Abstract
Aims: This study aims to investigate the association between serum small dense low-density lipoprotein (sdLDL) cholesterol level and the development of coronary heart disease (CHD) in a Japanese community.
Methods: A total of 3,080 participants without prior cardiovascular disease, aged 40 years or older, were followed up for 8 years. The participants were divided into the quartiles of serum sdLDL cholesterol levels. The risk estimates were computed using a Cox proportional hazards model.
Results: During the follow-up period, 79 subjects developed CHD. Subjects in the highest quartile had a 5.41-fold (95% confidence interval, 2.12–13.82) higher risk of CHD than those in the lowest quartile after controlling for confounders. In the analysis classifying the participants into four groups according to the levels of serum sdLDL cholesterol and serum low-density lipoprotein (LDL) cholesterol levels, the risk of CHD almost doubled in subjects with sdLDL cholesterol of ≥ 32.9 mg/dL (median), regardless of serum LDL cholesterol levels, as compared with subjects with serum sdLDL cholesterol of < 32.9 mg/dL and serum LDL cholesterol of < 120.1 mg/dL (median). When serum sdLDL cholesterol levels were incorporated into a model with known cardiovascular risk factors, c-statistics was significantly increased (from 0.77 to 0.79; p = 0.02), and the net reclassification improvement was also significant (0.40; p < 0.001).
Conclusions: The present findings suggest that the serum sdLDL cholesterol level is a relevant biomarker for the future development of CHD that offers benefit beyond the serum LDL cholesterol level and a possible therapeutic target to reduce the burden of CHD in a Japanese community.
Keywords: Small dense LDL cholesterol, Coronary heart disease, Risk assessment, Prospective study
See editorial vol. 27: 641–643
Introduction
Coronary heart disease (CHD) is the leading cause of death worldwide and places a major economic and resource burden on private and public healthcare systems1). Globally, an estimated 7.4 million people died from CHD in 2015 2). CHD is mainly caused by atherosclerosis and the concomitant low-grade inflammation of the coronary arteries, which develop due to the deposition of atherogenic lipoprotein in the vessel walls3). Growing evidence from observational studies and clinical trials has demonstrated that elevated serum low-density lipoprotein (LDL) cholesterol is a significant risk factor for the development of CHD, and lowering the serum LDL cholesterol level with medications, such as statins, has a beneficial effect on the reduction of coronary risk through the prevention of atherothrombosis and plaque rupture in coronary arteries4–9). Nevertheless, it has been acknowledged that a relatively high proportion of individuals with serum LDL cholesterol in the normal range still develop CHD10).
LDL particles are heterogeneous with respect to size, density, and composition11). Recently, the difference in the atherogenic effect across LDL particles has attracted attention12). Small dense LDL (sdLDL), which is small and highly dense among LDL particles, has high atherogenic potential due to its increased susceptibility to oxidation, high endothelial permeability, and decreased hepatic LDL receptor affinity13, 14). Until recently, however, there have been few prospective studies with a large sample size investigating the influence of sdLDL on coronary risk independent of other cardiovascular risk factors probably due to the limited methods available for the measurement of sdLDL (principally ultracentrifugation15) or gradient gel electrophoresis) 16). These methods were not suitable for routine analysis because of their long assay times and expensive equipment. Over the last decade, a homogeneous method that allows the easy measurement of serum sdLDL cholesterol levels has been developed17, 18). Using this method, several prospective cohort studies have revealed a significant association between higher serum sdLDL cholesterol and the development of CHD19–21). However, few studies have addressed the predictive ability of serum sdLDL cholesterol level for future CHD risks.
Aim
The aim of the present study was to evaluate whether serum sdLDL cholesterol levels are a clinically relevant biomarker for CHD that offers benefit beyond serum LDL cholesterol levels and other known cardiovascular risk factors using the prospective longitudinal data from a general Japanese population.
Methods
Study Design and Participants
The Hisayama study, a population-based prospective cohort study of cardiovascular diseases, has been underway since 1961 in the town of Hisayama, which is located in a suburb of Fukuoka City on Kyushu Island in Japan. According to the national census and nutrition surveys, the age and occupational distributions of the Hisayama population have been similar to those in Japan as a whole since the 1960s22). The full community surveys of the health status of residents ≥ 40 years of age have been repeated annually since 1961 22). A screening survey for the present study was performed in 2007 and 2008, and a detailed description has been published previously23). Briefly, a total of 3,384 residents aged ≥ 40 years (78.2% of the total population of this age group) underwent the examination. After excluding eight subjects who did not consent to participate in the study, 223 subjects who had past history of stroke or CHD, and 73 subjects with no measurement of serum sdLDL cholesterol, the remaining 3,080 subjects (1,290 men and 1,790 women) were enrolled in the present study.
The study was approved by the Kyushu University Institutional Review Board for Clinical Research, and written informed consent was obtained from all the participants.
Follow-Up Survey
The subjects were followed up prospectively until November 2015 or their death (median, 8.3 years) by annual health examinations or by mail or telephone for any subject who did not undergo the examination or who moved out of the town. The development of CHD was also checked by a daily monitoring system organized by the study team, local physicians, and members of the Health and Welfare Office of the town. Subjects with suspected CHD events were evaluated for their detailed clinical information by study team physicians. When a subject died, an autopsy was performed at the Department of Pathology of Kyushu University, if consent for autopsy was obtained. During the follow-up period, autopsy examination was performed for 192 (57.8%) of 332 deceased subjects. In addition to the deceased cases, four subjects were lost to follow-up, all of whom were subjects who moved out of the town.
Measurement of the Serum sdLDL Cholesterol Level
At the screening examination, the portions of the plasma specimens were stored at −80°C until serum sdLDL cholesterol concentrations were measured in 2014. Serum sdLDL cholesterol concentrations were directly measured on a Hitachi 7180 automated chemistry analyzer using a homogeneous assay (sdLDL-EX “SEIKEN”; Denka Seiken, Tokyo)17, 18), which received Food and Drug Administration clearance on August 18, 2017. The subjects were divided into four groups according to the quartiles of serum sdLDL cholesterol levels: Q1, ≤ 24.4 mg/dL [≤ 0.63 mmol/L]; Q2, 24.5–32.8 mg/dL [0.63–0.84 mmol/L]; Q3, 32.9–43.6 mg/dL [0.85–1.12 mmol/L]; and Q4, ≥ 43.7 mg/dL [≥ 1.13 mmol/L].
Outcomes
The primary outcome of the present analysis was CHD. The criteria for the diagnosis of CHD included first-ever fatal and nonfatal myocardial infarction, silent myocardial infarction, sudden cardiac death within 1 h after the onset of acute illness, coronary angioplasty, and bypass grafting. We counted the incident cases of CHD during the follow-up period as events, regardless of the presence or absence of stroke before the onset of CHD. The diagnosis of myocardial infarction was based on detailed clinical information and at least two of the following findings: typical clinical symptoms, electrocardiogram evidence of myocardial infarction, elevated cardiac enzymes, or morphologic findings including echocardiographic, scintigraphic, or angiographic abnormalities compatible with myocardial injury or myocardial necrosis or scars more than 1 cm in diameter at autopsy24, 25).
