C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrythmia. Clin Toxicol (Phila) 2006;44:173–175. doi: 10.1080/15563650500514558. [DOI] [PubMed] [Google Scholar]
- 20.Morgan N.D., Patel S.V., Dvorkina O. Suspected hydroxychloroquine-associated QT-interval prolongation in a patient with systemic lupus erythematous. J Clin Rheumatol. 2013;19:286–288. doi: 10.1097/RHU.0b013e31829d5e50. [DOI] [PubMed] [Google Scholar]
- 21.Choi Y., Lim H.S., Chung D., Choi J.G., Yoon D. Risk evaluation of azithromycin-induced QT prolongation in real-world practice. Biomed Res Int. 2018:1574806. doi: 10.1155/2018/1574806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chorin E., Dai M., Shulman E. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat Med. 2020;26:808–809. doi: 10.1038/s41591-020-0888-2. [DOI] [PubMed] [Google Scholar]
- 23.Saleh M., Gabriels J., Chang D. The effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol. 2020 Apr 29 doi: 10.1161/CIRCEP.120.008662. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bessière F., Roccia H., Delinière A. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020;(May 1) doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercuro N.J., Yen C.F., Shim D.J. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;(May 1) doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drew B.J., Ackerman M.J., Funk M. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121:1047–1060. doi: 10.1161/CIRCULATIONAHA.109.192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Bruin M.L., Langendijk P.N., Koopman R.P., Wilde A.A., Leufkens H.G., Hoes A.W. In-hospital cardiac arrest is associated with use of non-antiarrhythmic QTc prolonging drugs. Br J Clin Pharmacol. 2007;63:216–223. doi: 10.1111/j.1365-2125.2006.02722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration Hydroxychloroquine or Chloroquine for COVID-19: Drug Safety Communication - FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. https://www.fda.gov/safety/medical-product-safety-information/hydroxychloroquine-or-chloroquine-covid-19-drug-safety-communication-fda-cautions-against-use Available at:
- 29.Roden D.M., Harrington R.A., Poppas A., Russo A.M. Consideration for drug interactions on QTc in exploratory COVID-19 (Coronavirus Disease 2019) treatment. Circulation. 2020;141:e906–e907. doi: 10.1161/CIRCULATIONAHA.120.047521. [DOI] [PubMed] [Google Scholar]
- 30.Tisdale J.E., Jayes H.A., Kingery J.R. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–487. doi: 10.1161/CIRCOUTCOMES.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenigsberg D.N., Khanal S., Kowalski M., Krishnan S.C. Prolongation of the QTc interval is seen uniformly during early transmural ischemia. J Am Coll Cardiol. 2007;49:1299–1305. doi: 10.1016/j.jacc.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz P.J., Spazzolini C., Priori S.G. Who are the long-QT syndrome patients who receive an implantable cardioverter-defibrillator and what happens to them?: Data from the European Long-QT Syndrome Implantable Cardioverter-Defibrillator (LQTS ICD) Registry. Circulation. 2010;122:1272–1282. doi: 10.1161/CIRCULATIONAHA.110.950147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.