Abstract
Aim: The purpose of this study is to investigate the impact of arterial stiffness assessed using Cardio-ankle Vascular Index (CAVI) on long-term outcome after acute coronary syndrome (ACS).
Methods: A total of 387 consecutive patients (324 males; age, 64 ± 11 years) with ACS were enrolled. We examined CAVI and brachial-ankle pulse wave velocity (ba PWV) as the parameters of arterial stiffness. The patients were divided into two groups according to the cut-off value of CAVI determined using the receiver operating characteristic curve for the prediction of major adverse cardiovascular events (MACE): low-CAVI group, 177 patients with CAVI < 8.35; high-CAVI group, 210 patients with CAVI ≥ 8.35. The primary endpoint was the incidence of MACE (cardiovascular death, recurrence of ACS, heart failure requiring hospitalization, or stroke).
Results: A total of 62 patients had MACE. Kaplan-Meier analysis demonstrated a significantly higher probability of MACE in the high-CAVI group than in the low-CAVI group (median follow-up: 62 months; log-rank, p < 0.001). Multivariate analysis suggested that CAVI was an independent predictor of MACE (hazard ratio [HR], 1.496; p = 0.02) and cardiovascular death (HR, 2.204; p = 0.025), but ba PWV was not. We investigated the incremental predictive value of adding CAVI to the GRACE score (GRS), a validated scoring system for risk assessment in ACS. Stratified by CAVI and GRS, a significantly higher rate of MACE was seen in patients with both higher CAVI and higher GRS than the other groups (p < 0.001). Furthermore, the addition of CAVI to GRS enhanced net reclassification improvement (NRI) and integrated discrimination improvement (IDI) (NRI, 0.337, p = 0.034; and IDI, 0.028, p = 0.004).
Conclusion: CAVI was an independent long-term predictor of MACE, especially cardiovascular death, adding incremental clinical significance for risk stratification in patients with ACS.
Keywords: Arterial stiffness, Cardio-ankle vascular index, Prognosis, GRACE risk score, Acute coronary syndrome
See editorial vol. 27: 639–640
Introduction
Acute coronary syndrome (ACS) is one of the leading causes of morbidity and mortality in most developed countries even in the era of optimal reperfusion therapy1, 2). Therefore, proper risk stratification is crucial for the assessment of long-term prognosis after ACS. Recent studies showed that arterial stiffness has attracted attention as a potentially useful parameter for risk stratification of patients with ACS3, 4). Cardio-ankle vascular stiffness index (CAVI) is a noninvasive measure of arterial stiffness that is not influenced by blood pressure at the time of examination5, 6). We previously reported that high CAVI was an independent predictor of cardiovascular (CV) events and nonfatal ischemic stroke in patients with ACS3). However, because of relatively short follow-up duration (15 months), we could not evaluate the effect of CAVI on long-term outcomes and CV mortality.
The Global Registry of Acute Coronary Events risk score (GRS) is an established and validated risk scoring system recommended by current guidelines7, 8). Although the scoring system has a good predictive value for adverse CV disease events in ACS, it does not include novel risk factors such as arterial stiffness.
Aim
In this study, we explored the effect of arterial stiffness assessed using CAVI on CV events in patients with ACS during a long-term period. Furthermore, we investigated whether CAVI could improve the prognostic performance of the GRS.
Methods
Study Population
We studied 447 patients with ACS who underwent early coronary angiography in Yokohama City University Medical Center between April 2012 and February 2016. ACS was defined as ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, and unstable angina pectoris according to the current guideline7). The presence of hypertension was defined as blood pressure > 140/90 mm Hg9) or treatment with oral antihypertensive drugs. Dyslipidemia was defined as plasma levels of fasting triglycerides ≥ 150 mg/dL and/or fasting total cholesterol ≥ 200 mg/dL and/or low-density lipoprotein cholesterol ≥ 130 mg/dL10). Diabetes mellitus was determined using the following criteria: medical history, fasting glucose value of ≥ 126 mg/dL, casual plasma glucose value of ≥ 200 mg/dL, or diabetic pattern based on 75-g oral glucose tolerance tests. We excluded patients with any of the following characteristics: (1) persistent atrial fibrillation (n = 19), (2) severe aortic insufficiency (n = 4), (3) peripheral arterial disease (Ankle Brachial index ≤ 0.9 or previous history of lower extremity bypass and/or endovascular therapy) (n = 25), (4) CAVI data not available (n = 3), or (5) informed consent was not given (n = 6). A total of 390 patients with ACS met the eligibility criteria and were enrolled. Three patients were lost to followup, so we analyzed the cases of 387 patients in the current study (Fig. 1). All patients underwent calculation of GRS at admission8), and a high GRS was defined as > 140 based on previous reports7). The study protocol was approved by the Yokohama City University Medical Center Institutional Review Board, and all patients provided written informed consent (UMIN-CTR ID: UMIN000016651).
Fig. 1.
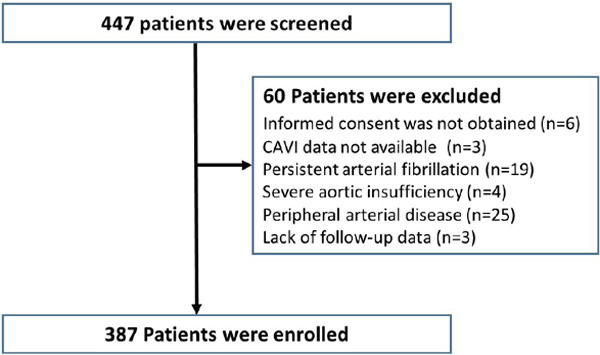
Study protocol flow chart
CAVI indicates cardio-ankle vascular index.
Two-Dimensional Echocardiography
A standardized complete echocardiographic examination was performed at the same phase as CAVI measurement by experienced sonographers blinded to the clinical data and CAVI following a standard protocol11). All images were obtained using a commercial ultrasound system (Vivid q or Vivid E9, GE Healthcare). The left ventricular ejection fraction was calculated using the biplane modified Simpson method. The peak early diastolic velocity (E) was imaged at the tip of the mitral leaflets from the apical four-chamber view. Tissue Doppler imaging was applied to the apical four-chamber view to determine peak early (e′) velocities at the septal mitral valve annulus. E/e′ (septal) was calculated as an index of left ventricular diastolic function.
