Abstract
Aim: The degradation of the vascular extracellular matrix is important for atherosclerosis. The cysteine protease legumain was shown to be upregulated in atherosclerotic plaques, especially unstable plaques. However, no study has reported blood legumain levels in patients with coronary artery disease (CAD).
Methods: We investigated plasma legumain and C-reactive protein (CRP) levels in 372 patients undergoing elective coronary angiography.
Results: CAD was found in 225 patients. Compared with patients without CAD, those with CAD had higher CRP levels (median 0.60 [0.32, 1.53] vs. 0.46 [0.22, 0.89] mg/L, P < 0.001), but no difference was found in legumain levels between patients with and without CAD (median 5.08 [3.87, 6.82] vs. 4.99 [3.84, 6.88] ng/mL). A stepwise increase in CRP was found depending on the number of > 50% stenotic vessels: 0.55 mg/L in 1-vessel, 0.71 mg/L in 2-vessel, and 0.86 mg/L in 3-vessel diseases (P < 0.001). However, legumain did not differ among 1-, 2-, and 3-vessel diseases (5.20, 4.93, and 5.01 ng/mL, respectively). Of 225 patients with CAD, 40 (18%) had complex lesions. No difference was found in CRP levels between patients with CAD with and without complex lesions (0.60 [0.34, 1.53] vs. 0.60 [0.32, 1.51] mg/L). Notably, legumain levels were higher in patients with CAD with complex lesions than without such lesions (6.05 [4.64, 8.64] vs. 4.93 [3.76, 6.52] ng/mL, P < 0.01). In multivariate analysis, legumain levels were not a factor for CAD, but were a factor for complex lesions. The odds ratio for complex lesions was 2.45 (95% CI = 1.26–4.79) for legumain > 5.5 ng/mL.
Conclusion: Plasma legumain levels were associated with the presence of complex coronary lesions.
Keywords: Coronary artery disease, Legumain, Plaque instability
Introduction
Atherosclerosis is a chronic inflammatory disease, and macrophages play a key role in atherosclerotic-related inflammation1). The degradation and turnover of the vascular extracellular matrix (ECM) is an important part of atherosclerosis. Proteolytic enzymes, such as matrix metalloproteinases (MMPs) and cathepsin cysteine proteases, which are localized mainly in macrophages, play a major role in the degradation of the ECM and the progression and instability of atherosclerotic plaques2, 3). Legumain is a lysosomal cysteine protease4) and can also activate other proteases, including MMP-2, cathepsins-L and -S, and fibronectin5–7). Papaspyridonos et al.8) first demonstrated the upregulation of legumain mRNA and protein in unstable regions of human carotid atherosclerotic plaques, thus suggesting a possible role for this enzyme in plaque instability. Sun et al.9) also showed that legumain expression was upregulated in cultured macrophages treated with oxidized low-density lipoprotein (LDL). Moreover, Mattock et al.10) reported increased protein and expression levels of both legumain and active cathepsin-L in unstable carotid plaques. Cathepsin-L is processed prominently by legumain, and it has potent collagenolytic and elastinolytic activities11, 12). Therefore, legumain has been recognized to play a role in plaque instability.
Recently, Lunde et al.13) reported plasma legumain levels to be higher in patients with carotid artery stenosis than in healthy controls. Regarding coronary artery disease (CAD), Clerin et al.14) demonstrated increased legumain mRNA expression in human coronary atherosclerotic lesions. However, no study has evaluated plasma legumain levels in patients with CAD. Therefore, our study was performed to elucidate the association between plasma legumain levels and the presence, severity, and lesion morphology of CAD in 372 patients undergoing coronary angiography.
