Abstract
Aim: This study focused on the expression pattern of long non-coding RNA maternally expressed gene 3 (MEG3) and its value in ischemic stroke (IS).
Methods: The expression pattern and the roles of MEG3 in the development of IS were explored in mice IS model and human brain microvascular endothelial cells (hBMECs). A case-control study, including 215 IS patients and 153 controls, was also conducted to investigate its prognostic value.
Results: In vivo study showed that MEG3 increased significantly in the IS group (P = 0.004), and its level remained stable within 3 to 48h after the onset of IS. Besides, the survival time of the mouse in the high MEG3 group was significantly lower than that in the low MEG3 group (P = 0.042). In vitro study showed that oxygen–glucose deprivation (OGD) treatment significantly up-regulated expressions of MEG3, Bax, and cleaved caspase-3, and further promoted apoptosis of hBMECs, while si-MEG3 blocked these effects. A human study showed that MEG3 increased markedly within 48h of IS onset and was positively associated with the National Institutes of Health Stroke Scale (r = 0.347, P < 0.001), modified Rankin Scale (r = 0.385, P < 0.001), high-sensitivity C-reactive protein (r = 0.221, P = 0.002) level, and infarct volume (r = 0.201, P = 0.006). Overall survival analysis showed that patients with higher MEG3 expression within 48h had a relatively poor prognosis (P < 0.001). Meanwhile, multivariate analysis revealed that MEG3 was an independent prognostic marker for unfavorable functional outcome and death in IS patients.
Conclusions: This study suggested that MEG3 might be considered as an intervention point and potential prognostic indicator for IS.
Keywords: Ischemic stroke, Prognosis, Long non-coding RNA, Maternally expressed gene 3
Introduction
Ischemic stroke (IS) is the most common type of stroke, accounting for 60%–70% of all strokes1). The mortality rate of IS in China is about 10%, the recurrence rate of survivors is about 40%, and the disability rate is more than 50%1). IS diagnosis is based primarily on imaging and neurological impairment. Unfortunately, current intervention and treatment of IS are still limited, including the following aspects: (1) Ischemic damage in brain tissue is not obvious within 24h after ischemic stroke by computed tomography (CT), and CT is mainly used to quickly eliminate intracranial tumors or cerebral hemorrhage2). (2) Magnetic resonance imaging (MRI) has higher sensitivity and specificity than CT, but most medical institutions in China cannot conduct MRI examinations2). (3) A recent systematic review suggested that the survival rate of IS patients who can receive thrombolytic therapy within 6h of symptom onset can be increased by 46.0%, and the disability rate caused by IS can be reduced by 42.1%3). Unfortunately, only about 2% of patients are able to receive treatment in a timely manner3). An easily accessible test to reflect the severity and prognosis of the disease is pivotal for optimized care and allocation of health care resources to improve the outcome.
Increasing evidence has pointed to a relationship between long non-coding RNA (lncRNA) and cardiovascular disease, including atherosclerosis-related smooth muscle cell (SMC)4), endothelial cell (EC)5), and lipid metabolism regulation6). LncRNA maternally expressed gene 3 (MEG3) is a newly discovered non-coding RNA lying on the strongest genetic susceptibility locus for cardiovascular disease in the chromosome 14q9 7). Previous studies showed that MEG3 is highly expressed in EC, SMC, and immune cells, and may be involved in the atherosclerotic process such as in plaque destabilization7). However, there are a few reports on the basic and clinical evaluation of the prognostic value of MEG3 in IS.
In the present study, oxygen–glucose deprivation (OGD) in human brain microvascular endothelial cells (hBMECs) and middle cerebral artery occlusion (MCAO) in mice were performed to simulate an IS model. MEG3 expression and its prognostic value in IS patients were also investigated. The purpose of this study was to explore potential biomarkers for prognosis assessment of IS.
