Abstract
PURPOSE
To compare cisplatin plus fluorouracil (FU) versus carboplatin plus paclitaxel in chemotherapy-naïve advanced anal cancer to establish the optimal regimen.
PATIENTS AND METHODS
Patients who had not received systemic therapy for advanced anal cancer were randomly assigned 1:1 to intravenous cisplatin 60 mg/m2 (day 1) plus FU 1,000 mg/m2 (days 1-4) every 21 days or carboplatin (area under the curve, 5; day 1) plus paclitaxel 80 mg/m2 (days 1, 8, and 15) every 28 days for 24 weeks, until disease progression, intolerable toxicity, or withdrawal of consent. Primary end point was objective response rate (ORR). Primary and secondary end points were assessed in a hierarchic model to compare the regimens and pick the winner.
RESULTS
We conducted an international multicenter randomized phase II study in 60 centers between December 2013 and November 2017. Median follow-up was 28.6 months. A total of 91 patients were randomly assigned: 46 to cisplatin plus FU and 45 to carboplatin plus paclitaxel. ORR was 57% (95% CI, 39.4% to 73.7%) for cisplatin plus FU versus 59% (95% CI, 42.1% to 74.4%) for carboplatin plus paclitaxel. More serious adverse events were noted in the cisplatin plus FU arm (62%) compared with the carboplatin plus paclitaxel arm (36%; P = .016). Median progression-free survival was 5.7 months (95% CI, 3.3 to 9.0 months) for cisplatin plus FU compared with 8.1 months (95% CI, 6.6 to 8.8 months) for carboplatin plus paclitaxel. Median overall survival was 12.3 months for cisplatin plus FU (95% CI, 9.2 to 17.7 months) compared with 20 months (95% CI, 12.7 months to not reached) for carboplatin plus paclitaxel (hazard ratio, 2.00; 95% CI, 1.15 to 3.47; P = .014).
CONCLUSION
This is the first international randomized trial to our knowledge conducted in chemotherapy-naïve advanced anal cancer. Although there was no difference in ORR, the association with clinically relevant reduced toxicity and a trend toward longer survival suggest that carboplatin plus paclitaxel should be considered as a new standard of care.
INTRODUCTION
Anal cancer is rare and accounts for < 3% of all GI malignancies.1 However, incidence has risen over the past decades. The number of new cases in the United States was reported as 1.9 per 100,000 men and women between 2012 and 2016.2,3 A majority of patients present with localized or locally advanced disease, where radical chemoradiotherapy (with concurrent mitomycin C with fluorouracil [FU] or capecitabine) is the standard of care administered with curative intent.4-9 Local failure rates after chemoradiotherapy approach 30%, and for some, salvage surgery is feasible.5,7,10
Metastatic dissemination occurs in 10% of patients after chemoradiotherapy, whereas < 10% present with metastatic disease de novo.4,5,7 For those patients with inoperable or metastatic disease, prognosis remains poor, with relative 5-year survival rates of approximately 30%.4 Palliative chemotherapy is routinely offered to these patients.11 To date, no randomized clinical trial has been conducted in this setting to inform the optimal chemotherapy regimen. International guidelines suggest a platinum agent combined with fluoropyrimidine for the first-line treatment of advanced anal cancer12,13 on the basis of limited evidence from single-arm phase II studies; response rates of between 34% and 75% and median overall survival (OS) ranging from 12 to 34 months have been reported in retrospective studies and single-arm phase II trials.14-16 Although these data are limited, this regimen has been adopted internationally. Paclitaxel was first reported as treatment for advanced anal cancer in 2011 and was recently combined with carboplatin in a retrospective series.16-18 Response rates of 69% and median survival of 12 months have been reported.17 This observed efficacy has led to some clinicians employing carboplatin and paclitaxel as first-line treatment for advanced anal cancer.
The International Rare Cancers Initiative Anal Cancer Working Group recognized the evidence gap in clinical decision making for patients with advanced anal cancer as an area of unmet clinical need, prompting this global clinical trial comparing cisplatin plus FU versus carboplatin plus paclitaxel to set a standard of care and establish the cytotoxic backbone for future clinical trials.
PATIENTS AND METHODS
Study Design and Participants
InterAAct (ClinicalTrials.gov identifier: NCT02051868) was an international open-label multicenter randomized phase II trial that recruited patients from 60 centers from the United Kingdom, Australia, Norway, and the United States. Eligible patients were age ≥ 18 years with histologic confirmation of epidermoid anal squamous carcinoma, locally recurrent inoperable or metastatic disease, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2, and measurable disease according to RECIST (version 1.1). HIV-positive patients were included provided they were receiving highly active antiretroviral therapy and CD4 count was ≥ 200/μL or CD4 count was ≤ 200/μL (ie, undetectable plasma HIV-positive viral load). Previous definitive chemoradiotherapy was permitted provided progression occurred ≥ 6 months, but no previous systemic treatment for advanced disease was permitted. Patients were required to have adequate organ function and adequate cardiac and respiratory function. Exclusion criteria included resectable recurrent localized disease, brain metastases, and major surgery ≤ 28 days, or palliative radiotherapy completed ≤ 7 days.
The trial was approved by the National Research Ethics Committee London Riverside (13/LO/1463), the Medicines and Healthcare Products Regulatory Agency, and the institutional review boards of all centers. In the United States, the study was approved by the National Cancer Institute Cancer Therapy Evaluation Program via ECOG (EA2133).
