Abstract
Aim: We aimed to clarify actual food and nutrient intakes in Japanese patients with dyslipidemia. We also compared food and nutrient intakes between patients with and without low-density lipoprotein cholesterol (LDL-C) lowering drug therapy.
Methods: Food and nutrient intakes were assessed employing 3-day weighted dietary records in this cross-sectional study of 104 Japanese outpatients with dyslipidemia, age 30–65 years, not given dietary counseling. Anthropometric and biochemical parameters were measured after an overnight fast. Food and nutrient intakes were compared between patients with versus without LDL-C lowering drug prescriptions. Stepwise multiple regression analysis was performed to identify relationships between the serum LDL-C concentrations and food intakes.
Results: Of the 104 patients, 43.3% were prescribed LDL-C lowering drugs, primarily statins. Of the total patients, 83% had lipid intakes over 25% of total energy consumption (%E), exceeding the recommendation for dyslipidemia by the Japan Atherosclerosis Society. Similarly, 77% had saturated fatty acid intakes over 7%E, and 88% had cholesterol intakes over 200 mg per day. Dietary fiber consumption was low (< 25 g) in 97% of patients. Those taking LDL-C lowering drugs consumed less “meat, poultry and processed meat products” and “cereals”, and more “fish”, “fruits” and “nuts”, than patients not taking these drugs (p < 0.05). Food intakes correlating with LDL-C concentrations independently of drug therapy differed between patients taking versus not taking these medications.
Conclusion: Our results support the necessity of diet therapy for patients with dyslipidemia regardless of whether LDL-C lowering drugs are prescribed.
The clinical trial registration number: UMIN000022955
Keywords: Diet, Nutrients, Food intake, Dyslipidemia, Statins
Introduction
Dyslipidemia is a risk factor for atherosclerotic cardiovascular disease (ASCVD) and its treatment is of great public health and clinical importance. For primary prevention of ASCVD, lifestyle modifications, such as cessation of smoking, diet therapy and exercise should generally precede or accompany the administration of drug therapy1). The Japan Atherosclerosis Society recommends, in the guidelines for prevention of ASCVD, limiting total energy intake and maintaining an appropriate body weight, limiting the percentage of energy derived from fat and saturated fatty acids (SFA), decreasing cholesterol intake, and increasing the intakes of n-3 polyunsaturated fatty acids (PUFA) and dietary fiber, as the foundation of diet therapy1). In addition, based on evidence obtained from epidemiological studies, a low-salt Japanese dietary pattern (The Japan Diet) with reduced fat from meat and animal fats, and the consumption of a diet combining soy, fish, vegetables, seaweed, mushrooms, fruits, and unrefined grains is highly recommended1, 2). Maruyama et al. demonstrated the anti-atherosclerotic effects of The Japan Diet in a pilot intervention study involving middle-aged Japanese men in whom no disease had been detected3).
The Japanese dietary pattern has been considered to be anti-atherosclerotic as compared to those of western countries, but the increasing Westernization of eating habits has also been noted in Japan2, 4–9). However, recent dietary intakes of Japanese patients with dyslipidemia have not, to our knowledge, been reported. We have thus been unable to accurately ascertain the current state of food intakes that are recommended as diet therapy for dyslipidemia. Evaluation of the dietary nutrient sources for diet therapy would allow us to develop an effective therapeutic intervention for dyslipidemia.
In patients with hypercholesterolemia, lipid-lowering drugs, especially statins, can have an immediate impact on abnormal lipid profiles. Therefore, the importance of diet therapy might appear to be diminished. Food and nutrient intakes while taking lipid-lowering drugs have been studied in other countries, but the results have been inconsistent10–13). Although the implementation of dietary intake interventions and medication status differ among countries, no studies on the association between low-density lipoprotein-cholesterol (LDL-C) lowering drug use and diet have been conducted in Japan.
Aim
We investigated actual food and nutrient intakes in Japanese patients with dyslipidemia who had not received dietary counseling, to clarify the contributions of food to the nutrient intakes targeted in diet therapy for dyslipidemia. One further objective was to compare food and nutrient intakes between patients with and without LDL-C lowering drug therapy.
Methods
Study Design and Subjects
This was a cross-sectional study of dyslipidemic patients who agreed to participate in “A randomized clinical trial to assess the effects of the Japan Diet intake on serum lipid and chronic inflammation parameters”. Outpatients with dyslipidemia who had not received dietary counseling were recruited from the Teramoto Medical and Dental Clinic (Tokyo, Japan), Shizuoka City Shizuoka Hospital (Shizuoka, Japan) and Tokorozawa Heart Center (Saitama, Japan) between September 2016 and September 2018. All subjects were Japanese and had documented dyslipidemia, based on laboratory examinations. The inclusion criteria were fasting serum LDL-C ≧ 140 mg/dL and/or triglyceride (TG) ≧ 150 mg/dL, age between 30 and 65 years, body mass index (BMI) over 18.5 kg/m2, receiving no medication or treated with the same drug for at least 3 months, and determined by a doctor to be able to participate in the study. The exclusion criteria were being a smoker, habitual dietary supplement or health food product user, pregnant, homozygous familial hypercholesterolemia, past history of ASCVD, renal dysfunction (estimated glomerular filtration rate < 60 ml/min/1.73 m2), hemoglobin A1c (HbA1c) ≧8.0% and hypothyroidism.
The present study was conducted according to the guidelines of the Declaration of Helsinki, and all procedures were approved by the ethical committee for experimental research involving human subjects of Japan Women's University (No.246). We obtained written informed consent from all patients prior to participation. The clinical trial registration number is UMIN000022955.
