Abstract
The current challenge of the COVID-19 pandemic is complicated by the limited therapeutic options against the virus, with many being anecdotal or still undergoing confirmatory trials, underlining the urgent need for novel strategies targeting the virus. The pulmotropic virus causes loss of oxygenation in severe cases with acute respiratory distress syndrome (ARDS) and need for mechanical ventilation. This work seeks to introduce placental extract-derived biologically active components as a therapeutic option and highlights their mechanism of action relevant to COVID-19 virus. Human placenta has been used in clinical practice for over a century and there is substantial experience in clinical applications of placental extract for different indications. Aqueous extract of human placentacontains growth factors, cytokines/chemokines, natural metabolic and other compounds, anti-oxidants, amino acids, vitamins, trace elements and biomolecules, which individually or in combination show accelerated cellular metabolism, immunomodulatory and anti-inflammatory effects, cellular proliferation and stimulation of tissue regeneration processes.
Placental extract treatment is proposed as a suitable therapeutic approach consideringthe above properties which could protect against initial viral entry and acute inflammation of alveolar epithelial cells, reconstitute pulmonary microenvironment and regenerate the lung. We reviewed useful therapeutic information of placental biomolecules in relation to COVID-19 treatment. We propose the new approach of using placental growth factors, chemokines and cytokine which will execute antiviral activity in coordination with innate and humoral immunity and improve patient's immunological responses to COVID-19. Executing a clinical trial using placental extract as preventive, protective and/or therapeutic approach for COVID-19treatment could advance the development of a most promising therapeutic candidate that can join the armamentaria against the COVID-19 virus.
Keywords: Novel coronavirus COVID-19, Placental extract, Immunomodulatory, Anti-inflammatory, Cellular proliferation, Tissue regeneration
Highlights
-
•
The SARS-2 coronavirus has high morbidity and mortality and challenged health care services in many countries.
-
•
Clinical applications of placental extracts have been shown an acute need for researchers to satisfiy the scientific temperament.
-
•
This review aims to underline generating an early, protective immune response against SARS CoV-2.
-
•
Well-designed, outcome-oriented clinical trials to test placental extract as an antiviral agent could provide the answers the world seeks.
1. Introduction of COVID-19
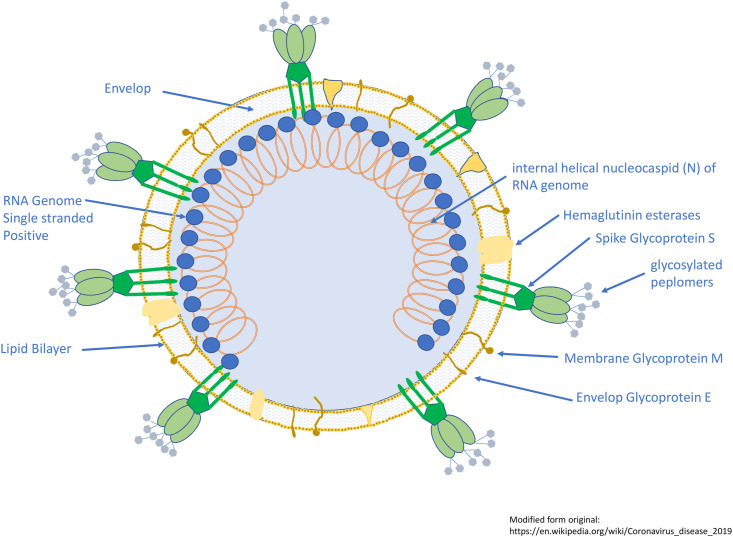
Tyrrell and Bynoein 1965 identified a virus responsible for common cold from human embryonic tracheal organ cultures and they named it as B814(1). In 1967, Almeida and Tyrrell performed electron microscopy study on fluids from organ cultures infected with B814and found particles sized (80–150 nm) that resembled the infectious bronchitis virus of chickens(2). In 1975, Tyrrell and Almeida named new group of virus as coronavirus (corona denoting the crown-like appearance of the surface projections) and was later officially accepted as a new genus of viruses(3). Coronaviruses belong to the Coronaviridae family in the Nidovirales order. Corona virus comprises a single-stranded RNA as nucleic material, size ranging from 26 to 32 kbs in length (Fig. 1 ). The subgroups of coronaviruses family are alpha (α), beta (β), gamma (γ) and delta (δ) coronavirus.