Random Assignment
Patients were randomly allocated at a 1:1 ratio to receive either cisplatin plus FU or carboplatin plus paclitaxel. Treatment was assigned centrally by computer by the trials unit at the Institute for Cancer Research Trials Centre using a minimization algorithm.19,20 Stratification was by ECOG PS (0-1 v 2), disease status (locally advanced v metastatic), HIV status (positive v negative), and region (United Kingdom v Australia v Europe v United States). Sites were informed electronically of treatment allocation after random assignment.
Study Procedures
Patients received either intravenous cisplatin (60 mg/m2; day 1) and FU (1,000 mg/m2; days 1-4) every 21 days or carboplatin (area under the curve, 5; day 1) and paclitaxel (80 mg/m2; days 1, 8, and 15) every 28 days. All patients were treated for 24 weeks or until disease progression, intolerable toxicity, or withdrawal of consent.
Adverse events (AEs) were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0), from random assignment to 30 days after administration of the last study treatment. Serious AEs (SAEs) were reported from informed consent up to 30 days after the last study treatment or after, if deemed related to trial treatment.
Response to treatment was assessed by each investigator site as per RECIST (version 1.1) criteria using computed tomography or magnetic resonance imaging scans obtained pretreatment and at 12 weeks, at 24 weeks, and every 12 weeks thereafter until disease progression. No central review was undertaken to confirm radiographic response. Quality of life (QOL) was evaluated before treatment and then at 7, 12, 24, and 48 weeks using the European Organisation for Research and Treatment of Cancer Quality of Life Core 30 Questionnaire and EuroQol ED-5D5L.
The exploratory translational substudy collecting blood and tissue was optional. Archival diagnostic tumor biopsies were retrieved, and optional tumor biopsies on progression were collected upon further consent.
A research sample of 35 mL of whole blood was collected from patients pretreatment, at 12 weeks, and at disease progression. Plasma was isolated from blood samples that were collected pre- and posttreatment (at 12 weeks) following standard protocols for cell-free DNA isolation. Circulating human papillomavirus (HPV) DNA was measured in plasma using an amplicon-based next-generation sequencing panel interrogating for 8 high-risk HPV subtypes (16, 18, 31, 33, 35, 45, 52, and 58). To classify HPV-positive and -negative samples using the NGS panel, we set a threshold whereby a sample was classified positive if there were 3 reads present from > 10 different HPV amplicons for each HPV subtype.
Outcomes
The primary end point was best overall response rate (ORR), defined as the percentage of patients achieving confirmed partial (PR) or complete response (CR) as per RECIST (version 1.1). Secondary end points included the feasibility of international setup and recruitment; progression-free survival (PFS; time from random assignment to the date of confirmed clinical/radiologic progression or death resulting from any cause); OS (time from start of treatment to death resulting from any cause); disease control rate at 12 and 24 weeks posttreatment, defined as CR, PR, or stable disease; and assessments of AEs and QOL. Exploratory objectives included evaluation of circulating HPV DNA in pre- and posttreatment plasma samples and correlation with radiographic response.
Statistical Analysis
On the basis of an ORR estimate of 40% in the cisplatin plus FU arm, a clinically relevant difference in ORR between groups was defined as 10%. Using the selection trial pick-the-winner design for phase II randomized trials,21 the trial was originally designed to require 40 patients (accounting for 10% dropout rate) to be recruited to each arm (total, 80) to detect a 10% difference in ORR between the arms with 80% power.
Because approximately 17% of patients were initially nonassessable for the primary end point, the study sample size was increased up to a maximum of 90 patients to allow 36 assessable patients per arm. On the basis of the selection trial design, primary and secondary study end points were assessed in a hierarchic model to compare the 2 regimens and pick the winner. According to this model, the regimen with the higher ORR would be declared the winner. If there were no difference in ORR, the regimen with the lower rate of grade 3 to 4 toxicities or AEs would be selected. If there were no difference in either ORR or toxicities, the regimen with superior QOL data would be chosen. If no winner were picked after assessing activity, toxicity, and QOL, a strong recommendation on which regimen should be used could not be made.
The primary analysis of best response was based on the modified intention-to-treat (ITT) population, defined as all patients randomly assigned in the study who were eligible, received at least 1 cycle of chemotherapy, were assessable for response, or had evidence of clinical progression. AEs were assessed in the safety population, consisting of all patients who had received at least 1 cycle of randomly assigned treatment.
ORR was reported by arm with 95% CIs and compared between arms using a χ2 test. In addition, a logistic regression model was fitted to adjust for the stratification variables and calculate an odds ratio (with associated 95% CI) between arms. Kaplan-Meier curves were plotted for PFS and OS; treatment effect hazard ratios (HRs; with 95% CIs and P values) were obtained from Cox proportional hazards regression models, which included HRs adjusted for random assignment stratification factors.