Methods
Study Patients
We investigated plasma legumain levels in 372 consecutive patients undergoing elective coronary angiography for suspected CAD at Tokyo Medical Center from July 2008 to February 2017. Any patients with acute coronary syndrome (ACS), defined as acute myocardial infarction and class III unstable angina at rest by Braunwald's classification15), those with a history of percutaneous coronary intervention or cardiac surgery, or those with a history of heart failure or aortic diseases were excluded from this study. Patients with liver cirrhosis, renal failure, or inflammatory diseases were also excluded. Hypertension was defined as blood pressures of ≥ 140/90 mmHg or on drugs, and 228 (61%) patients were taking anti-hypertensive drugs. Hyperlipidemia was defined as an LDL-cholesterol level of > 140 mg/dL or on drugs, and 146 (39%) were taking statins. Diabetes mellitus (a fasting plasma glucose level of ≥ 126 mg/dL or on treatment) was present in 105 (28%) patients, and 138 (37%) were smokers (≥ 10 pack-years). Our study was performed in accordance with the Declaration of Helsinki and was approved by the institutional ethics committee of our hospital (R07-054/R15-056). After written informed consent was obtained, overnight-fasting blood samples were taken on the morning of the day when angiography was performed.
Measurement of Plasma Legumain and C-Reactive Protein Levels
Blood samples were collected in EDTA-containing tubes. The plasma was stored at −80°C. Plasma legumain levels were measured by an enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (Human Legumain ELISA Kit, Ray Biotech, GA, USA) at Ochanomizu University, according to the manufacturer's instructions. The intra- and inter-assay coefficients of variation were < 10% and < 12%, respectively. According to the data supplied by the manufacturer, this kit shows no cross-reactivity with cathepsin-L, and it measures the total concentration of both the precursor and active forms of legumain. Plasma high-sensitivity C-reactive protein (hsCRP) levels were also measured by a BNII nephelometer (Dade Behring, Tokyo, Japan).
Coronary Angiography
Angiograms were recorded on a cineangiogram system (Philips Electronics Japan, Tokyo, Japan). CAD was defined as at least one coronary artery having > 50% luminal diameter stenosis on angiograms. The severity of CAD was represented as the number of > 50% stenotic vessels and the numbers of > 50% and > 25% stenotic segments. According to Ambrose's classification16, 17), stenotic lesions were classified as complex if they had sharp overhanging edges, irregular borders, ulcerations, or intraluminal lucency. Coronary artery segments were divided into 29 segments according to the Coronary Artery Surgery Study classification. All angiograms were evaluated by a single cardiologist (Y.M.), blinded to the clinical and laboratory data.
Statistical Analysis
The differences between the 2 groups were evaluated by an unpaired t-test for parametric variables, the Mann–Whitney U test for nonparametric variables, and the chi-squared test for categorical variables. Differences among ≥ 3 groups were evaluated by analysis of variance with Scheffe's test for parametric variables, the Kruskal–Wallis test with Steel–Dwass test for nonparametric variables, and the chi-squared test for categorical variables. Since the distributions of the measured legumain and hsCRP levels were considered to be highly skewed and to be nonparametric variables by the Shapiro–Wilk test, the results were presented as the median value. Correlations between legumain levels and the numbers of stenotic segments were evaluated by Spearman's rank correlation test. To determine the cutoff point of legumain levels for complex lesions, a relative cumulative frequency distribution curve was created. The optimal cutoff point was determined as the point where the sensitivity and specificity curves intersected. Multiple logistic regression analysis was used to elucidate the independent associations between legumain levels and CAD or complex lesions. Regarding the cutoff point of hsCRP levels, the previously reported cutoff point of 1.0 mg/L for CAD was used18, 19). All statistical analyzes were carried out using the SPSS software package (IBM SPSS version 25, Tokyo, Japan). A P value of < 0.05 was considered statistically significant. Results are presented as the mean ± standard deviation (SD) or the median value.
Results
Of the 372 study patients, CAD was found in 225 (1-vessel disease [1-VD], n = 92; 2-vessel disease [2-VD], n = 72; 3-vessel disease [3-VD], n = 61). Compared with 147 patients without CAD, 225 with CAD were older, predominantly male, and had a higher prevalence of hypertension, diabetes, and hyperlipidemia, and higher hsCRP levels (median 0.60 vs. 0.46 mg/L, P < 0.001) (Table 1). However, there was no signficant difference in plasma legumain levels between patients with and without CAD (median 5.08 vs. 4.99 ng/mL, P = 0.81) (Fig. 1). A stepwise increase in hsCRP levels was found depending on the number of > 50% stenotic coronary vessels: 0.46 mg/L in CAD(-), 0.55 mg/L in 1-VD, 0.71 mg/L in 2-VD, and 0.86 mg/L in 3-VD (P < 0.001). However, there was no marked difference in plasma legumain levels among the 1-VD, 2-VD, and 3-VD groups (5.20, 4.93, and 5.01 ng/mL, respectively; P = 0.96). No signifinant correlations were found between legumain levels and the numbers of > 50% and > 25% stenotic coronary segments (P = 0.95 and 0.65, respectively).