Other Risk Factor Measurements
Each subject completed a self-administered questionnaire covering medical history, medication for hypertension, diabetes, and dyslipidemia, smoking habits, alcohol intake, and physical activity. Smoking and drinking habits were categorized as either current use or not. Current smoking was defined as smoking at least one cigarette per day. Current drinking was defined as drinking at least one alcoholic beverage per month. The subjects engaging in sports or other forms of exertion ≥ 3 times a week during their leisure time made up a regular exercise group. The body height and weight were measured in light clothing without shoes, and the body mass index (BMI) was calculated (kg/m2). The blood pressure was measured three times in a sitting position using an automated sphygmomanometer (BP-203 RVIIIB; Omron Healthcare), and the mean of three measurements was used for the analysis. Hypertension was defined as blood pressure ≥ 140/90 mmHg and/or current use of antihypertensive agents. Electrocardiogram abnormalities were defined as left ventricular hypertrophy (Minnesota code, 3-1), ST depression (4-1, 2, 3), or atrial fibrillation/flutter (8-3).
The blood samples were collected from an antecubital vein. Plasma glucose levels were measured by the hexokinase method, and serum insulin levels were determined by using an electro-chemiluminescence immunoassay. Diabetes mellitus was defined as fasting plasma glucose levels ≥ 126 mg/dL, 2-h post load or casual glucose levels of at least 200 mg/dL, or the current use of glucose-lowering agents (i.e., oral glucose-lowering agents or insulin). Hemoglobin A1c (HbA1c) was measured by latex aggregation immunoassay using determiner HbA1c (Kyowa Medix, Tokyo). The value for HbA1c was estimated as a National Glycohemoglobin Standardization Program equivalent value calculated with the following formula26): HbA1c (%) = 1.02 × HbA1c (Japan Diabetes Society) (%) + 0.25%. Insulin resistance was estimated by the homeostasis model assessment of insulin resistance (HOMA-IR) values, which were calculated as follows27): HOMA-IR=fasting plasma glucose (mg/dL) × fasting serum insulin (IU/mL)/405. Serum LDL cholesterol and high-density lipoprotein (HDL) cholesterol levels and serum creatinine were measured enzymatically. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation with a Japanese coefficient of 0.813 28). Serum high-sensitivity C-reactive protein (hs-CRP) concentrations were measured using the frozen serum portion thawed in 2004 by a modified version of the Behring Latex-Enhanced CRP assay on a Behring Nephelometer BN-100 (Behring Diagnostics, Westwood, MA).
Statistical Analysis
The trends in the means (standard deviations [SDs]) and the frequencies of risk factors across the quartiles of sdLDL cholesterol were tested by a linear and a logistic regression analysis, respectively, in which serum sdLDL cholesterol levels were assigned ordinal values of 1, 2, 3, and 4 for the first (Q1), second (Q2), third (Q3), and fourth (Q4) quartiles, respectively. Serum HOMA-IR, triglycerides, and hs-CRP levels were shown as medians and interquartile ranges in the baseline characteristics of the population and were log-transformed in the analyses because their distributions were skewed. The incidence rate of CHD was calculated using the person-year method after adjusting for age and sex by means of the direct method. The Cox proportional hazards model was used to estimate the hazard ratio (HR) and its 95% confidence intervals (CIs) for the development of CHD for the quartiles or the cutoff levels of serum sdLDL cholesterol levels or per 1 SD increment in log-transformed serum sdLDL cholesterol levels (as a continuous variable). The trends in the age- and sex-adjusted incidence rates or HRs across the serum sdLDL cholesterol levels were tested by a Cox proportional hazards model including serum sdLDL cholesterol levels assigned ordinal numbers of 1, 2, 3, or 4 as categorical variables and the relevant covariates. In the multivariable analysis, the risk estimates were adjusted for potential confounding factors at baseline, namely, age, sex, systolic blood pressure, use of antihypertensive agents, HbA1c, use of glucose-lowering agents, serum HDL cholesterol, lipid-modifying agents, BMI, eGFR, electrocardiogram abnormalities, current smoking, current drinking, and regular exercise. Additionally, we made an another multivariable-adjusted model that included the covariates selected from these potential confounders using the backward elimination method at p < 0.20 for the remaining variables. The interactions in the association between the subgroups of sex, hypertension, diabetes, BMI (< 25 or ≥ 25 kg/m2), serum LDL cholesterol (< 120.1 or ≥ 120.1 mg/dl), use of lipid-lowering agents, current smoking, and eGFR (< 60 or ≥ 60 ml/min/1.73 m2) were tested by adding multiplicative interaction terms to the relevant Cox model. The proportions of missing values were less than 0.1% for all the variables included in the model. To compare the accuracy of risk assessment for the development of CHD between the models including known cardiovascular disease risk factors with and without serum sdLDL cholesterol level or serum LDL cholesterol level, the increase in the Harrell's c-statistics among models was evaluated and tested using a method described by Newson29). Moreover, the increased predictive ability of the serum sdLDL cholesterol level or the serum LDL cholesterol level was further estimated by the net reclassification improvement (NRI) and integrated discrimination improvement (IDI)30), where the individual probabilities were estimated by using the Cox proportional hazards model. The cutoff value of serum sdLDL cholesterol levels that optimizes the discriminating ability for the risk of incident CHD was determined as the point closest to (0,1) on the receiver-operating characteristic curve, min 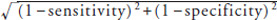 , where sensitivity = the number of subjects above the cutoff value of serum sdLDL cholesterol level/total number of subjects among subjects who developed CHD, and specificity = the number of subjects below the cutoff value/total number of subjects among subjects who did not develop CHD31). A two-sided value of p < 0.05 was considered statistically significant in all analyses. Statistical analyses were conducted using Statistical Analysis Software (SAS) version 9.4 (SAS Institute, Cary, NC), and Stata version 14.0 (StataCorp, College Station, TX).
, where sensitivity = the number of subjects above the cutoff value of serum sdLDL cholesterol level/total number of subjects among subjects who developed CHD, and specificity = the number of subjects below the cutoff value/total number of subjects among subjects who did not develop CHD31). A two-sided value of p < 0.05 was considered statistically significant in all analyses. Statistical analyses were conducted using Statistical Analysis Software (SAS) version 9.4 (SAS Institute, Cary, NC), and Stata version 14.0 (StataCorp, College Station, TX).
Results
Table 1 exhibits the baseline characteristics of the study population according to the quartiles of serum sdLDL cholesterol. The mean values of systolic blood pressure, HbA1c, serum total cholesterol, serum LDL cholesterol, BMI, and eGFR; the median values of HOMA-IR and serum hs-CRP; and the frequencies of male sex, use of statins, current smoking, and current drinking increased significantly with high serum sdLDL cholesterol levels. Conversely, the mean values of age and serum HDL cholesterol decreased significantly with high serum sdLDL cholesterol levels.