Materials and Methods
MCAO Model Establishment
Twenty-four ICR mice (30–35 g, 12 males and 12 females) were purchased from the Experimental Animal Center of Zhejiang University School of Medicine (SCXK [Z] 2017-0008; Laboratory animal welfare ethics committee approval number: L201804011). In the process of feeding and experiments with the animals, humane care was given according to the 3R principle. Twenty-four mice were randomly divided into the MCAO group (n = 12) and control group (n = 12). The MCAO group was intraperitoneally injected with 10 mg/kg xylazine. The left middle cerebral artery was permanently blocked by a single-wire nylon suture through the external carotid artery into the internal carotid artery to the origin of the middle cerebral artery. The control group underwent the same surgical procedure except for the suture ligation of the middle cerebral artery. Mice cerebral blood flow was detected by B-ultrasound, and the reduction of hemi-cerebral blood flow confirmed the successful MCAO modeling. At different times after successful modeling, 40-µL tail vein blood of each mouse was collected. Subsequently, both groups were observed for 8 weeks.
Cell Culture and OGD
hBMECs were obtained from the Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences Institute of Neuroscience, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in high-glucose Dulbecco's Modified Eagle Medium (DMEM, Gibco) supplemented with 4 mM glutamine, sodium pyruvate (Sigma, St. Louis, MO, USA), and 10% fetal bovine serum (Gibco, Rockville, MD, USA), and grew in a 37°C incubator with 5% CO2. Cells were treated with OGD exposure, as previously described8). Briefly, cells were cultured in DMEM without glucose (DMEM, Gibco) and were placed in a hypoxia incubator (Thermo Scientific, USA) with 1% O2, 5% CO2, and 94% N2 for different times. Cells maintained under normoxic conditions were used as controls.
Cell Transfection
siRNA for MEG3 and negative control were purchased from Genepharm Co., (Shanghai, China). The transfections were performed using Lipofectamine 2000 (Invitrogen, NY, USA) according to the manufacturer's instructions. Cells were cultured, and the level of MEG3 was examined 48h post-transfection.
Western Blot Analysis
Cells were lysed using Radioimmunoprecipitation Assay Lysis Buffer (Beyotime, China). Equal amounts of proteins (30 mg) were separated by 10% Sodium dodecyl sulfate–Polyacrylamide gel electrophoresis and then transferred to a polyvinyl difluoride membrane. After soaking in Protein-Free Rapid Block Buffer (Beyotime, China), the membrane was incubated overnight at 4 °C with one of the following antibodies: rabbit anti-Bax monoclonal antibody and rabbit anti-β-catenin monoclonal antibody (1:500; Affinity, USA). Then the membrane was incubated with a secondary goat anti-rabbit antibody (1:4000; Affinity, USA) for 1h at room temperature. Finally, the membrane was visualized by ECL-PLU (Amersham Biosciences. Sweden).
Flow Cytometry
Apoptosis was evaluated with Annexin V fluorescein isothiocyanate (FITC)/apoptosis detection kit (Sigma, USA). Cell suspensions were exposed to 5 µL annexin V-FITC and 10 µL propidium iodide for 15 min at 15°C–25°C. Stained cells were carefully analyzed using a BD FACS Calibur™ flow cytometer (BD Biosciences, USA).
Immunofluorescence
hBMECs were fixed with 4% formaldehyde for 15 min, washed with phosphate-buffered saline (PBS) twice, then incubated with 5% bovine serum albumin for 30 min at room temperature and then with rabbit monoclonal antibody against cleaved caspase-3 (1:200; Cell Signaling Technology, USA) overnight at 4°C. After incubating with goat FITC-conjugated anti-rabbit IgG (1:100, Google biological technology, China) for 2h in a dark room, the cells were then incubated with 4′,6-diamidino-2-phenylindole for 2 min at room temperature, washed twice with PBS, and observed under a laser scanning confocal microscope.
Human Study Subjects
We recruited 215 patients (138 men and 77 women; median age: 73, interquartile range [IQR]: 64–81) who were diagnosed as IS between October 2016 and May 2018 at Zhejiang Provincial People's Hospital, Hangzhou, China. The National Institutes of Health Stroke Scale (NIHSS) score was assessed9). IS was classified into three groups: large-artery atherosclerosis (LAA), cardioembolism (CE), and small-vessel occlusion (SVO). MRI with diffusion-weighted imaging was performed in some patients. We also collected 153 age- and gender-matched controls (101 men and 52 women; median age: 71, IQR: 65–76) from the physical examination center. Blood samples were collected after the admission (within 4–12h [n = 64], 12–24h [n = 108], 24–36h [n = 32], 36–48h [n = 11] from symptom onset). Blood samples of controls were collected at the time of physical examination.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Zhejiang Provincial People's Hospital (Hangzhou, China). Written informed consent was provided in accordance with the Declaration of Helsinki.
RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR (qRT-PCR)
The expression level of MEG3 was detected on the Bio-Rad CFX96 (Inc., Hercules, CA, USA) using SYBR Green qPCR Mix according to the manufacturer's procedures. The expression of MEG3 was normalized to Glyceraldehyde-3-Phosphate Dehydrogenase. All the experiments were in triplicates. MEG3 level was calculated using the 2−ΔCt method.
Follow-Up and Endpoints
The prognosis of patients was obtained within 6 months after hospitalization according to the modified Rankin Scale (mRS)10). An unfavorable functional outcome was defined as an mRS score of more than 4. The primary endpoint was an unfavorable functional outcome after 6 months. The secondary endpoint was death within 6 months follow-up.
Statistical Analysis
Statistical analyses were performed using SPSS version 19.0. All data were presented as mean ± standard deviation or median (IQR) or rate (%). The differences between normally distributed numeric variables were evaluated by Student's t-test, whereas non-normally distributed variables were analyzed by Mann–Whitney U-test. Categorical variables were analyzed by χ2 test. Correlations were analyzed using Spearman correlation. Association between IS and levels of MEG3 was calculated through binary logistic regression analysis. The survival curve was calculated by the Kaplan–Meier method. P < 0.05 was considered to be statistically significant.
Results
MEG was Up-Regulated in MCAO Mice
Fig. 1A provides images of mouse brain sections in response to MCAO. After hypoxia-induced ischemic infarct in mice, tail vein blood was collected and analyzed. The production of MEG3 was detected using the qRT-PCR method. In this study, we found that the MEG3 expression in the MCAO group was significantly higher than that in the control group (P = 0.004, Fig. 1B). Besides, in the MCAO group, the survival time of the high MEG3 group was significantly lower than that of the low MEG3 group (Logrank test P = 0.042, Fig. 1C). The expression pattern of MEG3 after the onset of IS showed a rapid increase in the first 3h, then keeping a relatively stable and slow-upward trend within 3 to 48h (Fig. 1D).
Fig. 1.
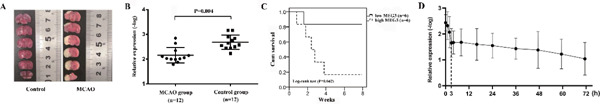
The expression level and prognostic value of maternally expressed gene 3 (MEG3) in mice with middle cerebral artery occlusion (MCAO) (A). Images of mouse brain sections in response to MCAO. (B). MEG3 level in MCAO was significantly higher than that in controls at 48h after ischemic stroke (IS) model establishment. (C). In the MCAO group, the survival time of the high MEG3 group was significantly shorter than that of the low MEG3 group. (D). The expression pattern of MEG3 after IS model establishment.
MEG3 Promoted hBMECs Apoptosis
OGD treatment significantly up-regulated expressions of MEG3, Bax, and cleaved caspase-3, and further promoted apoptosis of hBMECs, while si-MEG3 blocked these effects (Figs. 2A, B, C). MEG3 expression in hBMECs during OGD treatment showed a similar pattern as that in IS mice: increasing sharply in the first 4h, then maintaining stability and gradually increasing within 4 to 48h (Fig. 2D).
Fig. 2.
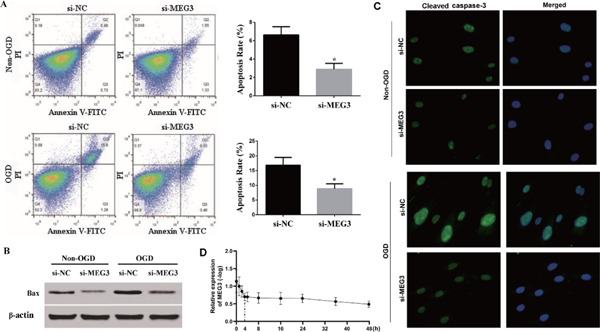
Apoptosis of human brain microvascular endothelial cells (hBMECs) in response to various disturbances (A). Apoptosis of hBMECs in response to various disturbances assessed by flow cytometry. (B). Images of western blot showed that oxygen– glucose deprivation (OGD) treatment up-regulated the expression level of Bax, while it was reversed by si- maternally expressed gene 3 (MEG3). (C). Images of immunofluorescence showed that OGD treatment up-regulated the expression level of cleaved caspase-3, while it was reversed by si-MEG3. (D).The expression pattern of MEG3 after OGD treatment.
