Abstract
Aim: A high-risk strategy has been implemented for lipid-lowering therapy in the primary prevention of cardiovascular disease. However, atherosclerosis and cardiovascular events are common among individuals with low cardiovascular risk. This study aimed to determine whether the small dense low-density lipoprotein cholesterol (sdLDLC) level can predict carotid atherosclerosis progression and identify high-risk individuals.
Methods: Baseline sdLDLC and low-density lipoprotein cholesterol (LDLC) were measured in 808 particip ants from the Chinese Multi-provincial Cohort Study, aged 45–74 years. Adjusted relative risk was calculated using a modified Poisson regression model to assess the relationship between sdLDLC and 5-year atherosclerosis progression, as indicated by the progression, incidence, and multi-territorial extent of carotid plaque.
Results: The 5-year atherosclerosis progression increased significantly with increased sdLDLC. Baseline sdLDLC was significantly associated with the short-term risk of plaque progression after multivariable adjustment, even in participants with low LDLC or a 10-year estimated cardiovascular risk. sdLDLC predicted plaque progression (relative risk 2.05; 95% confidence interval 1.43–2.93) in participants with LDLC < 130 mg/dL. Furthermore, participants with the highest sdLDLC but intermediate or low cardiovascular risk (accounting for 16% of the cohort) had double the risk of plaque progression, which was comparable to those with the same sdLDLC and high cardiovascular risk, relative to those with the lowest sdLDLC levels and low cardiovascular risk.
Conclusions: sdLDLC is independently associated with the progression of carotid atherosclerosis, which may provide a basis for clinicians to reclassify individuals believed to be at low cardiovascular risk into the high-risk category, and those with high sdLDLC may benefit from more aggressive cholesterol-lowering treatment.
Keywords: Small dense low-density lipoprotein cholesterol, Carotid atherosclerosis, Community-based cohort study, High-risk strategy, Risk reclassification
Introduction
An important strategy in the primary prevention of cardiovascular disease (CVD) focuses on reducing the incidence of future events in high-risk individuals through the appropriate management of modifiable risk factors. Low-density lipoprotein cholesterol (LDLC) is considered as one of the most important modifiable risk factors for CVD and remains the primary target for current cardiovascular risk reduction strategies1–3). However, although a majority of CVD-free asymptomatic individuals maintain low levels of LDLC4), they have a high prevalence of subclinical atherosclerosis5). In addition, a number of individuals who have experienced cardiovascular events demonstrate low levels of LDLC6, 7). Thus, LDLC, which is widely measured in routine clinical practice, may not be considered an appropriate target for prevention strategies in individuals with low LDLC values, particularly in those at low cardiovascular risk. Instead, clarification of the potential biomarkers for the prediction of atherosclerosis should contribute to a more precise identification of individuals at high cardiovascular risk and enable the early prevention of CVD events in individuals who have atherosclerosis but low LDLC.
In addition to other cardiovascular risk factors, there may be other low-density lipoprotein (LDL)-related factors that are responsible for the considerable residual cardiovascular risk. Accumulating evidence suggests that the development and progression of atherosclerosis are dependent not only and not so much on the amount as on the specific properties of LDL. LDL can be classified into multiple distinct particles of differing size, density, and composition, and their atherogenicity seems to differ accordingly. However, it is as yet unclear to what extent LDL subfraction measurements improve the clinical assessment of cardiovascular risk over the standard assay of LDLC8). The findings of experimental studies have suggested that small dense LDL may acce lerate atherosclerosis by stimulating macrophage foam cell formation9, 10). Several observational studies have demonstrated that the high small dense LDL cholesterol (sdLDLC) level is strongly associated with CVD events11–14), even more so than the LDLC level11, 12). Subclinical atherosclerosis underlies most cardiovascular events15, 16), and its early detection can improve risk stratification among asymptomatic individuals17). However, it remains unclear whether high levels of sdLDLC can promote the development of carotid atherosclerosis, irrespective of the LDLC level, and whether screening for this biomarker in individuals with differing degrees of cardiovascular risk could identify those with even grea ter cardiovascular risk, and assist in the identification of appropriate targets for therapeutic intervention in such individuals.
In this study, we measured the LDLC and sdLDLC levels in a CVD-free population. We tested the hypotheses that sdLDLC is associated with the progression of carotid atherosclerosis and that this association is preserved under low LDLC level or low cardiovascular risk, using asymptomatic participants in a community-based prospective cohort study.
Materials and Methods
Study Participants
Study participants were recruited from the Chinese Multi-provincial Cohort Study Project18, 19). Analysis of carotid atherosclerosis was performed in participants in the Chinese Multi-provincial Cohort Study–Beijing Project19, 20). Briefly, 1324 participants, aged 45–74 years, underwent analysis of their demographic characteristics, measurements of traditional risk factors, and carotid ultrasonography, in 2002. All the participants were then followed up to identify any occurrence of CVD conducted every 1–2 years from baseline until now, and invited to a repeat examination to identify risk factors and to undergo a second carotid ultrasonography in 2007. After excluding participants for the following reasons, that is, loss to follow-up (n = 62), death (n = 15), lack of participation at the re-examination (n = 228), presence of established CVD (n = 68), blood sample for the measurement of sdLDLC in 2015 not available (n = 129), and incomplete data at baseline (n = 14), 808 (359 men and 449 women) participants for whom complete data had been obtained from both examinations were eligible for inclusion in the final analysis.
All participants gave their written informed consent, and this study was approved by the Ethics Committee of Beijing An Zhen Hospital, Capital Medical University, and was performed in accordance with the Declaration of Helsinki.
Risk Factor Survey
In all the surveys, demographic characteristics and personal medical history were collected using a standardized questionnaire. Anthropometric measurements and blood pressure were recorded during the physical examination. Body mass index was calculated as weight in kilograms divided by height squared in meters. The survey method and definitions of risk factors, including hypertension, diabetes mellitus, obesity, and current smoking, have been previously described19, 20).
Laboratory Assays
Venous blood samples were collected after at least 8 h of fasting. Lipid profile, fasting blood glucose, and high-sensitivity C-reactive protein (hs-CRP) were measured in fresh samples on the day of collection, in 2002, according to previously described methods19, 20). The remaining sample was then aliquoted and stored at −80°C. Total cholesterol, triglyceride, and fasting blood glucose levels were determined by enzymatic methods (Human Diagnostics, Wiesbaden, Germany). LDLC and high-density lipoprotein cholesterol levels were assayed by homogeneous methods (Daiichi, Tokyo, Japan). Non-high-density lipoprotein cholesterol was calculated by subtracting high-density lipoprotein cholesterol from total cholesterol. Hs-CRP was measured using a particle-enhanced immunoturbidimetric method (DiaSys, Holzheim, Germany). The cholesterol content of small dense LDL was measured using an automated homogeneous assay, as described previously21) (Denka Seiken Co., Ltd., Tokyo, Japan), and analyzed on a Hitachi 7180 automatic analyzer, in 2015. The coefficient of variation was 3.25% for low-range controls and 4.18% for high-range controls.
Carotid Atherosclerosis Assessment
Carotid atherosclerosis was assessed using high-resolution B-mode ultrasonography. The method and measurement validation have been described previously19, 20). Briefly, the presence of a plaque was defined by an intima–media thickness of ≥ 1.5 mm, or the presence of a focal structure encroaching into the arterial lumen of ≥ 0.5 mm, or ≥ 50% of the thickness of the surrounding intima–media. The presence of carotid plaque was inspected over an area that included six segments: the far and near walls of the bilateral common carotid arteries, their bifurcations, and the internal carotid arteries. Progression of a carotid plaque was defined by the appearance of at least one plaque at re-examination in a previously plaque-free arterial segment. In participants who had no plaque at baseline (n = 649), the incidence of carotid plaques was defined by the appearance of at least one new plaque at re-examination in one of the six segments. The multi-territorial extent of a new carotid plaque was defined according to the number of vascular sites with evidence of disease, including a detectable plaque by the total number of the six segments for which an ultrasonographic image was available (with a maximum of six). Participants were classified as disease-free (no vascular sites affected) or having focal (one vascular site) or generalized (≥ two vascular sites) atherosclerosis. The total plaque area of maximum plaques was also used to evaluate the severity of the carotid atherosclerosis. Participants with no carotid plaque at re-examination were assigned 0 mm2 for the area of new plaque. Participants were classified into three ordinal groups (0, 0.1−14.9, and ≥ 15.0 mm2), using the medium of new plaque area (15 mm2) in participants with a new plaque at re-examination for the cut-off point.
