Abstract
Purpose
Elderly individuals have comorbidities that can adversely affect surgical outcomes. Some studies reported that elderly patients with hepatocellular carcinoma (HCC) have higher liver- and non-liver–related deaths. Therefore, palliative treatments are preferred in these patients. We compared surgical treatment outcomes between young and old age groups.
Methods
In total, 233 liver resections were performed in patients with HCC from March 2012 to December 2018. We retrospectively reviewed medical records. The old age group was defined as patients aged more than 70 years. We compared perioperative characteristics and surgical outcomes and analyzed the prognostic factors for disease-free survival (DFS) and overall survival (OS) rates.
Results
The young and old age group included 184 and 49 patients, respectively. Preoperative characteristics were similar. Major liver resection rate was similar (young age group, 26.1% vs. old age group, 20.4%), but the operation time was a little bit shorter in old age group. Major postoperative complications were 23 (12.5%) and 9 (18.4%) in the young and old age group (P = 0.351). Median non-liver–related overall survival were 80 and 76 months (P = 0.889) and liver-related OS were 76 and 76 months (P = 0.514) in the young and old age groups, respectively. Age was not an independent risk factor for DFS and OS.
Conclusion
Elderly patients showed similar non-liver- and liver-related OS rates as young patients after liver resection. Postoperative complications were also similar. If elderly patients are well selected, they can receive curative treatment and show good surgical outcomes.
Keywords: Hepatocellular carcinoma, Non-liver–related overall survival, Old age, Young age
INTRODUCTION
Hepatocellular carcinoma (HCC) is a common cause of death worldwide. The number of elderly patients with HCC has been increasing with a longer life expectancy among this population [1]. According to the National Cancer Information Center in Korea, HCC was the second leading cause of cancer mortality in Korea in 2017 [2]. South Korea utilizes the National Cancer Screening Programs as a cancer management policy at the national level. The patients with HCC can be diagnosed and treated relatively early [3]. However, the early detection and diagnosis of HCC did improve with this effort, and there are still many difficulties in treating patients with HCC. Especially, older people have coexisting medical morbidities that can adversely affect surgical outcomes and may become the reason for selecting palliative treatment modalities, including transarterial chemoembolization (TACE).
Recent advances in surgical techniques and perioperative management could reduce the age-related complications of liver surgery [4] and many elderly patients with HCC are receiving aggressive management [5]. However, there are some debates regarding the prognosis of elderly patients. Disease-free survival (DFS) was similar in such patients but overall survival (OS) was worse than younger patients, and especially, non-liver–related OS was also poorer in elderly patients [6]. Recent studies have shown that surgical resection or liver transplantation show better results than other therapeutic options such as radiofrequency ablation, TACE, and systemic chemotherapy. Unfortunately, there are significant obstacles to curative liver resection such as coexisting extrahepatic metastasis, underlying liver disease, and the concern of a patient's older age [7,8]. The aim of this study was to evaluate clinical outcomes and safety of the surgical approach and compare these in older and younger patients with HCC as well as to compare the non-liver- and liver-related OS rates between the old and young groups.
METHODS
Patients
From March 2012 to December 2018, 233 patients underwent liver resection by a single surgeon and were pathologically diagnosed with HCC after liver resection. This study was approved by Institutional Review Board of Wonju Severance Christian Hospital (No. CR314023). We prospectively collected medical records of all patients, and they were enrolled in this study. Patients were divided into 2 groups according to their age at admission; young (patients aged <70 years) and old (patients aged ≥70 years) age groups.
Patient selection and treatment of HCC
Hepatologists regularly checked high-risk patients based on their α-FP level and ultrasonography (US) according to the Korea Practice Guideline for the Management of HCC [9]. A dynamic contrast enhanced CT scan was performed when there were α-FP increases or suspicious nodules on US. Additionally, an MRI and 18F-fluoro-2-deoxy-D-glucose PET CT scans were performed to evaluate intra- or extrahepatic metastasis. A multidisciplinary team including surgeons, radiologists, and internal medicine doctors evaluated patients' eligibility for liver resection. The operation was determined based on the tumor extent, residual liver function, and performance scale of patients. The Eastern Cooperative Oncology Group (ECOG) performance scale was used for this evaluation. The ECOG scales of 0 and 1 were regarded as appropriate for patients to undergo liver resection. The indocyanine retention rate of 15 minutes (ICG R15) was used to evaluate residual liver function. If there was enough remnant liver volume or multiple tumors were located in the same segment or section that was to be removed, we did not limit the size and number of tumors. We usually performed anatomical liver resection and achieved wide resection margin in patients with a well-preserved liver function. Major liver resection was defined as liver resection of more than 2 segments.