Table 1. Clinical characteristics and plasma legumain levels.
| CAD (−) | P value | CAD | Complex lesion (−) | Complex lesion | Complex lesion (+) | |
|---|---|---|---|---|---|---|
| (n = 147) | CAD (−) vs. CAD | (n = 225) | (n = 185) | (+) vs. (−) | (n = 40) | |
| Age (years) | 66 ± 10 | < 0.001 | 69 ± 10 | 69 ± 10 | 0.49 | 68 ± 10 |
| Gender (male) | 88 (60%) | < 0.001 | 174 (77%) | 142 (77%) | 0.90 | 32 (80%) |
| Hypertension | 89 (61%) | < 0.001 | 176 (78%) | 148 (80%) | 0.30 | 28 (70%) |
| SBP (mmHg) | 130 ± 21 | 0.20 | 133 ± 20 | 133 ± 21 | 0.76 | 132 ± 19 |
| Diabetes mellitus | 22 (15%) | < 0.001 | 83 (37%) | 70 (38%) | 0.80 | 13 (33%) |
| Smoking | 46 (31%) | 0.10 | 92 (41%) | 78 (42%) | 0.80 | 14 (35%) |
| Hyperlipidemia | 67 (46%) | < 0.025 | 132 (59%) | 109 (59%) | 0.98 | 23 (58%) |
| Statin | 45 (31%) | < 0.01 | 101 (45%) | 88 (48%) | 0.20 | 13 (33%) |
| LDL-C (mg/dL) | 113 ± 28 | 0.72 | 114 ± 32 | 111 ± 32 | < 0.005 | 127 ± 28 |
| HDL-C (mg/dL) | 60 ± 14 | < 0.001 | 51 ± 13 | 51 ± 14 | 0.99 | 51 ± 13 |
| hsCRP levels | 0.46 | < 0.001 | 0.60 | 0.60 | 0.93 | 0.60 |
| (mg/L) | [0.22, 0.89] | [0.32, 1.53] | [0.32, 1.51] | [0.34, 1.53] | ||
| > 1.0mg/L | 33 (22%) | < 0.025 | 78 (35%) | 65 (35%) | 0.90 | 13 (33%) |
| Legumain levels | 4.99 | 0.81 | 5.08 | 4.93 | < 0.01 | 6.05 |
| (ng/mL) | [3.84, 6.88] | [3.87, 6.82] | [3.76, 6.52] | [4.64, 8.64] | ||
| > 5.5 ng/mL | 58 (39%) | 0.90 | 92 (41%) | 68 (37%) | < 0.025 | 24 (60%) |
Data are presented as the mean ± SD or the number (%) of patients, except for hsCRP and legumain levels, which are presented as the median value and interquartile range.
SBP indicates systolic blood pressure; LDL-C, LDL-cholesterol; and HDL-C, HDL-cholesterol.
Fig. 1.

Plasma legumain levels and the presence of CAD and complex coronary lesions
Plasma legumain levels did not differ markedly between patients with and without CAD (P = NS) (left). Notably, legumain levels were significantly higher in patients with CAD with complex lesions than in those without such lesions and in patients without CAD (P < 0.05) (right). The central line represents the median and the box represents the 25th to 75th percentiles. The whiskers represent the lowest and highest values in the 25th percentile minus 1.5 interquartile range (IQR) and 75th percentile plus 1.5 IQR, respectively.