Dietary Data Collection
Each patient completed a 3-day weighted dietary record. When they agreed to participate in the study, the patients were instructed as to how to keep their dietary records, and were provided recording sheets. They were asked to weigh and record all foods and beverages consumed on each day of recording. When weighing was difficult, the patients were instructed to take a picture and record the size and quantity of the foods they ate. The three recording days consisted of one weekend day and two weekdays during the one-week period before the examinations. Three-day dietary records that had been kept by the patients were confirmed and collected by a registered dietitian. Energy and nutrient intakes were calculated employing Excel-Eiyokun Version 8.0 software (Kenpakusha Co., Ltd., Tokyo, Japan) based on the Standard Tables of Food Composition in Japan -2015- (Seventh revised edition) (Ministry of Education, Culture, Sports, Science and Technology, Japan)14). In the Standard Tables of Food Composition, soluble and insoluble dietary fiber types are not listed separately for the “seaweed” category, such that we evaluated total dietary fiber intake. The food intakes were calculated based on division into food groups. Food groups based on the classification of the Standard Tables of Food Composition in Japan, were partially modified in accordance with nutrient content similarities and dietary counseling instructions. The “seafood other than fish and processed fish products (other seafood)” category consists of shellfish, prawns, shrimp, squid, octopus, crab, fish eggs and viscera and fish paste products. Canned fish was categorized as “fish”, rather than as “processed fish products”. Foods contained in the original “pulses” group in the Standard Tables of Food Composition were divided into “soybeans and soy products” and “other beans”. Miso (fermented soybean paste), a fermented seasoning made from soybeans, was not included in the “soybeans and soy products” category, instead being included among the “seasonings and spices”, the same as the classification in the Standard Tables of Food Composition. This was because miso is generally used as a seasoning in Japan, and its salt content is high. The “seaweed, mushrooms and konjac” category is comprised of foods very low in energy and high in fiber, foods that have traditionally been widely consumed in Japan. Therefore, “seaweed, mushroom and konjac” were categorized into a food group different from that of vegetables. The “total vegetables” category includes vegetable juice intake, the “fruits” category that of fruit juices. “High fat seasonings” consist of mayonnaise, oil-based dressings and roux. The alcoholic beverage intake values were calculated as pure ethanol amount in grams. For nutrient and food group intakes, the average intake for 3 days was taken as the subject's daily intake. To compare between groups, the amount of food intake was adjusted by energy intake, based on 1,000 kcal, to minimize differences in energy and food intakes due to body size and physical activity. The contribution rate, by food group, to each nutrient intake was calculated as the ratio of the nutrient intake from that food group to the total nutrient intake.
Confirmation of Medication Status
Prescribed drugs were confirmed based on the descriptions in the patients' medical records. Drugs prescribed by medical institutions other than the research facilities in the present study, were selfreported by the patients using a self-administered questionnaire. We classified statins, cholesterol absorption inhibitors and probucol as LDL-C lowering drugs, and fibrate and n-3 PUFA as TG-lowering drugs in this analysis.
Measurements
Anthropometric measurements and fasting blood collection were conducted in the morning following a 12-hour overnight fast. Body height and weight were measured, and BMI was calculated as weight (kg) divided by the square of height (m). A BMI of 22 kg/m2 was regarded as corresponding to the standard body weight (SBW). Blood pressure was measured with the patient in the seated position. Plasma and serum samples were obtained and the following concentrations were measured: serum total cholesterol (TC) (cholesterol dehydrogenase UV method), LDL-C (enzymatic method (direct method)), high-density lipoprotein-cholesterol (HDL-C) (enzymatic method (direct method)), TG (enzymatic method), HbA1c (latex coagulating method), plasma glucose (hexokinase UV method), serum insulin (chemiluminescent enzyme immunoassay (CLEIA) method), and uric acid (enzymatic method) at the laboratory of BML Inc., Tokyo, Japan. Non HDL-C was calculated as TC minus HDL-C.
Statistical Analysis
Statistical analyses were carried out using IBM SPSS Statistics (Version 22; IBM Japan, Ltd., Tokyo, Japan). The patients were divided into two groups depending on whether or not LDL-C lowering drugs had been prescribed. The normality of the distribution was assessed be applying the Shapiro-Wilk test. To test differences between groups, we performed the unpaired t-test for normal distributions and the Mann-Whitney U test for non-normal distributions. Categorical variables were analysed with the Chisquared test or Fisher's exact test. Stepwise multiple regression analyses were performed with serum LDL-C concentrations as the dependent variable, while food intakes (expressed as amount per 1,000 kcal of energy intake), age, gender (coded as women = 0, men = 1), BMI, and prescribed medications (coded as not prescribed = 0, prescribed = 1) were the independent variables. A value of p < 0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of Subjects
This study included 104 patients (53 women / 51 men) with dyslipidemia. The mean age was 53 ± 8 (Mean ± SD) years. Overall, 38.5% of the patients were categorized as being obese, with BMI ≧ 25 kg/m2. Patients taking lipid-lowering drugs accounted for 56.7%. Forty-five patients (43.3%) were prescribed LDL-C lowering drugs, and 88.9% of these 45 were taking statins while 24.4% were taking a cholesterol absorption inhibitor. Patients taking LDL-C lowering drugs were older (p < 0.001), tended to more often be female (p = 0.107), had more frequently been prescribed hypoglycemic drugs (p = 0.016), and fewer were taking anti-hyperuricemic drugs (p = 0.014), than those not taking LDL-C lowering drugs (Table 1).
Table 1. Clinical characteristics of Japanese patients with dyslipidemia.
| All patients (n = 104) |
Patients not taking LDL-C lowering drugs (n = 59) |
Patients taking LDL-C lowering drugs (n = 45) |
p value | |
|---|---|---|---|---|
| Age (years) | 53 ± 8 | 50 ± 9 | 57 ± 6 | < 0.001 |
| Men / Women | 51 / 53 | 33 / 26 | 18 / 27 | 0.107 |
| Prescribed lipid-lowering drug | 59 (56.7) | 14 (23.7) | 45 (100.0) | < 0.001 |
| Statin | 40 (38.5) | 0 (0.0) | 40 (88.9) | — |
| Cholesterol absorption inhibitor | 11 (10.6) | 0 (0.0) | 11 (24.4) | — |
| Probucol | 2 (1.9) | 0 (0.0) | 2 (4.4) | — |
| Fibrate | 13 (12.5) | 11 (18.6) | 2 (4.4) | 0.030 |
| n-3 polyunsaturated fatty acid | 7 (6.7) | 4 (6.8) | 3 (6.7) | 0.649 |
| Hypertension | 48 (46.2) | 29 (49.2) | 19 (42.2) | 0.482 |
| Prescribed antihypertensive drug | 29 (27.9) | 19 (32.2) | 10 (22.2) | 0.261 |
| Diabetes | 15 (14.4) | 6 (10.2) | 9 (20.0) | 0.157 |
| Prescribed hypoglycemic drug | 10 (9.6) | 2 (3.4) | 8 (17.8) | 0.016 |
| Prescribed anti-hyperuricemic drug | 11 (10.6) | 10 (16.9) | 1 (2.2) | 0.014 |
n = 104, Values are presented as means ± standard deviations or number (%).
P values were calculated using the unpaired t-test, the chi-square test or Fisher's exact test for comparisons between patients with versus without LDL-C-lowering drug therapy.