Statistical Analysis
Continuous variables, expressed as mean (± standard deviation) in the case of normally distributed variables, or as median (interquartile range), were compared using the unpaired Student's t-test, the Mann–Whitney U-test, or one-way analysis of variance, where appropriate. Categorical variables, expressed as number (percentage), were compared using the chi-square test. Correlations were determined using the Spearman rank method, after adjusting for age and sex.
An estimate of the proportion of sdLDLC in the entire mass of LDLC (sdLDLC/LDLC ratio) was obtained by calculating the ratio of sdLDLC to total LDLC. sdLDLC-related parameters were analyzed as quartiles (sdLDLC: < 33, 33–47, 47–62, and ≥ 63 mg/dL; sdLDLC/LDLC ratio: < 27, 27–34, 35–42, and ≥ 43%). Relative risk (RR) (95% confidence interval, CI) values for the 5-year progression and incidence of carotid plaque within subgroups of sdLDLC-related parameters were calculated using a modified Poisson regression model, after adjustment for known CVD risk factors (age, sex, body mass index, systolic blood pressure, fasting blood glucose, high-density lipoprotein cholesterol, and current smoking), lipid-lowering treatment, and large buoyant LDL cholesterol. The relationships of sdLDLC-related parameters with the multi-territorial extent and total area of new plaque were evaluated using multivariable ordinal logistic regression models. Risk estimates were calculated for each subgroup, using the lowest quartile as a reference. The relationships between sdLDLC-related parameters and progression risk were further analyzed after dichotomization for LDLC (< 130 mg/dL versus ≥ 130 mg/dL). Risk estimates were then calculated for participants in each combined subgroup of sdLDLC-related parameters and LDLC, using the lowest quartile of sdLDLC-related parameters in the LDLC < 130 mg/dL category as the reference group. The relationships between sdLDLC-related parameters and the risk of plaque progression were also analyzed after dichotomization for non-high-density lipoprotein cholesterol (< 160 mg/dL versus ≥ 160 mg/dL). To explore whether heterogeneity existed in the plaque progression associated with high sdLDLC between subgroups of known CVD risk factors, a subgroup × high sdLDLC interaction was determined using a modified Poisson regression model, including terms for high sdLDLC (with the lowest quartile as the reference), subgroups of known CVD risk factors, and subgroup × high sdLDLC interaction.
Cardiovascular risk was a lso calculated according to the 2016 Chinese guidelines for the management of dyslipidemia in adults1). A multivariable model was fitted to include both sdLDLC quartile and differing cardiovascular risk. Each combined subgroup of sdLDLC and cardiovascular risk was compared with the lowest sdLDLC quartile and low risk as a reference.
Sensitivity analyses were performed after additional adjustment for baseline hs-CRP, and 5-year changes in systolic blood pressure, total cholesterol, and fasting blood glucose, and reanalyzed after the exclusion of participants with diabetes mellitus. Furthermore, to test whether missing data would introduce potential bias, comparisons were performed between participants who were eligible for the final analyses and those who were unavailable for re-examination, and no significant differences were observed with respect to lipid-related biomarkers (Table 1).
Table 1. Baseline Characteristics of the Study Participants Who Were Eligible and Unavailable for Re-examination.
| Variables | Eligible participants (n = 808) | Participants unavailable for re-examination (n = 516) | P value |
|---|---|---|---|
| Age, year | 59.0 ± 7.8 | 61.5 ± 7.8 | 0.001 |
| Men, n (%) | 359 (44.4) | 262 (50.8) | 0.024 |
| Lipids and lipoproteins | |||
| Total cholesterol, mg/dL | 215.47 ± 39.93 | 214.42 ± 38.93 | 0.662 |
| LDL cholesterol, mg/dL | 135.88 ± 36.05 | 135.73 ± 35.45 | 0.739 |
| HDL cholesterol, mg/dL | 58.61 ± 13.31 | 57.82 ± 12.89 | 0.103 |
| Triglycerides, mg/dL | 114.0 (83.0, 166.0) | 122.0 (86.5, 176.0) | 0.330 |
| Lipid-lowering treatment, n (%) | 94 (11.6) | 72 (14.0) | 0.214 |
| Blood pressure | |||
| Systolic blood pressure, mmHg | 128.9 ± 18.3 | 130.9 ± 19.6 | 0.057 |
| Diastolic blood pressure, mmHg | 80.6 ± 9.4 | 81.1 ± 10.7 | 0.350 |
| Hypertension, n (%) | 367 (45.4) | 282 (54.7) | 0.001 |
| Fasting blood glucose, mmol/L | 4.87 ± 1.00 | 5.08 ± 1.43 | 0.001 |
| Diabetes, n (%) | 42 (5.2) | 49 (9.4) | 0.001 |
| Body mass index, kg/m2 | 25.0 ± 3.2 | 24.9 ± 3.2 | 0.665 |
| Obesity, n (%) | 140 (17.3) | 79 (15.3) | 0.335 |
| High-sensitivity CRP, mg/L | 0.82 (0.37, 1.76) | 1.02 (0.47, 2.05) | 0.002 |
| Current smoking, n (%) | 82 (10.1) | 44 (8.5) | 0.327 |
CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Data are expressed as number (percent) for categorical variables or mean ± standard deviation for continuous variables, except for hs-CRP and triglycerides, which are presented as median (interquartile range). Current smoking was defined as ≥ 1 cigarettes per day. Hypertension was defined as blood pressure ≥ 140 mm Hg systolic, ≥ 90 mm Hg diastolic, and/or current antihypertensive treatment. Diabetes was defined as fasting blood glucose ≥ 7.0 mmol/L or previously diagnosed diabetes. Obesity was defined as body mass index ≥ 28 kg/cm2.
Statistical analyses were performed using SAS software (v9.3, SAS Institute Inc., Cary, NC, USA). P < 0.05 was considered to represent statistical significance. Sample size was estimated using PASS software (v8.0, NCSS, Kaysville, UT, USA).
Results
Baseline Characteristics
The baseline median sdLDLC was 47.9 (32.7, 63.2) (range, 8.0–134.0) mg/dL, and thus a mean of 33.9% of total LDLC was sdLDLC. The distribution of sdLDLC was approximately normal (Supplementary Fig. 1). Although sdLDLC displays a strong positive correlation with LDLC (partial-r: 0.738) (Supplementary Table 1), there were substantial variations in sdLDLC among the participants when they were stratified according to their circulating LDLC level (Supplementary Fig. 2). The baseline median sdLDLC values were 24.7 (19.3, 32.2) (range, 8.0–86.0) mg/dL, 35.5 (29.0, 48.6) (range, 15.0–84.0) mg/dL, 51.3 (42.3, 62.2) (range, 25.0–103.0) mg/dL, and 71.1 (59.0, 85.7) (range, 35.0–134.0) mg/dL in participants with LDLC levels of < 100 mg/dL, 100–129 mg/dL, 130–159 mg/dL, and ≥ 160 mg/dL, respectively. The baseline characteristics of the participants, when stratified into the sdLDLC quartile, are shown in Table 2. Statistically significant trends were present for all the known CVD risk factors, except for current smoking, with increasing sdLDLC quartile (Table 2).
Supplementary Fig. 1.
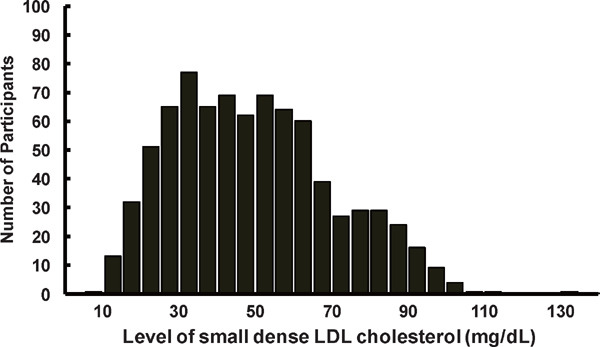
Distribution of Small Dense Low-density Lipoprotein Cholesterol
LDL, low-density lipoprotein.