Among the 225 patients with CAD, 40 (18%) were found to have complex coronary lesions. No difference was found in hsCRP levels between patients with CAD with and without complex lesions (0.60 vs. 0.60 mg/L, respectively, P = 0.93). Notably, legumain levels were significantly higher in patients with CAD with complex lesions than in those without such lesions and in patients without CAD (6.05 vs. 4.93 and 4.99 ng/mL, respectively, P < 0.01) (Fig. 1). To determine the cutoff point of legumain levels for complex lesions, a relative cumulative frequency distribution curve was created. As shown in Fig. 2, the optimal cutoff point was found to be around 5.5 ng/mL. The prevalence of legumain level > 5.5 ng/mL was higher in patients with CAD with complex lesions than in those without such lesions and in patients without CAD (60% vs. 37% and 39%, respectively, P < 0.025).
Fig. 2.
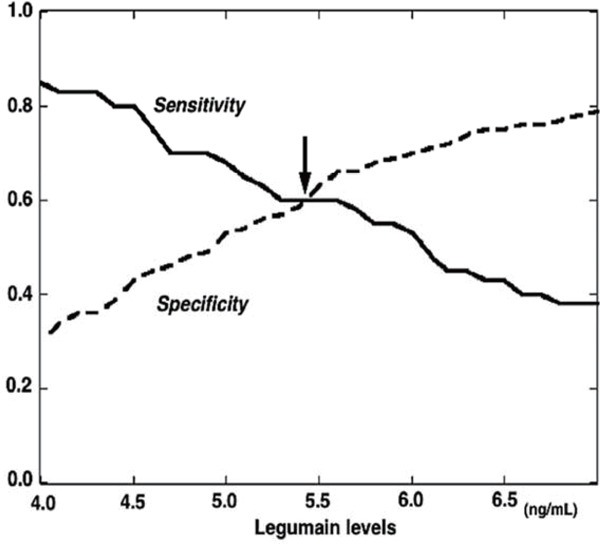
The relative cumulative frequency distribution curve for the optimal cutoff point of legumain levels for complex lesions
The curve indicates that the optimal cutoff point of legumain levels for complex lesions is around 5.5 ng/mL. The arrow indicates the optimal cutoff point of legumain levels.
To elucidate the independent associations between legumain level and CAD or complex lesions, variables (age, gender, hypertension, hyperlipidemia, statin use, diabetes, smoking, and hsCRP and legumain levels) were entered into a multiple logistic regression model for the 372 study patients (Table 2). Legumain levels were not a significant factor for CAD, but they were the only factor associated with complex lesions independent of atherosclerotic risk factors. The odds ratio for complex lesions was 2.45 (95% confidence interval [CI] = 1.26–4.79) for a legumain level > 5.5 ng/mL (P < 0.01). Furthermore, in 225 patients with CAD, legumain levels were also the factor for complex lesions independent of atherosclerotic risk factors (Table 3). The odds ratio for complex lesions was 2.58 (95% CI = 1.28–5.20) a for legumain level > 5.5 ng/mL (P < 0.01).
Table 2. Factors associated with CAD and complex coronary lesions (Multiple logistic regression analysis of the 372 study patients).
| Odds ratio | (95% CI) | P value | |
|---|---|---|---|
| CAD | |||
| Age (per 10 years increase) | 1.36 | (1.08–1.72) | < 0.02 |
| Male gender | 2.61 | (1.56–4.36) | < 0.001 |
| Hypertension | 1.67 | (1.01–2.75) | < 0.05 |
| Hyperlipidemia | 1.82 | (1.13–2.92) | < 0.02 |
| Diabetes mellitus | 2.64 | (1.53–4.56) | < 0.01 |
| Complex coronary lesions | |||
| Legumain level > 5.5 ng/mL | 2.45 | (1.26–4.79) | < 0.01 |
The dependent variable was the presence of CAD or complex coronary lesions.
The analysis included age, gender, hypertension, hyperlipidemia, statin use, diabetes mellitus, smoking, and hsCRP (> 1.0 mg/L) and legumain (> 5.5 ng/mL) levels.
Table 3. Factors associated with complex coronary lesions (Multiple logistic regression analysis of the 225 patients with CAD).
| Odds ratio | (95% CI) | P value | |
|---|---|---|---|
| Complex coronary lesions | |||
| Legumain level > 5.5 ng/mL | 2.58 | (1.28–5.20) | < 0.01 |
The dependent variable was the presence of complex coronary lesions.