Table 1. Baseline characteristics of participants according to quartiles of serum small dense low-density lipoprotein cholesterol levels (the Hisayama Study, 2007–2008).
| Serum sdLDL cholesterol level, mg/dL | |||||
|---|---|---|---|---|---|
| Variables | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p for trend |
| ≤ 24.4 | 24.5–32.8 | 32.9–43.6 | ≥ 43.7 | ||
| (n = 763) | (n = 775) | (n = 768) | (n = 774) | ||
| Age (years) | 65 (15) | 63 (12) | 64 (11) | 61 (10) | < 0.001 |
| Men (%) | 35.0 | 38.3 | 42.1 | 52.1 | < 0.001 |
| Systolic blood pressure (mmHg) | 127 (20) | 129 (19) | 134 (19) | 136 (18) | < 0.001 |
| Use of antihypertensive agents (%) | 30.9 | 27.6 | 31.6 | 27.9 | 0.49 |
| HbA1c (%) | 5.3 (0.7) | 5.5 (0.7) | 5.5 (0.8) | 5.7 (0.9) | < 0.001 |
| Use of glucose-lowering agents (%) | 5.1 | 6.8 | 6.9 | 7.1 | 0.14 |
| HOMA-IR | 1.1 (0.7–1.5) | 1.2 (0.8–1.8) | 1.4 (0.9–2.1) | 1.8 (1.2–2.7) | < 0.001 |
| Serum total cholesterol (mg/dL) | 179.7 (26.6) | 204.7 (25.5) | 216.8 (30.9) | 237.2 (34.2) | < 0.001 |
| Serum LDL cholesterol (mg/dL) | 92.4 (19.4) | 116.2 (20.3) | 131.1 (24.5) | 148.3 (31.0) | < 0.001 |
| Serum HDL cholesterol (mg/dL) | 72.9 (18.2) | 71.6 (18.0) | 65.7 (17.0) | 58.8 (15.0) | < 0.001 |
| Serum triglycerides (mg/dL) | 71 (55–92) | 89 (68–115) | 107 (83–139) | 163 (120–232) | < 0.001 |
| Use of lipid-modifying agents (%) | 16.1 | 12.9 | 14.5 | 12.1 | 0.064 |
| Use of statins (%) | 15.7 | 12.9 | 13.8 | 10.3 | 0.005 |
| BMI (kg/m2) | 21.9 (3.5) | 22.3 (3.1) | 23.5 (3.5) | 24.3 (3.3) | < 0.001 |
| eGFR (ml/min/1.73 m2) | 73.5 (14.9) | 75.5 (12.4) | 75.0 (11.7) | 77.1 (11.1) | < 0.001 |
| Electrocardiogram abnormalities (%) | 16.4 | 12.9 | 15.8 | 16.3 | 0.65 |
| Serum hs-CRP (mg/L) | 0.3 (0.1–0.7) | 0.4 (0.2–0.7) | 0.4 (0.2–0.8) | 0.6 (0.3–1.0) | < 0.001 |
| Current smoking (%) | 15.1 | 18.5 | 19.4 | 26.7 | < 0.001 |
| Current drinking (%) | 39.7 | 45.4 | 47.5 | 57.8 | < 0.001 |
| Regular exercise (%) | 11.6 | 12.5 | 11.3 | 12.0 | 0.98 |
SI conversion factors: To convert mg/dL values to mmol/L, multiply serum sdLDL cholesterol, serum total cholesterol, serum LDL cholesterol, and HDL cholesterol values by 0.02586, and multiply serum triglycerides values by 0.01129.
Data are presented as the mean values (standard deviation), percentages, or medians (interquartile range).
The trends in the means and the frequencies of risk factors across the quartiles of sdLDL cholesterol were tested by a linear and a logistic regression analysis. Serum HOMA-IR, triglycerides, and hs-CRP levels were log-transformed in the analyses due to skewed distribution.
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; sdLDL, small dense low-density lipoprotein.
During the follow-up period, a total of 79 subjects had a first-ever CHD event. Among them, 45 subjects experienced myocardial infarction, 33 subjects experienced coronary angioplasty, and one subject experienced bypass grafting. Fig. 1 shows that the age- and sex-adjusted cumulative incidence of coronary heart disease increased significantly with the elevating serum sdLDL cholesterol levels (p for trend < 0.001). Table 2 demonstrates the age- and sexadjusted incidence rates and multivariable-adjusted HRs for the development of CHD according to the quartiles of serum sdLDL cholesterol levels. The incidence rates increased linearly with high serum sdLDL cholesterol levels: 1.04, 3.37, 4.67, and 5.58 per 1000 person-years from the first to the fourth quartile groups, respectively (p < 0.001 for trend). The HRs of the development of CHD increased significantly with high serum sdLDL cholesterol levels after adjusting for age, sex, systolic blood pressure, use of antihypertensive agents, HbA1c, use of glucose-lowering agents, serum HDL cholesterol, use of lipid-modifying agents, BMI, eGFR, electrocardiogram abnormalities, current smoking, current drinking, and regular exercise (model 1, p for trend < 0.001): subjects in the highest quartile had a 5.41-fold (95% CI, 2.12–13.82) higher risk of CHD than those in the lowest quartile. These associations remained significant even after additional adjustments for serum LDL cholesterol levels (p for trend = 0.03), serum hs-CRP levels (p for trend < 0.001), or HOMA-IR (p for trend < 0.001) in addition to the abovementioned confounding factors. We also performed a multivariable-adjusted analysis including age, sex, HbA1c, serum HDL cholesterol, electrocardiogram abnormalities, and regular exercise as covariates, which were selected with the backward elimination method from the aforementioned covariates (model 2). Consequently, the HRs of the development of CHD also increased significantly with high serum sdLDL cholesterol levels (p for trend < 0.001). Moreover, the sensitivity analysis including the use of statins as an adjustment factor instead of the use of lipid-modifying agents in the multivariable-adjusted models or among subjects who were not using lipid-modifying agents were not altered substantially although the association did not reach the statistically significant level in the multivariable-adjusted model including serum LDL cholesterol among subjects who were not using lipid-modifying agents (p for trend = 0.07) (Supplementary Table 1 and Supplementary Table 2).
Fig. 1.
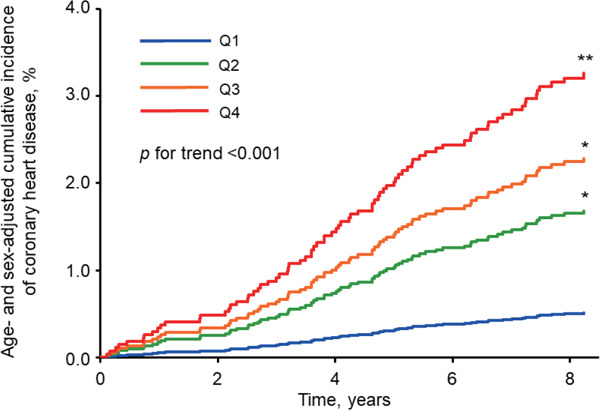
Age- and sex-adjusted cumulative incidence of coronary heart disease according to quartiles of serum small dense low-density lipoprotein cholesterol
*p < 0.05, **p < 0.001 versus the lowest quartile of serum small dense low-density lipoprotein cholesterol.