MEG3 Expression in Peripheral Blood Leukocytes was Significantly Up-Regulated in IS Patients
We first analyzed the expression level of MEG3 in IS patients admitted to the hospital at different times. Results showed that there was no significant difference of MEG3 levels among different times (P > 0.001, Fig. 3A). MEG3 expression in IS patients was significantly higher than that in controls (P < 0.001, Fig. 3B). Further research indicated that MEG3 expression in the LAA group was higher than that in CE, SVO, and the controls (LAA vs. CE, P = 0.003; LAA vs. SVO, P < 0.001; LAA vs. the controls, P < 0.001, Fig. 3C), and MEG3 level in CE and SVO was significantly higher than that in the controls (CE vs. the controls, P < 0.001; SVO vs. the controls, P < 0.001, Fig. 3C). However, upon the comparison of the level in the CE and SVO groups, no marked difference was found. Of note, when MEG3 expression was divided into quartiles, we also found that the proportion of LAA strokes increased according to the elevated level of MEG3 (P < 0.001, Table 1).
Fig. 3.
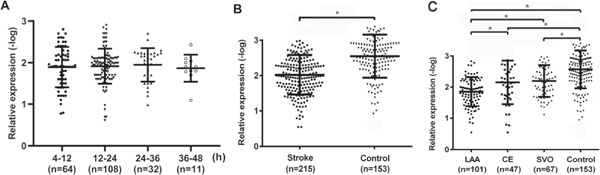
The maternally expressed gene 3 (MEG3) expression in peripheral blood leukocytes among subgroups (A). The expression level of MEG3 of IS patients admitted to hospital at different times. (B). MEG3 expression in ischemic stroke (IS) patients was significantly higher than that in the controls. (C). MEG3 expression in large-artery atherosclerosis (LAA) was significantly higher than that in cardioembolism (CE), small-vessel occlusion (SVO), and the controls, and MEG3 expression in CE and SVO was significantly higher than that in the controls. *P < 0.05.
Table 1. Participants' characteristics according to the quartiles of MEG3.
| Subject groups | Quartiles of MEG3, range, relative expression (-log), N = 368 |
||||
|---|---|---|---|---|---|
| 1st, < 1.722 | 2nd, 1.722–2.065 | 3rd, 2.065–2.455 | 4th, > 2.455 | P | |
| LAA | 40 (43.5%) | 33 (35.9%) | 24 (26.0%) | 4 (4.3%) | < 0.001 |
| CE | 11 (12.0%) | 9 (9.8%) | 11 (12.0%) | 16 (17.5%) | 0.458 |
| SVO | 14 (15.2%) | 18 (19.6%) | 15 (16.3%) | 20 (21.7%) | 0.648 |
| Control | 27 (29.3%) | 32 (34.7%) | 42 (45.7%) | 52 (56.5%) | 0.001 |
Abbreviation: LAA: large-artery atherosclerosis; CE: cardioembolism; SVO: small-vessel occlusion.
In univariate analysis, increased MEG3 (P < 0.001, odds ratio [OR] = 1.409, 95% confidence interval [CI]: 1.144–1.675) level showed significant association with IS. After adjusting for relevant clinical and laboratory variables, elevated MEG3 (P < 0.001, adjusted OR = 1.268, 95% CI: 1.088–1.450) remained significant with regard to increased odds of having IS.
Correlation between MEG3 Expression and Clinical Variables
We analyzed the correlation between MEG3 expression and clinical parameters in the 215 IS patients. As shown in Fig. 4, the MEG3 expression was significantly correlated with NIHSS scores (r = 0.347, P < 0.001, Fig. 4A), mRS (r = 0.385, P < 0.001, Fig. 4C) and high-sensitivity C-reactive protein (hs-CRP; r = 0.221, P = 0.002, Fig. 4D). Moreover, there was a significant positive association between MEG3 level and infarct volume (r = 0.201, P = 0.006, Fig. 4B).