Outcomes
We compared baseline characteristics of patients and pathologic characteristics of tumors between the young and old age groups and analyzed prognostic factors for DFS and OS and identified whether age was an independent prognostic factor for DFS and OS. Finally, we compared OS rates between the young and old age groups with respect to liver-related mortality, non-liver–related mortality, and OS, including liver-related, non-liver–related, and operative mortality.
Statistics
Statistical analyses were performed using IBM SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA). All continuous variables were presented as median and range. All categorical results were presented as numbers and percentages. Categorical and continuous variables were compared using Fisher exact test and Student t-test, respectively. OS and DFS rates were analyzed using the Kaplan-Meier test. Univariate and multivariate analyses of DFS and OS rates were conducted using Cox proportional hazard model (forward stepwise) to identify prognostic factors. P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
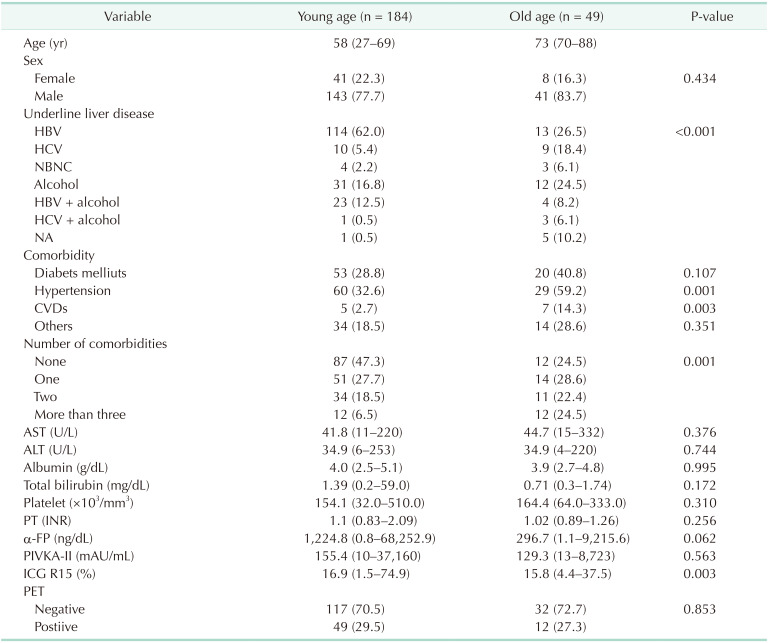
A total of 233 patients with HCC underwent liver resection. Forty-nine patients (21.0%) were included in the old group and 184 (79.0%) in the young group. Baseline characteristics of patients in the 2 groups are summarized in Table 1. The percentage of patients with HBV infection was significantly lower in the old age group (34.7% vs. 74.5%, P < 0.001). Alcohol and HCV infection were significantly more common causes of underlying liver disease in the old age group. Among the comorbidities, the prevalence of hypertension and cerebrovascular disease and the number of patients with more than 2 comorbidities were significantly higher in the old age group. There were no significant differences between the 2 groups regarding preoperative liver function tests.
Table 1. Baseline characteristics of young and old age groupsa).
Values are presented as median (range) or number (%).
NA, not analyzed; CVD, cerebrovascular disease; PT, prothrombin time; INR, international normalized ratio; PIVKA-II, PT induced by vitamin K absence-II; ICG R15, indocyanine retention rate at 15 minutes.
a)Young age group included patients aged <70 years and old age group included those aged ≥70 years.