The LDL-C concentrations of patients taking LDL-C lowering drugs averaged 113 ± 27 mg/dL, a value lower than that of patients not taking these drugs (135 ± 29 mg/dL) (p < 0.001) (Table 2). Those with LDL-C ≧ 120 mg/dL accounted for 56.7% of all patients, 33.3% of LDL-C lowering drug-treated patients and 74.6% of those not taking these drugs. The TG concentration tended to be lower in patients taking LDL-C lowering drugs (p = 0.073). Those with TG ≧ 150mg/dL accounted for 27.9% of all patients, 22.2% of patients taking LDL-C lowering drugs and 32.2% of those not taking these drugs.
Table 2. Anthropometric variables, blood pressure and biochemical parameters of Japanese patients with dyslipidemia.
| All patients (n = 104) |
Patients not taking LDL-C lowering drugs (n = 59) |
Patients taking LDL-C lowering drugs (n = 45) |
p value | |
|---|---|---|---|---|
| Body height (cm) | 163.2 ± 8.3 | 164.2 ± 8.6 | 161.9 ± 7.8 | 0.155 |
| Body weight (kg) | 65.3 ± 13.9 | 67.3 ± 15.4 | 62.7 ± 11.4 | 0.205 |
| Body Mass Index (kg/m2) | 24.3 ± 3.8 | 24.7 ± 4.2 | 23.8 ± 3.2 | 0.327 |
| Systolic blood pressure (mmHg) | 127 ± 17 | 129 ± 15 | 124 ± 19 | 0.148 |
| Diastolic blood pressure (mmHg) | 79 ± 12 | 81 ± 12 | 76 ± 13 | 0.023 |
| Total cholesterol (mg/dL) | 220 ± 34 | 231 ± 30 | 204 ± 33 | < 0.001 |
| non HDL-cholesterol (mg/dL) | 158 ± 35 | 172 ± 31 | 140 ± 31 | < 0.001 |
| LDL-cholesterol (mg/dL) | 126 ± 30 | 135 ± 29 | 113 ± 27 | < 0.001 |
| HDL-cholesterol (mg/dL) | 62 ± 17 | 59 ± 18 | 64 ± 16 | 0.129 |
| Triglyceride (mg/dL) | 102 [76 – 155] | 111 [76 – 174] | 88 [70 – 135] | 0.073 |
| HbA1c (%) | 5.7 ± 0.6 | 5.6 ± 0.5 | 5.8 ± 0.6 | 0.061 |
| Glucose (mg/dL) | 100 ± 20 | 96 ± 13 | 105 ± 25 | 0.113 |
| Insulin (µU/mL) | 7.0 ± 4.8 | 7.0 ± 5.1 | 7.1 ± 4.4 | 0.445 |
| Uric acid (mg/dL) | 5.5 ± 1.3 | 5.5 ± 1.3 | 5.5 ± 1.2 | 0.756 |
n = 104, Values are presented as means ± standard deviations or median [interquartile range].
HDL: High-density lipoprotein, LDL: Low-density lipoprotein.
P values were calculated using the Mann-Whitney U test or the unpaired t-test for comparisons between patients with versus without LDL-C-lowering drug therapy.
Energy and Nutrient Intakes
Total energy intake, energy intakes derived from lipids and SFA, and cholesterol intakes did not differ between the patients with and without LDL-C lowering drug therapy (Table 3).
Table 3. Daily energy and nutrient intakes of Japanese patients with dyslipidemia.
| All patients (n = 104) |
Patients not taking LDL-C lowering drugs (n = 59) |
Patients taking LDL-C lowering drugs (n = 45) |
p value | |
|---|---|---|---|---|
| Energy (kcal) | 1895 ( 773, 3156) | 1869 ( 773, 2866) | 1927 ( 901, 3156) | 0.707 |
| Energy (kcal/SBWkg) | 32.5 (14.3, 54.7) | 30.7 (14.3, 41.9) | 33.4 ( 16.7, 54.7) | 0.493 |
| Lipid (g) | 60.9 (15.0, 120.2) | 60.9 (15.0, 110.0) | 61.6 ( 24.4, 120.2) | 0.392 |
| (%E) | 29.8 (17.5, 46.1) | 29.9 (17.5, 44.1) | 29.8 ( 20.0, 46.1) | 0.348 |
| SFA (g) | 17.6 ( 4.6, 41.5) | 18.4 ( 4.6, 33.8) | 16.6 ( 7.5, 41.5) | 0.966 |
| (%E) | 8.6 ( 4.4, 14.0) | 8.7 ( 4.4, 12.9) | 8.5 ( 4.8, 14.0) | 0.738 |
| MUFA (g) | 23.6 ( 5.6, 47.9) | 24.4 ( 5.6, 41.0) | 23.3 ( 8.8, 47.9) | 0.552 |
| n-3PUFA (g)§ | 2.4 ( 0.7, 11.5) | 2.2 ( 0.7, 5.3) | 2.7 ( 0.7, 11.5) | 0.006 |
| EPA+DHA (g)§ | 0.6 ( 0.0, 3.3) | 0.4 ( 0.0, 2.2) | 0.8 ( 0.0, 3.3) | 0.002 |
| n-6PUFA (g) | 10.9 ( 1.9, 23.4) | 11.0 ( 1.9, 20.6) | 10.7 ( 4.1, 23.4) | 0.893 |
| Cholesterol (mg) | 334 ( 50, 767) | 346 ( 50, 767) | 333 ( 115, 726) | 0.659 |
| Carbohydrate (g) | 235.0 (81.1, 408.0) | 236.5 (81.1, 408.0) | 232.9 (133.1, 356.0) | 0.855 |
| (%E) | 55.2 (34.4, 68.1) | 55.6 (37.2, 68.1) | 54.6 (34.4, 67.3) | 0.216 |
| Total dietary fiber (g) | 14.2 ( 5.5, 32.0) | 13.8 ( 6.5, 32.0) | 15.4 ( 5.5, 27.6) | 0.095 |
| (g/1,000 kcal) | 7.7 ( 3.4, 16.5) | 7.5 ( 3.4, 16.5) | 7.9 ( 3.6, 14.0) | 0.250 |
| Protein (g) | 70.2 (27.8, 135.8) | 68.6 (27.8, 126.6) | 73.9 ( 31.8, 135.8) | 0.219 |
| (%E) | 14.9 (11.4, 25.6) | 14.7 (11.4, 25.6) | 15.3 ( 11.6, 20.4) | 0.323 |
| Salt equivalents (g) | 10.0 ( 3.2, 16.4) | 9.6 ( 3.6, 16.4) | 10.2 ( 3.2, 15.3) | 0.962 |
n = 104, Values are presented as medians (minimum, maximum).