Measurement of Arterial Stiffness (CAVI and Brachial-Ankle Pulse Wave Velocity)
CAVI was measured automatically using VaSera VS-1000 (Fukuda Denshi, Tokyo, Japan). Cuffs were placed bilaterally on the upper arms and ankles while the subjects were lying supine and their heads held in midline position. Electrocardiography electrodes were placed on both wrists, and a microphone was placed to detect heart sounds over the sternum.
CAVI was determined using the following equation:
where a and b are constants, ρ is the blood density, ΔP is Ps − Pd, Ps is the systolic blood pressure, Pd is the diastolic blood pressure, and PWV is the pulse wave velocity.
The actual measurement method of CAVI has been previously reported5). In the current study, CAVI was measured more than 1 week after admission in a stable condition. The average of the right and left CAVI values was adopted for analyses.
The brachial-ankle pulse wave velocity (ba PWV) was measured using a volume-plethysmography device (BP-203RPEII, OMRON COLIN Co., Ltd., Tokyo, Japan). The details of the measurements and the reproducibility of this automatic method have been previously described12). The average of the right and left ba PWV values was used for analyses.
Follow-Up
Major adverse outcomes were evaluated for a median follow-up of 62 months (interquartile range [IQR], 60 to 64 months). The primary endpoint was the occurrence of major adverse CV events (MACE), defined as CV death, recurrence of ACS, heart failure (HF) requiring hospitalization, or stroke. CV death was defined as death from acute myocardial infarction, HF, stroke, or documented sudden death without apparent cardiovascular cause13). Stroke was defined as documented focal neurologic deficit and clinically relevant brain imaging of infarction or hemorrhage. The most severe endpoint was selected for primary endpoint analysis if > 1 MACE endpoint occurred during follow-up. We defined CV death for the most severe endpoint, HF requiring hospitalization for the second, stroke for the third and recurrence of ACS was the mildest. All events were followed by hospital visits or telephone interviews with experienced cardiovascular physicians who were not informed of the clinical details and results.
Statistical Analysis
Statistical analyses were performed using JMP pro 12 (SAS Institute, Inc., Cary, NC) and EZR, which is a graphical user interface for R (version 3.1.2, The R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were expressed as mean ± standard deviation for normally distributed variables and as median (IQR) for skewed distributed variables. Differences between the two groups were tested using the t-test for normally distributed variables, Mann–Whitney U test for skewed distributed variables, and chi-square test or Fisher exact test as appropriate for categorical variables. Receiver operating characteristic (ROC) curves were analyzed to assess the cut-off value of CAVI for the prediction of MACE. The optimal cut-off value was defined as the value that gives the maximal sum of sensitivity and specificity. The area under the curve (AUC) of CAVI was 0.663, sensitivity = 0.790, and 1-specificity = 0.495. Accordingly, we divided the patients into two groups by CAVI = 8.35: low-CAVI group, 177 patients with CAVI < 8.35; high-CAVI group, 210 patients with CAVI ≥ 8.35. We used Cox proportional-hazards models to estimate hazard ratios (HR) for endpoints and 95% confidence intervals (CI) using univariate and multivariate models. Coronary risk factors (age, male sex, hypertension, body mass index (BMI), current smoker, dyslipidemia, diabetes mellitus, and estimated glomerular filtration rate (eGFR)), GRS, ba PWV, and CAVI were entered in the univariate analysis. Baseline variables that were considered clinically relevant were entered into the multivariate analysis (forced inclusion model) together with CAVI (model 1: age, male sex, current smoker, hypertension, dyslipidemia, diabetes mellitus, BMI, eGFR, ba PWV and CAVI; model 2: GRS and CAVI). GRS was not included in multivariate analysis model 1 because of its high covariance with the coronary risk factors. The cumulative incidence of a clinical event was assessed using the Kaplan–Meier method and compared using the log-rank test. We evaluated whether the combination of CAVI and GRS gives a better predictive accuracy for MACE compared with each parameter alone. In addition, the incremental effect of adding CAVI to GRS to predict future deaths and CV events was evaluated using the net reclassification index (NRI) as previously described14). As a sensitivity analysis, we used a logistic regression model on MACE using the combination of CAVI and GRS as a predictor variable. The cumulative incidence for all analyses (p < 0.05) was considered statistically significant.
Results
Baseline Characteristics
The baseline characteristics of all patients are listed in Table 1. Patients in the high-CAVI group were older and had smaller BMI than those in the low-CAVI group. There was no significant difference in systolic blood pressure at admission between the two groups. However, diastolic blood pressure at admission was slightly high in the low-CAVI group. Hypertension was more prevalent in patients in the high-CAVI group than those in the low-CAVI group. Current smokers were less frequently observed in the low-CAVI group, and lipid profile was better in patients with high CAVI than in those with low CAVI. Patients in the high-CAVI group had lower eGFR at admission and lower left ventricular (LV) diastolic function evaluated by E/e′ than those in the low-CAVI group.