Fig. 4.
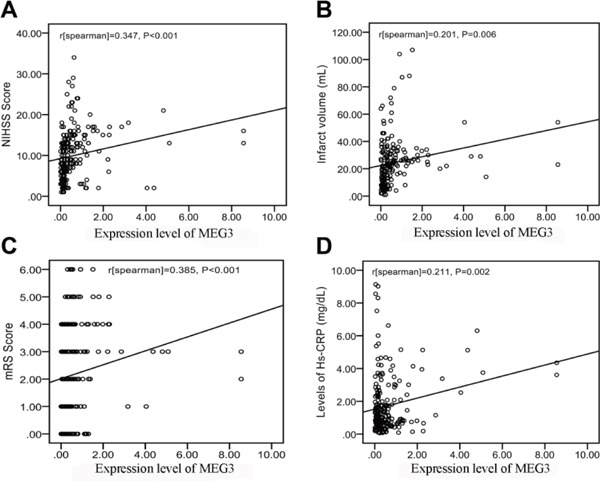
Correlation between maternally expressed gene 3 (MEG3) level and other predictors (A) Correlation between MEG3 level and the National Institutes of Health Stroke Scale (NIHSS) score. (B) Correlation between MEG3 level and infarct volume. (C) Correlation between MEG3 level and the modified Rankin Scale (mRS) score. (D) Correlation between MEG3 level and the level of high-sensitivity C-reactive protein (hs-CRP).
MEG3 Expression and 6-Month Functional Outcome
In our study, an unfavorable functional outcome was found in 84 patients (39.1%) with a median mRS score of 4 (IQR 3–5). In the 84 patients with an unfavorable functional outcome, MEG3 level was higher compared with those in patients with a favorable outcome (Fig. 5A). In univariate logistic regression analysis, MEG3 level, as compared with Hs-CRP, the NIHSS score, and other risk factors, are presented in Table 2. With an unadjusted OR of 2.504 (95% CI: 1.147–5.466, P < 0.001), MEG3 had a strong association with unfavorable functional outcome. After adjusting for all other significant outcome predictors, MEG3 remained an independent unfavorable outcome predictor with an adjusted OR of 1.998 (95% CI 1.144–3.491, P < 0.001). In addition, the NIHSS score and hs-CRP remained significant outcome predictors, unlike all others assessed (Table 2).
Fig. 5.
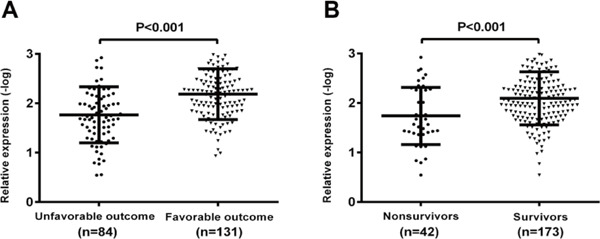
The maternally expressed gene 3 (MEG3) expression in peripheral blood leukocytes among subgroups (A). MEG3 level in patients with unfavorable outcomes was significantly higher than that in patients with favorable outcomes. (B). MEG3 level in non-survivors was significantly higher than that in survivors.
Table 2. Univariate and multivariate logistic regression analyses for outcome and mortality.