Perioperative and pathologic characteristics
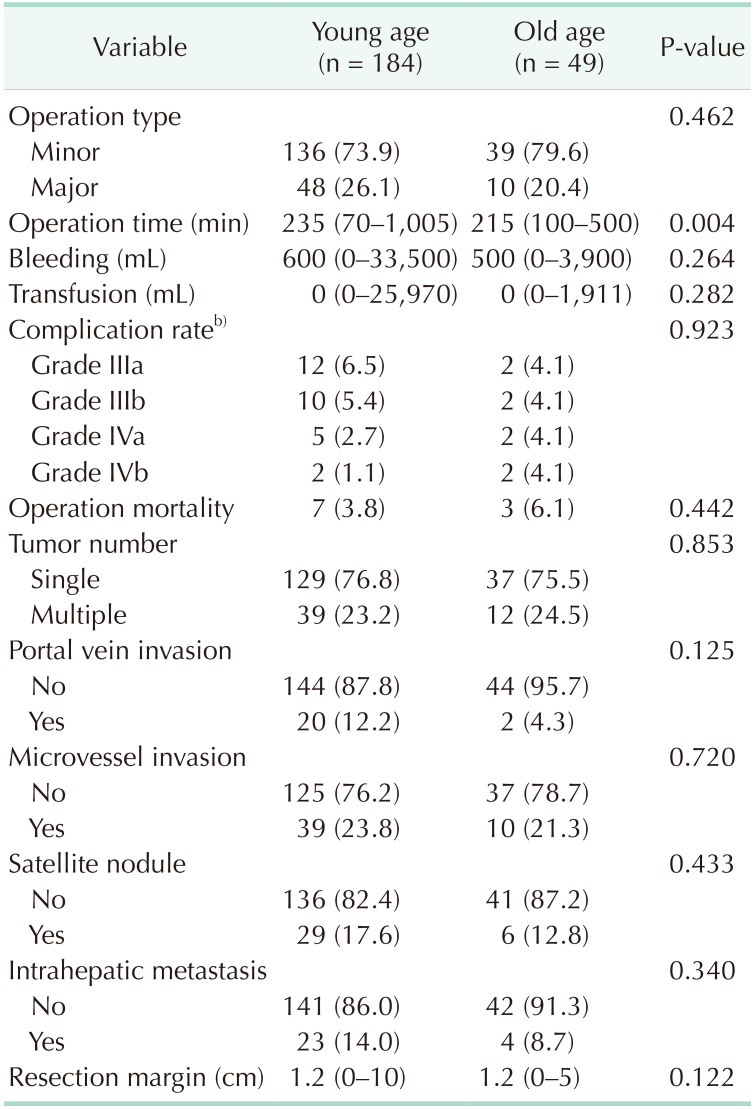
Operative and pathologic characteristics of patients in the 2 groups are summarized in Table 2. There was no difference in the operation types between the 2 groups (the ratio of major operations was 26.1% and 20.4% in the young and old age group, P = 0.462). Old age group suffered from wound infection and dehiscence as grade 3 and aspiration pneumonia and pulmonary embolism as grade 4 and young age group suffered from liver-related complications. The complication rate of a Clavien-Dindo classification of >III showed no significant difference between the groups. The operative mortality rate was also similar between the groups. However, the reason of mortality was mainly liver-related death in young age group and infection-related death in old age group. There was no difference in pathologic characteristics between the groups.
Table 2. Perioperative and pathological characteristics of patientsa).
Values are presented as number (%) or median (range).
a)Young age group included patients aged <70 years and old age group included those aged ≥70 years. b)Complication grade was defined using Clavien-Dindo classification.
Surgical outcomes
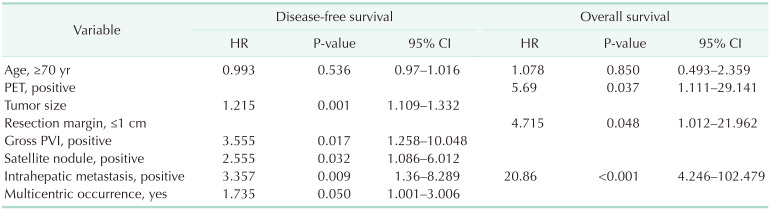
The median DFS was 50 months in all patients and 46 and 67 months in the young and old age groups, respectively; however, this did not show a significant difference (P = 0.722). The median OS was 84 months in all patients and 84 and 72 months in the young and old age groups, respectively. According to multivariate analysis, age was not an independent risk factor for DFS and OS. Tumor size, gross portal vein invasion, satellite nodule, intrahepatic metastasis, and multicentric occurrence were significant prognostic factors for DFS, whereas positivity of PET, a resection margin of ≤1 cm, and intrahepatic metastasis were significant prognostic factors for OS (Table 3).
Table 3. Multivariate analysis for disease-free survival and overall survival.
HR, hazard ratio; CI, confidential interval; PVI, portal vein invasion.
