Abstract
Aim: So far, the mechanisms behind the cardiovascular benefits of sodium/glucose cotransporter 2 (SGLT2) inhibitors have not been fully clarified.
Methods: In order to evaluate the effects of SGLT2 inhibitors on systemic hemodynamics, glucose metabolism, lipid profile, and endothelial function, 50 diabetic patients with established coronary artery disease (CAD) were included in this analysis and were given empagliflozin 10 mg/d. Cookie meal testing (carbohydrates: 75 g, fats: 28.5 g), endothelial function testing using flow-mediated dilatation (FMD), and body composition evaluation were performed before and after six months of treatment. Changes in %FMD between the treatment periods and its association with metabolic biomarkers were evaluated.
Results: After six months of treatment, the body weight and body fat percentage decreased significantly, while the body muscle percentage increased significantly. The hemoglobin A1c level and fasting and postprandial plasma glucose levels were significantly decreased with treatment. Postprandial insulin secretion was also significantly suppressed and the insulin resistance index was significantly decreased. Furthermore, the fasting and postprandial triglyceride (TG) levels decreased significantly, while total ketone bodies increased significantly after the six-month treatment. While the plasma brain natriuretic peptide level was not changed, the C-reactive protein level was decreased and FMD was significantly improved after the six-month treatment. Multiple regression analysis showed that the strongest predictive factor of FMD improvement is change in the plasma TG levels.
Conclusion: SGLT2 inhibitors improve multiple metabolic parameters. Of these, a reduction in plasma TGs was strongly associated with endothelial function recovery in diabetic patients with CAD, and this reduction may be related to the cardiovascular benefits of SGLT2 inhibitors.
Keywords: Diabetes mellitus, Sodium/glucose transporter 2 inhibitors, Triglycerides, Endothelial function, Flow-mediated dilatation
See editorial vol. 27: 637–638
Introduction
Type 2 diabetes mellitus (T2DM) is a prevalent disease that is recognized as a major risk factor for coronary artery disease (CAD)1, 2). The selective inhibitors of sodium/glucose cotransporter 2 (SGLT2) are glucose-lowering agents that target the kidney to reduce the reabsorption of glucose and promote urinary glucose excretion, and they have been approved for T2DM treatment in numerous countries3). In previous large studies, BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME)4) and the Canagliflozin Cardiovascular Assessment Study (CANVAS) trial5), it has been demonstrated that SGLT2 inhibitors were the first glucose-lowering agents to reduce both cardiovascular deaths and the high risk of cardiovascular diseases in patients with T2DM.
Because the EMPA-REG OUTCOME and CANVAS trials were not designed to determine the mechanism underlying their results, several explanations for the reduction in cardiovascular deaths with SGLT2 inhibitors have been proposed, including improved glycemic and lipid control, reduction in body weight, lowering of blood pressure, and amelioration of albuminuria6–8). However, the precise mechanisms behind the cardiovascular benefits of SGLT2 inhibitors have not been fully clarified.
Impaired endothelial function may play an important role in the initiation and progression of atherosclerosis9). Flow-mediated dilatation (FMD) in the brachial artery, which is a noninvasive parameter, is considered to be the current gold-standard surrogate marker of coronary endothelial function, and it has been demonstrated in a previous study that brachial FMD is a predictor of the severity of CAD, coronary plaque vulnerability, and cardiovascular events10–13).
Aim
In this study, we surrogated brachial FMD as the incidence of cardiovascular events and conducted an exploratory study to evaluate the effects of an SGLT2 inhibitor, empagliflozin, on systemic hemodynamics, glucose metabolism, lipid profile, and endothelial function.
Methods
Participants
This study was an open-label, single-arm, prospective observational study. It was approved by the Ethics Committee of Hyogo Prefectural Himeji Cardiovascular Center and complied with the Declaration of Helsinki. Informed consent was obtained from all eligible patients before participation.
A total of 56 Japanese patients with T2DM with documented chronic CAD were enrolled in this study from our outpatients between April 2017 and March 2018. The inclusion criteria were as follows: (1) SGLT2 inhibitor-naïve patients with T2DM who had been treated for more than 12 weeks with some antidiabetic drugs or diet and exercise; (2) patients with a hemoglobin A1c (HbA1c) (National Glycohemoglobin Standardization Program, NGSP) level of < 8.0%; and (3) males and females aged 40–90. CAD was defined as stenosis of > 50% of the diameter of a coronary artery on angiography or on computed tomography coronary angiography, or a history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass surgery. T2DM was diagnosed according to the Japan Diabetes Society criteria (HbA1c ≥ 6.4%, fasting plasma glucose [PG] ≥ 126 mg/dL, and PG ≥ 200 mg/dL at 2 h after the oral glucose tolerance test).
The exclusion criteria were as follows: (1) patients who, within 12 weeks of enrollment, had started or had had the dose changed or had been on any concomitant drug, such as antidiabetic drugs, statins, or antihypertensive drugs; (2) patients treated with insulin or glucagon-like peptide-1 agonists; (3) patients with type 1 DM or secondary DM; (4) patients with systemic diseases, including hepatic diseases, renal diseases (serum creatinine level > 2.0 mg/dL), collagen diseases, infections, malignancies, or acute coronary syndrome; and (5) patients with permanent implanted pacemakers, implanted cardiac defibrillators, or cardiac resynchronization therapy devices.
All patients met, at least once, with a dietician for nutritional guidance and were encouraged to start and maintain a low-calorie diet and mild-to-moderate exercise levels before enrollment in the study. At enrollment, we added empagliflozin 10 mg/d to the regimen, which was maintained for six months. At each visit, we asked the participants about any adverse events and medication compliance. In addition, all participants were prohibited from changing the dose of the concomitant drugs or adding any other drugs throughout the study period if no adverse effect occurred.
Measurements of the Body Composition
Body composition was measured using InBody 770 (InBody Co., Ltd., Seoul, South Korea) at enrollment and after six months of treatment. InBody 770 sends a very weak alternating current through the body, which is resisted by the body's tissues. This machine, InBody 770, divides the human body into five cylinders (right and left arms, right and left legs, and trunk) to increase the accuracy of the measurement and delivers currents of 50–1,000 kHz14, 15). Participants are asked to stand barefoot on the balance scale and to hold the handles of the machine. The output of the measurements, which takes approximately 15 s, is then printed.
Blood Biochemistry
Blood samples were collected from all patients after an overnight fast and were used to determine PG, HbA1c, 1,5-anhydroglucitol, immunoreactive insulin (IRI), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), remnant-like particle cholesterol (RLP-C) as a marker of postprandial hyperlipidemia16), total ketone body fraction, brain natriuretic peptide (BNP) as a marker of heart failure, C-reactive protein (CRP) as an inflammatory marker, and plasminogen activator inhibitor-1 (PAI-1) as a marker of thrombotic diathesis.
The change in plasma volume can be used for prognostication in acute decompensated heart failure, and whole body plasma volume loss may be one of the possible mechanisms for the beneficial effects of SGLT2 inhibitors for cardiovascular diseases17, 18). The Strauss–Davis–Rosenbaum formula is an easily accessible marker of estimating the percentage change in plasma volume in the clinical setting. Therefore, we calculated the change in plasma volume according to the following formula: Change in plasma volume=([(baseline hemoglobin level/hemoglobin level after six months of treatment) × (100 − hematocrit value after six months of treatment)/(100 − baseline hematocrit value)] − 1) × 10019).
All biochemical analyzes were performed using a commercially available kit. HbA1c levels were measured using high-performance liquid chromatography. IRI and BNP concentrations were measured using a chemiluminescent enzyme immunoassay. RLP-C was measured using an assay kit (Japan Immunoresearch Laboratories Co., Ltd., Takasaki, Japan). The total ketone body fraction level was measured by enzyme circulating methods (Kinos Inc., Tokyo, Japan). PAI-1 was measured using a latex photometric immunoassay.