Table 1. Baseline Clinical and Angiographic Characteristics of Patients with Acute Coronary Syndrome Who Underwent Cardio-Ankle Vascular Stiffness Index Measurement.
| Low CAVI group | High CAVI group | p value | |
|---|---|---|---|
| (n = 177) | (n = 210) | ||
| Age, years | 57 ± 10 | 71 ± 8 | < 0.001 |
| Male, n (%) | 155 (87) | 168 (80) | 0.054 |
| BMI, kg/m2 | 25.8 ± 3.9 | 23.6 ± 3.2 | < 0.001 |
| Current smoker, n (%) | 98 (55) | 75 (35) | < 0.001 |
| Hypertension, n (%) | 90 (50) | 136 (65) | 0.005 |
| Systolic BP at admission, mmHg | 146 ± 34 | 148 ± 34 | 0.641 |
| Diastolic BP at admission, mmHg | 90 ± 24 | 85 ± 22 | 0.023 |
| Dyslipidemia, n (%) | 86 (48) | 77 (36) | 0.022 |
| LDL cholesterol, mg/dl | 134 ± 38 | 122 ± 34 | < 0.001 |
| HDL cholesterol, mg/dl | 44 ± 11 | 46 ± 11 | 0.013 |
| Triglycerides, mg/dl | 168 (151–186) | 137 (114–161) | 0.032 |
| Diabetes mellitus, n (%) | 63 (35) | 94 (44) | 0.077 |
| HbA1c, % | 6.2 ± 1.3 | 6.1 ± 1.0 | 0.479 |
| History of cerebral infarction, % | 2 (1) | 10 (4) | 0.043 |
| History of myocardial infarction, % | 15 (8) | 21 (10) | 0.606 |
| Clinical presentation | 0.993 | ||
| Unstable angina pectoris, n (%) | 13 (7) | 15 (7) | |
| ST-segment elevation MI, n (%) | 132 (74) | 153 (72) | |
| Non-ST-segment elevation MI, n (%) | 32 (18) | 41 (19) | |
| Culprit artery, n (%) | 0.321 | ||
| Left anterior descending coronary artery | 86 (48) | 119 (56) | |
| Right coronary artery | 65 (36) | 66 (31) | |
| Left circumflex coronary artery | 22 (12) | 18 (8) | |
| No. of diseased vessels, n (%) | 0.550 | ||
| 1 | 136 (60) | 85 (55) | |
| 2 | 58 (25) | 47 (30) | |
| 3 | 30 (13) | 20 (13) | |
| In-hospital procedure, n (%) | |||
| In-hospital PCI, % | 167 (94) | 189 (90) | 0.116 |
| In-hospital CABG, % | 8 (4) | 10 (4) | 0.902 |
| hs-CRP at admission, mg/dl | 0.521 (0.303–0.739) | 0.593 (0.340–0.845) | 0.679 |
| eGFR at admission, ml/min/1.73 m2 | 75.9 ± 19.0 | 67.6 ± 18.3 | < 0.001 |
| peak CK-MB, IU/l | 175 (144–206) | 181 (154–208) | 0.755 |
| LVEF, % | 52.3 ± 10.5 | 52.1 ± 11.6 | 0.841 |
| E/e' | 10.1 ± 3.3 | 12.2 ± 3.8 | < 0.001 |
| Medications on discharge, n (%) | |||
| Aspirin | 172 (97) | 200 (95) | 0.192 |
| Thienopyridine | 155 (88) | 181 (86) | 0.584 |
| β-blocker | 106 (59) | 133 (63) | 0.483 |
| ACEI or ARB | 134 (75) | 162 (77) | 0.739 |
| Statin | 165 (93) | 194 (92) | 0.750 |
Values are the mean ± standard deviation (SD), n (%), or median (interquartile range) as appropriate. ACEI, angiotensin-converting enzyme-inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass grafting; CAVI, cardio-ankle vascular stiffness index; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LVEF, left ventricular ejection fraction; LDL, low-density lipoprotein; MI, myocardial infarction; PCI, percutaneous intervention.
ROC Analysis
ROC curves were constructed for the prediction of MACE and CV death. ROC analysis for MACE demonstrated that CAVI (AUC 0.663: 95% CI, 0.591–0.729, p < 0.001) was a significant predictor of MACE (Fig. 2A). ROC analysis for CV death demonstrated that CAVI (AUC 0.831: 95% CI, 0.715–0.906, p < 0.001) was a significant predictor of CV death (Fig. 2B).
Fig. 2.
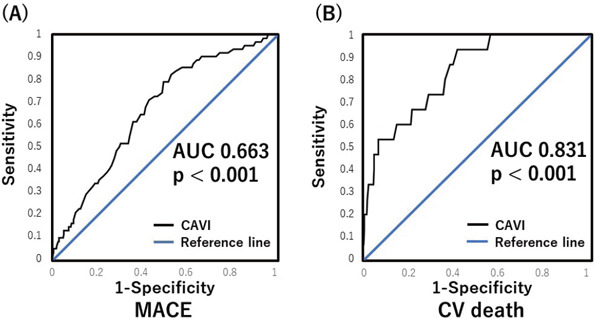
Receiver-operating characteristic (ROC) curves for MACE and cardiovascular (CV) death
(A) ROC analysis for MACE demonstrated that CAVI (AUC 0.663. p < 0.001) was significant predictors of MACE. (B) ROC analysis for CV death demonstrated that CAVI (AUC 0.831. p < 0.001) was significant predictors of CV death. AUC, area under the curve; CAVI, cardio-ankle vascular stiffness index; MACE, major adverse cardiovascular events; CV, cardiovascular.
Follow-Up and Major Adverse Cardiovascular Events
During the median follow-up of 62 months, 62 patients (16%) experienced MACE: 15 (3.8%) had cardiovascular death, 24 (6.3%) had recurrence of ACS, 10 (2.5%) had heart failure, and 13 (3.3%) had nonfatal stroke. MACE occurred less frequently in the low-CAVI group than in the high-CAVI group (7.3% vs 23.3%, p < 0.001). The average CAVI values were 9.0 ± 1.1 in the group with MACE and 8.4 ± 1.2 in the group without MACE. CAVI was significantly higher in the MACE group than in the without-MACE group (p < 0.001).
Kaplan–Meier Estimate for Event-Free Rate
Kaplan–Meier curves for patients with high and low CAVI values are shown in Fig. 3. MACE more frequently occurred in patients with high CAVI than in those with low CAVI (log-rank, p < 0.001) (Fig. 3A). CV death also occurred more often in patients with high CAVI than in those with low CAVI (log-rank, p < 0.001) (Fig. 3B).
Fig. 3.