Data are shown as number for the distribution of small dense LDL cholesterol among total study participants.
Supplementary Table 1. Correlations between Small Dense LDL Cholesterol and Other Cardiovascular Risk Factors.
| Variables | sdLDL cholesterol | sdLDLC/LDLC ratio |
|---|---|---|
| sdLDLC/LDLC ratio | 0.830*** | |
| Large buoyant LDL cholesterol | 0.219*** | −0.296*** |
| Total cholesterol | 0.691*** | 0.222*** |
| Triglycerides | 0.716*** | 0.829*** |
| LDL cholesterol | 0.738*** | 0.276*** |
| High-density lipoprotein cholesterol | −0.336*** | −0.496*** |
| Non high-density lipoprotein cholesterol | 0.792*** | 0.426*** |
| Systolic blood pressure | 0.178*** | 0.210*** |
| Diastolic blood pressure | 0.200*** | 0.251*** |
| Fasting blood glucose | 0.218*** | 0.205*** |
| Body mass index | 0.219*** | 0.245*** |
| High-sensitivity C-reactive protein | 0.180*** | 0.157*** |
LDL, low-density lipoprotein; sdLDL, small dense low-density lipoprotein; sdLDLC/LDLC ratio, ratio of small dense low-density lipoprotein cholesterol to total low-density lipoprotein cholesterol.
Correlation coefficients were estimated after adjustment for age and sex. *P < 0.050; **P < 0.010; ***P < 0.001.
Supplementary Fig. 2.
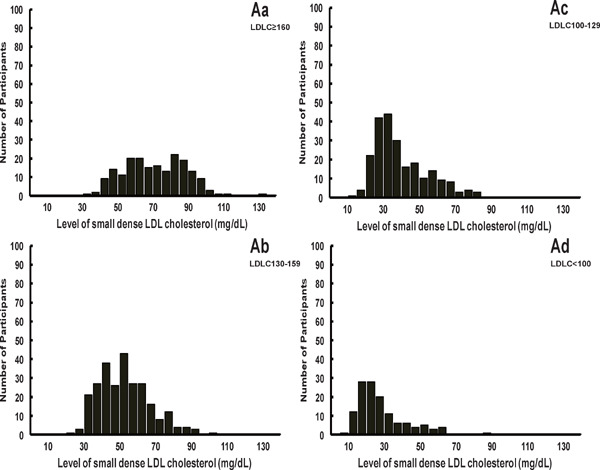
Distribution of Small Dense LDL Cholesterol in Participants Stratified According to LDL Cholesterol Level
LDL, low-density lipoprotein.
Data are shown as number for the distribution of small dense LDL cholesterol among the subgroup of LDL cholesterol level ≥ 160 mg/dL (A), 130–159 mg/dL (B), 100–129 mg/dL (C), and < 100 mg/dL (D).
Table 2. Baseline Characteristics of the Participants when Divided into Quartiles of Small Dense LDL Cholesterol Level.
| Variables | sdLDL cholesterol, mg/dL |
P value for trend | |||
|---|---|---|---|---|---|
| < 33 | 33–47 | 48–62 | ≥ 63 | ||
| Number | 195 | 201 | 204 | 208 | |
| Age, year | 57.2 ± 7.9 | 59.3 ± 7.7 | 59.5 ± 7.7 | 59.8 ± 7.6 | 0.001 |
| Men, n (%) | 74 (37.9) | 90 (44.8) | 94 (46.1) | 101 (48.6) | 0.036 |
| Lipids and lipoproteins | |||||
| Total cholesterol, mg/dL | 180.33 ± 27.94 | 208.51 ± 27.46 | 220.42 ± 29.42 | 250.28 ± 38.13 | < 0.001 |
| LDL cholesterol, mg/dL | 98.45 ± 20.45 | 130.20 ± 20.36 | 143.68 ± 26.31 | 168.83 ± 33.05 | < 0.001 |
| HDL cholesterol, mg/dL | 63.12 ± 15.48 | 60.74 ± 13.30 | 55.98 ± 12.47 | 54.92 ± 9.91 | < 0.001 |
| Triglycerides, mg/dL | 73.0 (59.0, 97.0) | 97.0 (78.5, 118.0) | 140.5 (108.0, 177.8) | 188.0 (138.0, 247.8) | < 0.001 |
| Non-HDL cholesterol, mg/dL | 120.88 ± 21.93 | 152.38 ± 21.37 | 169.08 ± 22.96 | 201.23 ± 36.65 | < 0.001 |
| sdLDL cholesterol, mg/dL | 25.5 (20.7, 29.3) | 39.2 (35.6, 43.8) | 54.8 (51.1, 59.1) | 77.4 (66.2, 86.1) | < 0.001 |
| lbLDL cholesterol, mg/dL | 75.2 (63.0, 85.1) | 90.7 (78.9, 103.2) | 89.1 (72.8, 107.5) | 89.7 (69.6, 107.6) | < 0.001 |
| sdLDLC/LDLC ratio, % | 24.5 (22.1, 27.2) | 29.8 (27.3, 33.7) | 37.6 (33.7, 43.5) | 47.1 (41.5, 52.9) | < 0.001 |
| Lipid-lowering treatment, n (%) | 8 (4.1) | 20 (10.0) | 26 (12.7) | 40 (19.2) | < 0.001 |
| Fasting blood glucose, mmol/L | 4.67 ± 0.84 | 4.77 ± 0.72 | 4.93 ± 0.79 | 5.13 ± 1.42 | < 0.001 |
| Diabetes, n (%) | 6 (3.1) | 7 (3.5) | 14 (6.9) | 15 (7.2) | 0.024 |
| Blood pressure | |||||
| Systolic blood pressure, mmHg | 123.1 ± 17.5 | 127.5 ± 17.1 | 132.8 ± 18.8 | 131.7 ± 18.2 | < 0.001 |
| Diastolic blood pressure, mmHg | 77.4 ± 9.5 | 79.3 ± 9.6 | 82.4 ± 9.3 | 83.1 ± 10.3 | < 0.001 |
| Hypertension, n (%) | 68 (34.9) | 93 (46.3) | 104 (51.0) | 102 (49.0) | 0.003 |
| Body mass index, kg/m2 | 24.1 ± 3.4 | 24.6 ± 2.9 | 25.2 ± 3.1 | 25.9 ± 3.2 | < 0.001 |
| Obesity, n (%) | 24 (12.3) | 28 (13.9) | 36 (17.6) | 52 (25.0) | < 0.001 |
| Hs-CRP, mg/L | 0.59 (0.31, 1.28) | 0.72 (0.27, 1.57) | 0.90 (0.40, 1.88) | 1.03 (0.52, 2.13) | < 0.001 |
| Current smoking, n (%) | 18 (9.2) | 20 (10.0) | 19 (9.3) | 25 (12.0) | 0.412 |
| High cardiovascular risk, n (%) | 11 (5.6) | 18 (9.0) | 34 (16.7) | 79 (38.0) | < 0.001 |
HDL, high-density lipoprotein; Hs-CRP, high-sensitivity C-reactive protein; lbLDL, large buoyant low-density lipoprotein; LDL, low-density lipoprotein; sdLDL, small dense low-density lipoprotein; sdLDLC/LDLC ratio, ratio of small dense LDL cholesterol to LDL cholesterol.
Data are expressed as number (percent) for categorical variables, as mean (standard deviation) for normally distributed continuous variables, and as median (interquartile range) otherwise.
Relationships of Carotid Atherosclerosis Progression With Sdldlc and Ldlc Levels
Of the 808 participants, 44.1% developed new plaque during the 5-year period of follow-up (Table 3). As baseline sdLDLC increased, a significant increase was found in the rate of plaque progression, from 29% in the 10–19 mg/dL subgroup to 71% in the ≥ 90 mg/dL subgroup (P < 0.001) (Supplementary Fig. 3). A progressive increase was also identified for the number of vascular sites affected by new plaque in participants who had no baseline plaque. A subgroup analysis was performed to explore the distribution of plaque progression, stratified according to sdLDLC quartile, among participants with an LDLC level of ≥ 160 mg/dL or < 100 mg/dL (Fig. 1). For those in the highest quartile of sdLDLC, the progression rate among participants with LDLC < 100 mg/dL was similar to those with LDLC ≥ 160 mg/dL.