| Parameter | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI)a | P | OR (95% CI)a | P | |
| Predictor: functional outcome | ||||
| Age | 1.001 (0.970, 1.032) | 0.995 | ||
| Male sex | 1.450 (0.658, 3.193) | 0.356 | ||
| Family history | 3.747 (0.763, 18.403) | 0.104 | ||
| BMI (kg/m2) | 1.060 (0.923, 1.217) | 0.410 | ||
| NIHSS | 1.641 (1.323, 1.979) | 0.021 | 1.297 (1.184, 1.425) | 0.015 |
| MEG3b | 2.504 (1.147, 5.466) | < 0.001 | 1.998 (1.144, 3.491) | < 0.001 |
| Hypertension | 1.663 (0.779, 3.549) | 0.189 | ||
| Hypercholesterolemia | 1.559 (0.695, 3.495) | 0.281 | ||
| Diabetes mellitus | 0.786 (0.518, 1.380) | 0.077 | ||
| Smoking | 1.121 (0.473, 2.655) | 0.796 | ||
| Alcoholism | 0.766 (0.306, 1.915) | 0.568 | ||
| Leucocyte count (× 109 /L) | 1.077 (0.952, 1.161) | 0.402 | ||
| Hs-CRP (mg/dL) | 1.927 (1.510, 2.466) | < 0.001 | 1.433 (1.168, 1.754) | 0.011 |
| Predictor: death | ||||
| Age | 0.999 (0.962, 1.038) | 0.972 | ||
| Male sex | 0.788 (0.401, 1.534) | 0.480 | ||
| Family history (Yes) | 2.776 (0.545, 14.151) | 0.219 | ||
| BMI (kg/m2) | 1.029 (0.875, 1.211) | 0.728 | ||
| NIHSS | 1.008 (1.002, 1.015) | 0.014 | 1.002 (0.997, 1.009) | 0.181 |
| MEG3b | 1.638 (1.202, 2.232) | 0.002 | 1.512 (1.058, 2.159) | 0.023 |
| Hypertension | 1.023 (0.995, 1.035) | 0.152 | ||
| Hypercholesterolemia | 1.236 (0.467, 3.272) | 0.670 | ||
| Diabetes mellitus | 0.979 (0.362, 2.650) | 0.967 | ||
| Smoking | 0.924 (0.335, 2.547) | 0.879 | ||
| Alcoholism | 2.032 (0.603, 6.846) | 0.253 | ||
| Leucocyte count (× 109 /L) | 1.056 (0.951, 1.174) | 0.450 | ||
| Hs-CRP (mg/dL) | 1.261 (1.061, 1.499) | 0.009 | 1.276 (1.040, 1.567) | 0.020 |
Abbreviation: OR: odds ratio; CI: confidence interval; BMI: body mass index; NIHSS: National Institutes of Health Stroke Scale; Hs-CRP: high sensitivity C-reactive protein. P < 0.05 was considered statistically significant.
Note that the odds ratio corresponds to a unit increase in the explanatory variable
Log-transformed to achieve normal distribution. Note that the odds ratio corresponds to a log unit increase in the explanatory variable
MEG3 Expression and 6-Month Mortality
At 6 months, 42 patients (19.5%) had died. Nonsurvivors had significantly higher MEG3 levels than survivors (Fig. 5B). In univariate logistic regression analysis, MEG3 level, as compared with hs-CRP, the NIHSS score, and other risk factors, are presented in Table 2. With an unadjusted OR of 1.638 (95% CI 1.202–2.232, P = 0.002), MEG3 level was an independent predictor for mortality. After adjusting for all other significant outcome predictors, MEG3 remained an independent mortality predictor with an adjusted OR of 1.512 (95% CI 1.058–2.159, P = 0.023). In addition, the NIHSS score and hs-CRP remained significant outcome predictors, unlike all others assessed (Table 2).
To answer whether MEG3 expression correlated to the death of IS patients, the 215 IS patients were divided into two groups: high MEG3 group (n = 108, MEG3 expression ≥ median) and low MEG3 group (n = 107, MEG3 expression < median) according to the median expression of MEG3. Time to death was calculated by the Kaplan–Meier method and log-rank test. Significantly, patients in the high-MEG3 group had a higher risk of death compared with the low-MEG3 group (Log-rank test P < 0.001, Fig. 6).
Fig. 6.
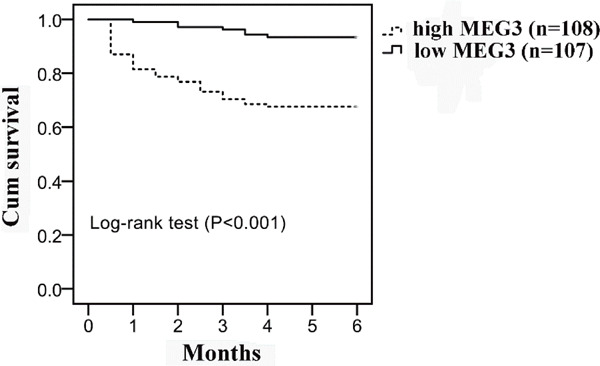
Kaplan–Meier survival curves for maternally expressed gene 3 (MEG3) Patients in the high MEG3 group had a higher risk of death compared with low MEG3 group. Data analyzed using log-rank test.