Cookie Meal Test
A cookie meal test (CMT) was performed before and six months after empagliflozin treatment to evaluate the changes in postprandial glucose, insulin, and fat metabolic dynamics. The cookie consisted of carbohydrates (75 g; 85% flour starch, 15% maltose), butter fat (28.5 g), and protein (8 g), with an overall caloric value of 592 kcal (Saraya Corp., Osaka, Japan). As previously reported, the CMT is sufficient for providing information regarding glucose intolerance and postprandial hypertriglyceridemia20).
All participants completed a 12 h overnight fast before the CMT, as previously described17). Briefly, the cookie was ingested with water within a 20 min period, and blood samples for PG, IRI, and TG measurement were obtained at 0, 1, and 2 h after the participant had ingested half the volume of the cookie. These values were reported as PG-1h, PG-2h, IRI-1h, IRI-2h, TG-1h, and TG-2h, respectively.
The homeostatic model assessment ratio (HOMA-R= fasting IRI × fasting PG/405) was used as an index of insulin resistance.
We also calculated the area under the response curves for PG (AUC-PG), IRI (AUC-IRI), and TG (AUC-TG) using the trapezoid rule. As an index of postprandial hyperglycemia, we calculated the incremental AUC-PG (AUC-PG − fasting PG × 2). In order to assess the postprandial TG change, we calculated incremental AUC-TG (AUC-TG − fasting TG × 2).
Measurement of Endothelial Function
Before undergoing FMD testing, the participants were instructed to fast for > 12 h and to abstain from any medications, smoking, alcohol, caffeine, and antioxidant vitamins during that time. All the participants rested for at least 15 min in a seated position in a quiet dark air-conditioned room (22–25°C) before the FMD measurements, as previously described12, 21). In brief, a longitudinal image of the right or left brachial artery was recorded at baseline using high-resolution ultrasonography and a 10 MHz linear array transducer probe (UNEX, Nagoya, Japan). A forearm cuff was inflated for 5 min at 50 mmHg above the systolic blood pressure just prior to the FMD measurement. After deflating the cuff, the diastolic diameter of the brachial artery was semiautomatically and continuously recorded for 2 min using a softwareequipped instrument that can monitor the arterial diameter. The %FMD was estimated as the percent change in the vessel diameter, which corresponded to the maximum dilatation reached during reactive hyperemia divided by the baseline value. Because the %FMD value is highly dependent on the baseline diameter of the vessel, we compared the baseline diameters and absolute changes in the diameters of the brachial artery for each group. We have also previously confirmed that there is excellent intra- and interobserver agreement for the %FMD measurement12, 21, 22).
Primary Endpoints
The primary endpoint of this study was the change in %FMD at enrollment and after six months of empagliflozin treatment. We also evaluated the association between changes in %FMD and changes in metabolic biomarkers.
Statistical Analysis
The sample size was determined by the paired samples t-test power analysis using previously reported data with the following assumptions: a type I error of 0.05 (two-tailed), a type II error of 0.1, a 1.0% difference in %FMD before and after empagliflozin treatment, and a standard deviation of 2.0%23). Therefore, a minimum of 44 patients are required to yield 90% power to detect a significant difference between before and after treatment.
All statistical analyzes were performed using MedCalc (Version 9.3; MedCalc Software, Mariakerke, Belgium), and a two-tailed P-value of < 0.05 was considered statistically significant.
Continuous variables with a normal distribution were reported as mean ± standard deviation, or otherwise as median and interquartile ranges. Intergroup comparisons of normally distributed data were performed using the unpaired Student's t-test. The Mann–Whitney U test was used if the variables were not normally distributed. Comparison of categorical variables was performed using the χ2 test. Differences in normally distributed biochemical data before and after treatment were compared using the paired t-test; otherwise, the Wilcoxon rank-sum test was used. Multivariable regression analyzes were used to identify independently associated factors of changes in %FMD. Potential factors of interest with a univariate P-value of < 0.05 were entered into the multivariate models.
Results
Study Population
A total of 56 diabetic patients with CAD were enrolled in this study. Of these, one patient had hypoglycemic attacks, one had a gastrointestinal disorder, and four withdrew their consent before the second CMT. Therefore, six patients were excluded from the final analyzes, leaving 50 patients.
Table 1 shows the baseline characteristics of the participants. The concomitant coronary risk factors were hypertension (90.0%), dyslipidemia (98.0%), and smoking (8.0%). The combined medications were statins (98.0%), n-3 polyunsaturated fatty acids (32.0%), angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (72.0%), dipeptidyl peptidase-4 inhibitors (52.0%), metformin/thiazolidine (20.0%), sulfonylurea agent (12.0%), and alpha glucosidase inhibitors (10.0%). Notably, all patients had CAD and multiple coronary risk factors (two or more coronary risk factors).
Table 1. Baseline characteristics.
| Variables | n = 50 |
|---|---|
| Age (y.o) | 66.5 ± 9.4 |
| Sex (Male, n, %) | 44 (88.0%) |
| Coronary risk factor | |
| Hypertension (n, %) | 45 (90.0%) |
| Dyslipidemia (n, %) | 49 (98.0%) |
| Current smoker (n, %) | 4 (8.0%) |
| Combined drugs | |
| Statins (n, %) | 49 (98.0%) |
| n-3 polyunsaturated fatty acids (n, %) | 16 (32.0%) |
| Calcium channel blocker (n, %) | 20 (40.0%) |
| Angiotensin converting enzyme inhibitors / angiotensin-receptor blocker (n, %) | 36 (72.0%) |
| Dipeptidyl peptidase 4 inhibitors (n, %) | 26 (52.0%) |
| Metformin / thiazolidine (n, %) | 10 (20.0%) |
| Sulfonylurea agent (n, %) | 6 (12.0%) |
| Alpha glucosidase inhibitors (n, %) | 5 (10.0%) |
y.o: years old
Changes in Anthropometric Measurements and Body Composition
As shown in Table 2, the body weight and body mass index were significantly decreased after the sixmonth empagliflozin treatment (P < 0.0001 and P < 0.001, respectively). Although the total fat quantity, fat percentage, total muscle quantity, and whole body water volume (P < 0.001, P < 0.0001, P < 0.0001, and P < 0.0001, respectively) were also decreased significantly, the extracellular water volume did not change and the muscle percentage was significantly increased (P < 0.0001). Furthermore, the systolic and diastolic blood pressures were significantly decreased during the six months (P = 0.03, P = 0.03).