The analysis included age, gender, hypertension, hyperlipidemia, statin use, diabetes mellitus, smoking, and hsCRP (> 1.0 mg/L) and legumain (> 5.5 ng/mL) levels.
Discussion
In the present study, plasma legumain levels did not differ between patients with and without CAD. They did not correlate with the severity of CAD and were not a significant factor for CAD. However, legumain levels were found to be high in patients with CAD with complex coronary lesions and to be an independent factor for complex lesions.
Legumain is a cysteine protease that is expressed predominantly in macrophages in atherosclerotic plaques4, 14), and it also promotes ECM degradation by activating MMP-2, cathepsin-L, and fibronectin5–7). Several studies reported the upregulation of legumain mRNA and protein in unstable regions of carotid atherosclerotic plaques, thus suggesting a role of legumain in plaque instability8–10). However, regarding blood legumain levels in patients with atherosclerotic diseases, Lunde et al.13) reported recently that plasma legumain levels were higher in 254 patients with carotid artery stenosis than in 91 healthy controls, with no correlation found between legumain levels and the degree of carotid stenosis. However, they found no significant differences in legumain levels among 117 patients with stroke, transitory ischemic attack, or amaurosis fugax within the last 2 months; 38 patients with such symptoms during the previous 2–6 months, and 99 asymptomatic patients. While increased levels of legumain mRNA and protein in coronary atherosclerotic lesions was reported14), no study has reported plasma legumain levels in patients with CAD. Our present study is the first to report that plasma legumain levels did not differ markedly between patients with and without CAD and that they did not correlate with the severity of CAD.
In the present study, patients with ACS, defined as acute myocardial infarction and class III unstable angina by Braunwald's classification15), were not included. However, 40 (18%) of 225 patients with CAD had complex coronary lesions on angiograms. Complex lesions are often seen in patients with ACS (approximately 70%), but such lesions are also found in patients with stable CAD (10–20%)20). As in our study, Rupprecht HJ et al.21) reported complex lesions in 39 (20%) of 200 patients with stable CAD, and Chester MR et al.22) found such lesions in 52 (23%) of 222 patients with stable CAD. Angiographic complex coronary lesions are recognized to be associated with plaque instability16, 23–25). Complex lesions are also reported to be associated with coronary events26, 27) and the rapid progression of coronary stenosis22). We previously measured serum MMP-1 levels in 185 patients undergoing coronary angiography28). Of the 128 patients with stable CAD, 32 (25%) had complex lesions. We showed MMP-1 levels to be higher in patients with CAD with complex lesions than in those without such lesions. Recently, we also evaluated plasma osteoglycin levels in 245 patients with stable CAD, of whom 41 (17%) had complex lesions29). Osteoglycin, which is a biologically active component of the ECM, was shown to be downregulated in unstable carotid plaques30). We reported osteoglycin levels to be lower in patients with CAD with complex lesions than in those without such lesions. Our present study first reported that plasma legumain levels were significantly higher in patients with CAD with complex coronary lesions than in those without complex lesions and in patients without CAD. A high plasma legumain level was a significant factor for the presence of complex coronary lesions independent of atherosclerotic risk factors. Therefore, our results suggest that plasma legumain levels may be a potential biomarker for complex coronary lesions and that high legumain levels in patients with CAD may reflect coronary plaque instability. However, as shown in Fig. 1, there was a substantial overlap in legumain levels between patients with CAD with and without complex lesions. Plasma legumain levels in patients with CAD may reflect plaque instability not only in coronary arteries but also in other vascular beds.
Wang et al.31) reported 12-week atorvastation treatment to reduce legumain mRNA expression in monocytes in 40 patients with CAD. They suggested that legumain reduction may be one of pleiotropic effects of statin. In our study, 45% of patients with CAD were taking statin, as expected. The percentage of patients taking statin was higher in patients with CAD than in those without CAD, but statin use was not an independent factor for CAD. However, this may have confounded the results of our study. Furthermore, the percentage of patients taking statin did not differ markedly between patients with CAD with and without complex lesions, and legumain levels were found to be a significant factor for complex lesions independent of atherosclerotic risk factors and statin use.