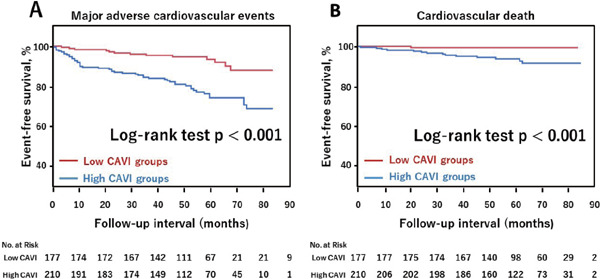
Kaplan-Meier estimates for major adverse events according to CAVI
Kaplan-Meier estimates are shown for the rate of major adverse cardiovascular events (CV death, recurrence of ACS, heart failure requiring hospitalization or stroke) (A) and CV death (a death from acute myocardial infarction, heart failure, stroke, or documented sudden death without apparent non-cardiovascular cause) (B) according to CAVI. CAVI, cardio-ankle vascular index; ACS, acute coronary syndrome.
By multiple adjusted Cox proportional-hazards analysis with coronary risk factors and CAVI and ba PWV, CAVI could predict MACE (HR, 1.494; 95% CI, 1.067–2.120; p = 0.021, Model 1 in Table 2) and CV death (HR, 2.173; 95% CI, 1.126–4.500; p = 0.027, Model 1 in Table 3). When adjusted for GRS, CAVI was also found to be an independent predictor of MACE (HR, 1.399; 95% CI, 1.093–1.802; p = 0.008, Model 2 in Table 2) and CV death (HR, 2.899; 95% CI, 1.755–5.109; p < 0.001, Model 2 in Table 3). In multivariate analysis, although CAVI and ba PWV were adopted as variables, both of them are index of arterial stiffness and may become a confounding factor. Therefore, we additionally performed a multivariate analysis with either ba PWV or CAVI separately. When we performed multivariate analysis of MACE without ba PWV, CAVI was a significant predictor of MACE after adjustment for the traditional coronary risk factors (HR, 1.549; 95% CI, 1.149–2.109; p = 0.004, Model 1 in Supplementary Table 1). Although there was a similar trend for ba PWV, it did not reach statistical significance (HR, 1.000; 95% CI, 0.990–1.001; p = 0.082, Model 2 in Supplementary Table 1). When adjusted for GRACE risk score, ba PWV was also not an independent predictor of MACE (HR, 1.000; 95% CI, 0.999–1.001; p = 0.051, Model 3 in Supplementary Table 1). In multivariate analysis of CV death without ba PWV, CAVI was an independent predictor of CV death after adjustment for coronary risk factors (HR, 2.309; 95% CI, 1.273–4.487; p = 0.008, Model 1 in Supplementary Table 2). However, ba PWV was not a significant predictor of CV death in multivariate analysis without CAVI (HR, 1.001; 95% CI, 0.999–1.003; p = 0.109, Mode 2 in Supplementary Table 2). When adjusted for GRACE risk score, ba PWV was an independent predictor of CV death (HR, 1.002; 95% CI, 1.000–1.003; p < 0.001, Model 3 in Supplementary Table 2).
Table 2. Univariate and multivariate logistic regression analysis-estimate hazard ratio for major adverse cardiovascular events.
| Variable | Univariate |
Multivariate (Model 1) |
Multivariate (Model 2) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Age, per 1 year | 1.031 | 0.854–1.006 | 0.014 | 0.989 | 0.953–1.026 | 0.569 | |||
| Male | 1.036 | 0.513–2.276 | 0.924 | 1.194 | 0.550–2.797 | 0.666 | |||
| Current smoker | 0.848 | 0.543–1.631 | 0.829 | 1.227 | 0.660–2.281 | 0.514 | |||
| Hypertension | 0.909 | 0.526–1.584 | 0.734 | 0.721 | 0.391–1.329 | 0.293 | |||
| Dyslipidemia | 0.845 | 0.479–1.465 | 0.533 | 1.015 | 0.557–1.833 | 0.958 | |||
| Diabetes mellitus | 1.069 | 0.611–1.849 | 0.811 | 1.015 | 0.562–1.811 | 0.957 | |||
| BMI, per 1 kg/m2 | 0.879 | 0.806–0.953 | 0.001 | 0.900 | 0.817–0.988 | 0.030 | |||
| eGFR, per 1 mL/min per 1.73 m2 | 0.986 | 0.971–1.001 | 0.073 | 0.988 | 0.971–1.005 | 0.198 | |||
| ba PWV | 1.001 | 1.000–1.001 | 0.004 | 1.000 | 0.999–1.001 | 0.658 | |||
| GRACE risk score | 1.015 | 1.008–1.023 | < 0.001 | 1.012 | 1.004–1.020 | 0.002 | |||
| mean CAVI | 1.595 | 1.271–2.022 | < 0.001 | 1.494 | 1.067–2.120 | 0.021 | 1.399 | 1.093–1.802 | 0.008 |
ba PWV, brachial-ankle pulse wave velocity; BMI, body mass index; eGFR, estimated glomerular filtration rate; GRACE, global registry of acute coronary events; HR, hazard ratio; 95% CI, 95% confidence interval.
Table 3. Univariate and multivariate logistic regression analysis-estimate hazard ratio for cardiovascular death.