AEs were reported as the worst grade per patient per event, and comparison between groups by a test of proportions was calculated with χ2 or Fisher’s test. QOL scores and differences from baseline over time by treatment were tabulated. Only variations of ≥ 10 points compared with baseline were considered clinically significant.22
RESULTS
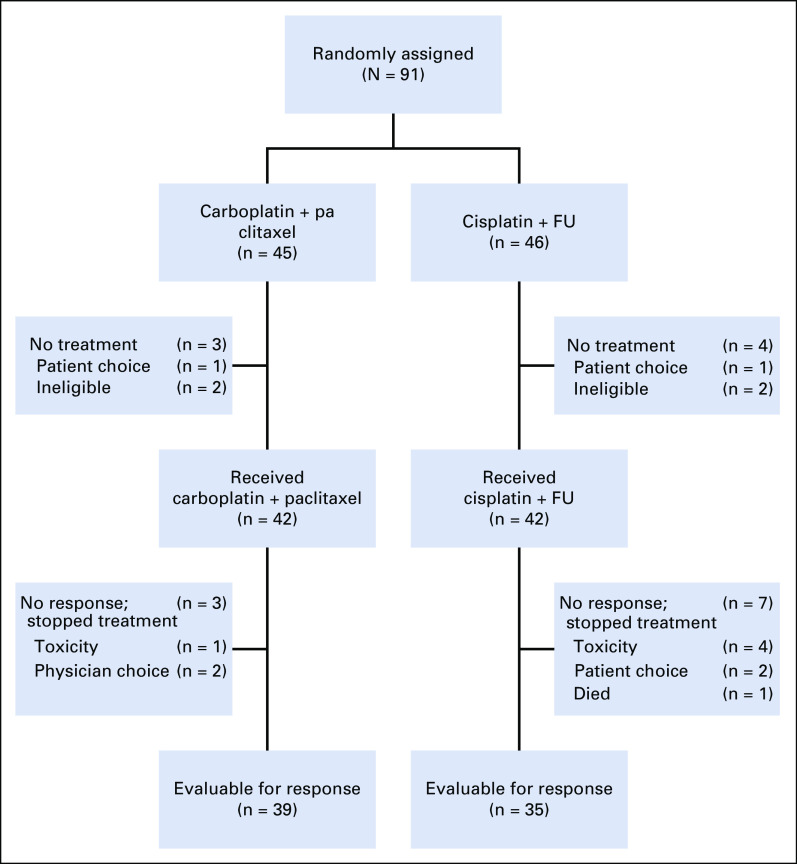
A total of 91 patients were recruited to the study between December 2013 and November 2017 from 31 centers; 45 were randomly assigned to carboplatin plus paclitaxel and 46 to cisplatin plus FU (Fig 1).
FIG 1.
CONSORT diagram. FU, fluorouracil.
Patient demographics were well balanced between both treatment arms at baseline (Table 1). A majority of patients had metastatic disease, had ECOG PS of 0 to 1, and were HIV negative. Of those who had previously received treatment for localized disease, a majority had received chemoradiotherapy.
TABLE 1.
Patient Demographic and Clinical Characteristics
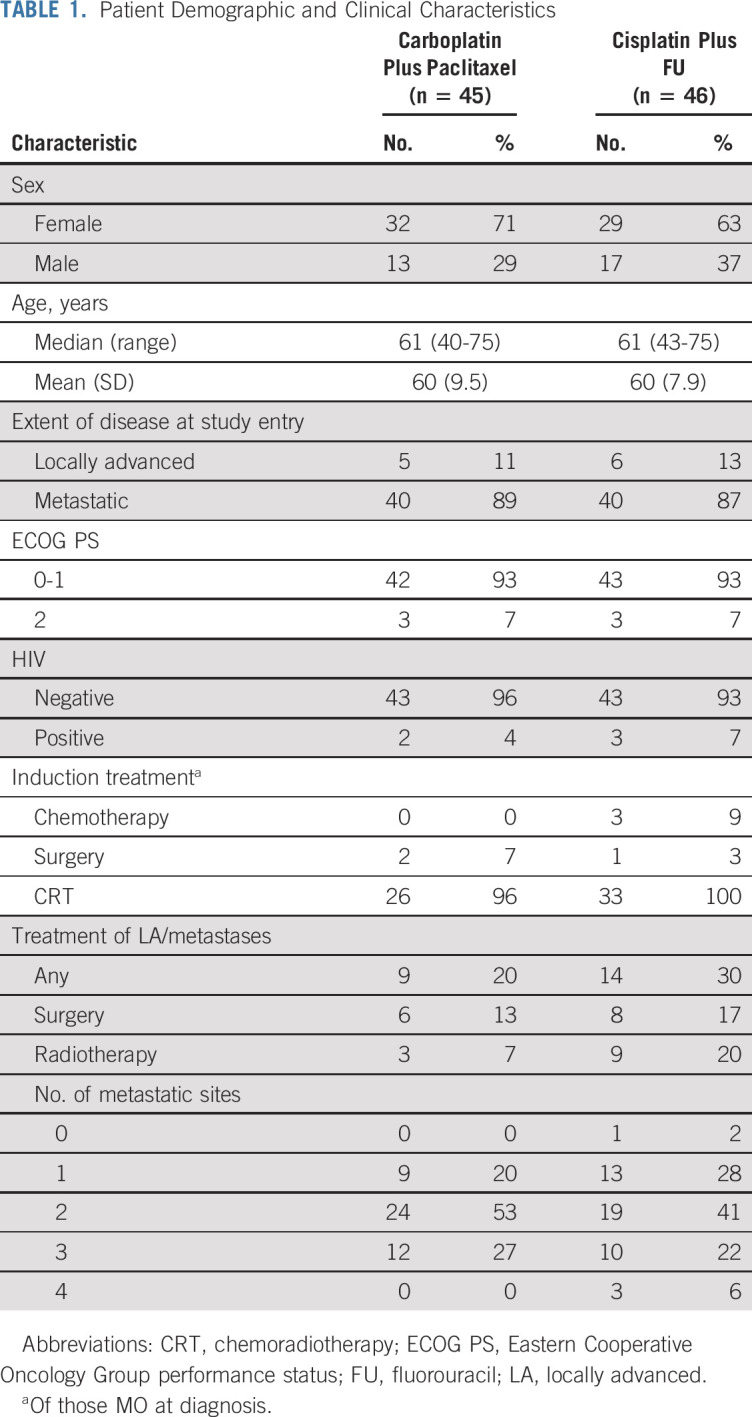
Of the randomly assigned patients, 42 in each arm received study treatment (Fig 1). The modified ITT population comprised 39 patients in the carboplatin plus paclitaxel arm and 35 in the cisplatin plus FU arm. The safety population consisted of 42 patients in each cohort.