DHA: docosahexaenoic acid, EPA: eicosapentaenoic acid, MUFA: monounsaturated fatty acids, PUFA: polyunsaturated fatty acids, SBW: standard body weight, SFA: saturated fatty acids, %E: % energy.
P values were calculated using the Mann-Whitney U test or the unpaired t-test for comparisons between patients with versus without LDL-C-lowering drug therapy.
The values do not include prescribed n-3 polyunsaturated fatty acid-based preparations.
Median energy intake was 32.5 kcal/SBW kg, but 36% of all patients consumed 35 kcal/SBW kg or more, which is regarded as the upper limit of energy intake for normal activity, and 10% of all patients consumed 40 kcal/SBW kg or more. Maximum energy intake was 54.7 kcal/SBW kg and this value was observed in the patients treated with LDL-C lowering drugs.
The median energy intake derived from lipids was 29.8% of total energy (%E), with 83% of patients consuming 25%E or more which is the upper limit of the dietary recommendation for dyslipidemia, 49% consuming 30%E or more which is the upper limit of dietary reference intakes for the Japanese population15), and the maximum was 46.1%E. The median energy intake derived from SFA was 8.6%E, and patients whose intake was 7%E or more accounted for 77%. Patients taking LDL-C lowering drugs had higher total eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) intakes than those not taking these drugs (p = 0.002), resulting in higher total n-3 PUFA intake in the former as compared to the latter (p = 0.006). Some patients consumed neither EPA nor DHA, and 30% consumed less than 300 mg of EPA and DHA. The median cholesterol intake was 334 mg, but cholesterol intakes varied from a minimum of 50 mg to a maximum of 767 mg, with 88% of patients consuming 200 mg or more and 58% consuming 300 mg or more.
Energy intake derived from carbohydrates between 50 and 60%E was consumed by 65% of all patients. Median total dietary fiber intake was 14.2 g, and less than 3% of patients consumed 25 g or more daily, which is considered to be a desirable intake, and minimum intake was only 3.4 g/1,000 kcal.
More than 95% of patients consumed the equivalent of more than 6 g of salt daily.
Food Intakes
Food intakes are shown in Table 4. The food intake amount is presented as the crude intake (g) for all patients, and adjusted intake as per 1,000 kcal of energy intake (g/1,000 kcal) for comparison between patients with and without LDL-C lowering drug therapy.
Table 4. Daily food intakes of Japanese patients with dyslipidemia.
| Food intakes (g) |
Food intakes (g/1,000 kcal) |
|||
|---|---|---|---|---|
| Food groups | All patients (n = 104) |
Patients not taking LDL-C lowering drugs (n = 59) |
Patients taking LDL-C lowering drugs (n = 45) |
p value |
| Cereals | 357.2 (99.0, 854.8) | 207.2 (102.8, 351.1) | 179.6 (64.1, 340.3) | 0.004 |
| Refined cereals | 318.6 (19.0, 854.8) | 183.1 ( 23.7, 298.3) | 173.4 (29.7, 340.3) | 0.113 |
| Unrefined cereals | 3.6 ( 0.0, 263.0) | 8.6 ( 0.0, 218.4) | 0.0 ( 0.0, 186.2) | 0.072 |
| Meat, poultry and processed meat products | 89.2 ( 0.0, 301.2) | 51.3 ( 13.7, 137.3) | 44.0 ( 0.0, 102.9) | 0.010 |
| Eggs | 32.3 ( 0.0, 130.5) | 17.6 ( 0.0, 57.7) | 15.5 ( 0.8, 60.3) | 0.630 |
| Milk and dairy products | 74.8 ( 0.0, 449.4) | 34.9 ( 0.0, 186.8) | 44.3 ( 0.0, 173.9) | 0.295 |
| Fish | 39.0 ( 0.0, 151.7) | 16.1 ( 0.0, 76.2) | 30.2 ( 0.0, 69.7) | 0.004 |
| Other seafood | 15.5 ( 0.0, 139.2) | 8.7 ( 0.0, 56.2) | 8.8 ( 0.0, 70.1) | 0.935 |
| Soybeans and soy products | 39.4 ( 0.0, 325.9) | 23.2 ( 0.0, 156.6) | 18.6 ( 0.0, 164.5) | 0.992 |
| Other beans | 0.0 ( 0.0, 32.3) | 0.0 ( 0.0, 6.3) | 0.0 ( 0.0, 13.9) | 0.524 |
| Nuts | 0.2 ( 0.0, 35.3) | 0.1 ( 0.0, 2.5) | 0.3 ( 0.0, 21.0) | 0.018 |
| Potatoes and other starches | 21.3 ( 0.0, 150.8) | 10.6 ( 0.0, 42.3) | 13.3 ( 0.0, 86.4) | 0.406 |
| Total vegetables | 255.9 (79.2, 731.2) | 151.4 ( 30.9, 301.5) | 131.6 (79.0, 386.1) | 0.903 |
| Green and yellow vegetables | 73.9 ( 3.9, 440.3) | 38.9 ( 4.6, 122.9) | 44.1 ( 1.9, 232.5) | 0.987 |
| Other vegetables | 148.5 (14.3, 335.3) | 68.4 ( 18.8, 191.1) | 83.3 (11.5, 183.0) | 0.735 |
| Salted or pickled vegetables | 5.5 ( 0.0, 85.9) | 1.2 ( 0.0, 40.0) | 4.8 ( 0.0, 35.3) | 0.008 |
| Seaweed, mushrooms and konjac | 22.4 ( 0.2, 100.3) | 11.0 ( 0.1, 55.9) | 15.5 ( 1.2, 51.1) | 0.143 |
| Fruits | 44.4 ( 0.0, 426.7) | 7.1 (0.0, 142.4) | 37.8 ( 0.0, 216.4) | 0.001 |
| Animal fats, SFA-rich vegetable oils and margarine | 1.3 ( 0.0, 15.5) | 0.6 ( 0.0, 6.4) | 0.6 ( 0.0, 5.3) | 0.845 |
| Vegetable oils and high fat seasonings | 20.2 ( 1.5, 47.6) | 10.3 ( 2.2, 23.2) | 9.0 ( 1.1, 27.0) | 0.191 |
| Seasonings and spices | 49.6 ( 3.9, 116.7) | 25.8 ( 2.4, 65.5) | 25.2 ( 8.9, 45.0) | 0.682 |
| Confections and sweets | 43.6 ( 1.1, 276.5) | 20.5 ( 0.7, 86.8) | 28.1 ( 0.6, 119.3) | 0.311 |
| Sugar-sweetened beverages | 0.0 ( 0.0, 641.7) | 0.0 ( 0.0, 282.4) | 0.5 ( 0.0, 339.6) | 0.365 |
| Alcoholic beverages§ | 0.0 ( 0.0, 71.0) | 0.0 ( 0.0, 33.9) | 0.0 ( 0.0, 33.6) | 0.583 |
n = 104, Values are presented as medians (minimum, maximum) in grams for all patients, and in grams per 1,000 kcal of energy intake for patients with versus without LDL-C lowering drug therapy.