Fig. 1.
Schematic of the coronavirus.The viruses are pleomorphic spherical particles with bulbous surface projections (~80–90 nm). Viral particles enclose a positive single stranded RNA genome complexed with the basic nucleocapsid (N) phosphoprotein. The virus consists of a lipid bilayer that anchors the membrane (M), envelope (E) and spike (S) proteins. A subset of coronaviruses have a shorter spike-like surface protein called hemagglutinin esterase. Spike glycoprotein (S), the type I glycoprotein forms glycosylated peplomers giving it a crown-like morphology. It provides the virus its bulbous surface projections. It interacts with its compliment host cell receptor in determining the tissue tropism and infectivity. The membrane glycoprotein (M), is highly hydrophobic, and has a short N-terminal ectodomain and a cytoplasmic tail. It spans the membrane three times. Small Envelop Glycoprotein (E), a membrane-spanning protein, is a highly hydrophobic protein. It has a short ectodomain, a transmembrane domain, and a cytoplasmic tail. The lipid bilayer envelope, membrane glycoproteins, and nucleocapsid shield the virus when it is outside the host.
These viruses, severe acute respiratory syndrome coronavirus (SARS-CoV), H5N1 influenza A, H1N1 2009 and Middle East respiratory syndrome coronavirus (MERS-CoV) cause acute lung injury and acute respiratory distress which leads to pulmonary failure and result in mortality. Evidence showed that wild animals and bats are the natural reservoir hosts and play a crucial role in transmitting various viruses. The SARS-CoV and MERS-CoV originated from bats, then transmitted to human via intermediate hosts, civets and camels(4). Chan et al. [5] reported a case of five patients in a family cluster, which confirmed Person-to-person transmission of corona viruses. Possible evidence of transmission was long chain of 4 “generations” (a person who originally contracted the virus from source infected animals someone else, who infected another individual, who then infected another individual), suggesting sustained human-to-human transmission [6]. To date the infection was believed to be transmitted through airborne respiratory droplets and physical contact(7).The recent detection of corona virus in the faeces of confirmed patients in Wuhan, Shenzhen and the first case in the United States, indicates that the virus can replicate in the digestive tract and exist, suggesting a potential forfaeco-oral transmission [8]. In December 2019, Wuhan, China experienced an outbreak of a novel coronavirus that killed more than thirty three hundred and infected over eighty two thousand individual still date. This virus was reported to be a member of the β group of coronaviruses. The novel virus was named as Wuhan coronavirus or 2019 novel coronavirus (2019-nCoV) by the Chinese researchers which was subsequently renamed the CoVID-19 virus(9).