Recruitment took longer than expected initially; however, in the last 24 months of recruitment, the target of 25 to 30 patients per year was achieved. Examining recruitment feasibility, 31 (52%) of 60 open centers recruited at least 1 patient. The regional distribution of patient recruitment for the 91 patients was as follows: United Kingdom (n = 68), Europe (n = 8), North America (n = 12), and Australia (n = 3).
Median number of cycles provided was 4.5 in the cisplatin plus FU arm versus 6 in the carboplatin plus paclitaxel arm; 47% received the planned 24 weeks versus 30%, respectively. Dose delays occurred in 64% versus 76% of patients and dose reductions were required in 67% versus 69% of patients in the cisplatin plus FU arm versus carboplatin plus paclitaxel arm, respectively. Mean dose-intensity (± standard deviation) was similar for both arms—cisplatin plus FU arm: cisplatin, 80.4% (± 17.4%) and FU, 80.8% (± 23.4%); carboplatin plus paclitaxel arm: carboplatin, 82% (± 13.5%) and paclitaxel, 83.7% (± 19.4%).
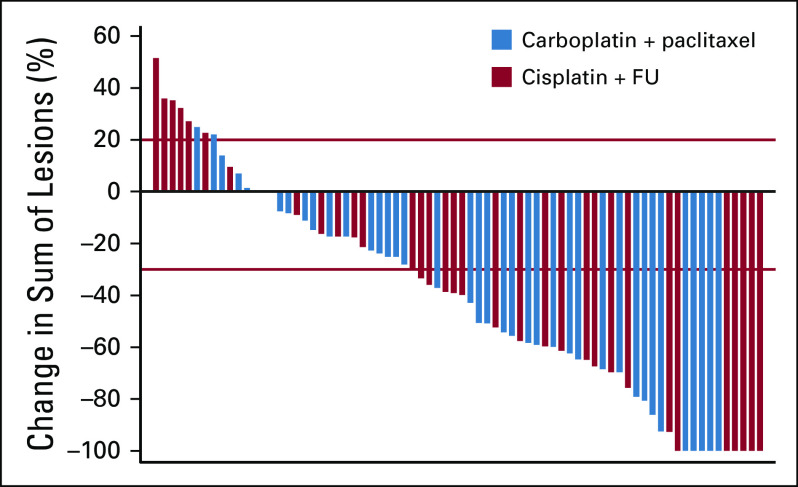
As of May 2019, median follow-up for all patients was 28.6 months. ORR was 57% (95% CI, 39.4% to 73.7%; CR, 17%; PR, 40%) for cisplatin plus FU versus 59% (95% CI, 42.1% to 74.4%; CR, 12.8%; PR, 46.2%) for carboplatin plus paclitaxel (Table 2; Fig 2). CR was observed regardless of disease burden or stage. At the time of analysis, 8 patients (22.9%) in the cisplatin plus FU arm had experienced disease progression compared with 6 (15.4%) in the carboplatin plus paclitaxel arm.
TABLE 2.
Summary of Objective Response
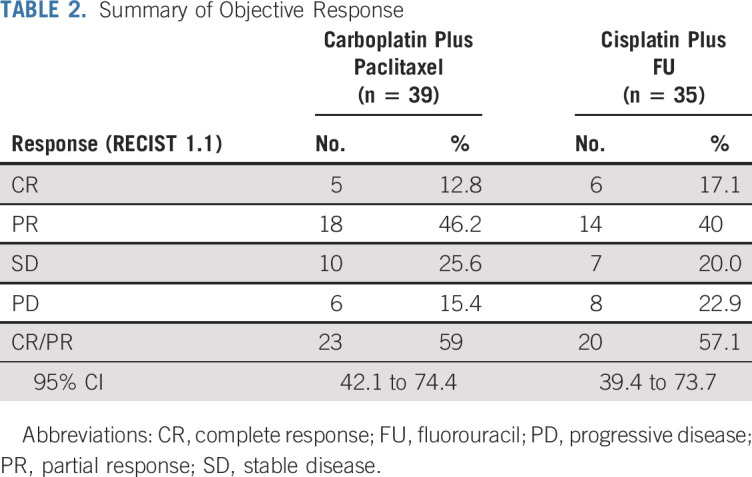
FIG 2.
Waterfall plot of objective response. FU, fluorouracil.