Beverages without sugar and soup stocks were omitted from the table.
P values were calculated using the Mann-Whitney U test for comparisons between patients with versus without LDL-C-lowering drug therapy.
Values are calculated as pure ethanol amount in grams.
The median intake of “refined cereals” was 318.6 g, though 46% consumed no “unrefined cereals” such that the median intake of the entire group was very low, at 3.6 g. “Cereals” intake was lower in patients with than in those without LDL-C lowering drug therapy (p = 0.004).
The median intake of “meat, poultry and processed meat products” was 89.2 g, with a maximum intake of 301.2 g. Among these categories, the median intake of “fatty meat, poultry and processed meat products”, containing 10 g of lipids per 100 g or more, was 69.9 g, which accounted for about 80% of the total “meat, poultry and processed meat products” intake. Intake of “meat, poultry and processed meat products” was lower in patients with than in those without LDL-C lowering drug therapy (p = 0.010). “Egg” intakes did not differ between patients with and without LDL-C lowering drug therapy, and the median intake was 32.3 g with some patients consuming no eggs at all. “Fish” intake was less than that of “meat, poultry and processed meat products”, and the median intake was 39.0 g, with 10% of patients consuming no “fish”. “Fish” intake by patients taking LDL-C lowering drugs was approximately double that of patients not taking these drugs (p = 0.004). Although 11% of patients did not consume “other seafood”, the foods mainly eaten were fish paste products, shrimp, shellfish and squid, and the maximum intake was approximately 140 g, showing no difference between patients with and without LDL-C lowering drug therapy. The median intake of “soybeans and soy products” was 39.4 g, but the maximum was high, at 325.9 g, and there was no difference between the two groups. The median intake of “nuts” was low, only 0.2 g, and 25% of patients ate no nuts during the period of keeping dietary records. “Nuts” intake was higher in patients with than in those without LDL-C lowering drug therapy (p = 0.018), and the maximum intake was 21.0 g/1,000 kcal in the former, but only 2.5 g/1,000 kcal in the latter.
The “total vegetables” intake was low, 79.2 g at a minimum, which is approximately one serving. The median intake was 255.9 g, and only 28% of patients consumed 350 g or more of vegetables, as recommended in the diet for dyslipidemia. The minimum intake of “green and yellow vegetables” was 3.9 g, the median was 73.9 g, and only 13% of patients consumed more than the recommended amount of 150 g. The median intake of “seaweed, mushrooms and konjac” was 22.4 g, but 7%, 15%, and 61% of patients, respectively, did not consume these three food items.
The median intake of “animal fats, SFA-rich vegetable oils and margarine” was 1.3 g, but the maximum was high, at 15.5 g. The median and maximum total intakes of “vegetable oil and high fat seasonings” were 20.2 g and 47.6 g, respectively. Median “confections and sweets” intake was 43.6 g and there was no difference between the two groups. Nearly two-thirds (65%) of patients consumed no alcoholic beverages.
Contributions of Food Groups to Nutrient Intakes
The largest contribution to total energy intake was that of “cereals”, at 36%, followed by “meat, poultry and processed meat products”, “confections and sweets” and “vegetable oil and high fat seasonings” (Fig. 1). The “meat, poultry and processed meat products” and “vegetable oil and high fat seasonings” categories accounted for 50% of lipid intake, followed by “confections and sweets”. The “meat, poultry and processed meat products” category made the largest, at 34%, contribution to SFA intake and, together with “confections and sweets” and “milk and dairy products”, accounted for about 60% of the total. The “animal fats, SFA-rich vegetable oils and margarine” category accounted for 5% of the SFA intake. The contributions to n-3 PUFA intake were 36% for the “vegetable oil and high fat seasonings”, 27% for the “fish”, and 10% for the “soybeans and soy products” categories. Three-quarters of total EPA and DHA intake was derived from “fish” and “other seafood”. As to n-6 PUFA intake, “vegetable oil and high fat seasonings” accounted for 35%, followed by “soybeans and soy products” and “meat, poultry and processed meat products”.
Fig. 1.
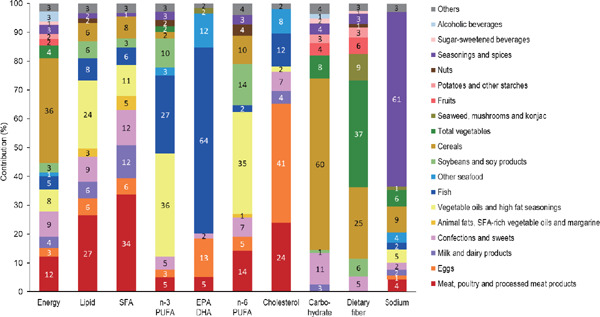
Contributions of food groups to nutrient intakes in Japanese patients with dyslipidemia
n = 104, The values are average contributions to nutrient intakes, calculated as the nutrient intake amounts from each food group divided by the total nutrient intake.
Food groups contributing less than 1% are included in “others”.
DHA: docosahexaenoic acid, EPA: eicosapentaenoic acid, PUFA: polyunsaturated fatty acids, SFA: saturated fatty acids
The contributions to cholesterol intake were 41% for “eggs”, 24% for “meat, poultry and processed meat products”, 12% for “fish”, and 8% for “other seafood”.
The “cereals” category made the largest contribution to carbohydrate intake, at 60%, followed by “confections and sweets” at 11%. The “fruits” and “sugar-sweetened beverages” accounted for 4% and 2%, respectively, of carbohydrate intake. The “total vegetables” and “cereals” categories accounted for 60% of total dietary fiber intake, followed by 9% from “seaweed, mushrooms and konjac”, 6% from “soybeans and soy products”, and 6% from “fruits”.
The “seasonings and spices” category accounted for 61% of sodium intake.
Relationships between LDL-C Concentrations and Food Intakes
Stepwise multiple regression analysis was performed with LDL-C concentrations as the dependent variable, while food intakes per 1,000 kcal of energy intake, age, gender, BMI and presence or absence of prescribed drugs served as independent variables (Table 5).