1.1. Mechanism of human COVID-19 infection
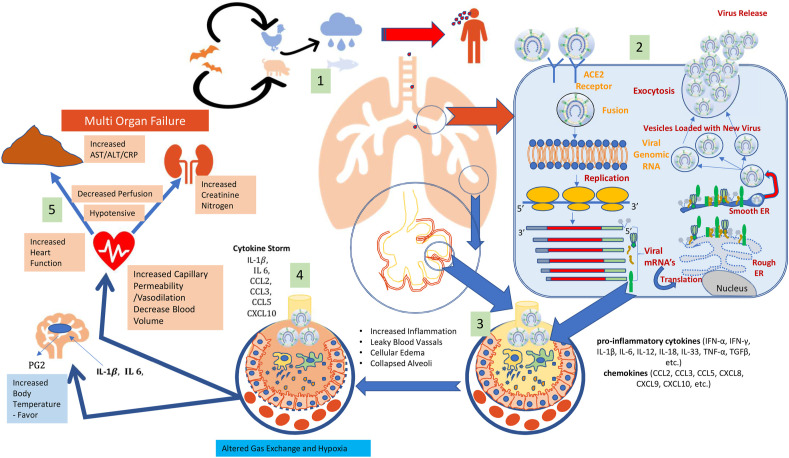
The mechanism of CoVID-19of attachment, replication and cellular changes during the infection are presented in Fig. 2 .Coronaviruses replication is facilitated by specific genes in ORF1 downstream regions that also encode proteins for nucleocapsid and spikes formation(10). The virus attaches to host cell through glycoprotein spikes on the outer surface (Fig. 1).Corona virus infect multiple hosts due to loosely attached receptor-binding domain (RBD). SARS-CoV caused systemic spread of severe lower respiratory disease with 10% mortality. Recently, another human coronavirus-Erasmus Medical Center (hCoV-EMC)) was recognized in severe lower respiratory tract infectionhttps://www.nature.com/articles/nature12005 - ref-CR4patients. Viral genome of hCoV-EMC is similar to coronaviruses found in bats. The dipeptidyl peptidase 4 (DPP4) act as a functional receptor for hCoV-EMC. Expression of DPP4 protein provides clues about the host range potential of hCoV-EMC(11). The entry mechanism of a coronavirus depends upon cellular proteases present in human airway such as trypsin-like protease (HAT), cathepsins and transmembrane protease serine 2 (TMPRSS2) that split the spike protein and establish further penetration changes. Coronavirus require angiotensin-converting enzyme 2 (ACE2) as a key receptor for cellular penetration. Researchers have confirmed that severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) uses SARS‐CoV receptor angiotensin‐converting enzyme 2 (ACE2) for host cell entry(12).
Fig. 2.
The Mechanism of human CoVID-19 infection: 1: infected animals can infect Humans followed by human-to-human transmission through aerial droplets and contact. 2: life cycle begins with S protein binds to the cellular receptor ACE2. After receptor binding, S protein facilitates viral envelope fusion with the cell membrane through the endosomal pathway. Then CoVID-19 releases RNA into the host cell. Viral genomic RNA is translated into viral replicase polyproteins, which are then cleaved into small viral proteases. The RNA polymerase generates a series of sub genomic mRNAs and finally translated into all viral proteins. Viral genome RNA and proteins are subsequently assembled into virions in the endoplasmic reticulum, then to Golgi and transported via vesicles and released out of the cell. 3: CoVID-19 infection results in activation of epithelial cells, macrophages and dendritic cells. Antigens will be presented to the antigen presentation cells (APC), which triggers body's anti-viral immunity and uncontrolled systemic inflammatory response resulting from the release of large amounts of pro-inflammatory cytokines (IFN-α, IFN-γ, IL-1β, IL-6, IL-12, IL-18, IL-33, TNF-α, TGFβ, etc.) and chemokines (CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10, etc.) by immune effector cells. 4: The cytokine storm will trigger a violent attack of immune system to the body, results in leaky blood vessels, cellular oedema and collapsed alveolar function. 5: Increased amount of IL6 and IL-1β results in activation of prostaglandins which results in increased body temperature. 5: System inflammation leads to decreased blood volume and increased hat ? function. Finally, it results in multiple organ failure, and may lead to death in severe cases.