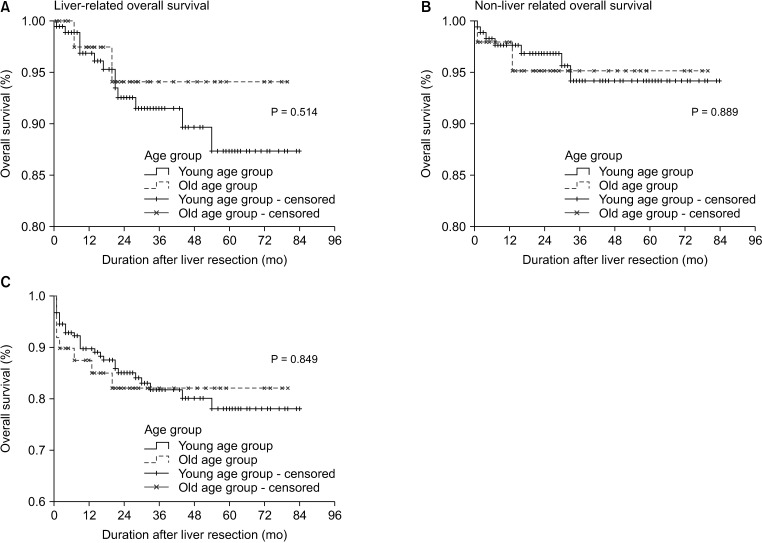
We analyzed OS according to the cause of death: liver-related, non-liver–related, and all causes. The median OS of the young and old age groups was 84 and 72 months in OS of all causes, which was not significant (Fig. 1).
Fig. 1. Overall survival rates in the young and old age groups according to the cause of death. (A) Liver-related overall survival between both groups (P = 0.514). (B) Non-liver–related overall survival between both groups (P = 0.889). (C) Overall survival by liver-related, non-liver–related, and operative mortality between both groups (P = 0.849) did not show a significant difference.
DISCUSSION
HCC shows a poor prognosis and its incidence is increasing in Korea, especially in the old age population [3]. If liver resection and perioperative management can be performed safely, elderly patients with HCC who undergo liver resection can be managed via curative treatment modalities. Generally, elderly patients with HCC have more complications than younger patients because of comorbidities such as cardiovascular and respiratory diseases.
There are several treatment choices for patients with HCC: curative treatments include liver resection, ablation therapy, or liver transplantation, and palliative treatments include palliative chemotherapy, regional therapy such as TACE, percutaneous ethanol injection, palliative radiotherapy, or pain management only [10]. Older patients tend less often to receive curative treatments, which accounted for the poorer result than younger patients [11].
An older age could be an important risk factor for surgery. According to Turrentine et al. [5], patients aged ≥70 years have increased morbidity without increase of preoperative risk factors. Many elderly patients tend to receive other treatments including TACE or medical management instead of surgical management because of their misunderstanding of surgical risks. The major liver resection type and transfusion were known as prognostic factors; however, some reports reported that conservative liver resection did not influence the outcomes [12,13]. In our study, although the frequency of minor resection was similar between both groups, young age group received actually more bisegmentectomies including anterior and posterior sectionectomy (43 cases [24.4%] vs. 7 cases [14.3%]) and it induced significant difference of the operation time. Like other studies, these conservative surgical approaches did not influence the surgical outcomes of older patients. This might be due to our patient selection, which was based on the performance or liver function, not based on their age. Other studies showed similar results [1,14,15,16,17,18]. There were several studies that predicted surgical outcomes in elderly patients who underwent liver resection [19,20,21,22]. Among them, the performance status included common prognostic factors to predict postoperative complications or mortality. Additionally, there was one study that determined the effect of the medical insurance system on surgical outcomes. In that study, medical insurance positively affected postoperative complications and oncologic outcomes [23]. In Korea, all individuals must be enrolled in the National Health Insurance Service. The performance score, underlying disease, and preoperative liver function should be the most important factors, rather than age or treatment selection, in elderly patients. If patients are carefully selected and perioperative management for older patients is conducted, we expect to improve surgical outcomes, especially for elderly patients with comorbidities.
The outcome of HCC resection has improved through advances in diagnostic imaging studies, surgical techniques, and postoperative care [4,24]. We observed no differences in DFS or OS between the younger and older patients, as in previous studies [25,26,27]. Age was not an independent risk factor that determined a prognosis. Additionally, some recent studies have emphasized that elderly patients showed an inferior non-liver–related OS [1,28]. However, in our study, there were no differences of liver-related, non-liver–related, and OS between the older and younger age groups. The old age group showed a similar OS in all causes.