Table 2. Changes in anthropometric measurements and body compositions, %flow-mediated dilatation and blood chemistry data.
| Variable | Baseline | 6-month | P-value |
|---|---|---|---|
| Body composition data and flow-mediated dilatation data | |||
| Weight (kg) | 68.6 ± 9.9 | 65.3 ± 9.7 | < 0.0001 |
| Body mass index (kg/m2) | 24.9 ± 3.0 | 23.7 ± 3.1 | < 0.0001 |
| Body fat quantity (kg) | 18.3 ± 5.1 | 15.9 ± 5.2 | < 0.0001 |
| Body fat percentage (%) | 26.9 ± 5.7 | 24.6 ± 6.3 | < 0.0001 |
| Muscle quantity (kg) | 46.9 ± 7.2 | 45.8 ± 6.9 | < 0.0001 |
| Muscle percentage (%) | 69.1 ± 5.5 | 71.3 ± 6.1 | < 0.0001 |
| Whole body water volume (L) | 36.5 ± 5.9 | 35.7 ± 5.4 | < 0.0001 |
| Extracellular water volume (L) | 13.9 ± 2.1 | 13.7 ± 2.0 | 0.17 |
| %change plasma volume (%) | −6.9 (−11.0, −1.3) | ||
| Systolic blood pressure (mmHg) | 133.8 ± 15.1 | 130.6 ± 14.3 | 0.03 |
| Diastolic blood pressure (mmHg) | 80.3 ± 10.3 | 78.4 ± 9.5 | 0.03 |
| %flow-mediated dilatation (%) | 3.5 (2.4, 5.5) | 4.7 (3.6, 5.6) | 0.001 |
| Absolute change in diameter (mm) | 0.17 ± 0.08 | 0.19 ± 0.08 | 0.001 |
| Blood chemistry data | |||
| White blood cell (× 102/µL) | 61.6 ± 14.6 | 60.7 ± 15.4 | 0.67 |
| Red blood cell (× 104/µL) | 476.2 ± 36.9 | 498.0 ± 38.4 | < 0.0001 |
| Hemoglobin (g/dL) | 14.6 ± 1.1 | 15.0 ± 1.2 | < 0.0001 |
| Hematocrit (%) | 43.3 ± 2.9 | 44.8 ± 3.0 | 0.0001 |
| Platelet (× 104/µL) | 21.7 ± 4.6 | 20.5 ± 4.5 | 0.001 |
| Plasma creatinine (mg/dL) | 0.82 ± 0.16 | 0.84 ± 0.17 | 0.07 |
| e-GFR (mg/dL) | 73.4 ± 14.6 | 71.3 ± 14.0 | 0.06 |
| Urine albumin (mg/dL) | 16.8 (7.2, 52.7) | 11.3 (7.4, 25.1) | 0.01 |
| Uric acid (mg/dL) | 5.4 ± 1.3 | 4.7 ± 1.1 | < 0.0001 |
| Hemoglobin A1c (%) | 7.0 ± 0.5 | 6.6 ± 0.4 | < 0.0001 |
| 1,5-anhydroglucitol (µg/dL) | 11.1 ± 5.7 | 2.1 ± 2.0 | < 0.0001 |
| Plasma glucose level (mg/dL) | |||
| Fasting | 129.4 ± 16.1 | 114.8 ± 18.7 | < 0.0001 |
| After 1 hour | 238.7 ± 33.4 | 217.2 ± 33.6 | < 0.0001 |
| After 2 hours | 235.9 ± 46.4 | 213.7 ± 51.1 | 0.001 |
| Incremental AUC-PG (mg/dL/2 hr) | 162.6 ± 46.4 | 151.9 ± 43.4 | 0.07 |
| Immunoreactive insulin (µU/mL) | |||
| Fasting | 7.8 (4.6, 9.5) | 5.0 (3.2, 8.5) | < 0.0001 |
| After 1 hour | 40.9 (25.0, 60.3)3 | 3.5 (18.2, 59.8) | 0.0005 |
| After 2 hours | 49.2 (28.3, 79.9) | 43.0 (23.5, 60.5) | 0.0006 |
| Incremental AUC-IRI (µU/mL/2 hr) | 54.7 (30.7, 88.7) | 46.3 (25.8, 78.8) | 0.0002 |
| HOMA-R | 2.4 (1.4, 3.2) | 1.3 (0.9, 2.3) | < 0.0001 |
| Total cholesterol (mg/dL) | 164.9 ± 25.6 | 163.6 ± 29.7 | 0.62 |
| LDL cholesterol (mg/dL) | 90.6 ± 23.5 | 90.8 ± 24.1 | 0.92 |
| HDL cholesterol (mg/dL) | 51.1 ± 19.6 | 51.1 ± 9.4 | 0.99 |
| Plasma triglyceride (mg/dL) | |||
| Fasting | 119.9 ± 54.9 | 95.7 ± 33.5 | 0.0002 |
| After 1 hour | 142.5 ± 53.5 | 122.6 ± 41.0 | 0.001 |
| After 2 hours | 184.3 ± 70.2 | 153.5 ± 52.8 | < 0.0001 |
| Incremental AUC-TG (mg/dL/2 hr) | 54.7 ± 34.8 | 55.8 ± 32.6 | 0.80 |
| RLP cholesterol (mg/dL) | 5.0 (3.3, 7.0) | 3.8 (2.4, 4.9) | < 0.0001 |
| C reactive protein (mg/dL) | 0.11 (0.03, 0.22) | 0.07 (0.03, 0.13) | 0.003 |
| Brain natriuretic peptide (pg/mL) | 31.3 (14.4, 58.6) | 20.6 (9.2, 54.6) | 0.37 |
| PAI-1 (ng/mL) | 18.6 ± 7.8 | 13.8 ± 5.0 | < 0.0001 |
| Total ketone body (µmol/L) | 89.0 (61.0, 152.0) | 145.5 (94.0, 218.0) | 0.0002 |
e-GFR: estimate glomerular filtration rate, AUC-PG: area under the curve of plasma glucose, HOMA-R: homeostasis model assessment insulin resistance, AUC-IRI: area under the curve of immunoreactive insulin, LDL: low density lipoprotein, HDL: high density lipoprotein, AUC-TG; area under the curve of triglyceride, RLP: remnant like particle
Changes in Fasting and Postprandial Blood Chemistry Analysis and Endothelial Function
The fasting and postprandial blood chemistry data at baseline and at six months are also shown in Table 2. After six months of treatment, the HbA1c level, fasting PG, and IRI were significantly decreased; furthermore, a significant reduction in the insulin resistance index (HOMA-R) was observed, and a low 1,5-anhydroglucitol level indicated a deleterious effect.
Regarding the lipid profiles, fasting TG and RLP-C exhibited a significant reduction, although there were no significant changes in total cholesterol, HDL-C, and LDL-C.
Postprandial PG (PG-1h, PG-2h, and incremental AUC-PG) and postprandial IRI (IRI-1h, IRI-2h, and incremental AUC-IRI) were all decreased significantly. In a similar fashion, a significant reduction in postprandial TG (TG-1h, TG-2h) was observed, but the incremental AUC-TG did not exhibit a significant change.
Although the white blood cell count remained unchanged, significant increases were observed in the red blood cell count, hemoglobin level, and hematocrit level, whereas the platelet count decreased slightly. Plasma uric acid and urinary albumin level decreased significantly, whereas the serum creatinine level was slightly increased. No significant change was observed in the BNP.
The median baseline %FMD was 3.5%, and there was no significant association between the baseline %FMD and coronary risk factors and other baseline parameters. After six months of treatment, the %FMD, the primary endpoint of this study, improved significantly (median: 3.5–4.7%, P < 0.0001), whereas CRP and PAI-1 decreased significantly. Absolute change in brachial artery diameter was also increased significantly.
Regression Analysis of Factors Predicting %FMD Improvement
The univariate and multivariate regression analysis results are shown in Table 3. The univariate analysis revealed that changes in the HbA1c level, fasting and postprandial IRI and TG levels, and plasma total ketone body level were associated with the changes in %FMD. The multiple regression analysis revealed that the strongest independent factor predicting FMD changes was change in the TG level.