Table 2. Hazard ratios for the development of coronary heart disease according to serum small dense low-density lipoprotein cholesterol levels, 2007–2015.
| Serum sdLDL cholesterol level, mg/dL |
|||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p for trend | |
| ≤ 24.4 | 24.5–32.8 | 32.9–43.6 | ≥ 43.7 | ||
| No. of events/subjects | 6/763 | 18/775 | 24/768 | 31/774 | |
| Age-and sex-adjusted incidence rate (per 1,000 person-years) | 1.04 | 3.37 | 4.67 | 5.58 | < 0.001 |
| Hazard ratio (95% CI) | |||||
| Age-and sex-adjusted | 1.00 (Reference) | 3.34 (1.32–8.44) | 4.55 (1.85–11.20) | 6.53 (2.67–15.95) | < 0.001 |
| Model 1 | 1.00 (Reference) | 3.18 (1.25–8.09) | 4.15 (1.66–10.40) | 5.41 (2.12–13.82) | < 0.001 |
| Model 1 + serum LDL cholesterol | 1.00 (Reference) | 2.75 (1.06–7.14) | 3.22 (1.20–8.64) | 3.76 (1.28–10.99) | 0.03 |
| Model 1 + serum triglycerides | 1.00 (Reference) | 3.06 (1.19–7.84) | 3.93 (1.54–10.02) | 4.90 (1.78–13.43) | 0.003 |
| Model 1 + log hs-CRP | 1.00 (Reference) | 3.23 (1.27–8.21) | 4.21 (1.68–10.55) | 5.55 (2.17–14.20) | < 0.001 |
| Model 1 + log HOMA-IR | 1.00 (Reference) | 2.95 (1.16–7.50) | 3.93 (1.57–9.85) | 4.80 (1.87–12.33) | < 0.001 |
| Model 2 | 1.00 (Reference) | 3.04 (1.20–7.68) | 3.97 (1.60–9.83) | 4.85 (1.94–12.12) | < 0.001 |
SI conversion factors: To convert mg/dL values to mmol/L, multiply serum sdLDL cholesterol values by 0.02586 and multiply serum triglycerides values by 0.01129.
Abbreviations: CI, confidence interval; hs-CRP, high-sensitivity C reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; SD, standard deviation; sdLDL, small dense low-density lipoprotein.
Model 1: Adjusted for age, sex, systolic blood pressure, use of antihypertensive agents, hemoglobin A1c, use of glucose-lowering agents, high density lipoprotein cholesterol, use of lipid-modifying agents, body mass index, estimated glomerular filtration rate, electrocardiogram abnormalities, current smoking, current drinking, and regular exercise.
Model 2: Adjusted for age, sex, hemoglobin A1c, high density lipoprotein cholesterol, electrocardiogram abnormalities, and regular exercise, which were selected with the backward elimination method at p < 0.20 for the remaining variables.
Supplementary Table 1. Multivariable-adjusted hazard ratios for coronary heart disease according to serum small dense low-density lipoprotein cholesterol levels, 2007–2015.
| Serum sdLDL cholesterol level, mg/dL |
|||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p for trend | |
| ≤ 24.4 | 24.5–32.8 | 32.9–43.6 | ≥ 43.7 | ||
| Hazard ratio (95% CI) | |||||
| Model 1 | 1.00 (Reference) | 3.18 (1.25–8.06) | 4.12 (1.65–10.33) | 5.32 (2.08–13.59) | < 0.001 |
| Model 1 + serum LDL cholesterol | 1.00 (Reference) | 2.77 (1.07–7.22) | 3.28 (1.22–8.80) | 3.84 (1.31–11.24) | 0.03 |
| Model 1 + serum triglycerides | 1.00 (Reference) | 3.04 (1.19–7.77) | 3.86 (1.51–9.85) | 4.71 (1.72–12.93) | 0.003 |
| Model 1 + log hs-CRP | 1.00 (Reference) | 3.23 (1.27–8.21) | 4.19 (1.67–10.49) | 5.47 (2.14–14.00) | < 0.001 |
| Model 1 + log HOMA-IR | 1.00 (Reference) | 2.94 (1.15–7.47) | 3.88 (1.55–9.73) | 4.68 (1.82–12.04) | 0.0012 |
SI conversion factors: To convert mg/dL values to mmol/L, multiply serum sdLDL cholesterol values by 0.02586 and multiply serum triglycerides values by 0.01129.
Abbreviations: CI, confidence interval; hs-CRP, high-sensitivity C reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; SD, standard deviation; sdLDL, small dense low-density lipoprotein.
Model 1: Adjusted for age, sex, systolic blood pressure, use of antihypertensive agents, hemoglobin A1c, use of glucose-lowering agents, high density lipoprotein cholesterol, use of statins, body mass index, estimated glomerular filtration rate, electrocardiogram abnormalities, current smoking, current drinking, and regular exercise
Supplementary Table 2. Multivariable-adjusted hazard ratios for coronary heart disease according to serum small dense low-density lipoprotein cholesterol levels excluding subjects with use of lipid-modifying agents (n = 2,651), 2007–2015.
| Serum sdLDL cholesterol level, mg/dL |
|||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p for trend | |
| ≤ 24.4 | 24.5–32.8 | 32.9–43.6 | ≥ 43.7 | ||
| No. of events/subjects | 5/639 | 12/675 | 18/657 | 29/680 | |
| Age-and sex-adjusted incidence rate (per 1,000 person-years) | 1.01 | 2.38 | 4.38 | 5.51 | < 0.001 |
| Hazard ratio (95% CI) | |||||
| Age-and sex-adjusted | 1.00 (Reference) | 2.46 (0.87–7.01) | 3.92 (1.45–10.63) | 6.56 (2.49–17.28) | < 0.001 |
| Model 1 | 1.00 (Reference) | 2.46 (0.86–7.02) | 3.50 (1.27–9.66) | 5.25 (1.90–14.48) | < 0.001 |
| Model 1 + serum LDL cholesterol | 1.00 (Reference) | 1.97 (0.67–5.76) | 2.41 (0.81–7.11) | 3.06 (0.96–9.73) | 0.07 |
| Model 1 + serum triglycerides | 1.00 (Reference) | 2.45 (0.85–7.06) | 3.50 (1.24–9.86) | 5.27 (1.77–15.75) | 0.002 |
| Model 1 + log hs-CRP | 1.00 (Reference) | 2.52 (0.88–7.21) | 3.59 (1.30–9.91) | 5.51 (1.99–15.23) | < 0.001 |
| Model 1 + log HOMA-IR | 1.00 (Reference) | 2.29 (0.80–6.54) | 3.31 (1.20–9.13) | 4.74 (1.71–13.15) | 0.001 |
SI conversion factors: To convert mg/dL values to mmol/L, multiply serum sdLDL cholesterol values by 0.02586 and multiply serum triglycerides values by 0.01129.
Abbreviations: CI, confidence interval; hs-CRP, high-sensitivity C reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; SD, standard deviation; sdLDL, small dense low-density lipoprotein.
Model 1: Adjusted for age, sex, systolic blood pressure, use of antihypertensive agents, hemoglobin A1c, use of glucose-lowering agents, high density lipoprotein cholesterol, body mass index, estimated glomerular filtration rate, electrocardiogram abnormalities, current smoking, current drinking, and regular exercise
We also investigated the influence of serum sdLDL cholesterol levels on the risk of CHD according to serum LDL cholesterol levels. In this analysis, the subjects were divided into four groups according to sdLDL cholesterol levels (≥ 32.9 mg/dL [median] or < 32.9 mg/dL) and LDL cholesterol levels (≥ 120.1 mg/dL [median] or < 120.1 mg/dL). The multivariable-adjusted HR of CHD almost doubled in subjects with serum sdLDL cholesterol of ≥ 32.9 mg/dL, regardless of serum LDL cholesterol levels, as compared with subjects with serum sdLDL cholesterol of < 32.9 mg/dL and serum LDL cholesterol of < 120.1 mg/dL as a reference (Supplementary Fig. 1).