| Variable | Univariate |
Multivariate (Model 1) |
Multivariate (Model 2) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Age, per 1 year | 1.145 | 1.073–1.240 | < 0.001 | 1.076 | 0.990–1.181 | 0.097 | |||
| Male | 0.784 | 0.240–3.516 | 0.713 | 1.413 | 0.352–7.696 | 0.651 | |||
| Current smoker | 0.607 | 0.186–1.743 | 0.370 | 1.564 | 0.406–5.701 | 0.498 | |||
| Hypertension | 1.444 | 0.502–4.712 | 0.509 | 0.844 | 0.235–3.310 | 0.798 | |||
| Dyslipidemia | 0.487 | 0.133–1.453 | 0.225 | 0.689 | 0.167–2.375 | 0.572 | |||
| Diabetes mellitus | 1.710 | 0.601–4.973 | 0.309 | 1.522 | 0.467–4.980 | 0.479 | |||
| BMI, per 1 kg/m2 | 0.795 | 0.669–0.932 | 0.006 | 0.909 | 0.748–1.098 | 0.331 | |||
| eGFR, per 1 mL/min per 1.73 m2 | 0.968 | 0.940–0.996 | 0.027 | 0.991 | 0.955–1.025 | 0.617 | |||
| ba PWV | 1.002 | 1.001–1.003 | < 0.001 | 1.000 | 0.998–1.002 | 0.687 | |||
| GRACE risk score | 1.020 | 1.008–1.032 | < 0.001 | 1.011 | 0.998–1.025 | 0.086 | |||
| mean CAVI | 3.276 | 2.032–5.680 | < 0.001 | 2.173 | 1.126–4.500 | 0.027 | 2.899 | 1.755–5.109 | < 0.001 |
ba PWV, brachial-ankle pulse wave velocity; BMI, body mass index; eGFR, estimated glomerular filtration rate; GRACE, global registry of acute coronary events; HR, hazard ratio; 95% CI, 95% confidence interval.
Supplementary Table 1. Univariate and multivariate logistic regression analysis-estimate hazard ratio for major adverse cardiovascular events.
| Variable | Multivariate (Model 1) |
Multivariate (Model 2) |
Multivariate (Model 3) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Age, per 1 year | 0.990 | 0.955–1.027 | 0.618 | 1.004 | 0.971–1.039 | 0.796 | |||
| Male | 1.172 | 0.543–2.730 | 0.697 | 1.318 | 0.617–3.051 | 0.493 | |||
| Current smoker | 1.209 | 0.653–2.235 | 0.542 | 1.248 | 0.674–2.311 | 0.477 | |||
| Hypertension | 0.738 | 0.405–1.349 | 0.322 | 0.748 | 0.408–1.371 | 0.346 | |||
| Dyslipidemia | 1.015 | 0.557–1.832 | 0.958 | 1.009 | 0.555–1.816 | 0.974 | |||
| Diabetes mellitus | 1.014 | 0.562–1.807 | 0.962 | 1.120 | 0.628–1.979 | 0.695 | |||
| BMI, per 1 kg/m2 | 0.900 | 0.817–0.988 | 0.030 | 0.890 | 0.809–0.975 | 0.001 | |||
| eGFR, per 1 mL/min per 1.73 m2 | 0.988 | 0.971–1.005 | 0.179 | 0.988 | 0.971–1.005 | 0.188 | |||
| GRACE risk score | 1.014 | 1.007–1.022 | < 0.001 | ||||||
| ba PWV | 1.000 | 0.999–1.001 | 0.082 | 1.000 | 0.999–1.001 | 0.051 | |||
| mean CAVI | 1.549 | 1.149–2.109 | 0.004 | ||||||
Model 1 age, male, current smoker, hypertension, dyslipidemia, diabetes mellitus, BMI, eGFR and CAVI, Model 2 age, male, current smoker, hypertension, dyslipidemia, diabetes mellitus, BMI, eGFR and ba PWV, Model 3 GRACE risk score and ba PWV. GRACE, global registry of acute coronary events; HR, hazard ratio; 95% CI, 95% confidence interval; Other abbreviations as in Table 1
Supplementary Table 2. Univariate and multivariate logistic regression analysis-estimate hazard ratio for cardiovascular death.
| Variable | Multivariate (Model 1) |
Multivariate (Model 2) |
Multivariate (Model 3) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Age, per 1 year | 1.079 | 0.994–1.183 | 0.083 | 1.105 | 1.020–1.210 | 0.020 | |||
| Male | 1.381 | 0.347–7.423 | 0.670 | 1.534 | 0.403–7.826 | 0.560 | |||
| Current smoker | 1.524 | 0.398–5.485 | 0.520 | 1.559 | 0.411–5.613 | 0.496 | |||
| Hypertension | 0.884 | 0.249–3.410 | 0.851 | 0.880 | 0.262–3.204 | 0.839 | |||
| Dyslipidemia | 0.678 | 0.165–2.323 | 0.555 | 0.741 | 0.186–2.504 | 0.643 | |||
| Diabetes mellitus | 1.531 | 0.473–4.984 | 0.470 | 2.003 | 0.646–6.387 | 0.226 | |||
| BMI, per 1 kg/m2 | 0.907 | 0.748–1.094 | 0.314 | 0.875 | 0.730–1.044 | 0.141 | |||
| eGFR, per 1 mL/min per 1.73 m2 | 0.989 | 0.955–1.023 | 0.559 | 0.989 | 0.955–1.022 | 0.534 | |||
| GRACE risk score | 1.017 | 1.004–1.031 | 0.006 | ||||||
| ba PWV | 1.001 | 0.999–1.003 | 0.109 | 1.002 | 1.000–1.003 | < 0.001 | |||
| mean CAVI | 2.309 | 1.273–4.487 | 0.008 | ||||||
BMI, body mass index; eGFR, estimated glomerular filtration rate; ba PWV, brachial-ankle pulse wave velocity; GRACE, global registry of acute coronary events; HR, hazard ratio; 95% CI, 95% confidence interval.
CAVI and GRACE Risk Score
We evaluated the incremental effect of adding CAVI to the GRS for predicting MACE. When we divided all patients into four groups according to the cut-offs for CAVI and GRS > 140, significantly higher MACE rates were seen in patients with both higher CAVI and higher GRS (n = 35, 26.1% of all patients) than the other groups (p < 000.1) (Fig. 4). Furthermore, the inclusion of CAVI into the GRS model was associated with an NRI of 33.7% (p = 0.034), suggesting an effective reclassification. The integrated discrimination improvement (IDI) again showed that the diagnostic performance of the model was significantly improved by adding CAVI to the GRS (IDI: 0.028, p = 0.004). For the sensitivity analysis, CAVI, comparing to GRS, was more strongly and gradually associated with MACE, in which the odds ratios (95% CIs) of higher GRS and lower CAVI, lower GRS and higher CAVI, and higher GRS and higher CAVI against lower GRS and lower CAVI were 1.238 (0.359–3.891), 3.076 (1.246–8.088), and 4.816 (2.232–11.621), respectively.