1.2. Pathogenesis of COVID-19
The presently known pathogenesis of CoVID-19 infection is presented in (Fig. 2:3,4, and 5) with knowledge on this still evolving. It is important to note that the human immunological system is naive for this virus increasing the vulnerability of the species. The virus has a R0 of 2.5 which with its’ airborne transmission makes it a challenge to contain, even compared to influenza virus. It is also infectious when the index case is completely asymptomatic and can stay alive on fomites for few hours to days. The mechanisms of SARS-CoV and MERS-CoVpathogenesis is very similar and can give us information on the pathogenesis. Once the virus enters the cells, its antigen will be presented to the antigen presentation cells(APC), Macrophages and Dendritic cells which trigger anti-viral immunity. Antigenic peptides are presented by human leukocyte antigen (HLA) and then recognized by virus-specific cytotoxic T lymphocytes (CTLs). These cytotoxic cells start to release of large amounts of pro-inflammatory cytokines (IFN-α, IFN-γ, IL-1β, IL-6, IL-12, IL-18, IL-33, TNF-α, TGFβ, etc.) and chemokines (CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10, etc.) [[13], [14], [15]]. The uncontrolled systemic inflammatory responses resulting in cytokine storm, which releases large amounts of pro-inflammatory cytokines. The cytokine storm triggers a violent attack by the immune system to the body, cause acute respiratory distress syndrome (ARDS) which leads to pulmonary oedema and lung failure, and have liver, heart, and kidney damages and finally lead to death in severe cases of CoVID-19 The cytokine storm launches a violent attack on the body organs, causing ARDS as well as hepatic, cardiac and renal damage leading to the mortality seen in severe cases of CoVID-19(16) (see Fig. 4).
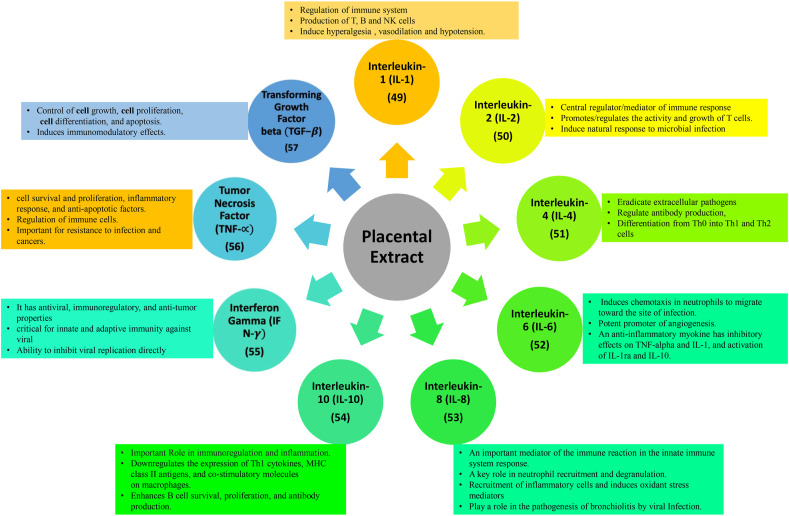
Fig. 4.
Functions of major cytokines and chemokines in placental extract.