Supplementary Fig. 1.
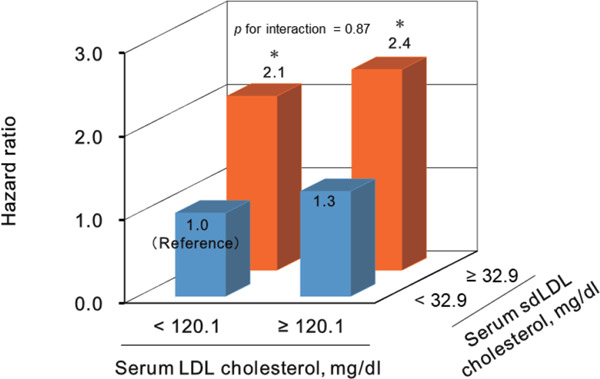
Multivariable-adjusted hazard ratios for the development of coronary heart disease according to serum low-density lipoprotein cholesterol and serum small dense low-density lipoprotein cholesterol
LDL, low-density lipoprotein; sdLDL, smalldense low-density lipoprotein.
*p < 0.05 vs. reference.
Hazard ratios were adjusted for age, sex, systolic blood pressure, antihypertensive drugs, hemoglobin A1c, antidiabetic medication, high density lipoprotein-cholesterol, lipid-lowering drugs, body mass index, estimated glomerular filtration rate, electrocardiogram abnormalities, current smoking, current drinking, and regular exercise.
In the subgroup analysis of various confounding factors, such as sex, hypertension, diabetes, BMI, serum LDL cholesterol, use of lipid-modifying agents, current smoking status, and eGFR, there was no evidence of heterogeneity in the magnitude of multivariable- adjusted HRs per 1 SD increment in log-transformed serum sdLDL cholesterol levels between the subgroups (all p for heterogeneity > 0.3; Supplementary Table 3).
Supplementary Table 3. Multivariable-adjusted hazard ratios for coronary heart disease per 1 SD increment in log-transformed small dense low-density lipoprotein-cholesterol levels in various subgroups of the study population, 2007–2015.
| Variables | Persons at risk | No. of events | HR (95% CI) per 1 SD increment in log (serum sdLDL cholesterol levels) | p for heterogeneity |
|---|---|---|---|---|
| Overall | 3,080 | 79 | 1.62 (1.23 to 2.16) | - |
| Sex | ||||
| Men | 1,290 | 49 | 1.48 (1.04 to 2.10) | 0.77 |
| Women | 1,790 | 30 | 1.93 (1.21 to 3.10) | |
| Hypertension | ||||
| No | 1,617 | 22 | 1.43 (0.86 to 2.39) | 0.76 |
| Yes | 1,463 | 57 | 1.63 (1.17 to 2.28) | |
| Diabetes | ||||
| No | 2,619 | 50 | 1.66 (1.17 to 2.34) | 0.61 |
| Yes | 461 | 29 | 1.57 (0.96 to 2.59) | |
| BMI | ||||
| < 25 kg/m2 | 2,294 | 53 | 1.67 (1.19 to 2.35) | 0.92 |
| ≥ 25 kg/m2 | 786 | 26 | 1.44 (0.89 to 2/34) | |
| Serum LDL cholesterol | ||||
| < 120.1 mg/dL | 1,538 | 33 | 2.01 (1.22 to 3.31) | 0.65 |
| ≥ 120.1 mg/dL | 1,542 | 46 | 1.45 (0.92 to 2.28) | |
| Use of lipid-modifying agents | ||||
| No | 2,651 | 64 | 1.66 (1.21 to 2.27) | 0.38 |
| Yes | 428 | 15 | 1.50 (0.78 to 2.90) | |
| Current smoking | ||||
| No | 2,465 | 58 | 1.67 (1.20 to 2.32) | 0.51 |
| Yes | 614 | 21 | 1.70 (0.97 to 2.96) | |
| eGFR | ||||
| ≥ 60 ml/min/1.73 m2 | 2,788 | 140 | 1.55 (1.15 to 2.09) | 0.70 |
| < 60 ml/min/1.73 m2 | 292 | 33 | 2.64 (1.13 to 6.18) |
SI conversion factors: To convert mg/dL values to mmol/L, multiply the serum LDL cholesterol value by 0.02586.
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LDL, low-density lipoprotein; SD, standard deviation; sdLDL, small dense low-density lipoprotein.
The model was adjusted for age, sex, systolic blood pressure, use of antihypertensive agents, hemoglobin A1c, use of glucose-lowering agents, serum high-density lipoprotein-cholesterol, use of lipid-modifying agents, body mass index, estimated glomerular filtration rate, electrocardiogram abnormalities, current smoking, current drinking, and regular exercise.
The variables relevant to the subgroup were excluded from the relevant Cox model.
In addition, we compared the c-statistics between the basic model including cardiovascular disease risk factors with and without sdLDL cholesterol to evaluate whether sdLDL cholesterol improves the accuracy of CHD risk assessment (Table 3). Adding the information on the serum sdLDL cholesterol level to the basic model including the aforementioned cardiovascular risk factors significantly increased the c-statistics (from 0.774 to 0.794; p = 0.02) and improved the accuracy of the risk assessment for CHD—namely, the continuous NRI was estimated as 0.40 (ZNRI 3.52, p < 0.001), and the IDI was estimated as 0.008 (ZIDI 2.40, p = 0.02). Meanwhile, adding the information on the serum LDL cholesterol level tended to increase the c-statistics, but the difference did not reach the level of statistical significance (from 0.774 to 0.793; p = 0.06) although the changes in continuous NRI and IDI were significant (NRI: 0.49, ZNRI 4.27, p < 0.001; IDI: 0.007, ZIDI 2.71, p = 0.01).
Table 3. Comparison of the accuracy of risk assessment for the development of coronary heart disease between the models adjusted for potential risk factors with and without small dense low-density lipoprotein cholesterol or low-density lipoprotein cholesterol.
| c-statistics (95% CI) | p value for difference in c-statistics | cNRI (95% CI) after adding sdLDL cholesterol or LDL cholesterol | p value for NRI | IDI (95% CI) after adding sdLDL cholesterol or LDL cholesterol | p value for IDI | |
|---|---|---|---|---|---|---|
| Basic model | 0.774 (0.730–0.817) |
reference | - | reference | - | reference |
| Basic model + log(serum sdLDL cholesterol) | 0.794 (0.754–0.835) |
0.02 | 0.40 (0.18–0.62) |
< 0.001 | 0.008 (0.001–0.01) |
0.02 |
| Basic model + serum LDL cholesterol | 0.793 (0.750–0.836) |
0.06 | 0.49 (0.27–0.70) |
< 0.001 | 0.007 (0.002–0.01) |
0.01 |
Abbreviations: CI, confidence interval; cNRI, continuous net reclassification improvement; IDI, integrated discrimination improvement; LDL, low-density lipoprotein; sdLDL, small dense low-density lipoprotein.