Discussion
lncRNA has drawn considerable attention for its effects on neurological disorders. We found that the level of MEG3 in the MCAO group was significantly higher than that in the control group, and the survival time of the high MEG3 group was significantly lower than that of the low MEG3 group. Results also suggested that OGD up-regulated the expressions of Bax and cleaved caspase-3, and promoted apoptosis of vascular ECs, while si-MEG3 reversed the effects of OGD treatment. Moreover, we also found that the MEG3 level in peripheral blood leukocytes was significantly higher in IS patients after adjustment for relevant covariates. When further considering the subtypes of IS, we documented that MEG3 had differential association patterns among stroke subtypes. LAA stroke was associated with the highest expression of MEG3, and MEG3 levels in CE and SVO were also significantly higher than that in the controls. Meanwhile, our data showed that MEG3 expression was related to infarct volume and stroke severity. In addition, compared with the low MEG3 group, the increased levels of MEG3 were associated with a poor prognosis and a shorter survival time.
It is generally accepted that genetic variants on chromosome 14q9 are associated with the risk of coronary artery disease. Of note, MEG3 expression has been elaborated to be affected by several polymorphisms in the 14q9 locus, and this differential expression may regulate the expression levels of genes involved in several atherogenic pathways, in turn, finally impact on the risk of cardiovascular disease11). An explanation interpretation of the expression profiling results is the role of MEG3 in coordinating vascular remodeling. In our study, we found that the expression level of MEG3 was associated with OGD, and with the time of treatment prolonged, the higher expression level was observed. In other words, MEG3 level may reflect the severity of ischemia, and this is in line with the results that MEG3 level is positively correlated with NIHSS scores, mRS, hs-CRP levels, and infarct volume in IS patients. Considering that the prognosis of IS is strongly associated with the severity of ischemia, these results all suggested that MEG3 may be used to indicate the prognosis of IS. Besides, our study indicated that the local environment chemicals, especially sugar content and oxygen content, regulated the expression of MEG3. Highly expressed MEG3 inhibits two important anti-apoptotic proteins, BCL-2-related protein A1 and baculoviral IAP repeat-containing 3 12) on the one hand, and the other hand, as our results showed, MEG3 can also stimulate expressions of apoptosis-related proteins, such as Bax and cleaved caspase-3, thus promoting apoptosis of vascular cells. Apoptosis of vascular cells can perform major changes in arterial architecture, especially when coordinated with matrix turnover and vascular cell proliferation. Besides, Huang et al. had proven that matrix turnover and vascular cell proliferation were also affected by MEG3 by regulating expressions of matrix metalloproteinase-3 and Heparin-binding epidermal growth factor-like growth factor11). Moreover, recent studies13) revealed that MEG3 may be also involved in some other aspects of stroke development, such as thrombogenesis and plaque destabilization.
Another valuable point we have found in this study is that the level of MEG3 was dramatically changed within 4h of the onset of IS, and maintaining a relatively stable value within 4 to 48h. This will allow us to have plenty of time to diagnose patients, and the feasibility and significance of detecting MEG3 were also guaranteed.
This study has several limitations. First, the sample size is relatively small; therefore, the present findings should be validated in trials with more cases in the future. Second, this study was conducted in a single hospital, thereby being prone to selection bias. Multi-center research is still needed to further validate our conclusions. Third, the current study did not explore the underlying mechanism of how MEG3 was regulated by OGD; thus, corresponding cell and animal experiments can be designed for further exploration.
Conclusion
The present study found that MEG3 expression was sharply up-regulated and maintained at a stable level within 4–48h of the onset of IS. We also found that MEG3 level in peripheral blood leukocytes was positively correlated with the severity of the disease and predicted poor prognoses of IS. The potential mechanism may be that OGD stimulated the expression of MEG3, and MEG3 further induced the expressions of apoptosis-related proteins, such as Bax and cleaved caspase-3, thus promoting the apoptosis of vascular ECs and aggravates hypoxia in brain tissue. These findings indicated that MEG3 could be served as an intervention target for IS therapy, and peripheral blood leukocytes MEG3 may be used as a novel prognostic marker for IS. Future studies are warranted to test the exact molecular mechanism and to verify the clinical values of MEG3 in IS.