Table 3. Characteristics of Carotid Atherosclerotic Plaque when Divided into Quartiles of Small Dense LDL Cholesterol Level.
| sdLDL cholesterol, mg/dL |
P value for trend | |||||
|---|---|---|---|---|---|---|
| Variables | Total | < 33 | 33–47 | 48–62 | ≥ 63 | |
| Number | 808 | 195 | 201 | 204 | 208 | |
| Prevalence of carotid plaque, n (%) | 159 (19.7) | 25 (12.8) | 39 (19.4) | 42 (20.6) | 53 (25.5) | 0.002 |
| Progression of carotid plaque, n (%) | 356 (44.1) | 54 (27.7) | 84 (41.8) | 96 (47.1) | 122 (58.7) | < 0.001 |
| Incidence of new plaque, n (%) | 274 (42.2) | 44 (25.9) | 67 (41.4) | 76 (46.9) | 87 (56.1) | < 0.001 |
| 1 Vascular site, n (%) | 133 (20.5) | 23 (13.5) | 38 (23.5) | 32 (19.8) | 40 (25.8) | < 0.001 |
| ≥ 2 Vascular sites, n (%) | 141 (21.7) | 21 (12.4) | 29 (17.9) | 44 (27.2) | 47 (30.3) | |
| Area < 15 mm2, n (%) | 152 (23.4) | 33 (19.4) | 42 (25.9) | 40 (24.7) | 37 (23.9) | < 0.001 |
| Area ≥ 15 mm2, n (%) | 158 (24.3) | 23 (13.5) | 36 (22.2) | 48 (29.6) | 51 (32.9) | |
sdLDL, small dense low-density lipoprotein.
Data are expressed as number (percent) for categorical variables, as mean (standard deviation) for normally distributed continuous variables, and as median (interquartile range) otherwise.
Supplementary Fig. 3.
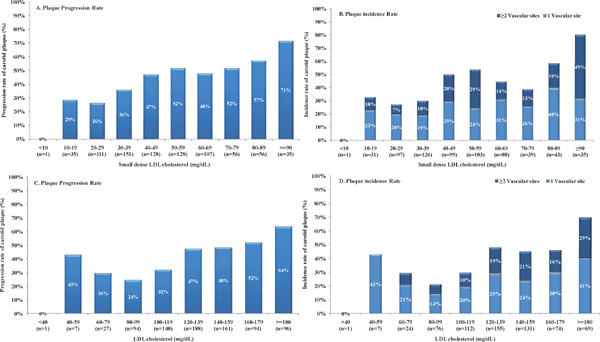
Progression of Carotid Atherosclerosis in Participants Stratified According to Small Dense LDL Cholesterol or LDL Cholesterol
LDL, low-density lipoprotein. Data are shown as progression and incidence of carotid plaque in participants stratified according to small dense LDL cholesterol (A and B), or LDL cholesterol (C and D), respectively.
Fig. 1.
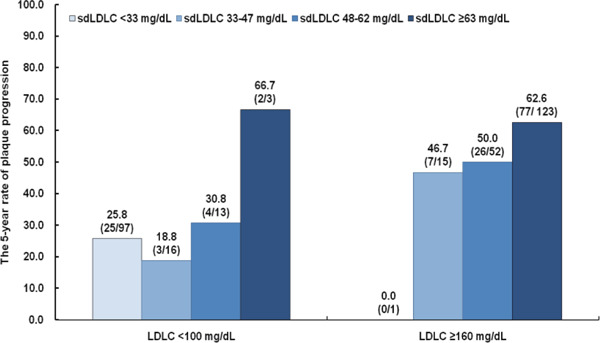
Plaque Progression Stratified According to Small Dense LDL Cholesterol Quartile in Participants with LDL Cholesterol Levels ≥ 160 mg/dL or < 100 mg/dL
LDLC, low-density lipoprotein cholesterol; sdLDLC, small dense low-density lipoprotein cholesterol.
Data are shown as the percentage (number of participants with plaque progression/total number of participants in the subgroup) demonstrating plaque progression among participants with LDLC levels ≥ 160 mg/dL or < 100 mg/dL.
The risk of plaque progression was significantly associated with the quartiles of sdLDLC-related biomarkers (Supplementary Table 2) and LDLC (RR [per 10 mg/dL] 1.12; 95% CI 1.08–1.17; P = 0.001) after multivariable adjustment. However, for participants with LDLC < 130 mg/dL (Table 4 and Supplementary Table 3), sdLDLC showed significant associations with plaque progression. Participants with the highest sdLDLC quartile had a 2.05-fold (95% CI 1.43–2.93; P < 0.001) higher risk of plaque progression than those with the lowest sdLDLC quartile (Table 4). In the multivariable model, high LDLC was not associated with the risk of plaque progression (RR [per 10 mg/dL] 1.11; 95% CI 0.97–1.27; P = 0.127). Participants with the lowest sdLDLC but LDLC ≥ 130 mg/dL had a comparable risk of plaque progression to those with the same sdLDLC and LDLC < 130 mg/dL (RR 0.89; 95% CI 0.28–3.62; P = 0.688). The significantly higher risk of plaque progression associated with the highest sdLDLC quartile was also found in participants with a non-high-density lipoprotein cholesterol level of < 160 mg/dL (RR 2.08; 95% CI 1.38–3.14; P = 0.001). Similar results were obtained after multivariable adjustment with respect to the risk of plaque incidence, multi-territorial extent, and total area of new plaque (Table 4 and Supplementary Table 3). Further adjustment for baseline hs-CRP, and 5-year changes in other risk factors, and reanalysis after the exclusion of participants with diabetes mellitus did not appreciably change these relationships (data not shown). An interaction model involving the sdLDLC quartile and subgroup was also fitted, but no significant interaction was found (Supplementary Fig. 4).
Supplementary Table 2. Relative Risk (95% Confidence Interval) of Carotid Atherosclerosis Progression According to Small Dense LDL Related Biomarkers Quartiles.
| Variables | No of participants | Plaque progression | Plaque incidence | Multi-territorial extent of new plaque | Total area of new plaque |
|---|---|---|---|---|---|
| sdLDLC quartile | |||||
| < 33 mg/dL | 195 | Reference | Reference | Reference | Reference |
| 33–47 mg/dL | 201 | 1.29 (0.98–1.70) | 1.33 (0.97–1.81) | 1.45 (0.95–2.23) | 1.43 (0.93–2.21) |
| 48–62 mg/dL | 204 | 1.38 (1.06–1.80) | 1.45 (1.07–1.96) | 2.08 (1.36–3.20) | 1.83 (1.19–2.82) |
| ≥ 63 mg/dL | 208 | 1.68 (1.31–2.17) | 1.70 (1.27–2.28) | 2.26 (1.46–3.49) | 2.42 (1.57–3.75) |
| sdLDLC/LDLC ratio quartile | |||||
| < 27% | 176 | Reference | Reference | Reference | Reference |
| 27–34% | 236 | 1.36 (1.05–1.76) | 1.30 (0.98–1.73) | 1.44 (0.95–2.18) | 1.36 (0.90–2.07) |
| 35–42% | 180 | 1.43 (1.09–1.87) | 1.43 (1.07–1.92) | 1.73 (1.11–2.68) | 1.62 (1.04–2.51) |
| ≥ 43% | 216 | 1.69 (1.29–2.21) | 1.77 (1.31–2.38) | 2.59 (1.65–4.06) | 2.85 (1.81–4.48) |
LDL, low-density lipoprotein; No, number; sdLDLC, small dense low-density lipoprotein cholesterol; sdLDLC/LDLC ratio, ratio of small dense LDL cholesterol to LDL cholesterol. Relative risk was calculated using a modified Poisson regression model after adjustment for known cardiovascular disease risk factors, lipid-lowering treatment, and large buoyant LDL cholesterol.