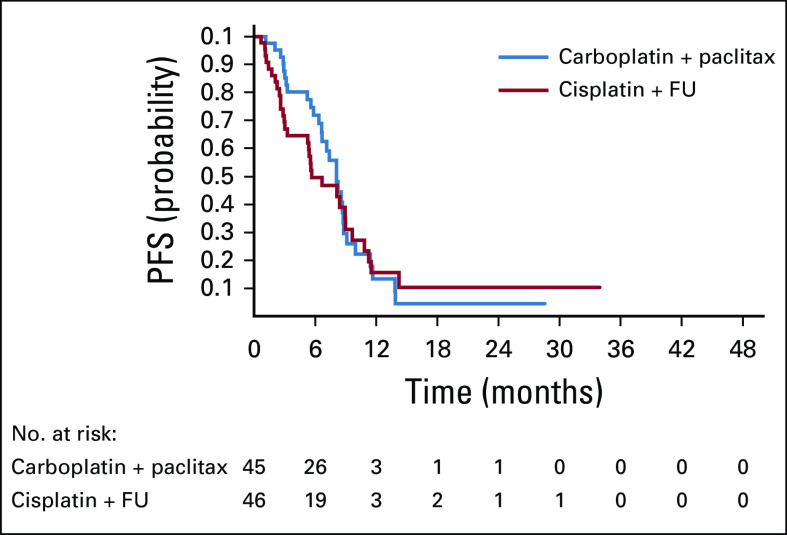
Median PFS was 5.7 months (95% CI, 3.3 to 9.0 months) for cisplatin plus FU compared with 8.1 months (95% CI, 6.6 to 8.8 months) for carboplatin plus paclitaxel and was not statistically significant (Fig 3). The unadjusted HR was 1.27 (95% CI, 0.75 to 2.14; P = .375). After adjusting for PS, HIV status, disease status, and region, the adjusted HR was 1.17 (95% CI, 0.68 to 2.02; P = .564).
FIG 3.
Kaplan-Meier analysis of progression-free survival (PFS). FU, fluorouracil.
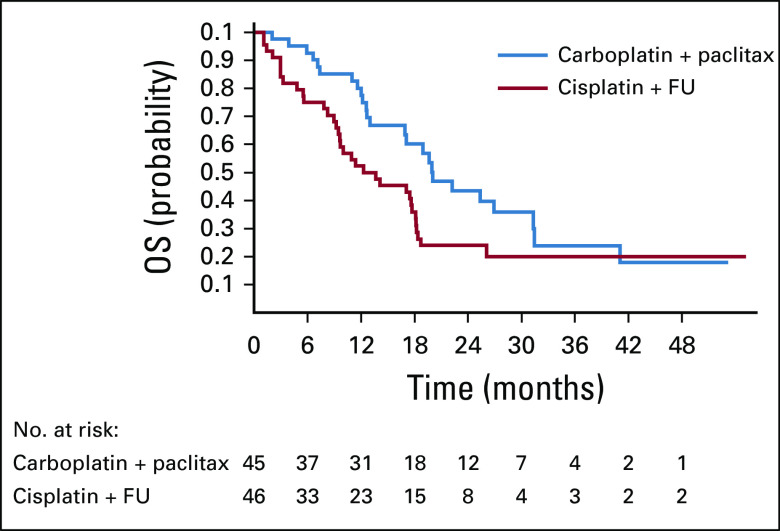
There was a trend toward a significant difference in OS favoring the carboplatin plus paclitaxel arm (Fig 4). Median OS was 12.3 months for cisplatin plus FU (95% CI, 9.2 to 17.7] compared with 20 months (95% CI, 12.7 months to not reached) for carboplatin plus paclitaxel, with an unadjusted HR of 2.00 (95% CI, 1.15 to 3.47; P = .014). After adjusting for stratification factors, the adjusted HR was 1.78 (95% CI, 0.98 to 3.23; P = .059).
FIG 4.
Kaplan-Meier analysis of overall survival (OS). FU, fluorouracil.
The AE profile is summarized in Table 3. There was more neutropenia and anemia with carboplatin plus paclitaxel but more nausea, vomiting, mucositis, and diarrhea with cisplatin plus FU. There were no grade 5 toxicities. Overall, there were more SAEs with cisplatin plus FU versus carboplatin plus paclitaxel (62% v 36%; P = .016).
TABLE 3.
Selected Grade ≥ 3 AEs
QOL assessment was limited because of poor compliance with return of the questionnaires. A minimal number of QOL questionnaires were returned after 12 weeks, limiting analysis. At baseline, 76% versus 67% of questionnaires were completed, reducing at 12 weeks to 46% versus 49%, for the cisplatin plus FU and carboplatin plus paclitaxel arms, respectively. Global health status score seemed to worsen (not significantly) for the cisplatin plus FU arm at 24 weeks but remained unchanged for carboplatin plus paclitaxel; however, data are limited because of small numbers.
Three patients in each cohort underwent surgery (metasesectomy, n = 2; palliative surgery, n = 1) and thus were balanced. After completion of treatment, 25 patients (54%) received subsequent anticancer therapy in the cisplatin plus FU cohort versus 17 (38%) in the carboplatin plus paclitaxel cohort, although overall, there were no statistical differences (Table 4), with crossover from each arm on progression. There were also no differences between cohorts in poststudy radiotherapy.
TABLE 4.