The basic model was adjusted for age, sex, systolic blood pressure, use of antihypertensive agents, hemoglobin A1c, use of glucose-lowering agents, high-density lipoprotein-cholesterol, lipid-modifying agents, body mass index, estimated glomerular filtration rate, electrocardiogram abnormalities, current smoking, current drinking, and regular exercise.
Finally, we investigated the cutoff value of sdLDL cholesterol that optimizes the discriminating ability for the risk of incident CHD. The point closest to (0, 1) on the receiver operating characteristic curve was 35 mg/dL (Supplementary Fig. 2). A total of 43.9% of subjects had a serum sdLDL cholesterol level above the cutoff value. Subjects with serum sdLDL cholesterol of ≥ 35 mg/dL were at a 2.09-fold (95% CI, 1.26–3.45) increased risk of the development of CHD after adjusting for the aforementioned confounding factors. In addition, the percentages of subjects with a serum sdLDL cholesterol level above the cutoff value, the sensitivities and specificities to detect subjects who developed CHD in 8 years, and the multivariableadjusted HRs of the serum sdLDL cholesterol level above the cutoff value compared with that below the cutoff value for the various cutoff levels of serum sdLDL cholesterol are shown in Supplementary Table 4.
Supplementary Fig. 2.
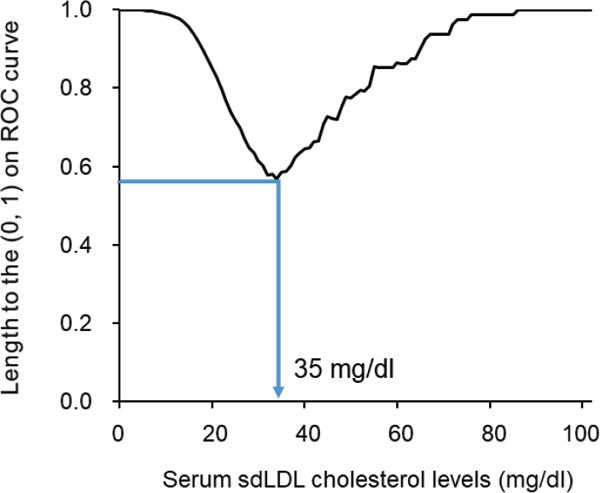
The cut-off value of serum small dense low-density lipoprotein cholesterol that optimizes the ability to discriminate the risk of coronary heart disease: the length to (0, 1) on the receiver operating characteristic curve
Abbreviations: ROC, receiver operating characteristic; sdLDL, small dense low-density lipoprotein
Length to the (0, 1) on ROC curve = 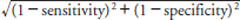
Supplementary Table 4. The sensitivity and specificity of detecting high-risk subjects who developed coronary heart disease during an 8-year follow-up for each cutoff value of serum small dense low-density lipoprotein cholesterol level.
| Cutoff value of serum sdLDL-C level (mg/dl) | Subjects with a serum sdLDL-C level above the cutoff value among 3,080 subjects |
Number of subjects with a serum sdLDL-C level above the cutoff value among 79 subjects who developed CHD during follo w-up | Sensitivity (%) | Specificity (%) | HR (95% CI) of a serum sdLDL-C level above the cutoff value vs. that below the cutoff valuea) | |
|---|---|---|---|---|---|---|
| Number | Frequency (%) | |||||
| 25 | 2,275 | 73.9 | 71 | 89.9 | 26.6 | 3.10 (1.45–6.63) |
| 30 | 1,806 | 58.6 | 59 | 74.7 | 41.8 | 1.98 (1.15–3.40) |
| 35 | 1,352 | 43.9 | 50 | 63.3 | 56.6 | 2.09 (1.26–3.45) |
| 40 | 1,018 | 33.1 | 36 | 45.6 | 67.3 | 1.52 (0.93–2.46) |
| 45 | 703 | 22.8 | 26 | 32.9 | 77.4 | 1.49 (0.89–2.49) |
| 50 | 513 | 16.7 | 19 | 24.1 | 83.5 | 1.50 (0.86–2.62) |
| 55 | 336 | 10.9 | 16 | 20.3 | 89.3 | 2.08 (1.14–3.78) |
| 60 | 242 | 7.9 | 12 | 15.2 | 92.3 | 2.04 (1.04–3.99) |
Abbreviations: CHD, coronary heart disease; sdLDL-C, small dense low-density lipoprotein-cholesterol.
SI conversion factors: To convert mg/dL values to mmol/L, multiply serum sdLDL cholesterol values by 0.02586.
Adjusted for age, sex, systolic blood pressure, use of antihypertensive agents, hemoglobin A1c, use of glucose-lowering agents, high density lipoprotein cholesterol, use of lipid-modifying agents, body mass index, estimated glomerular filtration rate, electrocardiogram abnormalities, current smoking, current drinking, and regular exercise.
Discussion
The present study demonstrated that higher serum sdLDL cholesterol levels were significantly associated with the development of CHD after adjustment for serum LDL cholesterol levels in addition to other potential cardiovascular risk factors. In addition, the ability to predict future CHD risk was significantly improved by adding the serum sdLDL cholesterol levels to the known cardiovascular risk factors. It has been well acknowledged that serum LDL cholesterol is an important therapeutic target to reduce the risk of CHD and is a good indicator for predicting CHD events19–21). Therefore, lipid-modifying therapy is widely accepted in clinical practice for the treatment of CHD32, 33). However, a relatively high percentage of individuals with serum LDL cholesterol in the normal range nonetheless go on to develop CHD10). Intriguingly, the present study found that the risk of CHD was significantly higher in subjects with higher serum sdLDL cholesterol level than those with lower serum sdLDL cholesterol level in subjects with serum LDL cholesterol levels below 120 mg/dL. These findings provide evidence that serum sdLDL cholesterol is a clinically valuable biomarker for estimating the future onset of CHD even in subjects with normal range serum LDL cholesterol and that serum sdLDL cholesterol is a possible therapeutic target for the prevention of CHD in general practice.
The recently developed assay for measuring serum sdLDL cholesterol would be readily adaptable to mass screening in general practice17, 18). Therefore, several prospective studies have assessed the association between sdLDL cholesterol and the risk of CHD. The Multi-Ethnic Study of Atherosclerosis (MESA) demonstrated a positive association between serum sdLDL cholesterol levels and the risk of CHD but found that higher serum sdLDL cholesterol was a significant risk factor for the development of CHD only in nondiabetic subjects20). In the present study, the risk of CHD was significantly increased in the group with high serum sdLDL cholesterol levels regardless of the status of diabetes. The Atherosclerosis Risk in Communities (ARIC) Study and the Suita Study also showed that subjects with higher serum sdLDL cholesterol levels were at a significantly increased risk of CHD compared with those with lower levels, but the significance of the association disappeared after additional adjustment for other lipid risk factors19, 21). In the present study, however, the association of serum sdLDL cholesterol with CHD was robust even after adjustment for known cardiovascular risk factors, including the serum LDL cholesterol level. Although the reasons for these discrepant findings are not entirely clear, they may be partly related to the different environmental and genetic backgrounds of the study populations (i.e., race, medical condition, and absolute coronary risk). The present study also demonstrated that high serum sdLDL cholesterol levels were significantly associated with the development of CHD in subjects with low and those with high LDL cholesterol levels. Similar findings were reported in the two aforementioned observational studies conducted in Western countries19, 20).