This study has several limitations. First, our data was restricted to patients who were treated at a single institution, which resulted in a smaller number of patients. Therefore, it would be effective to investigate a larger number of patients' data at multiple centers. Second, patients' quality of life post-surgery was not included in this data, which limited the outcomes examined for OS. Moreover, OS and years of life lost were not compared with a similarly aged population. Data collection was retrospective in nature and may have been accompanied with selection bias.
In conclusion, age alone should not be the determining risk factor for HCC. If elderly patients with HCC choose their treatment method, clinicians must consider their risk of liver function, assess their functional liver reserves, patient's general condition, and comorbidities including cardiovascular and pulmonary conditions, and should not present bias because of their age.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: SH Kim, SKB.
- Formal Analysis: ISS.
- Investigation: SH Kim, ISS, DGK, SH Kang.
- Methodology: DGK, SWC, SH Kim, MYK.
- Project Administration: SH Kim, ISS.
- Writing — Original Draft: ISS.
- Writing — Review & Editing: SH Kim, DGK, SWC, SH Kang, MYK, SKB.
References
- 1.Kaibori M, Yoshii K, Yokota I, Hasegawa K, Nagashima F, Kubo S, et al. Impact of advanced age on survival in patients undergoing resection of hepatocellular carcinoma: report of a Japanese nationwide survey. Ann Surg. 2019;269:692–699. doi: 10.1097/SLA.0000000000002526. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Information Center. Major cancer mortality [Internet] Goyang: National Cancer Information Center; c2019. [cited 2020 Jul 1]. Available from: https://www.cancer.go.kr/lay1/S1T645C646/contents.do. [Google Scholar]
- 3.Jung M. National Cancer Screening Programs and evidence-based healthcare policy in South Korea. Health Policy. 2015;119:26–32. doi: 10.1016/j.healthpol.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Kim SH, Kang DR, Lee JG, Kim DY, Ahn SH, Han KH, et al. Early predictor of mortality due to irreversible posthepatectomy liver failure in patients with hepatocellular carcinoma. World J Surg. 2013;37:1028–1033. doi: 10.1007/s00268-013-1959-z. [DOI] [PubMed] [Google Scholar]
- 5.Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203:865–877. doi: 10.1016/j.jamcollsurg.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Santambrogio R, Barabino M, Scifo G, Costa M, Giovenzana M, Opocher E. Effect of age (over 75 years) on postoperative complications and survival in patients undergoing hepatic resection for hepatocellular carcinoma. J Gastrointest Surg. 2017;21:657–665. doi: 10.1007/s11605-016-3354-1. [DOI] [PubMed] [Google Scholar]
- 7.Kutlu OC, Chan JA, Aloia TA, Chun YS, Kaseb AO, Passot G, et al. Comparative effectiveness of first-line radiofrequency ablation versus surgical resection and transplantation for patients with early hepatocellular carcinoma. Cancer. 2017;123:1817–1827. doi: 10.1002/cncr.30531. [DOI] [PubMed] [Google Scholar]
- 8.Ramesh H. Resection for hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(Suppl 3):S90–S96. doi: 10.1016/j.jceh.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019;13:227–299. doi: 10.5009/gnl19024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammad AY, Robbins JR, Turaga KK, Christians KK, Gamblin TC, Johnston FM. Palliative interventions for hepatocellular carcinoma patients: analysis of the National Cancer Database. Ann Palliat Med. 2017;6:26–35. doi: 10.21037/apm.2016.11.02. [DOI] [PubMed] [Google Scholar]
- 11.Guo H, Wu T, Lu Q, Dong J, Ren YF, Nan KJ, et al. Hepatocellular carcinoma in elderly: clinical characteristics, treatments and outcomes compared with younger adults. PLoS One. 2017;12:e0184160. doi: 10.1371/journal.pone.0184160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahiya D, Wu TJ, Lee CF, Chan KM, Lee WC, Chen MF. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: a 20-year experience. Surgery. 2010;147:676–685. doi: 10.1016/j.surg.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 13.Lim JH, Choi GH, Choi SH, Lee HS, Kim KS, Choi JS. Ventral segment-preserving right hepatectomy in patients with hepatocellular carcinoma. World J Surg. 2015;39:1034–1043. doi: 10.1007/s00268-014-2894-3. [DOI] [PubMed] [Google Scholar]