Summary of Subsequent Treatment Posttrial
Exploratory Correlatives
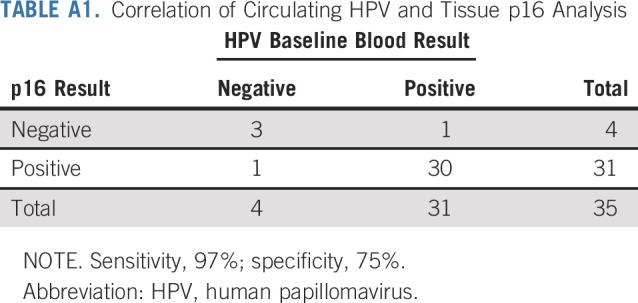
Of 91 patients, 35 (39%) had baseline HPV blood results and tissue P16 results available. Of 31 patients with p16-positive tumor tissue, 30 were positive for HPV DNA. For those 4 patients with p16-negative tumor tissue, 3 were negative by HPV DNA (sensitivity, 97%; specificity, 75%; Appendix Table A1, online only). There were 28 patients with blood samples collected at baseline, during treatment (12 weeks), or on progression. Of these, 5 patients converted from positive HPV DNA pretreatment to negative posttreatment by virtue of a reduction in HPV DNA below the threshold value of 3. For these 5 patients (CR, n = 2; PR, n = 3), median duration of response was 46 weeks (range, 38-64 weeks), with 1 still responding at last follow-up. All patients with radiologic progression had HPV DNA detectable at the time of progression.
DISCUSSION
InterAACT was the first international prospective randomized trial to our knowledge in advanced anal cancer. Although there was no difference in ORRs, carboplatin plus paclitaxel was associated with significantly fewer SAEs (36% v 62%) and fewer clinically relevant AEs, including mucositis, fatigue, and thromboembolism, and a trend toward longer OS that could be clinically meaningful. Furthermore carboplatin plus paclitaxel does not require prolonged infusion, unlike cisplatin plus FU. Therefore, as per the trial design, carboplatin plus paclitaxel was selected for future investigation.
Of note, more patients (n = 25; 54%) received subsequent systemic anticancer therapy in the cisplatin plus FU arm than in the carboplatin plus paclitaxel arm (n = 17; 38%). Furthermore, there were no clear differences in choice of subsequent systemic treatment or in lines of therapy administered between the 2 arms. This was similar regardless of subsequent radiotherapy, surgery, and radiofrequency ablation.
As per the trial design, survival was a secondary end point and exploratory and therefore should be interpreted with caution. Additionally, the number of events for OS was small, with broad CIs. Nevertheless, the efficacy data for the cisplatin plus FU arm are in keeping with previous historical reported series,14,23,24 whereas historical data for carboplatin and paclitaxel in this setting are limited.
Despite our ability to conduct and complete this trial successfully, it had its limitations. One primary limitation of InterAACT was the small randomized phase II design, although this is considered a rare cancer, with no previous reported randomized trials. It is noteworthy that a potentially meaningful difference in OS favoring carboplatin plus paclitaxel was reported, with no apparent difference in response rate or PFS. One explanation for this is that there was no independent central radiologic review of imaging in this open-label trial, which could have led to assessment bias. Additionally, although subsequent lines and types of systemic therapy were well balanced, the sequence of therapy may have differed and may have influenced OS. Finally, it is plausible that paclitaxel causes immunogenic modulation of tumor cells and microenvironment, causing increased sensitivity to antigen-specific cytotoxic T-cell killing. This has been demonstrated in preclinical studies and could account for the prolonged survival.25 This may provide a sound rationale for a combination approach of systemic chemotherapy with immunotherapy.
Furthermore, compliance with QOL questionnaires led to difficulties in interpretation of the QOL data. Our exploratory correlative analysis was also limited by sample size. Nevertheless, there was high sensitivity for HPV DNA detection at baseline when comparing with tumor tissue. The reduction of HPV DNA below the threshold level in some patients who demonstrated radiologic response is intriguing but needs further validation in a larger series.
Since the initiation of InterAACT, Kim et al26 have reported a single-arm multicenter study of docetaxel, cisplatin, and FU in patients with treatment-naïve metastatic anal cancer. The investigators observed an encouraging response rate of 83% and PFS of 11 months. However, 46 patients (70%) developed at least 1 grade 3 to 4 AE. Therefore, this regimen should be provided with caution and only be considered in patients with close follow-up and excellent PS.
Although conducting clinical trials in rare cancers can be challenging, our collaborative effort for the InterAACT trial proved successful. Initially, recruitment was slower than anticipated; however, we eventually successfully demonstrated the feasibility of international collaboration across multiple countries for a rare cancer and achieved target recruitment once all centers were activated. Furthermore, we now have an established international anal cancer network for future collaborative studies. After our presentation of the data at the European Society for Medical Oncology (ESMO) meeting, a revision to the 2019 National Comprehensive Cancer Network and ESMO guidelines now list carboplatin and paclitaxel as the preferred treatment option for patients with treatment-naïve metastatic anal cancer.
In summary, InterAACT, an international randomized phase II trial in the first-line setting of advanced anal cancer, demonstrated no difference in objective response between cisplatin plus FU versus carboplatin plus paclitaxel. However, carboplatin plus paclitaxel was associated with a more favorable toxicity profile and significant trend toward prolonged OS. These data support the consideration of carboplatin plus paclitaxel as a new standard of care in untreated advanced anal cancer and a cytotoxic platform for the development of future phase III trials.
ACKNOWLEDGMENT
The authors would like to acknowledge National Health Service funding to the National Institute for Health Research Biomedical Research Centre at the Royal Marsden NHS Foundation Trust and the Institute of Cancer Research.