Fig. 4.
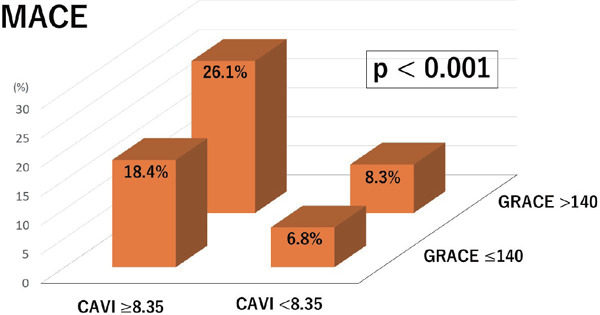
Relation of high or low CAVI and GRACE risk score
When we divided all patients into four groups according to the cut-offs for CAVI and GRACE risk score > 140, significantly higher MACE rates were seen in patients with both higher CAVI and higher GRACE risk score (n = 35, 26.1% of all patients) than the other groups (p < 000.1). CAVI, cardio-ankle vascular index; GRACE, global registry of acute coronary events; MACE, major adverse cardiovascular events.
Discussion
The main finding of the current study was that arterial stiffness assessed using CAVI was a significant and independent predictor of long-term incidence of MACE, especially CV death in patients with ACS. Furthermore, the results demonstrated that the prognostic performance of CAVI was independent of the GRS and the addition of CAVI to the GRS improved risk stratification in ACS patients. To the best of our knowledge, this is the first study to reveal the role of arterial stiffness evaluated using CAVI in long-term incidence of MACE and CV death in patients with ACS.
The Role of CAVI in the Prognosis
Previously, some studies reported that arterial stiffness was a significant predictor of adverse events in patients with coronary artery disease3, 4, 15, 16). However, the prognostic role of this parameter in patients with ACS has not been fully understood. In the current study, we demonstrated the independent predictive value of CAVI after adjustment for classic CV risk factors. In addition, the combined use of CAVI and GRS improved risk stratification in patients with ACS. These results suggested that CAVI has an adjunctive value in the assessment of traditional CV risk factors. This may be because CAVI reflects damage in the arterial wall over a long period. By contrast, blood pressure, glycemia, and lipids cannot reflect the genuine damage on the arterial wall because their values may fluctuate depending on the timing15). Therefore, we think that CAVI can serve as an additional prognostic tool to stratify patients with ACS at risk of the development of events, beyond the assessment of traditional atherosclerotic risk factors.
Organ damage caused by increased arterial stiffness may worsen the prognosis of patients with ACS. Previous studies showed that increased arterial stiffness determined using CAVI was associated with atherosclerotic diseases such as cerebral infarctions and chronic kidney disease (CKD)3, 17). In the current study, more patients with a high CAVI had a history of cerebral infarctions and renal insufficiency as compared with patients with a low CAVI. Previous studies revealed that cerebral infarctions and CKD were predictors of CV events in ACS8, 18). Hence, in the current study, atherosclerotic organ damage induced by increased arterial stiffness may adversely affect the prognosis of patients with ACS.
In addition, LV diastolic dysfunction induced by increased arterial stiffness may worsen the prognosis of patients with ACS. Previous studies reported that an increased LV afterload caused by elevated arterial stiffness induced higher LV filling pressure and diastolic dysfunction19). A number of studies demonstrated that diastolic dysfunction was associated with a poor prognosis in patients with ACS20, 21). In our study, E/e′, which was previously shown to be an indicator of LV diastolic dysfunction22), was significantly elevated in the high-CAVI group as compared to that in the low-CAVI group.
Comparison with Previous Studies
Previous studies investigated the effect of arterial stiffness on CV outcomes in patients with ACS. Tomiyama et al. reported that ba PWV was an independent predictor of the prognosis of patients with ACS23). In our study, there was a significant relationship between ba PWV and major adverse events in the univariate analysis but not in the multivariate analysis that included CAVI. In addition, when we performed multivariate analysis of major adverse events without ba PWV, CAVI was a significant predictor of MACE after adjustment for the established coronary risk factors. Although there was a similar trend for ba PWV, it did not reach statistical significance. These findings may be related to some as-yet undetermined effects on blood pressure at the time of the examination or the superiority of CAVI over ba PWV as a method of measuring arterial stiffness24).
Hans-Josef et al. reported that aortic PWV measured by cardiac magnetic resonance imaging (MRI) was an independent predictor of MACE comprising all-cause death, nonfatal myocardial reinfarctions, new congestive heart failure, and strokes4). Another study found that phase-contrast cardiac MRI could provide an accurate and reproducible method for the determination of PWV25). However, aortic stiffness measured by cardiac MRI requires more time and complex calculations. In addition, it can be performed in specific situations. Therefore, cardiac MRI is not suitable for all types of acutely hospitalized patients with ACS. However, CAVI can easily be measured in the clinical setting. Thus, we think that CAVI is a more useful predictive tool than cardiac MRI for all types of ACS.
As noted earlier, previous studies revealed the significance of arterial stiffness in the prognosis of patients with ACS. However, no previous studies examined the association between arterial stiffness and CV mortality in patients with ACS. Moreover, other previous studies were based on smaller study populations and shorter follow-up duration than ours. Furthermore, critically ill patients, such as those with HF or CKD, were excluded in the previous studies. In the current study, we could explore the role of arterial stiffness in such seriously ill patients using CAVI, which is an easy and noninvasive measurement of arterial stiffness. We believe that our study using CAVI as a marker of arterial stiffness is most reliable and reproductive in the current era.
Clinical Implications
The GRS is widely used, simple to assess, and effective in risk estimation of mortality and is recommended for all types of patients with ACS8). However, this score, which is based on common traditional risk factors for CV disease, was derived in the early 21st century. Since that time, many novel risk factors, including arterial stiffness, have been studied. The GRS does not include any of these new risk factors. Our study was the first to demonstrate that the addition of CAVI could improve risk stratification based on the GRS.