- 14.Bulathsinhala BKS, Tillekaratne MSB, Gunatilleke MB, Niriella MA, Wijegunawardena DGA, Siriwardana RC. Outcome of hepatic resection: First five-year experience in elderly and younger patients. Ceylon Med J. 2018;63:43–45. doi: 10.4038/cmj.v63i1.8617. [DOI] [PubMed] [Google Scholar]
- 15.Inoue Y, Tanaka R, Fujii K, Kawaguchi N, Ishii M, Masubuchi S, et al. Surgical outcome and hepatic regeneration after hepatic resection for hepatocellular carcinoma in elderly patients. Dig Surg. 2019;36:289–301. doi: 10.1159/000488327. [DOI] [PubMed] [Google Scholar]
- 16.Mastoraki A, Tsakali A, Papanikolaou IS, Danias N, Smyrniotis V, Arkadopoulos N. Outcome following major hepatic resection in the elderly patients. Clin Res Hepatol Gastroenterol. 2014;38:462–466. doi: 10.1016/j.clinre.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Zarour LR, Billingsley KG, Walker BS, Enestvedt CK, Orloff SL, Maynard E, et al. Hepatic resection of solitary HCC in the elderly: a unique disease in a growing population. Am J Surg. 2019;217:899–905. doi: 10.1016/j.amjsurg.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Zhao LY, Huo RR, Xiang X, Torzilli G, Zheng MH, Yang T, et al. Hepatic resection for elderly patients with hepatocellular carcinoma: a systematic review of more than 17,000 patients. Expert Rev Gastroenterol Hepatol. 2018;12:1059–1068. doi: 10.1080/17474124.2018.1517045. [DOI] [PubMed] [Google Scholar]
- 19.Hamaoka M, Kobayashi T, Ishiyama K, Ohira M, Tahara H, Kuroda S, et al. Evaluation of the risk factors and prognostic factors of hepatectomy for hepatocellular carcinoma in patients aged 80 years or more. J Hepatobiliary Pancreat Sci. 2017;24:58–64. doi: 10.1002/jhbp.413. [DOI] [PubMed] [Google Scholar]
- 20.Ide T, Miyoshi A, Kitahara K, Noshiro H. Prediction of postoperative complications in elderly patients with hepatocellular carcinoma. J Surg Res. 2013;185:614–619. doi: 10.1016/j.jss.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Ruzzenente A, Conci S, Ciangherotti A, Campagnaro T, Valdegamberi A, Bertuzzo F, et al. Impact of age on short-term outcomes of liver surgery: lessons learned in 10-years' experience in a tertiary referral hepato-pancreato-biliary center. Medicine (Baltimore) 2017;96:e6955. doi: 10.1097/MD.0000000000006955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Fuente SG, Bennett KM, Scarborough JE. Functional status determines postoperative outcomes in elderly patients undergoing hepatic resections. J Surg Oncol. 2013;107:865–870. doi: 10.1002/jso.23335. [DOI] [PubMed] [Google Scholar]
- 23.Jang JS, Shin DG, Cho HM, Kwon Y, Cho DH, Lee KB, et al. Differences in the survival of gastric cancer patients after gastrectomy according to the medical insurance status. J Gastric Cancer. 2013;13:247–254. doi: 10.5230/jgc.2013.13.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn SH, Kim SH, Choi GH, Choi JS, Kim KS. The optimal follow-up period in patients with above 5-year disease-free survival after curative liver resection for hepatocellular carcinoma. J Korean Surg Soc. 2013;85:269–274. doi: 10.4174/jkss.2013.85.6.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan JT, Zhao C, Peng NF, Yang Y, Zhong JH, Yang T, et al. Association between age and overall survival of patients with hepatocellular carcinoma after hepatic resection. J Surg Oncol. 2016;114:966–970. doi: 10.1002/jso.24434. [DOI] [PubMed] [Google Scholar]
- 26.Wei F. Does an extreme age (≥80 years) affect outcomes in patients after liver cancer surgery? A meta-analysis. ANZ J Surg. 2019;89:25–31. doi: 10.1111/ans.14676. [DOI] [PubMed] [Google Scholar]
- 27.Yamada S, Shimada M, Miyake H, Utsunomiya T, Morine Y, Imura S, et al. Outcome of hepatectomy in super-elderly patients with hepatocellular carcinoma. Hepatol Res. 2012;42:454–458. doi: 10.1111/j.1872-034X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 28.Cucchetti A, Sposito C, Pinna AD, Citterio D, Ercolani G, Flores M, et al. Effect of age on survival in patients undergoing resection of hepatocellular carcinoma. Br J Surg. 2016;103:e93–e99. doi: 10.1002/bjs.10056. [DOI] [PubMed] [Google Scholar]