Table 3. Regression analysis for predicting %flow-mediated dilatation improvement.
| variable | univariate |
multivariate |
||
|---|---|---|---|---|
| t | p-value | t | p-value | |
| Age | −0.119 | 0.91 | ||
| Sex | 0.892 | 0.38 | ||
| Changes in weight | −0.154 | 0.88 | ||
| Changes in body fat percentage | 0.593 | 0.56 | ||
| Changes in body muscle percentage | 0.919 | 0.36 | ||
| Changes in whole body water volume | 0.918 | 0.36 | ||
| %change plasma volume | 0.007 | 0.99 | ||
| Changes in hematocrit | −0.036 | 0.97 | ||
| Changes in urine albumin | 1.727 | 0.09 | ||
| Changes in e-GFR | 0.125 | 0.90 | ||
| Changes in uric acid | −0.074 | 0.94 | ||
| Changes in hemoglobin A1c | −2.438 | 0.02 | −1.568 | 0.12 |
| Changes in 1,5-anhydro-glucitol | −0.615 | 0.54 | ||
| Changes in fasting plasma glucose | −0.214 | 0.83 | ||
| Changes in incremental AUC-PG | 0.710 | 0.48 | ||
| Changes in fasting IRI | −2.433 | 0.02 | −1.444 | 0.16 |
| Changes in incremental AUC-IRI | −2.057 | 0.05 | ||
| Changes in HOMA-R | −1.769 | 0.08 | ||
| Changes in LDL cholesterol | −1.025 | 0.31 | ||
| Changes in HDL cholesterol | −0.107 | 0.92 | ||
| Changes in fasting triglyceride | −5.939 | < 0.0001 | −5.703 | < 0.0001 |
| Changing in incremental AUC-TG | −2.112 | 0.04 | −1.667 | 0.10 |
| Changes in RLP cholesterol | −4.971 | < 0.0001 | ||
| Changes in C-reactive protein | 0.299 | 0.77 | ||
| Changes in BNP | 1.618 | 0.11 | ||
| Changes in PAI-1 | −0.428 | 0.67 | ||
| Changing total ketone body | 2.057 | 0.04 | 1.267 | 0.21 |
e-GFR: estimate glomerular filtration rate, AUC-PG: area under the curve of plasma glucose, HOMA-R: homeostasis model assessment insulin resistance, AUC-IRI: area under the curve of immunoreactive insulin, LDL: low density lipoprotein, HDL: high density lipoprotein, AUC-TG; area under the curve of triglyceride, RLP: remnant like particle, BNP: brain natriuretic peptide, PAI-1: plasminogen activator inhibitor-1
Subgroup Analysis Based on the Patients Divided by the Median Values of Changes in %FMD
We divided the patients into two subgroups according to the median values of the changes in %FMD: lower Δ%FMD group (Δ%FMD ≤ 0.7%, n = 25 patients) and higher Δ%FMD group (Δ%FMD > 0.7%, n = 25 patients).
As shown in Table 4, we noted significant differences in the baseline fasting and postprandial TG and RLP-C levels between the two groups. We also noted that the baseline %FMD in the higher Δ%FMD group was significantly lower than that in the lower Δ %FMD group.
Table 4. Comparison of changes in body composition, flow-mediated dilatation and blood chemistry between lower and higher Δ %FMD group.
| Variable | Lower Δ%FMD group; n = 25 (Δ%FMD of ≤ 0.7%) |
Higher Δ%FMD group; n = 25 (Δ%FMD of > 0.7%) |
P-value | ||
|---|---|---|---|---|---|
| Baseline | 6-month | Baseline | 6-month | ||
| Body composition data | |||||
| Weight (kg) | 68.5 ± 9.7 | 65.2 ± 9.2* | 68.7 ± 10.2 | 65.4 ± 10.2* | 0.94 |
| Absolute Δ | −3.3 (−4.6, −1.9) | −2.7 (−4.4, −2.1) | 0.75 | ||
| Body mass index (kg/m2) | 24.9 ± 2.4 | 23.7 ± 2.5* | 24.9 ± 3.6 | 23.7 ± 3.7* | 0.97 |
| Absolute Δ | −1.1 (−1.6, −0.7) | −1.1 (−1.7, −0.7) | 0.72 | ||
| Body fat quantity (kg) | 18.0 ± 4.3 | 15.7 ± 4.2* | 18.6 ± 5.9 | 16.2 ± 6.3* | 0.72 |
| Absolute Δ | −1.7 (−3.8, −0.6) | −2.1 (−3.0, −1.4) | 0.54 | ||
| Body fat percentage (%) | 26.6 ± 5.6 | 24.3 ± 5.8** | 27.3 ± 6.0 | 24.9 ± 7.0* | 0.66 |
| Absolute Δ | −1.7 (−4.0, −0.3) | −2.5 (−3.3, −0.8) | 0.72 | ||
| Muscle quantity (kg) | 47.5 ± 8.2 | 46.5 ± 7.8** | 46.2 ± 6.0 | 45.1 ± 6.0** | 0.55 |
| Absolute Δ | −1.0 (−1.8, 0.2) | −1.1 (−1.5, −0.7) | 0.81 | ||
| Muscle percentage (%) | 69.4 ± 5.3 | 71.5 ± 5.6** | 68.7 ± 5.7 | 70.9 ± 6.7* | 0.67 |
| Absolute Δ | 1.6 (0.3, 3.8) | 2.4 (0.7, 3.1) | 0.82 | ||
| Whole body water volume (L) | 37.0 ± 6.4 | 36.3 ± 6.0** | 36.0 ± 4.6 | 35.2 ± 4.6** | 0.55 |
| Absolute Δ | −0.6 (−1.4, 0.2) | −0.9 (−1.1, −0.5) | 0.89 | ||
| Extracellular water volume (L) | 14.0 ± 2.5 | 13.9 ± 2.2 | 13.8 ± 1.6 | 13.5 ± 1.7 | 0.79 |
| Absolute Δ | −0.3 (−0.5, 0.2) | −0.3 (−0.4, −0.1) | 0.81 | ||
| %change plasma volume (%) | −6.99 (−10.59, −1.67) | −6.88 (−11.46, −1.22) | 0.83 | ||
| Systolic blood pressure (mmHg) | 130.1 ± 13.0 | 127.1 ± 11.7 | 136.9 ± 16.7 | 134.1 ± 15.9 | 0.15 |
| Absolute Δ | −3.0 (−8.0, 2.3) | −4.0 (−9.0, 5.3) | 0.96 | ||
| Diastolic blood pressure (mmHg) | 80.1 ± 10.7 | 78.2 ± 9.8 | 80.1 ± 10.1 | 78.6 ± 9.3*** | 0.88 |
| Absolute Δ | −1.0 (−6.0, 3.3) | −2.0 (−5.3, 2.0) | 0.56 | ||
| %flow-mediated dilatation (%) | 5.5 (3.4, 6.0) | 4.7 (2.8, 5.8) | 2.6 (1.9, 3.6) | 4.6 (3.8, 5.1)* | 0.0001 |
| Absolute Δ | −0.3 (−0.6, −0.1) | 1.7 (1.0, 2.4) | < 0.0001 | ||
| Absolute change in diameter (mm) | 0.19 (0.15, 0.26) | 0.18 (0.12, 0.24) | 0.11 (0.09, 0.16) | 0.19 (0.16, 0.24)* | < 0.0001 |
| Absolute Δ | −0.02 (−0.03, 0.00) | 0.07 (0.04, 0.11) | < 0.0001 | ||
| Blood chemistry data | |||||
| White blood cell (× 102/µL) | 60.1 ± 15.0 | 55.8 ± 12.9 | 63.0 ± 14.4 | 65.7 ± 16.2 | 0.50 |
| Absolute Δ | 0.0 (−10.5, 62.5) | 20.0 (−50.0, 90.0) | 0.14 | ||
| Red blood cell (× 104/µL) | 472.7 ± 42.1 | 491.0 ± 43.1* | 479.6 ± 31.2 | 505.0 ± 32.6* | 0.52 |