CAVI, which is not influenced by blood pressure at the time of the examination, integrates measurements of CV risk factors such as aging, hypertension, diabetes mellitus, and dyslipidemia5, 15). Therefore, it is reasonable that CAVI has better predicted values than each of these classic risk factors and shows the added value to GRS.
Otsuka et al. reported that persistent impairment of arterial stiffness assessed using CAVI was associated with future CV events, even after comprehensive management of traditional atherosclerotic risk factors in patients with stable coronary artery disease26). This suggested that serial assessments of arterial stiffness using CAVI could serve as an indicator of the need for treatment. Moreover, several interventional studies showed that nonpharmacological and pharmacological treatments were associated with an improvement in CAVI27–29). To determine the utility of CAVI in patients with ACS, more clinical and experimental studies are required. In addition, a therapeutic strategy for improving arterial stiffness in ACS needs to be developed.
Limitations
This study has limitations that need to be acknowledged. First, this study was based on a relatively small sample size at a single center. However, in comparison with previous studies, our study included the largest number of patients and had a longer follow-up duration so that we could clearly reveal the significance of arterial stiffness measured by CAVI. Second, we excluded patients with severe aortic insufficiency, peripheral artery disease, and persistent atrial fibrillation because it was difficult to accurately measure CAVI in these patients. It remains uncertain if our results apply to these subgroups of patients. Third, dyslipidemia, which is thought to be a conventional risk factor of recurrent atherosclerotic events, was less observed in the high-CAVI group than in the low- CAVI group. The relationship between CAVI and dyslipidemia has not been consistent with past studies30, 31). The higher proportion of elderly people in the high- CAVI group may affect the lipid profile32). Forth, compared to other recent studies, the rate of major adverse events in our study was relatively low2, 18). This better outcome may be explained by the extremely high proportion of patients who underwent percutaneous coronary intervention (92.1%) or coronary artery bypass grafting (4.8%).
Conclusion
Arterial stiffness determined using CAVI was an independent long-term predictor of MACE in patients with ACS. Furthermore, we demonstrated that the additional usefulness of CAVI can improve risk stratification based on the GRS.
Acknowledgements
Not applicable.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1). Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C and Stroke Statistics S : Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation, 2017; 135: e146-e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Ishihara M, Nakao K, Ozaki Y, Kimura K, Ako J, Noguchi T, Fujino M, Yasuda S, Suwa S, Fujimoto K, Nakama Y, Morita T, Shimizu W, Saito Y, Hirohata A, Morita Y, Inoue T, Okamura A, Uematsu M, Hirata K, Tanabe K, Shibata Y, Owa M, Tsujita K, Funayama H, Kokubu N, Kozuma K, Tobaru T, Oshima S, Nakai M, Nishimura K, Miyamoto Y, Ogawa H, Investigators JM : Long-Term Outcomes of Non-ST-Elevation Myocardial Infarction Without Creatine Kinase Elevation- The J-MINUET Study. Circ J, 2017; 81: 958-965 [DOI] [PubMed] [Google Scholar]
- 3). Gohbara M, Iwahashi N, Sano Y, Akiyama E, Maejima N, Tsukahara K, Hibi K, Kosuge M, Ebina T, Umemura S, Kimura K: Clinical Impact of the Cardio-Ankle Vascular Index for Predicting Cardiovascular Events After Acute Coronary Syndrome. Circ J, 2016; 80: 1420-1426 [DOI] [PubMed] [Google Scholar]
- 4). Feistritzer HJ, Klug G, Reinstadler SJ, Reindl M, Niess L, Nalbach T, Kremser C, Mayr A, Metzler B: Prognostic Value of Aortic Stiffness in Patients After ST-Elevation Myocardial Infarction. J Am Heart Assoc, 2017; 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Shirai K, Utino J, Otsuka K, Takata M: A novel blood pressure-independent arterial wall stiffness parameter; cardio- ankle vascular index (CAVI). J Atheroscler Thromb, 2006; 13: 101-107 [DOI] [PubMed] [Google Scholar]
- 6). Shirai K, Suzuki K, Tsuda S, Shimizu K, Takata M, Yamamoto T, Maruyama M, Takahashi K: Comparison of Cardio-Ankle Vascular Index (CAVI) and CAVI0 in Large Healthy and Hypertensive Populations. J Atheroscler Thromb, 2019; 26: 603-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr., Ganiats TG, Holmes DR, Jr., Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ: 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 2014; 64: e139-e228 [DOI] [PubMed] [Google Scholar]
- 8). Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, Avezum A, Goodman SG, Flather MD, Anderson FA, Jr., Granger CB: Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ (Clinical research ed), 2006; 333: 1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Whelton PK, Carey RM, Aronow WS, Casey DE, Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Sr., Williamson JD, Wright JT, Jr., 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults : A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension, 2018; 71: e13-e115 [DOI] [PubMed] [Google Scholar]
- 10). Imano H, Noda H, Kitamura A, Sato S, Kiyama M, Sankai T, Ohira T, Nakamura M, Yamagishi K, Ikeda A, Shimamoto T, Iso H: Low-density lipoprotein cholesterol and risk of coronary heart disease among Japanese men and women: the Circulatory Risk in Communities Study (CIRCS). Prev Med, 2011; 52: 381-386 [DOI] [PubMed] [Google Scholar]
- 11). Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU: Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J cardiovascular Imaging, 2015; 16: 233-270 [DOI] [PubMed] [Google Scholar]