Some cross-sectional studies have shown that the size of serum LDL particles tended to be small in subjects with central obesity (i.e., higher visceral fat area) or insulin resistiance34, 35). Moreover, subjects with high serum sdLDL cholesterol levels had high serum hs-CRP levels in the present study. Therefore, the elevation in serum sdLDL cholesterol levels may reflect the insulin resistance and systemic inflammation, which could lead to progression of metabolic disorders and atherosclerosis in the coronary arteries. Meanwhile, the present study showed that the excess risk of CHD in subjects with high serum sdLDL cholesterol levels remained significant even after adjusting for serum hs-CRP levels or HOMA-IR in addition to known cardiovascular risk factors, including metabolic components. These findings raise the possibility that the other mechanisms of CHD development may exist. sdLDL particles have been considered to penetrate into the arterial wall easily because of their small size and to have a high affinity for proteoglycans in the arterial wall, but a low affinity for the hepatic LDL receptor, leading to prolonged residency in plasma36, 37). sdLDL particles also lack antioxidant substances, such as vitamin E, and thus are highly susceptible to oxidation38). These features of sdLDL particles would be a causal factor promoting atherosclerosis and thus the development of CHD.
In addition, we found that the cutoff value of sdLDL cholesterol that optimizes the ability to discriminate the risk of developing CHD was approximately 35 mg/dL when equal weight was given to sensitivity and specificity and ethical and cost constraints were ignored. On the other hand, approximately 40% of individuals in our community were assigned to the high-risk population for CHD by this cutoff level. The Food and Drug Administration cleared the assay with a serum sdLDL cholesterol cutoff level of 50 mg/dL for detecting subjects with a high risk of CHD based on the results from the MESA and ARIC Study, in which approximately 30% of US communitydwelling individuals had serum sdLDL cholesterol levels of ≥ 50 mg/dL, and the sensitivity and the specificity of this cutoff level to detect subjects who developed CHD were found to be 41% and 71%, respectively39). These findings are almost equivalent to those at the cutoff level of 40 mg/dL in the present study (Supplementary Table 4). Thus, it may be reasonable to set the cutoff of the high-risk population for CHD in a Japanese community at 35–40 mg/dL. On the other hand, the sensitivity and specificity of this cutoff value to detect subjects with incident CHD were not particularly high. Therefore, a combined risk assessment with sdLDL cholesterol and other cardiovascular risk factors may be necessary rather than the isolated use of sdLDL cholesterol to identify the high-risk population for CHD more effectively. The optimal cutoff level of serum sdLDL cholesterol should be validated in other population-based cohort studies, with due consideration given to the risk assessment method, ethical issues, cost-effectiveness, and racial differences.
The strengths of this study include its longitudinal population-based design, almost perfect follow-up of study subjects, and accurate diagnoses of CHD. However, some potential limitations of this study should be noted. First, the serum sdLDL cholesterol level and other risk factors were based on only one measurement at baseline. In addition, we were unable to obtain information about medical treatment during the follow-up period. Given that serum sdLDL cholesterol levels and other risk factors may have changed during follow-up, this limitation could lead to the misclassification of these variables, which would weaken the association found in the present study, biasing the results toward the null hypothesis. Second, the number of CHD events was insufficient for a more detailed analysis. Third, we measured serum sdLDL cholesterol levels using frozen serum samples collected as part of the survey in 2002 and stored at −80°C for seven years. However, it has been reported that there is no major difference in sdLDL cholesterol levels between fresh serum samples and stored ones17). Finally, the generalizability of our findings, including the cutoff value, may be limited, because these analyses were conducted in only one cohort of Japanese. Therefore, our findings should be validated in other cohorts of various ethnic populations.
Conclusion
The present study demonstrated that elevated sdLDL cholesterol was associated with the development of CHD regardless of LDL cholesterol levels. Moreover, the incorporation of sdLDL cholesterol values into a model with known risk factors improved the predictive ability of the risk of CHD in a general population. These findings suggest that the measurement of serum sdLDL cholesterol would be useful for assessing future risk of CHD even in patients with LDL cholesterol within a normal range. Therefore, it may be supposed that high serum sdLDL cholesterol levels should be lowered intensively to prevent the onset of CHD. However, further investigations are required to clarify whether sdLDL cholesterol would be a suitable interventional target for reducing the burden of CHD.
Acknowledgments
We would like to gratefully and sincerely thank Professor Yoshinao Oda, Professor Toru Iwaki, and their colleagues at the Department of Anatomic Pathology and Department of Neuropathology, Graduate School of Medical Sciences, Kyushu University, who provided expertise and insight into the autopsy findings that greatly assisted our research. The statistical analyses were carried out using the computer resources offered under the category of General Projects by the Research Institute for Information Technology, Kyushu University.
This study was supported in part by Grants-in-Aid for Scientific Research (A) (JP16H02692) and (B) (JP16H05850, JP17H04126, and JP18H02737) and (C) (JP17K09114, JP17K09113, JP17K01853, JP18K07565, JP18K09412, and JP19K07890) and (Early-Career Scientists) (JP18K17925, and JP18K17382) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by Health and Labour Sciences Research Grants of the Ministry of Health, Labour and Welfare of Japan (H29-Junkankitou-Ippan-003, and H30-Shokuhin-[Sitei]-005); and by the Japan Agency for Medical Research and Development (JP19dk0207025, JP19ek0210082, JP19ek0210083, JP19km0405202, JP19ek0210080, JP19fk0108075). In addition, this study was sponsored by DENKA SEIKEN Co., Ltd. (Tokyo, Japan). The sponsor of the study had no role in the study design, conduct of the study, data collection, data interpretation or preparation of the report.
Abbreviations
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- ECG
electrocardiogram
- eGFR
estimated glomerular filtration rate
- HbA1c
hemoglobin A1c
- HDL
high density lipoprotein
- HOMA-IR
homeostasis model assessment of insulin resistance
- HR
hazard ratio
- hs-CRP
high-sensitivity C-reactive protein
- IDI
integrated discrimination improvement
- LDL
low-density lipoprotein
- SD
standard deviation
- NRI
net reclassification improvement
- sdLDL
small dense low-density lipoprotein
Conflict of Interest
Toshiharu Ninomiya received research funding from DENKA SEIKEN Co., Ltd. The other authors declare that they have no conflicts of interest.