Table 4. Relative Risk (95% Confidence Interval) of Carotid Atherosclerosis Progression According to Small Dense LDL Cholesterol Quartile and Dichotomized LDL Cholesterol.
| Variables | No of participants | Plaque progression | Plaque incidence | Multi-territorial extent of new plaque | Total area of new plaque |
|---|---|---|---|---|---|
| LDLC < 130 mg/dL + sdLDLC < 33 mg/dL | 184 | Reference | Reference | Reference | Reference |
| LDLC < 130 mg/dL + sdLDLC 33–47 mg/dL | 97 | 1.24 (0.91–1.68) | 1.28 (0.91–1.81) | 1.28 (0.78–2.08) | 1.26 (0.77–2.05) |
| LDLC < 130 mg/dL + sdLDLC 48–62 mg/dL | 52 | 1.54 (0.76–1.71) | 1.30 (0.82–2.06) | 2.00 (1.10–3.62) | 1.95 (1.07–3.54) |
| LDLC < 130 mg/dL + sdLDLC ≤ 63 mg/dL | 24 | 2.05 (1.43–2.93) | 1.99 (1.26–3.14) | 3.83 (1.79–5.69) | 4.39 (2.03–5.52) |
| LDLC ≤ 130 mg/dL + sdLDLC < 33 mg/dL | 11 | 0.89 (0.28–3.62) | 0.60 (0.08–4.52) | 0.89 (0.15–5.20) | 0.86 (0.15–5.15) |
| LDLC ≤ 130 mg/dL + sdLDLC 33–47 mg/dL | 104 | 1.40 (0.99–1.97) | 1.32 (0.90–1.95) | 1.64 (0.90–2.98) | 1.56 (0.86–2.84) |
| LDLC ≤ 130 mg/dL + sdLDLC 48–62 mg/dL | 152 | 1.49 (1.11–2.00) | 1.45 (1.05–2.01) | 2.06 (1.24–3.42) | 1.69 (1.02–2.82) |
| LDLC ≤ 130 mg/dL + sdLDLC ≤ 63 mg/dL | 184 | 1.65 (1.26–2.17) | 1.62 (1.19–2.21) | 2.01 (1.24–3.26) | 2.09 (1.29–3.39) |
LDLC, low-density lipoprotein cholesterol; No, number; sdLDLC, small dense low-density lipoprotein cholesterol.
Relative risk was calculated using a modified Poisson regression model after adjustment for known cardiovascular disease risk factors, lipid-lowering treatment, and large buoyant LDL cholesterol.
Supplementary Table 3. Relative Risk (95% Confidence Interval) of Carotid Atherosclerosis Progression According to Small Dense LDL Cholesterol / LDL cholesterol Ratio Quartile and Dichotomized LDL Cholesterol.
| Variables | No of participants | Plaque progression | Plaque incidence | Multi-territorial extent of new plaque | Total area of new plaque |
|---|---|---|---|---|---|
| LDLC < 130 mg/dL + sdLDLC/LDLC ratio < 27% | 127 | Reference | Reference | Reference | Reference |
| LDLC < 130 mg/dL + sdLDLC/LDLC ratio 27–34% | 105 | 1.21 (0.87–1.69) | 1.17 (0.80–1.70) | 0.98 (0.57–1.67) | 1.02 (0.59–1.74) |
| LDLC < 130 mg/dL + sdLDLC/LDLC ratio 35–42% | 44 | 1.12 (0.69–1.81) | 1.25 (0.76–2.08) | 1.39 (0.71–2.75) | 1.42 (0.72–2.82) |
| LDLC < 130 mg/dL + sdLDLC/LDLC ratio ≥ 43% | 81 | 1.45 (0.95–2.11) | 1.60 (0.97–2.47) | 2.82 (1.53–5.20) | 3.11 (1.67–5.77) |
| LDLC ≥ 130 mg/dL + sdLDLC/LDLC ratio < 27% | 49 | 0.87 (0.51–1.51) | 0.75 (0.41–1.38) | 0.68 (0.30–1.54) | 0.61 (0.27–1.40) |
| LDLC ≥ 130 mg/dL + sdLDLC/LDLC ratio 27–34% | 131 | 1.43 (1.02–2.01) | 1.22 (0.84–1.77) | 1.71 (0.94–3.14) | 1.37 (0.74–2.52) |
| LDLC ≥ 130 mg/dL + sdLDLC/LDLC ratio 35–42% | 136 | 1.48 (1.08–2.02) | 1.35 (0.96–1.90) | 1.64 (0.95–2.82) | 1.43 (0.83–2.46) |
| LDLC ≥ 130 mg/dL + sdLDLC/LDLC ratio ≥ 43% | 135 | 1.65 (1.22–2.23) | 1.66 (1.19–2.30) | 2.03 (1.20–3.43) | 2.25 (1.33–3.81) |
LDL, low-density lipoprotein; LDLC, low-density lipoprotein cholesterol; No, number; sdLDLC/LDLC ratio, ratio of small dense LDLC to total LDLC. Relative risk was calculated using a modified Poisson regression model after adjustment for known cardiovascular disease risk factors, lipid-lowering treatment, and large buoyant LDLC.
Supplementary Fig. 4.
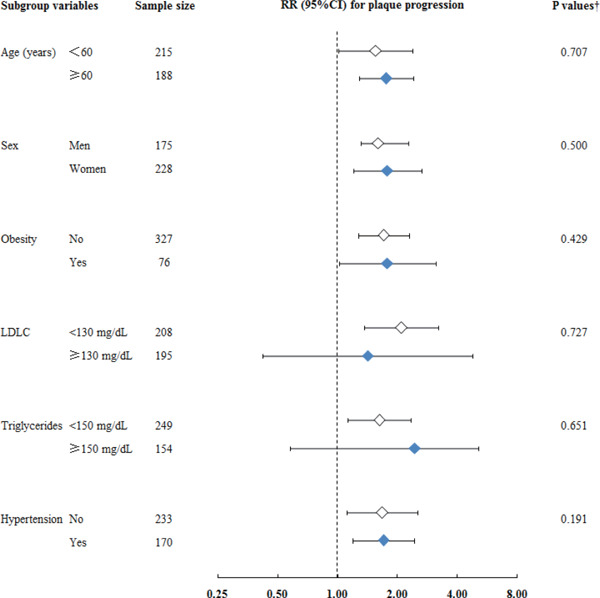
Relative Risk (95% Confidence Interval) of Plaque Progression Associated with the Highest Quartile of Small Dense LDL Cholesterol Compared with the Lowest Quartile in Subgroups of Known Cardiovascular Risk Factors
CI, confidence interval; LDL, low-density lipoprotein; LDLC, low-density lipoprotein cholesterol; RR, relative risk; sdLDL, small dense low-density lipoprotein.
Relative risk was calculated using a modified Poisson regression model after adjustment for known cardiovascular disease risk factors, lipid-lowering treatment, large buoyant LDL cholesterol. †P value for interaction analysis.
Relationships of Carotid Atherosclerosis Progression With Sdldlc and Differing Cardiovascular Risk
Briefly, 51.6% and 38.4% of participants at intermediate and low cardiovascular risk developed new plaque during the 5-year follow-up period, respectively. Baseline sdLDLC was significantly associated with the risk of atherosclerosis progression among participants with differing cardiovascular risk (Fig. 2 and Supplementary Fig. 5). The risk of plaque progression was twice as high among participants with the highest sdLDLC compared with those with the lowest sdLDLC, irrespective of cardiovascular risk (Fig. 2). However, participants with the lowest sdLDLC but high cardiovascular risk had only a non-significant RR of 1.43 (P = 0.371) when compared with those who h ad the same sdLDLC but low cardiovascular risk, possibly because of the limited number of participants involved. Furthermore, participants with the highest sdLDLC but intermediate or low cardiovascular risk (accounting for 16% of all the participants) had comparable risks to those with the same sdLDLC and high cardiovascular risk (P > 0.1). Similar results were also obtained for the risk of plaque incidence (Supplementary Fig. 5). Even for those participants with intermediate or low risk and an LDLC of < 130 mg/dL, sdLDLC was significantly associated with the risk of plaque progression (Supplementary Table 4). Further adjustment for baseline hs-CRP, and 5-year changes in other risk factors, and reanalysis after the exclusion of participants with diabetes mellitus did not appreciably change these relationships (data not shown).
Fig. 2.