Our study has several limitations. First, angiography was used to assess coronary atherosclerosis. Angiography cannot visualize plaques and shows only the severity and morphology of stenotic lesions. However, in our study, intravascular ultrasound (IVUS) or optical coherence tomography (OCT), which can visualize coronary plaques and evaluate plaque characteristics32), were not always performed. Second, we did not measure blood legumain levels in the coronary sinus. Our study did not provide any information on the main sources of plasma legumain in patients with complex coronary lesions. Third, legumain is synthesized as a 56-kDa proform and then requires activation and cleavage to a 36-kDa active form33). The ELISA kit used in our study measures the total concentrations of both the precursor and active forms. However, no ELISA kits available currently measure only the active form of legumain. Fourth, in our study, only 40 (18%) of 225 patients with CAD had complex coronary lesions. However, this prevalence was similar to that reported previously in patients with stable CAD20–22). The sample size of our study (225 patients with CAD) was found to be enough to show a 2.58-fold higher risk of complex lesions in patients with high legumain levels (> 5.5 ng/mL) with a statistical power of 80% and an α value of 0.05, because 196 patients were estimated as an adequate sample size with the prevalence of complex lesions (18%). However, the small numbers of study patients and patients with complex lesions are a major limitation. To confirm the findings of our study, a further study is needed with a larger number of study patients. Furthermore, no patient with ACS, who usually needs emergent coronary angiography, was included in our study. A further study on patients with ACS is needed to elucidate the potential role of legumain in ACS. Finally, our study was cross-sectional in nature and could not establish causality, since it only depicted some associations and proposed some hypotheses. To determine the prognostic values of legumain levels and complex lesions, a further prospective study is needed.
In conclusion, plasma legumain levels did not correlate with the severity of CAD and were not a significant factor for CAD. However, legumain levels were found to be characteristically high in patients with CAD with complex coronary lesions and to be an independent factor associated with complex lesions. Our results thus suggest that plasma legumain levels may be a potential biomarker for coronary plaque instability, such as complex coronary lesions.
Funding
This study was supported in part by a grant from the Honjo International Scholarship Foundation. Financial funding was also provided by Pfizer Japan, Inc.; however, these sponsors had no role in the design, analysis, or interpretation of our study.
Conflict of Interest
Our study has no conflict of interest to disclose.
References
- 1). Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell, 2001; 104: 503-516 [DOI] [PubMed] [Google Scholar]
- 2). Lutgens SP, Cleutjens KB, Daemen MJ, Heeneman S. Cathepsin cysteine proteases in cardiovascular disease. FASEB J, 2007; 21: 3029-3041 [DOI] [PubMed] [Google Scholar]
- 3). Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol, 2004; 24: 1359-1366 [DOI] [PubMed] [Google Scholar]
- 4). Dall E, Brandstetter H. Structure and function of legumain in health and disease. Biochimie, 2016; 122: 126-150 [DOI] [PubMed] [Google Scholar]
- 5). Chen JM, Fortunato M, Stevens RA, Barrett AJ. Activation of progelatinase A by mammalian legumain, a recently discovered cysteine proteinase. Biol Chem, 2001; 382: 777-783 [DOI] [PubMed] [Google Scholar]