Appendix
TABLE A1.
Correlation of Circulating HPV and Tissue p16 Analysis
SUPPORT
Funded by Cancer Research UK, AGITG and ECOG-ACRIN.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Sheela Rao, Francesco Sclafani, Cathy Eng, Richard A. Adams, David Sebag-Montefiore, Al Benson, Clare Peckitt, Eva Segelov, Amitesh Roy, Matt T. Seymour, Jack Welch, Rob Glynne-Jones, Dirk Arnold
Administrative support: Annette Bryant
Provision of study materials or patients: Sheela Rao, Cathy Eng, Marianne G. Guren, David Sebag-Montefiore, Al Benson, Eva Segelov, Amitesh Roy, Mark Saunders, Rebecca Muirhead, John Bridgewater, Dirk Arnold, David Cunningham
Collection and assembly of data: Sheela Rao, Francesco Sclafani, Annette Bryant
Data analysis and interpretation: Sheela Rao, Francesco Sclafani, Cathy Eng, Richard A. Adams, David Sebag-Montefiore, Al Benson, Clare Peckitt, Amitesh Roy, Mark P. Saunders, Rebecca Muirhead, Peter O’Dwyer, John Bridgewater, Shree Bhide, Rob Glynne-Jones, Dirk Arnold
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
International Rare Cancers Initiative Multicenter Randomized Phase II Trial of Cisplatin and Fluorouracil Versus Carboplatin and Paclitaxel in Advanced Anal Cancer: InterAAct
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sheela Rao
Consulting or Advisory Role: Bayer, Roche
Travel, Accommodations, Expenses: Incyte, Bayer
Francesco Sclafani
Research Funding: Bayer (Inst), AstraZeneca (Inst), Roche (Inst), Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: Bayer, Eli Lilly
Cathy Eng
Consulting or Advisory Role: Bayer Schering Pharma, Foundation of Medicine, Array BioPharma, Natera
Recipient: You
Richard A. Adams
Honoraria: Merck Serono, Servier, Amgen
Consulting or Advisory Role: Merck Serono, Amgen, Servier
Speakers’ Bureau: Merck Serono
Research Funding: Astra Zeneca (Inst), Merck Sharp & Dohme (Inst)
Travel, Accommodations, Expenses: Servier, Amgen, Merck Serono
Al Benson
Consulting or Advisory Role: Genentech/Roche, Bristol Myers Squibb, Guardant Health, Exelixis, Purdue Pharma, Rafael Pharmaceuticals, Eli Lilly, National Comprehensive Cancer Network, inVentive Health, Axio, Bayer, Merck, Astellas Pharma, Terumo, Thera Bionic, LSK Global Pharma
Research Funding: Advanced Accelerator Applications (Inst), Novartis (Inst), Infinity Pharmaceuticals (Inst), Merck Sharp & Dohme (Inst), Taiho Pharmaceutical (Inst), Bristol Myers Squibb, Acerta Pharma, Celgene, MedImmune, Xencor
Travel, Accommodations, Expenses: Genentech/Roche, Eli Lilly/ImClone, Bayer, Sanofi, Spectrum Pharmaceuticals, AVEO Pharmaceuticals, Gilead Sciences, Astellas Pharma, Boehringer Ingelheim, DAVA Oncology, TRM Oncology, Guardant Health, Helsinn, Guerbet, Boston Biomedical, Bristol Myers Squibb
Eva Segelov
Consulting or Advisory Role: Ipsen, Merck Sharp & Dohme, Athenex
Research Funding: Clinical Genomics (Inst)
Travel, Accommodations, Expenses: Amgen, Roche
Amitesh Roy
Honoraria: Bristol Myers Squibb, Ipsen, Roche
Honoraria: Merck Sharp & Dohme, Servier
Consulting or Advisory Role: Roche
Research Funding: Merck Serono (Inst)
Travel, Accommodations, Expenses: Ipsen, Bristol Myers Squibb
Matthew T. Seymour
Research Funding: Amgen (Inst)
John Welch
Employment: Theradex Oncology
Mark P. Saunders
Honoraria: Servier, Merck Serono
Travel, Accommodations, Expenses: Servier
Rebecca Muirhead
Honoraria: Boehringer Ingelheim
Travel, Accommodations, Expenses: Sirtex, Bayer/Onyx
Peter O’Dwyer
Consulting or Advisory Role: Genentech, Array BioPharma
Research Funding: Bristol Myers Squibb, Pfizer, Novartis, Genentech, Mirati Therapeutics, Celgene, GlaxoSmithKline, BBI Healthcare, Merck, Pharmacyclics, Bayer, Five Prime Therapeutics, Forty Seven, Amgen, H3 Biomedicine (Inst), Taiho Pharmaceutical (Inst), Array BioPharma (Inst), Eli Lilly/ImClone (Inst)
Expert Testimony: Bayer, Eli Lilly
John Bridgewater
Honoraria: Merck Serono, Servier
Consulting or Advisory Role: Merck Serono, Servier, Rochew, Bayer, AstraZeneca, Incyte, Basilea
Speakers’ Bureau: Celgene, Amgen, Bristol Myers Squibb
Research Funding: Amgen
Travel, Accommodations, Expenses: MSD Oncology, Merck Serono, Servier, Bristol-Myers Squibb/Medarex
Shree Bhide
Travel, Accommodations, Expenses: Elekta
Dirk Arnold
Honoraria: Bayer, Merck Serono, Roche/Genentech, Servier, Terumo, Bristol-Myers Squibb
Consulting or Advisory Role: Bayer, Merck Serono, Servier, Biocompatibles, Terumo, Bristol Myers Squibb, MSD Oncology
Research Funding: Roche/Genentech (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Bayer, Merck Serono
David Cunningham
Stock and Other Ownership Interests: OVIBIO
Consulting or Advisory Role: OVIBIO