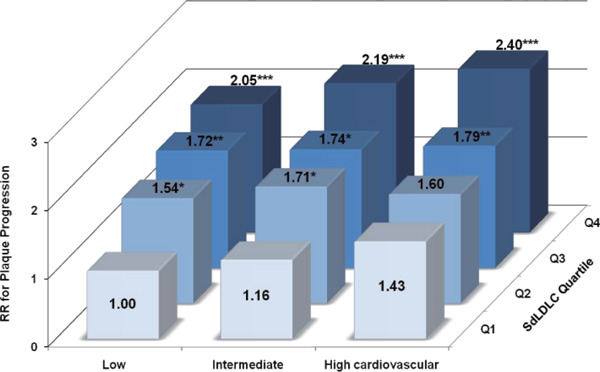
Relative Risk of Plaque Progression According to Subgroup of Small Dense LDL Cholesterol Quartile and Differing Cardiovascular Risk
RR, relative risk; sdLDLC, small dense low-density lipoprotein cholesterol.
Cardiovascular risk was calculated according to the 2016 Chinese guidelines for the management of dyslipidemia in adults. Relative risk was calculated using a modified Poisson regression model after adjustment for lipid-lowering treatment and large buoyant LDL cholesterol. *p < 0.050; **p < 0.010; ***p < 0.001.
Supplementary Fig. 5.
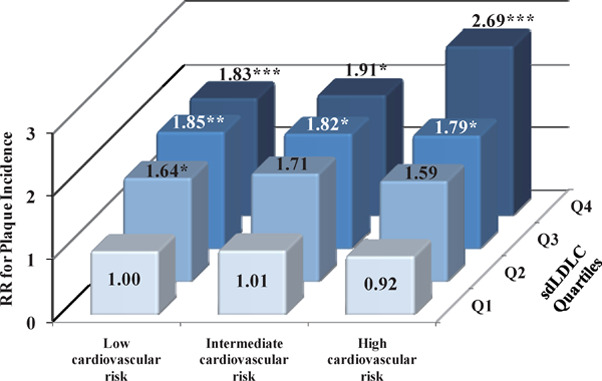
Relative Risk of Plaque Incidence According to Subgroup of Small Dense LDL Cholesterol Quartile and Differing Cardiovascular Risk
LDL, low-density lipoprotein; RR, relative risk; sdLDLC, small dense low-density lipoprotein cholesterol.
Cardiovascular risk was calculated according to the 2016 Chinese guideline for the management of dyslipidemia in adults. Relative risk was calculated using a modified Poisson regression model after adjustment for lipid-lowering treatment, and large buoyant LDL cholesterol in participants who had no plaque at baseline (n = 649). *P < 0.050; **P < 0.010; ***P < 0.001.
Supplementary Table 4. Relative Risk (95% Confidence Interval) of Carotid Atherosclerosis Progression According to Small Dense LDL Cholesterol Quartile and Dichotomized LDL Cholesterol in Participants with Intermediate or Low Cardiovascular Risk.
| Variables | No of participants | Plaque progression | Plaque incidence | Multi-territorial extent of new plaque | Total area of new plaque |
|---|---|---|---|---|---|
| LDLC < 130 mg/dL + sdLDLC < 33 mg/dL | 175 | Reference | Reference | Reference | Reference |
| LDLC < 130 mg/dL + sdLDLC 33–47 mg/dL | 88 | 1.28 (0.93–1.77) | 1.27 (0.89–1.82) | 1.31 (0.79–2.20) | 1.31 (0.79–2.20) |
| LDLC < 130 mg/dL + sdLDLC 48–62 mg/dL | 44 | 1.25 (0.81–1.93) | 1.44 (0.90–2.31) | 2.22 (1.17–4.18) | 2.12 (1.12–4.00) |
| LDLC < 130 mg/dL + sdLDLC ≥ 63 mg/dL | 20 | 2.05 (1.36–3.07) | 1.96 (1.15–3.34) | 3.46 (1.53–5.82) | 4.17 (1.83–5.52) |
| LDLC ≥ 130 mg/dL + sdLDLC < 33 mg/dL | 9 | 0.95 (0.26–3.48) | 0.57 (0.07–4.51) | 0.99 (0.17–5.93) | 1.01 (0.17–5.13) |
| LDLC ≥ 130 mg/dL + sdLDLC 33–47 mg/dL | 95 | 1.33 (0.89–2.00) | 1.34 (0.87–2.09) | 1.66 (0.84–3.25) | 1.74 (0.89–3.42) |
| LDLC ≥ 130 mg/dL + sdLDLC 48–62 mg/dL | 126 | 1.45 (1.04–2.02) | 1.46 (1.02–2.09) | 2.16 (1.22–3.81) | 1.87 (1.05–3.30) |
| LDLC ≥ 130 mg/dL + sdLDLC ≥ 63 mg/dL | 109 | 1.57 (1.15–2.14) | 1.40 (0.98–2.00) | 1.72 (1.01–2.95) | 1.76 (1.03–3.02) |
LDL, low-density lipoprotein; LDLC, low-density lipoprotein cholesterol; No, number; sdLDLC, small dense low-density lipoprotein cholesterol. Relative risk was calculated using a modified Poisson regression model after adjustment for known cardiovascular disease risk factors, lipid-lowering treatment, and large buoyant LDLC.
Discussion
In this prospective study of asymptomatic participants, we investigated whether measurement of sdLDLC would quantify the risk of subclinical atherosclerosis more effectively than standard lipid measures. The key findings are that high circulating sdLDLC was indicative of an approximately two-fold higher risk of progression of carotid atherosclerosis. This greater risk remained significant in individuals with low LDLC or non-high-density lipoprotein cholesterol levels, even in those with intermediate or low cardiovascular risk.
A high-risk strategy was implemented for lipid-lowering therapy in the primary prevention of CVD recommended by all guidelines1–3). Although the evidence obtained from major observational studies of general populations demonstrates a strong positive correlation between LDLC level and CVD risk, the Cooper Center Longitudinal Study found that only LDLC ≥ 160 mg/dL was independently associated with a higher RR of CVD or coronary heart disease mortality in a population at low estimated 10-year atherosclerotic CVD risk22). In addition, most CVD-free asymptomatic individuals maintain LDLC values of < 130 mg/dL. Therefore, clarifying the optimal biomarkers for use in cardiovascular prognosis should contribute to the precise identification of individuals with high cardiovascular risk and early prevention of CVD in those with intermediate or even low risk.
There are several observational studies that have identified associations between sdLDLC, and coronary heart disease incidence11, 12), or the presence of subclinical atherosclerosis23, 24). Our analyses have shown that sdLDLC was independently associated with atherosclerosis progression and carotid plaque progression, even when individuals had an sdLDLC ≥ 10 mg/dL. Furthermore, even in participants with LDLC levels < 130 mg/dL, high sdLDLC is associated with a significantly higher risk of plaque progression. Previous studies reported similar findings, where high sdLDLC is significantly associated with incident coronary heart disease in individuals with LDLC < 100 mg/dL in the Atherosclerosis Risk in Communities study12) and in normoglycemic, non-diabetic individuals in the Multi-Ethnic Study of Atherosclerosis11). Wilkins et al. also found that individuals with high apolipoprotein B and low LDLC, representing small dense LDL, had a 55% higher risk of the 25-year coronary artery calcium score in the Coronary Artery Risk Development in Young Adults study25). The present study extends these findings, making them more applicable to contemporary clinical practice by further investigating the association between sdLDLC and atherosclerosis progression in participants with differing cardiovascular risk, as defined in the 2016 Chinese guidelines for the management of dyslipidemia in adults. The highest sdLDLC doubled the risks of plaque development or progression compared with the lowest level in participants at intermediate or low cardiovascular risk, with a comparable impact in high-risk individuals; this association was also present in individuals with an intermediate or low risk and an LDLC level of < 130 mg/dL. Recently, Fernandez-Friera et al. showed that LDLC is independently associated with the presence and extent of atherosclerosis in both the cardiovascular risk factor-free and optimal groups in the Progression of Early Subclinical Atherosclerosis study5). We also identified the same trend in the association between baseline LDLC and the prevalence of carotid plaque in individuals with LDLC < 130 mg/dL and low cardiovascular risk (odds ratio [per 10 mg/dL] 1.19; P = 0.047). However, baseline sdLDLC, but not LDLC, was associated with the progression of carotid atherosclerosis in individuals with low LDLC and also in those with intermediate or low cardiovascular risk in our study. Tsai et al.11) reported the consistent finding that, among normoglycemic and CVD-free participants, LDLC ≥ 100 mg/dL is not associated with the risk of future coronary heart disease (hazard ratio 1.02; P = 0.93) in individuals with sdLDLC < 25.5 mg/dL. Therefore, it is apparent that the measurement of LDLC quantifies the cholesterol content of LDL particles but does not take into account the vast heterogeneity of LDL particles among individuals, and the use of LDLC to evaluate cholesterol-related cardiovascular risk may actually underestimate future risk in individuals who have a high level of circulating sdLDLC. Except for the atherogenic role of sdLDLC, it was also found that sdLDLC levels were strongly associated with atherosclerotic risk markers such as inflammation, thrombosis, hematologic markers26), and prediabetes27), which was further demonstrated in our study where sdLDLC levels were significantly correlated with hs-CRP and fasting blood glucose in the general population. Taking all these findings into account, the measurement of sdLDLC may therefore help clinicians to reclassify individuals thought to be at low risk into the high-risk category, for early intervention.