Table 5. Stepwise multiple regression analysis of serum LDL-C concentrations versus food intakes in Japanese patients with dyslipidemia.
| All patients § (n = 104) |
Patients not taking LDL-C lowering drugs† (n = 59) |
Patients taking LDL-C lowering drugs‡ (n = 45) |
||||||
|---|---|---|---|---|---|---|---|---|
| Independent variables | β | p value | Independent variables | β | p value | Independent variables | β | p value |
| LDL-C-lowering drug | −0.412 | < 0.001 | TG-lowering drug | −0.378 | 0.001 | Animal fats, SFA-rich | 0.328 | 0.028 |
| TG-lowering drug | −0.254 | 0.006 | Other seafood | 0.402 | < 0.001 | vegetable oils and | ||
| Other seafood | 0.178 | 0.046 | Nuts | −0.292 | 0.009 | margarine | ||
| Confections and sweets | 0.276 | 0.013 | ||||||
β: standardized coefficient, LDL-C: Low-density lipoprotein- cholesterol, SFA: saturated fatty acid, TG: triglyceride
Dependent variable: serum LDL-C concentrations
Independent variables: food intakes (g/1,000 kcal; cereals; meat, poultry and processed meat products; eggs; milk and dairy products; fish; other seafood; soybeans and soy products; nuts; potatoes and other starches; total vegetables; seaweed, mushrooms and konjac; fruits; animal fats, SFA-rich vegetable oils and margarine; vegetable oils and high fat seasonings; seasonings and spices; confections and sweets; sugar-sweetened beverages; alcoholic beverages), age, gender (coded as women = 0, men= 1), BMI and prescribed medications (coded as not prescribed = 0, prescribed = 1; LDLC-lowering drug, TG-lowering drug, antihypertensive drug, hypoglycemic drug, anti-hyperuricemic drug)
Adj R2 = 0.196, p < 0.001
Adj R2 = 0.385, p < 0.001
Adj R2 = 0.087, p = 0.028
In all patients, administration of LDL-C and TG lowering drugs correlated negatively with LDL-C concentrations, while the intake of “other seafood” showed a weak but independent positive correlation with LDL-C concentrations (Adj R2 = 0.196, p < 0.001). In patients not taking LDL-C lowering drugs, intakes of “other seafood” and “confections and sweets” correlated positively, while taking TG lowering drugs and “nuts” intake correlated negatively, with LDL-C concentrations (Adj R2 = 0.385, p < 0.001). In patients taking LDL-C lowering drugs, only intake of “animal fats, SFA-rich vegetable oils and margarine” showed a positive, though weak, correlation with LDL-C concentrations (Adj R2 = 0.087, p = 0.028).
Discussion
We studied Japanese patients with dyslipidemia who had not received dietary counseling. We found that there were many patients consuming excessive amounts of energy, lipids, SFA, cholesterol and sodium, while consumptions of dietary fiber and EPA and DHA were low, regardless of whether or not they were being treated with LDL-C lowering drugs.
“Meat, poultry and processed meat products” and “confections and sweets”, considered to cause hypercholesterolemia, made major contributions to the intakes of energy, lipids, SFA and cholesterol, which were high in our patients. On the other hand, EPA and DHA intakes were mostly derived from fish in our patients. Fish intake in Japan is among the highest in the world16), but the present patients included individuals who consumed neither EPA nor DHA and 30% of the patients had an EPA and DHA intake below 0.3 g. This is equivalent to the lowest quintile of intake according to an epidemiological study showing that subjects whose combined intakes of EPA and DHA exceeded 0.9 g had less coronary heart disease than those with the lowest quintile17).
Patients taking LDL-C lowering drugs consumed less “meat, poultry and processed meat products” and “cereals”, and more “fish”, “fruits” and “nuts”, which are considered to be healthy, than patients not taking these drugs. Some cross-sectional studies conducted in western countries found that statin users have a relatively preferable diet, that is, they endeavor to avoid fatty foods and also aim to eat foods with a high fiber content10), and have lower SFA intake11) than subjects not taking these drugs. Possible causes of these differences in food intakes may include so-called “medication behavior”, i.e. an increase in disease consciousness which might be actively practiced by obtaining information on treatments including dietary modifications. On the other hand, there are also reports of vegetable intake being lower12), and energy and lipid intakes increasing over time13) in statin users. These observations raise concern that diet might be neglected when a therapeutic effect is achieved, or assumed to be achieved, by drug therapy. In addition, patients taking LDL-C lowering drugs had a higher intake of “salted or pickled vegetables”, and may have had lower awareness of blood pressure management than those not taking these drugs.
The results of multiple regression analysis confirmed LDL-C concentrations to be reduced by lipidlowering drugs. Food intakes were shown to be associated with the LDL-C concentration independently of drug therapy, indicating the significance of diet therapy even when taking a LDL-C lowering drug. The food group which remained an independent variable in the analysis of LDL-C concentrations was “other seafood”, showing a positive association in all patients, and also in patients not taking LDL-C lowering drugs. The “other seafood” category includes food items that have low lipid contents but a high cholesterol content14). A diet containing 300 g of shrimp, which supplies 590 mg of dietary cholesterol, reportedly increased LDL-C in a randomized crossover trial18). Although the intake of “other seafood” in the present study was extremely small, the cholesterol intake accounted for 8% of total cholesterol consumption, and thus could not be ignored. However, in patients taking LDL-C lowering drugs, “other seafood” did not remain an independent variable, despite intakes not differing from those of patients not taking these drugs. A quarter of patients with LDL-C lowering drug therapy were taking a cholesterol absorption inhibitor, which inhibits the absorption of dietary cholesterol in the small intestine19). Thus, we can reasonably speculate that the effect of “other seafood” derived cholesterol on LDL-C concentrations might have been reduced by treatment with a cholesterol absorption inhibitor. However, the cross-sectional design of this study has limitations, and the impacts of seafood other than fish and processed fish products need further study.
In patients not taking LDL-C lowering drugs, a positive association of LDL-C with “confections and sweets” and a negative association with “nuts” were also recognized. The “confections and sweets” category is likely to be positively associated with LDL-C concentrations due to the high contribution of these foods to energy, lipid and SFA intakes. Consuming nuts reportedly lowers LDL-C levels, but this effect is recognized only at a dietary nut intake of at least 60 g per day20). In the present study, many patients did not consume nuts, and even the maximum intake was small, at an amount equivalent to 4.7 g in patients not taking LDL-C lowering drugs. Thus, further study is required to elucidate the relationship between consumption of nuts and the LDL-C concentration.