Research Funding: AstraZeneca (Inst), Amgen (Inst), Sanofi (Inst), Merrimack (Inst), Celgene (Inst), MedImmune (Inst), Bayer (Inst), 4SC (Inst), Clovis Oncology (Inst), Eli Lilly (Inst), Janssen (Inst), Merck (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Nelson VM, Benson AB., III Epidemiology of anal canal cancer. Surg Oncol Clin N Am. 2017;26:9–15. doi: 10.1016/j.soc.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 2.van der Zee RP, Richel O, de Vries HJ, et al. The increasing incidence of anal cancer: Can it be explained by trends in risk groups? Neth J Med. 2013;71:401–411. [PubMed] [Google Scholar]
- 3.Islami F, Ferlay J, Lortet-Tieulent J, et al. International trends in anal cancer incidence rates. Int J Epidemiol. 2017;46:924–938. doi: 10.1093/ije/dyw276. [DOI] [PubMed] [Google Scholar]
- 4. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. https://seer.cancer.gov/
- 5.Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I) Br J Cancer. 2010;102:1123–1128. doi: 10.1038/sj.bjc.6605605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–2539. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 7.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–2049. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 8.James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): A randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013;14:516–524. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 9.Epidermoid anal cancer. Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin—UKCCR Anal Cancer Trial Working Party, UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–1054. [PubMed] [Google Scholar]
- 10.Renehan AG, Saunders MP, Schofield PF, et al. Patterns of local disease failure and outcome after salvage surgery in patients with anal cancer. Br J Surg. 2005;92:605–614. doi: 10.1002/bjs.4908. [DOI] [PubMed] [Google Scholar]
- 11.Shridhar R, Shibata D, Chan E, et al. Anal cancer: Current standards in care and recent changes in practice. CA Cancer J Clin. 2015;65:139–162. doi: 10.3322/caac.21259. [DOI] [PubMed] [Google Scholar]
- 12. National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: Anal carcinoma (version 2). https://www.nccn.org/professionals/physician_gls/default.aspx.
- 13.Glynne-Jones R, Nilsson PJ, Aschele C, et al. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2014;40:1165–1176. doi: 10.1016/j.ejso.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Tanum G. Treatment of relapsing anal carcinoma. Acta Oncol. 1993;32:33–35. doi: 10.3109/02841869309083882. [DOI] [PubMed] [Google Scholar]
- 15.Faivre C, Rougier P, Ducreux M, et al. 5-fluorouracile and cisplatinum combination chemotherapy for metastatic squamous-cell anal cancer [in French] Bull Cancer. 1999;86:861–865. [PubMed] [Google Scholar]
- 16. Boland PM, Wang K, Kohen A: Systemic therapy for advanced anorectal squamous cell carcinomas: A single institutional experience. J Clin Oncol 34, 2016 (suppl; abstr 728) [Google Scholar]
- 17.Kim R, Byer J, Fulp WJ, et al. Carboplatin and paclitaxel treatment is effective in advanced anal cancer. Oncology. 2014;87:125–132. doi: 10.1159/000361051. [DOI] [PubMed] [Google Scholar]
- 18.Abbas A, Nehme E, Fakih M. Single-agent paclitaxel in advanced anal cancer after failure of cisplatin and 5-fluorouracil chemotherapy. Anticancer Res. 2011;31:4637–4640. [PubMed] [Google Scholar]
- 19.Altman DG, Bland JM. Treatment allocation by minimisation. BMJ. 2005;330:843. doi: 10.1136/bmj.330.7495.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown S, Thorpe H, Hawkins K, et al. Minimization: Reducing predictability for multi-centre trials whilst retaining balance within centre. Stat Med. 2005;24:3715–3727. doi: 10.1002/sim.2391. [DOI] [PubMed] [Google Scholar]
- 21.Simon R, Wittes RE, Ellenberg SS. Randomized phase II clinical trials. Cancer Treat Rep. 1985;69:1375–1381. [PubMed] [Google Scholar]
- 22.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 23.Sclafani F, Morano F, Cunningham D, et al. Platinum-fluoropyrimidine and paclitaxel-based chemotherapy in the treatment of advanced anal cancer patients. Oncologist. 2017;22:402–408. doi: 10.1634/theoncologist.2016-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eng C, Chang GJ, You YN, et al. The role of systemic chemotherapy and multidisciplinary management in improving the overall survival of patients with metastatic squamous cell carcinoma of the anal canal. Oncotarget. 2014;5:11133–11142. doi: 10.18632/oncotarget.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodge JW, Garnett CT, Farsaci B, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer. 2013;133:624–636. doi: 10.1002/ijc.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S, François E, André T, et al. Docetaxel, cisplatin, and fluorouracil chemotherapy for metastatic or unresectable locally recurrent anal squamous cell carcinoma (Epitopes-HPV02): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2018;19:1094–1106. doi: 10.1016/S1470-2045(18)30321-8. [DOI] [PubMed] [Google Scholar]