- 6). Shirahama-Noda K, Yamamoto A, Sugihara K, Hashimoto N, Asano M, Nishimura M, Hara-Nishimura I. Biosynthetic processing of cathepsins and lysosomal degradation are abolished in asparaginyl endopeptidase-deficient mice. J Biol Chem, 2003; 278: 33194-33199 [DOI] [PubMed] [Google Scholar]
- 7). Morita Y, Araki H, Sugimoto T, Takeuchi K, Yamane T, Maeda T, Yamamoto Y, Nishi K, Asano M, Shirahama-Noda K, Nishimura M, Uzu T, Hara-Nishimura I, Koya D, Kashiwagi A, Ohkubo I. Legumain/asparaginyl endopeptidase controls extracellular matrix remodeling through the degradation of fibronectin in mouse renal proximal tubular cells. FEBS Lett, 2007; 581: 1417-1424 [DOI] [PubMed] [Google Scholar]
- 8). Papaspyridonos M, Smith A, Burnand KG, Taylor P, Padayachee S, Suckling KE, James CH, Greaves DR, Patel L. Novel candidate genes in unstable areas of human atherosclerotic plaques. Arterioscler Thromb Vasc Biol, 2006; 26: 1837-1844 [DOI] [PubMed] [Google Scholar]
- 9). Sun W, Lin Y, Chen L, Ma R, Cao J, Yao J, Chen K, Wan J. Legumain suppresses OxLDL-induced macrophage apoptosis through enhancement of the autophagy pathway. Gene, 2018; 652: 16-24 [DOI] [PubMed] [Google Scholar]
- 10). Mattock KL, Gough PJ, Humphries J, Burnand K, Patel L, Suckling KE, Cuello F, Watts C, Gautel M, Avkiran M, Smith A. Legumain and cathepsin-L expression in human unstable carotid plaque. Atherosclerosis, 2010; 208: 83-89 [DOI] [PubMed] [Google Scholar]
- 11). Li W, Kornmark L, Jonasson L, Forssell C, Yuan XM. Cathepsin L is significantly associated with apoptosis and plaque destabilization in human atherosclerosis. Atherosclerosis, 2009; 202: 92-102 [DOI] [PubMed] [Google Scholar]
- 12). Liu J, Sukhova GK, Yang JT, Sun J, Ma L, Ren A, Xu WH, Fu H, Dolganov GM, Hu C, Libby P, Shi GP. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis, 2006; 184: 302-311 [DOI] [PubMed] [Google Scholar]
- 13). Lunde NN, Holm S, Dahl TB, Elyouncha I, Sporsheim B, Gregersen I, Abbas A, Skjelland M, Espevik T, Solberg R, Johansen HT, Halvorsen B. Increased levels of legumain in plasma and plaques from patients with carotid atherosclerosis. Atherosclerosis, 2017; 257: 216-223 [DOI] [PubMed] [Google Scholar]
- 14). Clerin V, Shih HH, Deng N, Hebert G, Resmini C, Shields KM, Feldman JL, Winkler A, Albert L, Maganti V, Wong A, Paulsen JE, Keith JC, Jr, Vlasuk GP, Pittman DD. Expression of the cysteine protease legumain in vascular lesions and functional implications in atherogenesis. Atherosclerosis, 2008; 201: 53-66 [DOI] [PubMed] [Google Scholar]
- 15). Braunwald E. A classification of unstable angina revisited. Circulation, 2000; 102: 118-122 [DOI] [PubMed] [Google Scholar]
- 16). Ambrose JA, Israel DH. Angiography in unstable angina. Am J Cardiol, 1991; 68: 78B-84B [DOI] [PubMed] [Google Scholar]
- 17). Dangas G, Mehran R, Wallenstein S, Courcoutsakis NA, Kakarala V, Hollywood J, Ambrose JA. Correlation of angiographic morphology and clinical presentation in unstable angina. J Am Coll Cardiol, 1997; 29: 519-525 [DOI] [PubMed] [Google Scholar]
- 18). Arima H, Kubo M, Yonemoto K, Doi Y, Ninomiya T, Tanizaki Y, Hata J, Matsumura K, Iida M, Kiyohara Y. High-sensitivity C-reactive protein and coronary heart disease in a general population of Japanese: The Hisayama Study. Arterioscler Thromb Vasc Biol, 2008; 28: 1385-1391 [DOI] [PubMed] [Google Scholar]
- 19). Momiyama Y, Kawaguchi A, Kajiwara I, Ohmori R, Okada K, Saito I, Konishi M, Nakamura M, Sato S, Kokubo Y, Mannami T, Adachi H, Kario K, Iso H, Ohsuzu F, Tsushima M. Prognostic value of plasma high-sensitivity C-reactive protein levels in Japanese patients with stable coronary artery disease: The Japan NCVC-Collaborative Inflammation Cohort Study. Atherosclerosis, 2009; 207: 272-276 [DOI] [PubMed] [Google Scholar]