References
- 1). Finegold JA, Asaria P, Francis DP: Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol, 2013; 168: 934-945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). World Health Organization: Fact-sheets of cardiovascular disease. http://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) Accessed on July 16, 2019
- 3). Sampson UK, Fazio S, Linton MF: Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep, 2012; 14: 1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Banach M, Serban C, Sahebkar A, Mikhailidis DP, Ursoniu S, Ray KK, Rysz J, Toth PP, Muntner P, Mosteoru S, García-García HM, Hovingh GK, Kastelein JJ, Serruys PW, Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group : Impact of statin therapy on coronary plaque composition: a systematic review and metaanalysis of virtual histology intravascular ultrasound studies. BMC Med, 2015; 13: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB: Prediction of coronary heart disease using risk factor categories. Circulation, 1998; 97: 1837-1847 [DOI] [PubMed] [Google Scholar]
- 6). Imamura T, Doi Y, Arima H, Yonemoto K, Hata J, Kubo M, Tanizaki Y, Ibayashi S, Iida M, Kiyohara Y: LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population: the Hisayama study. Stroke, 2009; 40: 382-388 [DOI] [PubMed] [Google Scholar]
- 7). Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH: A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA, 1996; 276: 882-888 [PubMed] [Google Scholar]
- 8). Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists' (CTT) Collaborators : Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet, 2005; 366: 1267-1278 [DOI] [PubMed] [Google Scholar]
- 9). Law MR, Wald NJ, Rudnicka AR: Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and metaanalysis. BMJ, 2003; 326: 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Sachdeva A, Cannon CP, Deedwania PC, Labresh KA, Smith SC, Jr, Dai D, Hernandez A, Fonarow GC: Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J, 2009; 157: 111-117. e2 [DOI] [PubMed] [Google Scholar]
- 11). Krauss RM, Burke DJ: Identification of multiple classes of plasma low density lipoproteins in normal human subjects. J Lipid Res, 1982; 23: 97-104 [PubMed] [Google Scholar]
- 12). Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN: Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases. Oxid Med Cell Longev, 2017; 2017: 1273042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). de Graaf J, Hak-Lemmers HL, Hectors MP, Demacker PN, Hendriks JC, Stalenhoef AF: Enhanced susceptibility to in vitro oxidation of the dense low density lipoprotein subfraction in healthy subjects. Arterioscler Thromb, 1991; 11: 298-306 [DOI] [PubMed] [Google Scholar]
- 14). Chancharme L, Therond P, Nigon F, Lepage S, Couturier M, Chapman MJ: Cholesteryl ester hydroperoxide lability is a key feature of the oxidative susceptibility of small, dense LDL. Arterioscler Thromb Vasc Biol, 1999; 19: 810-820 [DOI] [PubMed] [Google Scholar]
- 15). Swinkels DW, Hak-Lemmers HL, Demacker PN: Single spin density gradient ultracentrifugation method for the detection and isolation of light and heavy low density lipoprotein subfractions. J Lipid Res, 1987; 28: 1233-1239 [PubMed] [Google Scholar]
- 16). Nichols AV, Krauss RM, Musliner TA: Nondenaturing polyacrylamide gradient gel electrophoresis. Methods Enzymol, 1986; 128: 417-431 [DOI] [PubMed] [Google Scholar]
- 17). Hirano T, Ito Y, Saegusa H, Yoshino G: A novel and simple method for quantification of small, dense LDL. J Lipid Res, 2003; 44: 2193-2201 [DOI] [PubMed] [Google Scholar]
- 18). Ito Y, Fujimura M, Ohta M, Hirano T: Development of a homogeneous assay for measurement of small dense LDL cholesterol. Clin Chem, 2011; 57: 57-65 [DOI] [PubMed] [Google Scholar]
- 19). Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, Ballantyne CM: Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol, 2014; 34: 1069-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Tsai MY, Steffen BT, Guan W, McClelland RL, Warnick R, McConnell J, Hoefner DM, Remaley AT: New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol, 2014; 34: 196-201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, Okamura T, Miyamato Y: Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita Study. J Atheroscler Thromb, 2013; 20: 195-203 [DOI] [PubMed] [Google Scholar]
- 22). Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, Gotoh S, Fukuhara M, Ikeda F, Shikata K, Yoshida D, Yonemoto K, Kamouchi M, Kitazono T, Kiyohara Y: Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the Hisayama Study (1961–2009). Circulation, 2013; 128: 1198-1205 [DOI] [PubMed] [Google Scholar]
- 23). Sakata S, Hata J, Fukuhara M, Yonemoto K, Mukai N, Yoshida D, Kishimoto H, Ohtsubo T, Kitazono T, Kiyohara Y, Ninomiya T: Morning and evening blood pressures are associated with intima-media thickness in a general population: the Hisayama Study. Circ J, 2017; 81: 1647-1653 [DOI] [PubMed] [Google Scholar]
- 24). Thygesen K, Alpert JS, White HD: Joint ESC/ACCF/AHA/WHF task force for the redefinition of myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J, 2007; 28: 2525-2538 [DOI] [PubMed] [Google Scholar]
- 25). Ninomiya T: Japanese Legacy Cohort Studies: The Hisayama Study. J Epidemiol, 2018; 28: 444-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H: Committee on the Standardization of Diabetes Mellitus - Related Laboratory Testing of Japan Diabetes Society. International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig, 2012; 3: 39-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 1985; 28: 412-419 [DOI] [PubMed] [Google Scholar]
- 28). Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S: Modification of the CKD epidemiology collaboration (CKDEPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis, 2010; 56: 32-38 [DOI] [PubMed] [Google Scholar]
- 29). Newson RB: Compariong the predictive power of survival models using Harrell's C or Somers' D. Stata J, 2010; 10: 339-358 [Google Scholar]
- 30). Pencina MJ1, D'Agostino RB, Sr, Steyerberg EW: Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med, 2011; 30: 11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Perkins NJ, Schisterman EF: The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol, 2006; 163: 670-675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Prospective Studies Collaboration. Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R: Blood cholesterol and vascular mortality by age, sex, and blood pressure: a metaanalysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet, 2007; 370: 1829-1839 [DOI] [PubMed] [Google Scholar]
- 33). Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D: Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol, 2008; 52: 1769-1781 [DOI] [PubMed] [Google Scholar]
- 34). Okazaki M, Usui S, Ishigami M, Sakai N, Nakamura T, Matsuzawa Y, Yamashita S: Identification of unique lipoprotein subclasses for visceral obesity by component analysis of cholesterol profile in high-performance liquid chromatography. Arterioscler Thromb Vasc Biol, 2005; 25: 578-584 [DOI] [PubMed] [Google Scholar]
- 35). Goff DC, Jr, D'Agostino RB, Jr, Haffner SM, Otvos JD: Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. Results from the Insulin Resistance Atherosclerosis Study. Metabolism, 2005; 54: 264-270 [DOI] [PubMed] [Google Scholar]
- 36). Galeano NF, Al-Haideri M, Keyserman F, Rumsey SC, Deckelbaum RJ: Small dense low density lipoprotein has increased affinity for LDL receptor-independent cell surface binding sites: a potential mechanism for increased atherogenicity. J Lipid Res, 1998; 39: 1263-1273 [PubMed] [Google Scholar]
- 37). Anber V, Griffin BA, McConnell M, Packard CJ, Shepherd J: Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Atherosclerosis, 1996; 124: 261-271 [DOI] [PubMed] [Google Scholar]
- 38). Tribble DL, Rizzo M, Chait A, Lewis DM, Blanche PJ, Krauss RM, Tribble DL: Enhanced oxidative susceptibility and reduced antioxidant content of metabolic precursors of small, dense low-density lipoproteins. Am J Med, 2001; 110: 103-110 [DOI] [PubMed] [Google Scholar]
- 39). Food and Drug Administration: 510(k) Substantial equivalence determination decision summary assay only template. https://www.accessdata.fda.gov/cdrh_docs/reviews/K161679.pdf Accessed on July 16, 2019