| Absolute Δ | 15.0 (6.0, 30.0) | 21.0 (9.0, 45.0) | 0.47 | ||
| Hemoglobin (g/dL) | align="char" char="±"14.4 ± 1.0 | 14.9 ± 1.0** | 14.5 ± 1.3 | 15.1 ± 1.4** | 0.86 |
| Absolute Δ | 0.5 (0.2, 0.9) | 0.5 (0.2, 1.1) | 0.75 | ||
| Hematocrit (%) | 43.0 ± 2.6 | 44.5 ± 2.4** | 43.6 ± 3.2 | 45.1 ± 3.5*** | 0.48 |
| Absolute Δ | 1.7 (0.2, 2.6) | 1.6 (0.0, 3.3) | 0.84 | ||
| Platelet (× 104/µL) | 21.0 ± 4.0 | 19.7 ± 3.9*** | 22.3 ± 5.2 | 21.3 ± 5.0*** | 0.32 |
| Absolute Δ | −1.4 (−2.8, 0.6) | −1.3 (−2.5, 0.5) | 0.79 | ||
| Plasma creatinine (mg/dL) | 0.79 ± 0.12 | 0.81 ± 0.16 | 0.84 ± 0.16 | 0.87 ± 0.17 | 0.31 |
| Absolute Δ | 0.02 (−0.04, 0.06) | 0.02 (−0.04, 0.06) | 1.00 | ||
| e-GFR (mg/dL) | 75.1 ± 15.4 | 73.1 ± 13.4 | 71.6 ± 13.9 | 69.4 ± 14.5 | 0.40 |
| Absolute Δ | −2.2 (−7.1, 4.6) | −1.3 (−5.9, 1.1) | 0.95 | ||
| Urine albumin (mg/dL) | 13.8 (6.2, 62.8) | 10.8 (6.0, 23.6) | 18.0 (10.1, 46.6) | 12.4 (8.6, 26.9) | 0.72 |
| Absolute Δ | −2.1 (−17.4, 1.1) | −4.3 (−19.8, 2.8) | 0.79 | ||
| Uric acid (mg/dL) | 5.3 ± 1.4 | 4.5 ± 1.0** | 5.9 ± 1.2 | 4.8 ± 1.2** | 0.46 |
| Absolute Δ | −0.6 (−1.3, −0.2) | −0.8 (−1.5, −0.4) | 0.51 | ||
| HemoglobinA1c (%) | 7.2 ± 0.5 | 6.8 ± 0.4* | 6.8 ± 0.3 | 6.4 ± 0.4** | 0.11 |
| Absolute Δ | −0.4 (−1.0, −0.2) | −0.3 (−0.5, −0.1) | 0.26 | ||
| 1,5-anhydroglucitol (µg/dL) | 10.9 ± 6.2 | 2.5 ± 2.7* | 11.3 ± 5.4 | 1.8 ± 0.7* | 0.83 |
| Absolute Δ | −7.6 (−9.8, −4.2) | −9.7 (−12.2, −5.6) | 0.25 | ||
| Plasma glucose level (mg/dL) | |||||
| Fasting | 130.1 ± 16.6 | 114.0 ± 12.7* | 128.2 ± 15.8 | 115.6 ± 23.4* | 0.61 |
| Absolute Δ | −15.0 (−29.8, −6.8) | −16.0 (−23.3, −7.3) | 0.66 | ||
| After 1 hour | 242.7 ± 34.1 | 212.8 ± 28.3* | 234.7 ± 32.9 | 221.6 ± 34.4 | 0.40 |
| Absolute Δ | −32.0 (−53.5, −10.8) | −13.0 (−40.3, 1.5) | 0.16 | ||
| After 2 hours | 238.7 ± 51.0 | 204.8 ± 45.6** | 233.0 ± 42.2 | 222.7 ± 55.4 | 0.67 |
| Absolute Δ | −36.0 (−69.0, −1.0) | −13.0 (−45.5, 16.0) | 0.07 | ||
| Incremental AUC-PG (mg/dL/2hr) | 166.2 ± 46.0 | 144.1 ± 42.3* | 158.9 ± 41.3 | 159.6 ± 43.8 | 0.56 |
| Absolute Δ | −20.5 (−45.0, 0.9) | −0.5 (−24.8, 28.8) | 0.09 | ||
| Immunoreactive insulin (µU/mL) | |||||
| Fasting | 6.3 (4.1, 9.1) | 5.4 (3.5, 8.5) | 7.9 (4.8, 9.9) | 4.4 (3.0, 8.7)* | 0.35 |
| Absolute Δ | −0.7 (−2.3, 1.1) | −2.3 (−3.5, −0.5) | 0.03 | ||
| After 1 hour | 40.7 (22.9, 54.3) | 37.6 (18.1, 58.9) | 41.1 (25.7, 69.3) | 27.0 (19.9, 60.4)** | 0.51 |
| Absolute Δ | −1.9 (−8.0, 3.4) | −7.5 (−16.8, −3.8) | 0.01 | ||
| After 2 hours | 47.9 (27.4, 65.9) | 42.8 (22.6, 60.0) | 50.7 (32.1, 93.5) | 43.2 (27.0, 70.3) | 0.14 |
| Absolute Δ | −5.1 (−16.4, 2.9) | −8.0 (−31.0, −2.7) | 0.12 | ||
| Incremental AUC-IRI (µU/mL/2 hr) | 53.7 (30.6, 75.1) | 48.0 (23.7, 75.1) | 57.6 (35.8, 100.0) | 41.0 (29.3, 79.5)** | 0.31 |
| Absolute Δ | −6.6 (−9.2, 2.1) | −13.9 (−21.8, −0.1) | 0.04 | ||
| HOMA-R | 2.4 (1.4, 3.1) | 1.7 (1.4, 2.2)*** | 2.6 (1.5, 3.2) | 1.3 (0.8, 2.7)** | 0.53 |
| Absolute Δ | −0.5 (−1.2, 0.1) | −0.9 (−1.4, −0.3) | 0.21 | ||
| Total cholesterol (m/dL) | 157.6 ± 21.0 | 161.4 ± 22.6 | 172.2 ± 28.1 | 165.8 ± 38.8 | 0.06 |
| Absolute Δ | 4.0 (−9.2, 17.2) | −6.0 (−16.2, 3.8) | 0.09 | ||
| LDL cholesterol (mg/dL) | 88.1 ± 18.7 | 89.4 ± 17.8 | 93.1 ± 27.7 | 92.2 ± 29.4 | 0.46 |
| Absolute Δ | 3.0 (−8.0, 11.0) | −3.0 (−10.2, 6.3) | 0.34 | ||
| HDL cholesterol (mg/dL) | 48.4 ± 8.8 | 50.9 ± 9.8 | 53.8 ± 26.4 | 51.2 ± 9.2 | 0.33 |
| Absolute Δ | 0.0 (−3.0, 10.0) | 1.0 (−3.0, 8.3) | 0.97 | ||
| Plasma triglyceride (mg/dL) | |||||
| Fasting | 99.0 ± 33.7 | 96.2 ± 33.3 | 140.8 ± 64.0 | 95.1 ± 34.4* | 0.006 |
| Absolute Δ | −9.0 (−19.5, 16.8) | −36.0 (−67.0, −15.8) | 0.0002 | ||
| After 1 hour | 119.8 ± 32.9 | 124.2 ± 43.7 | 165.2 ± 60.7 | 121.0 ± 38.9* | 0.002 |
| Absolute Δ | 0.0 (−10.3, 20.8) | −38.0 (−56.5, −20.7) | < 0.0001 | ||
| After 2 hours | 155.6 ± 49.5 | 154.4 ± 57.9 | 212.9 ± 76.9 | 152.5 ± 48.3* | 0.003 |
| Absolute Δ | 4.0 (−20.5, 18.0) | −47.0 (−80.8, −34.8) | < 0.0001 | ||
| Incremental AUC-TG (mg/dL/2 hr) | 49.1 ± 30.1 | 57.1 ± 36.2 | 60.4 ± 38.8 | 52.5 ± 29.1* | 0.25 |
| Absolute Δ | 5.5 (−14.5, 22.8) | −9.5 (−15.9, 3.1) | 0.05 | ||
| RLP cholesterol (mg/dL) | 4.1 (2.6, 6.0) | 4.1 (2.6, 5.1) | 6.1 (4.2, 9.8) | 3.7 (2.4, 4.7)* | 0.01 |
| Absolute Δ | −0.4 (−1.2, 0.9) | −1.9 (−4.4, −1.0) | 0.0003 | ||
| C reactive protein (mg/dL) | 0.07 (0.03, 0.18) | 0.06 (0.03, 0.11) | 0.12 (0.04, 0.29) | 0.10 (0.04, 0.20)*** | 0.22 |
| Absolute Δ | −0.01 (−0.08, 0.02) | −0.02 (−0.07, 0.003) | 0.87 | ||
| Brain natriuretic peptide (pg/dL) | 31.4 (14.0, 59.2) | 19.4 (9.1, 39.7) | 28.8 (14.2, 59.0) | 22.0 (10.8, 58.0) | 0.93 |
| Absolute Δ | −3.1 (−17.8, 0.7) | 2.1 (−10.7, 9.4) | 0.17 | ||
| PAI-1 (ng/mL) | 16.9 ± 6.5 | 12.9 ± 4.8** | 20.3 ± 8.6 | 14.7 ± 5.1* | 0.13 |
| Absolute Δ | −4.0 (−8.3, 0.0) | −3.0 (−8.8, −2.0) | 0.44 | ||
| Total ketone body (µmol/L) | 95.0 (65.5, 143.0) | 134.0 (86.0, 152.5) | 80.0 (50.0, 138.5) | 191.0 (107.0, 336.0)* | 0.36 |
| Absolute Δ | 23.0 (−37.0, 76.0) | 82.5 (15.0, 170.5) | 0.02 | ||
P-values represent comparison of each values between groups at 6 months except for absolute Δ.