Acknowledgments
We thank all of the subjects enrolled in this study.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Zhejiang Provincial People's Hospital (Hangzhou, China). Written informed consent was provided in accordance with the Declaration of Helsinki. All animal experiments and surgical procedures were approved by the Experimental Animal Center of Zhejiang University School of Medicine (L201804011).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
None.
References
- 1). Kim BJ, Lee SH, Ryu WS, Kim CK, Yoon BW. Adipocytokines and ischemic stroke: differential associations between stroke subtypes. J Neurol Sci, 2012; 312: 117-122 [DOI] [PubMed] [Google Scholar]
- 2). Zhu M, Zuo J, Shen J, Jing W, Luo P, Li N, Wen X, Wang C, Yu M, Liang C, Tu J. Diagnostic Potential of Differentially Expressed Homer1 and Homer2 in Ischemic Stroke. Cell Physiol Biochem, 2016; 39: 2353-2363 [DOI] [PubMed] [Google Scholar]
- 3). Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, Cohen G. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet, 2012; 379: 2364-2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Motterle A, Pu X, Wood H, Xiao Q, Gor S, Ng FL, Chan K, Cross F, Shohreh B, Poston RN, Tucker AT, Caulfield MJ, Ye S. Functional analyses of coronary artery disease associated variation on chromosome 9p21 in vascular smooth muscle cells. Hum Mol Genet, 2012; 21: 4021-4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Kurian L, Aguirre A, Sancho-Martinez I, Benner C, Hishida T, Nguyen TB, Reddy P, Nivet E, Krause MN, Nelles DA, Esteban CR, Campistol JM, Yeo GW, Belmonte JCI. Identification of novel long noncoding RNAs underlying vertebrate cardiovascular development. Circulation, 2015; 131: 1278-1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Halley P, Kadakkuzha BM, Faghihi MA, Magistri M, Zeier Z, Khorkova O, Coito C, Hsiao J, Lawrence M, Wahlestedt C. Regulation of the apolipoprotein gene cluster by a long noncoding RNA. Cell Rep, 2014; 6: 222-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Bai Y, Zhang Q, Su Y, Pu Z, Li K. Modulation of the Proliferation/Apoptosis Balance of Vascular Smooth Muscle Cells in Atherosclerosis by lncRNA-MEG3 via Regulation of miR-26a/Smad1 Axis. Int Heart J, 2019; 60: 444-450 [DOI] [PubMed] [Google Scholar]
- 8). Mo ZT, Fang YQ, He YP, Zhang S. Beta-Asarone protects PC12 cells against OGD/R-induced injury via attenuating Beclin-1-dependent autophagy. Acta Pharmacol Sin, 2012; 33: 737-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Brott T, Marler JR, Olinger CP, Jr., Adams HP, Tomsick T, Barsan WG, Biller J, Eberle R, Hertzberg V, Walker M. Measurements of acute cerebral infarction: lesion size by computed tomography. Stroke, 1989; 20: 871-875 [DOI] [PubMed] [Google Scholar]
- 10). Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke, 1988; 19: 1497-1500 [DOI] [PubMed] [Google Scholar]
- 11). Huang P, Huang FZ, Liu HZ, Zhang TY, Yang MS, Sun CZ. LncRNA MEG3 functions as a ceRNA in regulating hepatic lipogenesis by competitively binding to miR-21 with LRP6. Metabolism, 2019; 94: 1-8 [DOI] [PubMed] [Google Scholar]
- 12). Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci, 2006; 43: 1-67 [DOI] [PubMed] [Google Scholar]
- 13). Holdt LM, Sass K, Gäbel G, Bergert H, Thiery J, Teupser D. Expression of Chr9p21 genes CDKN2B (p15(INK4b)), CDKN2A (p16(INK4a), p14(ARF)) and MTAP in human atherosclerotic plaque. Atherosclerosis, 2011; 214: 264-270 [DOI] [PubMed] [Google Scholar]