Although the modulation of LDL particle size has failed to show benefits in patients in terms of fewer CVD events and slower progression of atherosclerosis28), data from several clinical trials have supported the use of statin therapy for primary prevention in higher risk individuals. Previous studies have shown that high-intensity statin therapy significantly lowers not only total LDLC, but also sdLDLC, by about 50%29). Proteomic analysis has identified significant differences in the proteome of LDL from that of lower density of apoB-containing lipoproteins10, 30). These differences suggest that LDL particles acquire some proteins directly from the plasma, high-density lipoprotein particles, or peripheral cells, and not just from the lipolysis of triglyceride-rich lipoproteins. It is possible that some of these proteins have LDL-specific functions that might alter the metabolism of LDL subfractions, and this might provide an explanation for the higher atherogenicity of sdLDL relative to large buoyant LDL, which may involve lower binding affinity for the LDL receptor, longer plasma half-life, and weaker resistance to oxidative stress9, 31, 32). Oxidized LDL, rather than native LDL, could directly accelerate atherosclerosis by stimulating macrophage foam cell formation33) and increasing the risk of CVD events and subclinical atherosclerosis34). However, sdLDL may be prone to being oxidized and more effective at promoting macrophage lipid accumulation than the same quantity of mixed LDL35).
The present report is the first large-scale study conducted in a general Chinese population to assess the importance of sdLDLC. In this study, we investigated the relationship between sdLDLC and atherosclerotic plaque progression: we found that baseline sdLDLC was positively associated with the progression of carotid atherosclerosis and that the atherogenic impact of sdLDLC was found in individuals with low LDLC or non-high-density lipoprotein cholesterol, even in those with intermediate or low cardiovascular risk.
STUDY LIMITATIONS. Firstly, we measured sdLDLC using automated homogeneous assay, not the method most frequently used in previous studies, such as ultracentrifugation, gradient gel electrophoresis, and nuclear magnetic resonance imaging, which might limit between-study comparisons due to measurement divergence. The results using the previous methods lack particle standardization and reproducibility, demonstrate a wide range of variation (6%–93%)32, 36). The assay used in the present study, which was developed by Ito et al.21), can be used to quantify sdLDLC with high precision, selectivity, reliability, simplicity, throughput, and speed. It might be widely applicable in clinical laboratories and should be useful for the evaluation of the significance of sdLDL in the development of CVD and atherosclerosis in future clinical and epidemiologic studies. Secondly, our study was observational in design, which precluded causality inference. Although we adjusted for several confounding factors, we cannot exclude the impact of some unaccounted-for residual confounding factors that were imperfectly measured or unmeasured, such as the type of lipid-lowering medication being used. This is a common limitation of a non-randomized study, but it nevertheless highlights the need for further studies. Thirdly, our sample size was large, but only represented a proportion of the original cohort. To ascertain whether the missing data created potential bias, we compared the baseline characteristics of study participants who were eligible with those who were unavailable for re-examination, and found no significant lipid-related differences. Nevertheless, attempts should be made to replicate our findings and to validate them clinically in larger studies. Finally, the fact that our study participants were all Chinese may limit the generalizability of our findings, and therefore further investigations should be conducted in populations of other ethnicities.
Conclusions
Our findings indicate that sdLDLC level is independently associated with the progression of carotid atherosclerosis. The atherogenic impact of sdLDLC is substantial in individuals with low LDLC and in those with low cardiovascular risk. Our findings may have important ramifications for the prognosis and treatment of atherosclerosis, by reinforcing the potential importance of not all LDL particles being equally atherogenic and by showing that sdLDL is an important factor defining LDL particle functionality. Thus, sdLDLC has the potential to serve as a marker for the atherogenic function of LDL. Clinically, sdLDLC could be measured in individuals with intermediate or low risk, and those with higher sdLDLC may benefit from more aggressive cholesterol-lowering therapy. This possibility requires further investigations in large, prospective epidemiologic studies and clinical trials.
Acknowledgments
We gratefully acknowledge the contribution of all the investigators from the participating centers of the Chinese Multi-provincial Cohort Study for data collection. Special thanks go to Denka Seiken Co., Ltd. (Japan) for providing all the required reagents for small dense low-density lipoprotein cholesterol measurement; however, they were not involved in the study design, data analysis, or interpretation of the findings.
Abbreviations and Acronyms
- CI
confidence interval
- CVD
cardiovascular disease
- Hs-CRP
high-sensitivity C-reactive protein
- LDLC
low-density lipoprotein cholesterol
- RR
relative risk
- sdLDLC
small dense low-density lipoprotein cholesterol
Funding
This work was supported by grants from the National Science & Technology Pillar Program during the 12th 5-Year Plan Period of China (grant numbers 2011BAI09B01 and 2011BAI11B03), the National Natural Science Foundation of China (grant number 81570409), the National Key Research and Development Program of China (grant number 2016YFC0900902), and the High-level Technical Training Project Funding of Beijing Health System (grant number 2015-3-046).
Disclosures
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this work.