In the patients taking LDL-C lowering drugs, only “animal fats, SFA-rich vegetable oils and margarine” still showed a positive, though extremely weak, association with LDL-C concentrations. Although the median intake of “animal fats, SFA-rich vegetable oils and margarine” was small, only 1.3 g, the SFA intake accounted for 5%. Margarine and shortening are comprised of 0.5 to 1.2% trans fatty acids14). Statins, the most commonly taken drug in our patients, inhibit HMG-CoA reductase, suppress cholesterol synthesis, and promote LDL receptor synthesis, thereby lowering the blood LDL-C level21). On the other hand, SFA reduce expression of the LDL receptor and increase blood LDL-C levels22), and trans fatty acids increase apoB-100 23, 24). Therefore, we can reasonably speculate that SFA and trans-fatty acids, whose mechanisms of action in cholesterol metabolism differ from those of statins, were independently extracted as factors related to LDL-C concentrations in the patients taking LDL-C lowering drugs.
Another goal of dietary therapy for hypercholesterolemia is to increase dietary fiber intake, based on the expectation that cholesterol absorption will be inhibited while fecal excretion of bile acids in the intestinal tract is promoted25, 26). However, on multiple regression analysis, food sources of dietary fiber, such as “vegetables”, “cereals”, “seaweed, mushrooms and konjac”, “soybeans and soy products” and “fruits”, were not factors independently related to LDL-C concentrations. This was observed in patients both with and without LDL-C lowering drug therapy. A metaanalysis showed that a 3 g increase in soluble dietary fiber intake decreased LDL-C levels27). In statin users, an LDL-C-lowering effect was observed with supplemental administration of 15 g of psyllium28), and with consumption of more than 3 oz. (84 g) of whole grain equivalents per day29). In the present study, the intakes of dietary fiber and unrefined cereals, which are expected to be a source of dietary fiber, were much lower than those in prior studies, regardless of whether or not LDL-C lowering drugs were being taken, likely accounting for the lack of association with LDL-C levels.
Our present study has several limitations. First, although the food and nutrient intakes were ascertained employing three-day dietary records, a method which is considered to be the standard in this type of research, the possibilities of under-reporting and also of dietary records not reflecting habitual intake cannot be ruled out. In addition, we could not evaluate trans fatty acid, or the intakes of soluble and insoluble dietary fiber, due to the lack of these data in the Standard Tables of Food Composition in Japan14). Second, the small number of patients did not allow us to evaluate differences according to gender, age, or the types and amounts of medications being taken. Third, since the patients with dyslipidemia in the present study were limited to non-smokers and those who did not use supplements, an investigation with a larger number of patients with more variables is required.
While we acknowledge these limitations, in recent years, no studies have focused on the actual food and nutrient intakes of Japanese patients with dyslipidemia, by employing the dietary record method. The present results suggest the necessity of diet therapy for patients with dyslipidemia regardless of whether or not they are receiving LDL-C lowering drug therapy. Moreover, based on the actual dietary intake situation, we identified food groups warranting particular attention when providing diet therapy for dyslipidemia. Maruyama et al. conducted a pilot intervention study and reported an ameliorating effect on ASCVD risk factors in response to adopting the lowsalt Japanese dietary pattern (The Japan Diet)3, 30). The clinical significance of recommending this Japanese dietary pattern is also supported by the actual food and nutrient intakes of Japanese patients with dyslipidemia, as observed herein.
Conclusion
Among Japanese patients with dyslipidemia who had not received dietary counseling, we identified several who consumed excessive amounts of energy, lipids, SFA, cholesterol and sodium, while consuming relatively small amounts of dietary fiber, EPA and DHA, regardless of whether or not LDL-C lowering drugs had been prescribed. Food groups showing an independent correlation with LDL-C concentrations differed between patients with and without LDL-C lowering drug therapy. Furthermore, diet therapy while taking LDL-C lowering drugs, merits further consideration.
Acknowledgments
The authors thank all patients who participated in the present study, and all staff members who supported the survey. The authors also appreciate all members of the Laboratory of Nutrition Education and Clinical Nutrition of Japan Women's University, including Ms. Kanako Kamoshita, Ms. Seina Komine, Ms. Sayaka Hasegawa, Ms. Rina Ichiki, Ms. Kanako Chibai, Ms. Chieko Fukuda, Ms. Miyu Oshika, Ms. Sia SuHuai, Ms. Moe Matsumoto, Ms. Saaya Yamada, Ms. Mariko Nakazawa, Ms. Yui Nishikata, Ms. Sayuri Igawa, Ms. Hazuki Kitayama, Ms. Mayuka Kodama, Ms. Kirika Fujitani, Ms. Aoi Tokunaga, and Ms. Akari Yasuda, all of whom made major contributions to conducting this study.
Notice of Grant Support
This study was financially supported by a research grant from the SKYLARK Food Science Institute and Rice Stable Supply Support Organization. The SKYLARK Food Science Institute and Rice Stable Supply Support Organization had no role in the design, analysis or writing of this article.
COI
This study was financially supported by a research grant from the SKYLARK Food Science Institute and Rice Stable Supply Support Organization. The authors report the following disclosures: M. Waki has received clinical research funding from Sanofi and AstraZeneca; T. Teramoto has received honoraria from Sanofi, MSD, Bayer Yakuhin and Astellas, and scholarship grants from Takeda Pharmaceutical; none of the other authors has any potential conflicts of interest to disclose.