- 20). Ambrose JA. Prognostic implications of lesion irregularity on coronary angiography. J Am Coll Cardiol, 1991; 18: 675-676 [DOI] [PubMed] [Google Scholar]
- 21). Rupprecht HJ, Sohn HY, Kearney P, Bickel C, Nafe B, Meyer J. Clinical predictors of unstable coronary lesion morphology. Eur Heart J, 1995; 16: 1526-1534 [DOI] [PubMed] [Google Scholar]
- 22). Chester MR, Chen L, Kaski JC. The natural history of unheralded complex coronary plaques. J Am Coll Cardiol, 1996; 28: 604-608 [DOI] [PubMed] [Google Scholar]
- 23). Meuwissen M, van der Wal AC, Koch KT, van der Loos CM, Chamuleau SA, Teeling P, de Winter RJ, Tijssen JG, Becker AE, Piek JJ. Association between complex coronary artery stenosis and unstable angina and the extent of plaque inflammation. Am J Med, 2003; 114: 521-527 [DOI] [PubMed] [Google Scholar]
- 24). Levin DC, Fallon JT. Significance of the angiographic morphology of localized coronary stenoses: histopathologic correlations. Circulation, 1982; 66: 316-320 [DOI] [PubMed] [Google Scholar]
- 25). Waxman S, Mittleman MA, Zarich SW, Fitzpatrick PJ, Lewis SM, Leeman DE, Shubrooks SJ, Jr, Abela GS, Nesto RW. Plaque disruption and thrombus in Ambrose's angiographic coronary lesion types. Am J Cardiol, 2003; 92: 16-20 [DOI] [PubMed] [Google Scholar]
- 26). Wilson RF, Holida MD, White CW. Quantitative angiographic morphology of coronary stenoses leading to myocardial infarction or unstable angina. Circulation, 1986; 73: 286-293 [DOI] [PubMed] [Google Scholar]
- 27). Ellis S, Alderman EL, Cain K, Wright A, Bourassa M, Fisher L. Morphology of left anterior descending coronary territory lesions as a predictor of anterior myocardial infarction: a CASS Registry Study. J Am Coll Cardiol, 1989; 13: 1481-1491 [DOI] [PubMed] [Google Scholar]
- 28). Kato R, Momiyama Y, Ohmori R, Taniguchi H, Nakamura H, Ohsuzu F. Levels of matrix metalloproteinase-1 in patients with and without coronary artery disease and relation to complex and noncomplex coronary plaques. Am J Cardiol, 2005; 95: 90-92 [DOI] [PubMed] [Google Scholar]
- 29). Seki T, Saita E, Kishimoto Y, Ibe S, Miyazaki Y, Miura K, Ohmori R, Ikegami Y, Kondo K, Momiyama Y. Low levels of plasma osteoglycin in patients with complex coronary lesions. J Atheroscler Thromb, 2018; 25: 1149-1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Malaud E, Merle D, Piquer D, Molina L, Salvetat N, Rubrecht L, Dupaty E, Galea P, Cobo S, Blanc A, Saussine M, Marty-Ané C, Albat B, Meilhac O, Rieunier F, Pouzet A, Molina F, Laune D, Fareh J. Local carotid atherosclerotic plaque proteins for the identification of circulating biomarkers in coronary patients. Atherosclerosis, 2014; 233: 551-558 [DOI] [PubMed] [Google Scholar]
- 31). Wang ZH, Liu XL, Zhong M, Zhang LP, Shang YY, Hu XY, Li L, Zhang Y, Deng JT, Zhang W. Pleiotropic effects of atorvastatin on monocytes in atherosclerotic patients. J Clin Pharmacol, 2010; 50: 311-319 [DOI] [PubMed] [Google Scholar]
- 32). Matsumoto A, Yamamoto H, Matsuoka T, Kayama K, Onishi S, Matsuo N, Kihara S. Cystatin C-adiponectin complex in plasma associates with coronary plaque instability. J Atheroscler Thromb, 2017; 24: 970-979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Dall E, Brandstetter H. Activation of legumain involves proteolytic and conformational events, resulting in a context- and substrate-dependent activity profile. Acta Crystallogr Sect F Struct Biol Cryst Commun, 2012; 68: 24-31 [DOI] [PMC free article] [PubMed] [Google Scholar]