- 12). Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y: Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res, 2002; 25: 359-364 [DOI] [PubMed] [Google Scholar]
- 13). Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL: 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol, 2015; 66: 403-469 [DOI] [PubMed] [Google Scholar]
- 14). Cook NR: Comments on ‘Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond’ by M. J. Pencina et al., Statistics in Medicine. Stat Med, 2008; 27: 191-195 [DOI] [PubMed] [Google Scholar]
- 15). Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. European Network for Non-invasive Investigation of Large A: Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J, 2006; 27: 2588-2605 [DOI] [PubMed] [Google Scholar]
- 16). Akkus O, Sahin DY, Bozkurt A, Nas K, Ozcan KS, Illyes M, Molnar F, Demir S, Tufenk M, Acarturk E: Evaluation of arterial stiffness for predicting future cardiovascular events in patients with ST segment elevation and non-ST segment elevation myocardial infarction. Scientific-World Journal, 2013; 2013: 792693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Kubozono T, Miyata M, Ueyama K, Nagaki A, Hamasaki S, Kusano K, Kubozono O, Tei C: Association between arterial stiffness and estimated glomerular filtration rate in the Japanese general population. J Atheroscler Thromb, 2009; 16: 840-845 [DOI] [PubMed] [Google Scholar]
- 18). Daida H, Miyauchi K, Ogawa H, Yokoi H, Matsumoto M, Kitakaze M, Kimura T, Matsubara T, Ikari Y, Kimura K, Tsukahara K, Origasa H, Morino Y, Tsutsui H, Kobayashi M, Isshiki T, investigators P : Management and two-year long-term clinical outcome of acute coronary syndrome in Japan: prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ J, 2013; 77: 934-943 [DOI] [PubMed] [Google Scholar]
- 19). Sakane K, Miyoshi T, Doi M, Hirohata S, Kaji Y, Kamikawa S, Ogawa H, Hatanaka K, Kitawaki T, Kusachi S, Yamamoto K: Association of new arterial stiffness parameter, the cardio-ankle vascular index, with left ventricular diastolic function. J Atheroscler Thromb, 2008; 15: 261-268 [DOI] [PubMed] [Google Scholar]
- 20). Hillis GS, Ujino K, Mulvagh SL, Hagen ME, Oh JK: Echocardiographic indices of increased left ventricular filling pressure and dilation after acute myocardial infarction. J Am Soc Echocardiogr, 2006; 19: 450-456 [DOI] [PubMed] [Google Scholar]
- 21). Iwahashi N, Kimura K, Kosuge M, Tsukahara K, Hibi K, Ebina T, Saito M, Umemura S: E/e′ two weeks after onset is a powerful predictor of cardiac death and heart failure in patients with a first-time ST elevation acute myocardial infarction. J Am Soc Echocardiogr, 2012; 25: 1290-1298 [DOI] [PubMed] [Google Scholar]
- 22). Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ: Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation, 2000; 102: 1788-1794 [DOI] [PubMed] [Google Scholar]
- 23). Tomiyama H, Koji Y, Yambe M, Shiina K, Motobe K, Yamada J, Shido N, Tanaka N, Chikamori T, Yamashina A: Brachial -- ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circ J, 2005; 69: 815-822 [DOI] [PubMed] [Google Scholar]
- 24). Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, Matsuda S, Miyazaki Y, Hiratsuka A, Matsuzaki M: Cardio-ankle vascular index is superior to brachial- ankle pulse wave velocity as an index of arterial stiffness. Hypertens Res, 2008; 31: 1347-1355 [DOI] [PubMed] [Google Scholar]
- 25). Klug G, Metzler B: Assessing myocardial recovery following ST-segment elevation myocardial infarction: shortand long-term perspectives using cardiovascular magnetic resonance. Expert Rev Cardiovasc Ther, 2013; 11: 203-219 [DOI] [PubMed] [Google Scholar]
- 26). Otsuka K, Fukuda S, Shimada K, Suzuki K, Nakanishi K, Yoshiyama M, Yoshikawa J: Serial assessment of arterial stiffness by cardio-ankle vascular index for prediction of future cardiovascular events in patients with coronary artery disease. Hypertens Res, 2014; 37: 1014-1020 [DOI] [PubMed] [Google Scholar]
- 27). Hayashi K, Yamamoto T, Takahara A, Shirai K: Clinical assessment of arterial stiffness with cardio-ankle vascular index: theory and applications. J Hypertens, 2015; 33: 1742-1757 [DOI] [PubMed] [Google Scholar]
- 28). Kato M, Kumagai T, Naito R, Maeno K, Kasagi S, Kawana F, Ishiwata S, Narui K, Kasai T: Change in cardio-ankle vascular index by long-term continuous positive airway pressure therapy for obstructive sleep apnea. J Cardiol, 2011; 58: 74-82 [DOI] [PubMed] [Google Scholar]
- 29). Yamaguchi T, Shirai K, Nagayama D, Nakamura S, Oka R, Tanaka S, Watanabe Y, Imamura H, Sato Y, Kawana H, Ohira M, Saiki A, Shimizu N, Tatsuno I: Bezafibrate Ameliorates Arterial Stiffness Assessed by Cardio-Ankle Vascular Index in Hypertriglyceridemic Patients with Type 2 Diabetes Mellitus. J Atheroscler Thromb, 2019; 26: 659-669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Soska V, Dobsak P, Dusek L, Shirai K, Jarkovsky J, Novakova M, Brhel P, Stastna J, Fajkusova L, Freiberger T, Yambe T: Cardio-ankle vascular index in heterozygous familial hypercholesterolemia. J Atheroscler Thromb, 2012; 19: 453-461 [DOI] [PubMed] [Google Scholar]
- 31). Satoh N, Shimatsu A, Kato Y, Araki R, Koyama K, Okajima T, Tanabe M, Ooishi M, Kotani K, Ogawa Y: Evaluation of the cardio-ankle vascular index, a new indicator of arterial stiffness independent of blood pressure, in obesity and metabolic syndrome. Hypertens Res, 2008; 31: 1921-1930 [DOI] [PubMed] [Google Scholar]
- 32). Schoenenberger AW, Radovanovic D, Stauffer JC, Windecker S, Urban P, Niedermaier G, Keller PF, Gutzwiller F, Erne P, Investigators AP : Acute coronary syndromes in young patients: presentation, treatment and outcome. Int J Cardiol, 2011; 148: 300-304 [DOI] [PubMed] [Google Scholar]