As for absolute Δ of each values, P-values represent comparison between the two groups.
Values are presented as means ± standard deviation or medians and interquartile ranges, as indicated.
P < 0.0001 vs baseline
P < 0.001 vs baseline
P < 0.05 vs baseline
e-GFR; estimated glomerular filtration rate, AUC; area under the response curve, IRI; immunoreactive insulin, HOMA-R; homeostasis model assessment ratio, LDL; low density lipoprotein, HDL; high density lipoprotein, TG; triglyceride, RLP; remnant-like particle, PAI-1; plasminogen activator inhibitor type 1
After six months of treatment, both groups exhibited similar changes in anthropometric measurements and hemodynamic data. The %change plasma volume also exhibited a similar change.
Although both groups showed a significant reduction in HbA1c, fasting PG, and HOMA-R, the improvement of fasting and postprandial insulin secretion was significantly larger in the higher Δ%FMD group (Fig. 1). Among the lipid profiles, fasting and postprandial TG (e.g., incremental AUC-TG and RLP-C) were also significantly decreased only in the higher Δ%FMD group (Fig. 1). Furthermore, a decrease in the CRP level and a significant increment in total ketone bodies occurred only in the higher Δ %FMD group.
Fig. 1.
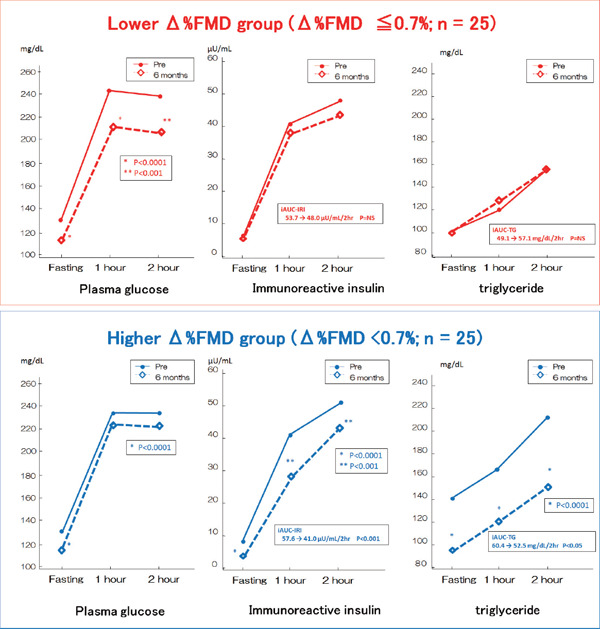
PG, IRI, and TG levels during the CMT at baseline and after six months
*P < 0.0001 versus baseline. **P < 0.001 versus baseline. Comparisons of data before and after treatment were performed using the paired t-test.
Discussion
In this study, we noted that six months of treatment with empagliflozin improved not only the glycemic control, but also multiple coronary risk factors, including blood pressure, obesity, insulin resistance, hypertriglyceridemia, and hyperuricemia. In addition, it ameliorated several atherosclerotic markers, such as inflammation, thrombogenicity, and endothelial function. Among these factors, an improvement in hypertriglyceridemia was the strongest independent factor predicting an improvement in endothelial function in diabetic patients with CAD treated with empagliflozin.
T2DM is a major risk factor for the progression of atherosclerosis and development of cardiovascular diseases. Among various oral glucose-lowering agents with different mechanisms of action, SGLT2 inhibitors are one of the few oral diabetes drugs that was able to achieve a significant reduction in major cardiovascular outcomes in large randomized controlled trials4, 5). As shown in several previous studies, the present study also revealed multiple favorable effects6–8, 24–27). However, the precise mechanisms underlying the cardiovascular benefits of SGLT2 inhibitors remain to be elucidated. We revealed that the reduction of both fasting and postprandial TG and insulin was significantly greater in the higher Δ%FMD group compared to the lower Δ%FMD group. In addition, the multivariate regression analysis revealed that an improvement in hypertriglyceridemia is the strongest independent factor predicting an improvement in endothelial function in diabetic patients with CAD. Similar to the present study, we previously demonstrated that α-glucosidase inhibitors, which have ameliorating effects on hyperglycemia without inducing of insulin secretion, improved hypertriglyceridemia and insulin resistance; these improvements were associated with the improvement of oxidative stress and endothelial function in diabetic patients with CAD28). Based on these observations, we are of the opinion that the mechanisms behind the cardiovascular benefits of SGLT2 inhibitors may be associated with the improvement in hypertriglyceridemia and insulin resistance.
In patients with insulin-resistant T2DM, the lipoprotein lipase activity is decreased and lipolysis is impaired, and there are few surface remnants available to be incorporated into the HDL particles. Consequently, the chylomicron remnant levels, which are highly atherogenic and act by enhancing systemic inflammation, platelet activation, coagulation, thrombus formation, and macrophage foam cell formation, are often elevated in such patients29, 30). Therefore, T2DM commonly demonstrates elevated TG, low HDL-C, and predominance of small dense LDL particles due to insulin resistance, and this type of dyslipidemia is considered to be atherogenic dyslipidemia31, 32). Sone et al. demonstrated that the serum TG level is a leading predictor of CAD, which is comparable to LDL-C in Japanese patients with T2DM33). On the other hand, since the report of Zilversmit34), postprandial hypertriglyceridemia has also been noticed to be a risk factor for atherosclerosis, especially CAD. Therefore, researchers are currently focusing on fasting and postprandial hypertriglyceridemia as a residual risk factor for cardiovascular events in statin-treated diabetic patients. Although some studies failed to show a relationship between hypertriglyceridemia and endothelial dysfunction and some controversy persists35, 36), there are many reports revealing that fasting and postprandial hypertriglyceridemia are closely associated with endothelial dysfunction and its amelioration link to the endothelial dysfunction recovery37, 38). Our data completely support these facts and suggest that the SGLT2 inhibitor empagliflozin may be a promising antiatherosclerotic drug; hence, it may contribute to cardiovascular benefits.