References
- 1). Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S. Committee for Epidemiology and Clinical Management of Atherosclerosis: Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Tada N, Maruyama C, Koba S, Tanaka H, Birou S, Teramoto T, Sasaki J: Japanese dietary lifestyle and cardiovascular disease. J Atheroscler Thromb, 2011; 18: 723-734 [DOI] [PubMed] [Google Scholar]
- 3). Maruyama C, Nakano R, Shima M, Mae A, Shijo Y, Nakamura E, Okabe Y, Park S, Kameyama N, Hirai S, Nakanishi M, Uchida K, Nishiyama H: Effects of a Japan Diet Intake Program on Metabolic Parameters in Middle-Aged Men: A pilot study. J Atheroscler Thromb, 2017; 24: 393-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MH, Kromhout D, Nedeljkovic S, Punsar S, Seccareccia F, Toshima H: The diet and 15-year death rate in the seven countries study. Am J Epidemiol, 1986; 124: 903-915 [DOI] [PubMed] [Google Scholar]
- 5). Kromhout D, Keys A, Aravanis C, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, Pekkarinen M, Simic BS, Toshima H: Food consumption patterns in the 1960s in seven countries. Am J Clin Nutr, 1989; 49: 889-894 [DOI] [PubMed] [Google Scholar]
- 6). Menotti A, Kromhout D, Blackburn H, Fidanza F, Buzina R, Nissinen A for the Seven Countries Study Research Group : Food intake patterns and 25-year mortality from coronary heart disease: cross-cultural correlations in the Seven Countries Study. Eur J Epidemiol, 1999; 15: 507-515 [DOI] [PubMed] [Google Scholar]
- 7). Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F, Giampaoli S, Jansen A, Karvonen M, Katan M, Nissinen A, Nedeljkovic S, Pekkanen J, Pekkarinen M, Punsar S, Rasanen L, Simic B and Toshima H : Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: the Seven Countries Study. Prev Med, 1995; 24: 308-315 [DOI] [PubMed] [Google Scholar]
- 8). Murakami K, Livingstone MBE, Sasaki S: Thirteen-Year Trends in Dietary Patterns among Japanese Adults in the National Health and Nutrition Survey 2003–2015: Continuous Westernization of the Japanese Diet. Nutrients, 2018; 10: 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Ministry of Health, Labor and Welfare, Japan: The National Health and Nutrition Survey in Japan, 2017. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/eiyou/h29-houkoku.html
- 10). Lytsy P, Burell G, Westerling R: Cardiovascular risk factor assessments and health behaviours in patients using statins compared to a non-treated population. Int J Behav Med, 2012; 19: 134-142 [DOI] [PubMed] [Google Scholar]
- 11). Johal S, Jamsen KM, Bell JS, Mc Namara KP, Magliano DJ, Liew D, Ryan-Atwood TE, Anderson C, Ilomaki J: Do statin users adhere to a healthy diet and lifestyle? The Australian Diabetes, Obesity and Lifestyle Study. Eur J Prev Cardiol, 2017; 24: 621-627 [DOI] [PubMed] [Google Scholar]
- 12). Lofgren I, Greene G, Schembre S, Delmonico MJ, Riebe D, Clark P: Comparison of diet quality, physical activity and biochemical values of older adults either reporting or not reporting use of lipid-lowering medication. J Nutr Health Aging, 2010; 14: 168-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Sugiyama T, Tsugawa Y, Tseng CH, Kobayashi Y, Shapiro MF: Different time trends of caloric and fat intake between statin users and nonusers among US adults: gluttony in the time of statins? JAMA Intern Med, 2014; 174: 1038-1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Ministry of Education, Culture, Sports, Science and Technology, Japan : Standard Tables of Food Composition in Japan -2015- (Seventh revised edition). 2015 [Google Scholar]
- 15). Ministry of Health, Labor and Welfare, Japan: Dietary Reference Intakes for Japanese (2015). https://www.mhlw.go.jp/file/05-Shingikai-10901000-Kenkoukyoku-Soumuka/0000114399.pdf
- 16). Micha R, Khatibzadeh S, Shi P, Andrews KG, Engell RE, Mozaffarian D, Global Burden of Diseases Nutrition and Chronic Diseases Expert Group : Global, regional and national consumption of major food groups in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys worldwide. BMJ Open, 2015; 5: e008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, Kokubo Y, Tsugane S, JPHC Study Group : Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan Public Health Center- Based (JPHC) Study Cohort I. Circulation, 2006; 113: 195-202 [DOI] [PubMed] [Google Scholar]
- 18). De Oliveira e, Silva ER, Seidman CE, Tian JJ, Hudgins LC, Sacks FM, Breslow JL: Effects of shrimp consumption on plasma lipoproteins. Am J Clin Nutr, 1996; 64: 712-717 [DOI] [PubMed] [Google Scholar]
- 19). Phan BA, Dayspring TD, Toth PP: Ezetimibe therapy: mechanism of action and clinical update. Vasc Health Risk Manag, 2012; 8: 415-427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Del Gobbo LC, Falk MC, Feldman R, Lewis K, Mozaffarian D: Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, metaanalysis, and dose-response of 61 controlled intervention trials. Am J Clin Nutr, 2015; 102: 1347-1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Stancu C, Sima A: Statins: mechanism of action and effects. J Cell Mol Med, 2001; 5: 378-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Fernandez ML, West KL: Mechanisms by which dietary fatty acids modulate plasma lipids. J Nutr, 2005; 135: 2075-2078 [DOI] [PubMed] [Google Scholar]
- 23). Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC: Trans fatty acids and cardiovascular disease. N Engl J Med, 2006; 354: 1601-1613 [DOI] [PubMed] [Google Scholar]
- 24). Matthan NR, Welty FK, Barrett PH, Harausz C, Dolnikowski GG, Parks JS, Eckel RH, Schaefer EJ, Lichtenstein AH: Dietary hydrogenated fat increases highdensity lipoprotein apoA-I catabolism and decreases low-density lipoprotein apoB-100 catabolism in hypercholesterolemic women. Arterioscler Thromb Vasc Biol, 2004; 24: 1092-1097 [DOI] [PubMed] [Google Scholar]
- 25). Chen JP, Chen GC, Wang XP, Qin L, Bai Y: Dietary Fiber and Metabolic Syndrome: A Meta-Analysis and Review of Related Mechanisms. Nutrients, 2018; 10: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Surampudi P, Enkhmaa B, Anuurad E, Berglund L: Lipid Lowering with Soluble Dietary Fiber. Curr Atheroscler Rep, 2016; 18: 75. [DOI] [PubMed] [Google Scholar]
- 27). Brown L, Rosner B, Willett WW, Sacks FM: Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr, 1999; 69: 30-42 [DOI] [PubMed] [Google Scholar]
- 28). Moreyra AE, Wilson AC, Koraym A: Effect of combining psyllium fiber with simvastatin in lowering cholesterol. Arch Intern Med, 2005; 165: 1161-1166 [DOI] [PubMed] [Google Scholar]
- 29). Vaidean GD, Manczuk M, Vansal SS, Griffith J: The cholesterol-lowering effect of statins is potentiated by whole grains intake. The Polish Norwegian Study (PONS). Eur J Intern Med, 2018; 50: 47-51 [DOI] [PubMed] [Google Scholar]
- 30). Shijo Y, Maruyama C, Nakamura E, Nakano R, Shima M, Mae A, Okabe Y, Park S, Kameyama N, Hirai S: Japan Diet Intake Changes Serum Phospholipid Fatty Acid Compositions in Middle-Aged Men: A Pilot Study. J Atheroscler Thromb, 2019; 26: 3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]