References
- 1). Joint committee for guideline revision. 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol, 2018; 15: 1-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Monique Verschuren WM, Vlachopoulos C, Wood DA, Luis Zamorano J, Cooney MT: 2016 ESC/EAS guidelines for the management of dyslipidaemias. Rev Esp Cardiol (Engl Ed), 2017; 70: 115. [DOI] [PubMed] [Google Scholar]
- 3). Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Jr., Sperling L, Virani SS, Yeboah J: 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol, 2019; 73: e285-e350 [DOI] [PubMed] [Google Scholar]
- 4). Zhang M, Deng Q, Wang L, Huang Z, Zhou M, Li Y, Zhao Z, Zhang Y: Prevalence of dyslipidemia and achievement of low-density lipoprotein cholesterol targets in chinese adults: a nationally representative survey of 163,641 adults. Int J Cardiol, 2018; 260: 196-203 [DOI] [PubMed] [Google Scholar]
- 5). Fernandez-Friera L, Fuster V, Lopez-Melgar B, Oliva B, Garcia-Ruiz JM, Mendiguren J, Bueno H, Pocock S, Ibanez B, Fernandez-Ortiz A, Sanz J: Normal ldl-cholesterol levels are associated with subclinical atherosclerosis in the absence of risk factors. J Am Coll Cardiol, 2017; 70: 2979-2991 [DOI] [PubMed] [Google Scholar]
- 6). Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, Amiya E, Ogawa T, Ozaki Y, Sakuma I, Nakagawa Y, Hibi K, Hiro T, Fukumoto Y, Hokimoto S, Miyauchi K, Yamazaki T, Ito H, Otsuji Y, Kimura K, Takahashi J, Hirayama A, Yokoi H, Kitagawa K, Urabe T, Okada Y, Terayama Y, Toyoda K, Nagao T, Matsumoto M, Ohashi Y, Kaneko T, Fujita R, Ohtsu H, Ogawa H, Daida H, Shimokawa H, Saito Y, Kimura T, Inoue T, Matsuzaki M R. N: High-dose versus low-dose pitavastatin in japanese patients with stable coronary artery disease (real-cad): a randomized superiority trial. Circulation, 2018; 137: 1997-2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Patel KV, Pandey A, de Lemos JA: Conceptual framework for addressing residual atherosclerotic cardiovascular disease risk in the era of precision medicine. Circulation, 2018; 137: 2551-2553 [DOI] [PubMed] [Google Scholar]
- 8). Krauss RM: Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol, 2010; 21: 305-311 [DOI] [PubMed] [Google Scholar]
- 9). Diffenderfer MR, Schaefer EJ: The composition and metaboli sm of large and small ldl. Curr Opin Lipidol, 2014; 25: 221-226 [DOI] [PubMed] [Google Scholar]
- 10). Thongtang N, Diffenderfer MR, Ooi EMM, Barrett PHR, Turner SM, Le NA, Brown WV, Schaefer EJ: Metabolism and proteomics of large and small dense ldl in combined hyperlipidemia: effects of rosuvastatin. J Lipid Res, 2017; 58: 1315-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, Hoefner DM, Remaley AT: New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol, 2014; 34: 196-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, Ballantyne CM: Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the atherosclerosis risk in communities (aric) study. Arterioscler Thromb Vasc Biol, 2014; 34: 1069-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Mora S, Caulfield MP, Wohlgemuth J, Chen Z, Superko HR, Rowland CM, Glynn RJ, Ridker PM, Krauss RM: Atherogenic lipoprotein subfractions determined by ion mobility and first cardiovascular events after random allocation to high-intensity statin or placebo: the justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (jupiter) trial. Circulation, 2015; 132: 2220-2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Shiffman D, Louie JZ, Caulfield MP, Nilsson PM, Devlin JJ, Melander O: LDL subfractions are associated with incident cardiovascular disease in the malmo prevention project study. Atherosclerosis, 2017; 263: 287-292 [DOI] [PubMed] [Google Scholar]
- 15). Chen PC, Jeng JS, Hsu HC, Su TC, Chien KL, Lee YT: Carotid atherosclerosis progression and risk of cardiovascular events in a community in taiwan. Sci Rep, 2016; 6: 25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Katakami N, Mita T, Gosho M, Takahara M, Irie Y, Yasuda T, Matsuoka TA, Osonoi T, Watada H, Shimomura I: Clinical utility of carotid ultrasonography in the prediction of cardiovascular events in patients with diabetes: a combined analysis of data obtained in five longitudinal studies. J Atheroscler Thromb, 2018; 25: 1053-1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Näslund Ulf, Ng Nawi, Lundgren Anna, Fhärm Eva, Grönlund Christer, Johansson Helene, Lindahl Bernt, Lindahl Prof Bertil, Lindvall Kristina, Nilsson Stefan K, Nordin Maria, Nordin Steven, Nyman Emma, Rocklöv Joacim, Vanoli Davide, Weinehall Lars, Wennberg Patrik, Wester Per, Norberg Margareta, VIPVIZA trial group : Visualization of asymptomatic atherosclerotic disease for optimum cardiovascular prevention (vipviza): a pragmatic, open-label, randomised controlled trial. LANCET, 2018; 393: 133-142 [DOI] [PubMed] [Google Scholar]
- 18). Liu J, Hong Y, D'Agostino RB, Sr., Wu Z, Wang W, Sun J, Wilson P W, Kannel WB, Zhao D: Predictive value for the chinese population of the framingham chd risk assessment tool compared with the chinese multi-provincial cohort study. JAMA, 2004; 291: 2591-2599 [DOI] [PubMed] [Google Scholar]
- 19). Qi Y, Fan J, Liu J, Wang W, Wang M, Sun J, Xie W, Zhao F, Li Y, Zhao D: Cholesterol-overloaded hdl particles are independently associated with progression of carotid atherosclerosis in a cardiovascular disease-free population: a community-based cohort study. J Am Coll Cardiol, 2015; 65: 355-363 [DOI] [PubMed] [Google Scholar]
- 20). Qi Y, Liu J, Wang W, Wang M, Zhao F, Sun J, Zhao D: Apolipoprotein E-containing high-density lipoprotein (hdl) modifies the impact of cholesterol-overloaded hdl on incident coronary heart disease risk: a community-based cohort study. J Clin Lipidol, 2018; 12: 89-98.e2 [DOI] [PubMed] [Google Scholar]
- 21). Ito Y, Fujimura M, Ohta M, Hirano T: Development of a homogen eous assay for measurement of small dense ldl cholesterol. Clin Chem, 2011; 57: 57-65 [DOI] [PubMed] [Google Scholar]
- 22). Shuaib M A, Laura F D, David L, Carolyn E B, Nina B R, Benjamin L W, Anand R, Darren K M, James A dL, Scott M G, Jarett D B, Khera A: Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease. Circulation, 2018; 138: 2315-2325 [DOI] [PubMed] [Google Scholar]
- 23). Maeda S, Nakanishi S, Yoneda M, Awaya T, Yamane K, Hirano T, Kohno N: Associations between small dense ldl, hdl subfractions (hdl2, hdl3) and risk of atherosclerosis in japanese-americans. J Atheroscler Thromb, 2012; 19: 444-452 [DOI] [PubMed] [Google Scholar]
- 24). Aoki T, Yagi H, Sumino H, Tsunekawa K, Araki O, Kimura T, Nara M, Ogiwara T, Nakajima K, Murakami M: Relationship between carotid artery intimamedia thickness and small dense low-density lipoprotein cholesterol concentrations measured by homogenous assay in japanese subjects. Clin Chim Acta, 2015; 442: 110-114 [DOI] [PubMed] [Google Scholar]
- 25). Wilkins JT, Li RC, Sniderman A, Chan C, Lloyd-Jones DM: Discordance between apolipoprotein b and ldl-cholesterol in young adults predicts coronary artery calcification: the cardia Study. J Am Coll Cardiol, 2016; 67: 193-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Hsu SH-J, Jang M-H, Torng P-L, Su T-C: Positive association b etween small dense low-density lipoprotein cholesterol concentration and biomarkers of inflammation, thrombosis, and prediabetes in non-diabetic adults. J Atheroscler Thromb, 2019; 26: 624-635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Hsu H, Hsu P, Cheng MH, Ito Y, Kanda E, Schaefer EJ, Ai M: Lipoprotein subfractions and glucose homeostasis in prediabetes and diabetes in taiwan. J Atheroscler Thromb, 2019; 26: 890-914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Rizzo M, Berneis K: Low-density lipoprotein size and cardiovascular risk assessment. QJM, 2006; 99: 1-14 [DOI] [PubMed] [Google Scholar]
- 29). Ai M, Otokozawa S, Asztalos BF, Nakajima K, Stein E, Jones P H, Schaefer EJ: Effects of maximal doses of atorvastatin versus rosuvastatin on small dense low-density lipoprotein cholesterol levels. Am J Cardiol, 2008; 101: 315-318 [DOI] [PubMed] [Google Scholar]
- 30). Dashty M, Motazacker MM, Levels J, de Vries M, Mahmoudi M, Peppelenbosch MP, Rezaee F: Proteome of human plasma very low-density lipoprotein and low-density lipoprotein exhibits a link with coagulation and lipid metabolism. Thromb Haemost, 2014; 111: 518-530 [DOI] [PubMed] [Google Scholar]
- 31). Hirano T: Pathophysiology of diabetic dyslipidemia. J Atheroscler Thromb, 2018; 25: 771-782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Hirayama S, Miida T: Small dense ldl: an emerging risk factor for cardiovascular disease. Clin Chim Acta, 2012; 414: 215-224 [DOI] [PubMed] [Google Scholar]
- 33). Gao S, Liu J: Association between circulating oxidized low-de nsity lipoprotein and atherosclerotic cardiovascular disease. Chronic Dis Transl Med, 2017; 3: 89-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Gao S, Zhao D, Qi Y, Wang W, Wang M, Sun J, Liu J, Li Y, Liu J: Circulating oxidized low-density lipoprotein levels independently predict 10-year progression of subclinical carotid atherosclerosis: a community-based cohort study. J Atheroscler Thromb, 2018; 25: 1032-1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Tani M, Kawakami A, Mizuno Y, Imase R, Ito Y, Kondo K, Ishii H, Yoshida M: Small dense ldl enhances thp-1 macrophage foam cell formation. J Atheroscler Thromb, 2011; 18: 698-704 [DOI] [PubMed] [Google Scholar]
- 36). Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN: Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid Med Cell Longev, 2017; 2017: 1273042. [DOI] [PMC free article] [PubMed] [Google Scholar]