On the other hand, Inzucchi et al. demonstrated that changes in markers such as the plasma volume, hematocrit, and hemoglobin are the most important mediator of a lower cardiovascular event rate18). Indeed, in the EMPA-REG OUTCOME and CANVAS trials, the greatest benefit of SGLT2 inhibitors was the reduction of hospitalization for heart failure4, 5). Our data also revealed a significantly decreased whole body water volume and significantly increased red blood cell count, hemoglobin level, and hematocrit level, along with a plasma volume reduction. Thus, the mitigating effect of SGLT2 inhibitors on volume overload probably contributed to the reduction of heart failure risks and might be one of the potential mechanisms for the reduction of cardiovascular events with SGLT2 inhibitors. However, the Hisayama study showed that both elevated and decreased hematocrit levels were associated with an increased incidence of cardiovascular diseases39). However, in our study, we observed that the hematocrit and hemoglobin levels in the higher Δ%FMD group were similar to those in the lower Δ%FMD group. Furthermore, the BNP level exhibited no changes in both groups. Therefore, we could not conclude that the only volume reduction effect of SGLT2 inhibitors was the most plausible mechanism of cardiovascular benefits of SGLT2 inhibitors.
Intriguingly, although the strongest independent factor of %FMD improvement was the reduction of plasma TG levels, the regression analysis also revealed that the total ketone body increment was associated with an improvement in endothelial function. Some researchers suggested that, under a mild hyperketonemia condition caused by SGLT2 inhibitors, the heart preferentially metabolizes ketone bodies instead of glucose, as an alternative fuel source, thereby improving the myocardial work efficiency and function40). In fact, Aubert et al. indicated that a hypertrophied and failing myocardium shifts to ketone bodies as a significant fuel source for oxidative adenosine triphosphate production in mouse models41). Our data also support this hypothesis; however, further large-sized, randomized, controlled study is warranted.
Limitations
Several limitations in the present study warrant consideration. First, this was a study with a single-arm design and no control group. Therefore, changes that might be observed without SGLT2 inhibitor treatment could not be compared in this study. However, in our previous study, we observed a not so significant change in %FMD among patients who underwent just dietary and exercise therapy for six months21). Thus, we believe that SGLT2 inhibitor therapy may have an additive beneficial influence on endothelial dysfunction in comparison with non-SGLT2-inhibitor therapy. Second, although we calculated the required sample size according to a previous study, the number of participants required to detect a possible association between changes in FMD and those of several biochemical markers was relatively small. Third, several concomitant drugs (e.g., statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers) were used, which had the potency of improving the endothelial function; therefore, some bias is likely to have occurred in our results. However, the participants were prohibited from changing the dose of concomitant drugs or adding any other drugs within 12 weeks of enrollment and throughout the study to minimize the effects of factors other than empagliflozin. Fourth, the plasma TG level gradually increases after a meal and reaches a peak at about 4 h after the meal, but we did not assess the plasma TG level 4 h after the cookie load in this study35, 36). This was due to difficulties associated with more multiple times blood sample obtainment for each outpatient. Therefore, the possibility of an inaccurate assessment of postprandial hypertriglyceridemia cannot be ruled out. Because Sato et al. demonstrated that RLP-C in the fasting period may be suitable for detecting postprandial hyperlipidemic subjects16), we used RLP-C as an alternative marker of postprandial hypertriglyceridemia. In addition, we calculated the incremental AUC-TG to assess the postprandial change of plasma TGs. We believe that we can evaluate postprandial hypertriglyceridemia meaningfully using these multiple markers.
Conclusions
In conclusion, empagliflozin improved not only glycemic control, but also insulin resistance and hypertriglyceridemia. These ameliorations of metabolic abnormalities were strongly associated with improvements in concomitant endothelial function among diabetic patients with CAD. Although the mechanism of the cardiovascular benefits of SGLT2 inhibitors might be multifactorial, reduction of plasma TGs may be mostly related to the cardiovascular benefits of SGLT2 inhibitors.
Acknowledgements
None.
Conflicts of Interest
None.
References
- 1). Mak KH, Moliterno DJ, Granger CB, Miller DP, White HD, Wilcox RG, Califf RM, Topol EJ. Influence of diabetes mellitus on clinical outcome in the thrombolytic era of acute myocardial infarction, GUSTO-I Investigators. Global Utilization of Streptokinase and Tissue Plasminogen Actibator for Occluded Coronary Arteries. J Am Coll Cardiol, 1997; 30: 171-179 [DOI] [PubMed] [Google Scholar]
- 2). Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndrome) Registry. Circulation, 2000; 102: 1014-1019 [DOI] [PubMed] [Google Scholar]
- 3). Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med, 2015; 66: 255-270 [DOI] [PubMed] [Google Scholar]
- 4). Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, EMPA-REG OUTCOME Investigators Empagliflozin, cardioavascular outcomes, and mortality in type 2 diabetes. N Engl J Med, 2015; 373: 2117-212826378978 [Google Scholar]
- 5). Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, Desai M, Matthews DR, CANVAS Program Collaborative Group Canagliflozin for primary and secondary prevention of cardiovascular events: Results from the CANVAS Program (Canagliflozi Cardiovascular Assessment Study). Circulation, 2018; 137: 323-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation, 2016; 134: 752-772 [DOI] [PubMed] [Google Scholar]
- 7). Nauck MA, Del Prato S, Meier JJ, Durán-García S, Rohwedder K, Elze M, Parikh SJ. Dapagliflozin versus glipzide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care, 2011; 34: 2015-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 inhibition and cardiovascular events: Why did EMPA-REG Outcomes surprise and what were the likely mechanisms? Diabetologia, 2016; 59: 1333-1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC. Close relation of endothelial function in the human coronary and peripheral circulation. J Am Coll Cardiol, 1995; 26: 1235-1241 [DOI] [PubMed] [Google Scholar]
- 10). Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Noninvasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet, 1992; 340: 1111-1115 [DOI] [PubMed] [Google Scholar]
- 11). Neunteufl T, Heher S, Katzenschlager R, Wölfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilatation in the brachial artery of patients with chest pain. Am J Cardiol, 2000; 86: 207-210 [DOI] [PubMed] [Google Scholar]
- 12). Sawada T, Emoto T, Motoji Y, Hashimoto M, Kageyama H, Terashita D, Mizoguchi T, Mizuguchi T, Iwasaki M, Taira K, Okamoto H, Matsuo Y, Kim SK, Takarada A, Yokoyama M. Possible association between noninvasive parameter of flow-mediated dilatation in brachial artery and whole coronary plaque vulnerability in patients with coronary artery disease. Int J Cardiol, 2013; 166: 613-620 [DOI] [PubMed] [Google Scholar]
- 13). Neunteufl T, Katzenschlager R, Hassan A, Klaar U, Schwarzacher S, Glogar D, Bauer P, Weidinger F. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis, 1997; 129: 111-118 [DOI] [PubMed] [Google Scholar]
- 14). Cha K, Chertow GM, Gonzalez J, Lazarus JM, Wilmore DW. Multifrequency bioelectrical impedance estimates the distribution of body water. J Appl Physiol, 1995; 79: 1316-1319 [DOI] [PubMed] [Google Scholar]
- 15). Kurinami N, Sugiyama S, Nishimura H, Morita A, Yoshida A, Hieshima K, Miyamoto F, Kajiwara K, Jinnouchi K, Jinnouchi T, Jinnouchi H. Clinical factors associated with initial decrease in body-fat percentage induced by add-on sodium-glucose co-transporter 2 inhibitors in patient with type 2 diabetes mellitus. Clin Drug Investig, 2018; 38: 19-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Sato I, Ishikawa Y, Ishimoto A, Katsura S, Toyokawa A, Hayashi F, Kawano S, Fujioka Y, Yamashita S, Kumagai S. Significance of measuring serum concentrations od remnant lipoproteins and Apolipoprotein B-48 in fasting period. J Atheroscler Thromb, 2009; 16: 12-20 [DOI] [PubMed] [Google Scholar]
- 17). Hudson SR, Chan D, Ng LL. Change in plasma volume and prognosis in acute decompenated heart failure: an observational cohort study. J R Soc Med, 2016; 109: 337-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, Hantel S, Bluhmki E, Lachin JM. How does empagliflozin reduce cardiovascular mortality? Insights from a medication analysis of the EMPA-REG OUTCOME Trial. Diabetes Care, 2018; 41: 356-363 [DOI] [PubMed] [Google Scholar]
- 19). Strauss M, Davis R, Rosenbaum J, Rossmeisl EC. Water diuresis produced during recumbency by the intravenous infusion of isotonic saline solution. J Clin Invest, 1951; 30: 862-868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Harano Y, Miyawaki T, Nabiki J, Shibachi M, Adachi T, Ikeda M, Ueda F, Nakano T. Development of cookie test for the simultaneous determination of glucose intolerance, hyperinsulinemia, insulin resistance and postprandial dyslipidemia. Endocr J, 2006; 53: 173-180 [DOI] [PubMed] [Google Scholar]
- 21). Sawada T, Tsubata H, Hashimoto N, Takabe M, Miyata T, Aoki K, Yamashita S, Oishi S, Osue T, Yokoi K, Tsukishiro Y, Onishi T, Shimane A, Taniguchi Y, Yasaka Y, Ohara T, Kawai H, Yokoyama M. Effects of 6-month eicosapentaenoic acid on postprandial hypertriglycemia, hyperlipidemia, insulin secretion ability, and concomitant endothelial dysfunction among newly-diagnosed impaired glucose metabolism patients with coronary artery disease. An open label, single blinded, prospective randomized controlled trial. Cardiovasc Diabetol, 2016; 15: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Emoto T, Sawada T, Hashimoto M, Kageyama H, Terashita D, Mizoguchi T, Mizuguchi T, Motodi Y, Iwasaki M, Taira K, Okamoto H, Matsuo Y, Kim SK, Takarada A, Yokoyama M. Effect of 3-month repeated administration of miglitol on vascular endothelial function in patients with diabetes mellitus and coronary artery disease. Am J Cardiol, 2012; 109: 42-46 [DOI] [PubMed] [Google Scholar]
- 23). Shigiyama F, Kumashiro N, Miyagi M, Ikehara K, Kanda E, Uchino H, Hirose T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol, 2017; 16: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Abdul-Ghani MA, Norton L, Defrozo RA. Role of sodium-glucose cotransporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes. Endocr Rev, 2011; 32: 515-531 [DOI] [PubMed] [Google Scholar]
- 25). Cai X, Ji L, Chen Y, Yang W, Zhou L, Han X, Zhang S, Ji L. Comparisons of weight changes between sodium-glucose cotranspoter 2 inhibitors treatment and glucagonlike peptide-1 analogs treatment in type 2 diabetes patients: a meta-analysis. J Diabetes Investig, 2017; 8: 510-517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Mazidi M, Rezaie P, Gao HK, Kengne AP. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: A systematic review and meta-analysis of 43 randomized control trials with 225228 patients. J Am Heart Assoc, 2017; 6: e005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Zhao Y, Xu L, Tian D, Xia P, Zheng H, Wang L, Chen L. Effects of sodium-glucose cotransporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes Metab, 2018; 20: 458-462 [DOI] [PubMed] [Google Scholar]
- 28). Sawada T, Shiotani H, Terashita D, Nagasawa Y, Kim SS, Koide M, Yokoyama M. Comparison of effects of alpha-glucosidase inhibitors and glinide drugs on endothelial dysfunction in diabetic patients with coronary artery disease. Circ J, 2014; 78: 248-255 [DOI] [PubMed] [Google Scholar]
- 29). Masuda D, Yamashita S. Postprandial Hyperlipidemia and Remnant Lipoprotein. J Atheroscler Thromb, 2017; 24: 95-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Fujioka Y, Ishikawa Y. Remnant lipoproteins as strong key particles to atherogenesis. J Atheroscler Thromb, 2009; 16: 145-154 [DOI] [PubMed] [Google Scholar]
- 31). Fruchart JC, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R, Chapman MJ, Dodson PM, Fioretto P, Ginsberg HN, Kadowaki T, Lablanche JM, Marx N, Plutzky J, Reiner Z, Rosenson RS, Staels B, Stock JK, Sy R, Wanner C, Zambon A, Zimmet P. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol, 2008; 102: 1K-34K [DOI] [PubMed] [Google Scholar]
- 32). Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism, 2014; 63: 1469-1479 [DOI] [PubMed] [Google Scholar]
- 33). Sone H, Tanaka S, Tanaka S, Iimuro S, Oida K, Yamasaki Y, Oikawa S, Ishibashi S, Katayama S, Ohashi Y, Akanuma Y, Yamada N, Japan Diabetes Complications Study Group Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complication Study (JDCS). J Clin Endocrinol Metab, 2011; 96: 3448-3456 [DOI] [PubMed] [Google Scholar]
- 34). Zilversmit DB. Atherosclerosis: a postprandial phenomenon. Circulation, 1979; 60: 473-485 [DOI] [PubMed] [Google Scholar]
- 35). Chowienczyk PJ, Watts GF, Wierzbicki AS, Cockcroft JR, Brett SE, Ritter JM. Preserved endothelial function in patients with severe hypertriglyceridemia and low functional lipoprotein lipase activity. J Am Coll Cardiol, 1997; 29: 964-968 [DOI] [PubMed] [Google Scholar]
- 36). Schnell GB, Robertson A, Houston D, Malley L, Anderson TJ. Impaired brachial artery endothelial function is not predicted by elevated triglycerides. J Am Coll Cardiol, 1999; 33: 2038-2043 [DOI] [PubMed] [Google Scholar]
- 37). Yunoki K, Nakamura K, Miyoshi T, Enko K, Kubo M, Murakami M, Hata Y, Kohno K, Morita H, Kusano KF, Ito H. Impact of hypertriglyceridemia on endothelial dysfunction during Statin ± Ezetimibe therapy in patients with coronary heart disease. Am J Cardiol, 2011; 108: 333-339 [DOI] [PubMed] [Google Scholar]
- 38). Noda Y, Miyoshi T, Oe H, Ohno Y, Nakamura K, Toh N, Kohno K, Morita H, Kusano K, Ito H. Alogliptin ameliorates postprandial lipemia and postprandial endothelial dysfunction in non-diabetic subjects: a preliminary report. Cardiovasc Diabetol, 2013; 12: 8. 10.1186/1475-2840-12-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Gotoh S, Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, Fukuhara M, Ikeda F, Ago T, Kitazono T, Kiyohara Y. Hematocrit and the risk of cardioivascular disease in a Japanese community: The Hisayama Study. Atherosclerosis, 2015; 242: 199-204 [DOI] [PubMed] [Google Scholar]
- 40). Mudalier S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardioneral outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care, 2016; 39: 1115-1122 [DOI] [PubMed] [Google Scholar]
- 41). Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circulation, 2016; 133: 698-705 [DOI] [PMC free article] [PubMed] [Google Scholar]