1.3. Immunology in coronavirus infections
Innate and adaptive immune systems function to tackle the daily exposure to pathogens the human body faces. Innate (non-specific, natural) immunity provides the initial and immediate response of the body based on broad pathogen specificity and is mediated by Dentritic cells, macrophages and B cells. Adaptive (learnt, specific) immunity responds to antigens/pathogens and is mediated by B cells, T-lymphocytes, Natural Killer Cells and effector lymphocytes. In both cases, the tissue-specific cells release small functional proteins called cytokines to attract immune system components to reach the site.Probable protective mechanism and involvement of cytokine and chemokines for COVID-19 infection is presented in Fig. 5. Cytokines play a key role in cell-to-cell communication within the immune system through receptors, at picomolar concentrations. They can have autocrine, paracrine or endocrine effects and display features of pleiotropy, redundancy, synergy and antagonism. Cytokines are variously described as interleukins, lymphokines, monokines, interferons (involved in anti-viral responses), colony-stimulating factors and chemokines (mediate chemotaxis or chemoattraction). Entry of the virus in the body activates initially the innate immunity with endocytosis of the infecting particle, phagocytosis and release of inflammatory mediators by Antigen Presenting Cells (macrophages, dendritic cells) mainly Type 1 interferons. Infected body cells expose Pathogen Associated Molecular Patterns (PAMPs) which are recognized by macrophages through surface receptors of the Pattern Recognition Receptor group, mainly the Toll Like Receptors (TLR) and RNA recognition proteins. Pathogen recognition initiates phagocytosis and release of cytokines Type 1 Interferons (IFN-α, IFN-β), TNF-α, Nitric Oxide and other cytokines(17). The macrophages then function as antigen presenting cells and also release proinflammatory cytokines IL-1, IL-6, and TNF-α as a pyrogenic response and drive expansion of T-lymphocyte populations through production of IL-10 (TH2 activation) and IL-12 (TH1 activation) [18]. The interferons released by the cells helps to restrict or stop viral replication through its actions on expression of relevant genes. Cytokine of the IL-1 family - IL-37 is expressed in the macrophages/monocytes and has ability to suppress immune response and inhibit inflammation by suppressing eventually IL-1β, IL-6, TNF and CCL2(19). As an inhibitor of both innate and adaptive immunity and inflammatory responses, IL-37 plays a pivotal role in the antimicrobial response, including antiviral infections. Its effect on IL-1β is of great interest with respect to coronavirus-19 as explained later.A similar IL-1 family cytokine -IL-38 is released by macrophages and B cells and inhibits proinflammatory IL-cytokines. This cytokine too inhibits inflammation caused by coronavirus-19, thus having therapeutic potential [19,20]. The recognition of antigens by surface membrane antibodies of B-lymphocytes initiates the B-cell differentiation into effector cells (Plasma cells) with copious antibody production. Neutralizing IgA antibodies bind with viral particles and prevent their binding to or entry into the tissue cells, not needing the presence of complement to function(21). These antigen-antibody complexes undergo opsonization and are phagocytosed by the macrophages and other cells. The DCs are the only antigen-presenting cells which can migrate to lymphoid tissue and engage with T-cells thus forming a link between innate and adaptive immunity. The DCs express Toll-like Receptors which play a key role in recognizing viral proteins and presenting the antigens to naïve T cells(22). The TLRs on binding with coronavirus 2019 releases pro-IL-1β which is cleaved by caspase-1, followed by inflammasome activation and production of active mature IL-1β which is a mediator of lung inflammation, fever and fibrosis.Other chemokines like IL-8 and inflammatory cytokines IL-1β and IL-12 are also released by the DCs(23). The naive T-Lymphocytes (CD4+) are then stimulated to an intermediate state termed TH0. The decision as to whether the TH0 will develop into an inflammatory TH1 cell, a helper TH2 cell, or a TH17 cell depends on cytokine environment at the site of priming [24,25]. CD4+ T-Lymphocytes have coreceptors for MHC-Class II proteins. The production of IFN-γ by NK cells may influence the CD4+ T cell response to infectious cells, and they differentiate into pro-inflammatory TH1 cells able to activate macrophages(26,27). Naïve T cells stimulated with TGF- β, and IL-6 differentiate in to TH17 cells. TH17 cells secrete important cytokines IL-17, IL-21, IL-22. IL-17 stimulates the production of inflammatory cytokines, such as IL-6, TNF-α, IL-1β, chemokines (CXCL1, CXCL3, CXCL5, CXCL6), and several growth factors G/GM-CSF, and VEGF. TH17 cell also produces other important effector molecules, such as IL-21, IL-22, IL-26, IL-6 and CCL20(28). Th17 cytokines (IL-17 especially) as a bridge between innate and adaptive immune responses in host defences against a variety of pathogens at the mucosal surfaces(29).Both TH1and TH2Helper cells regulate the functioning of each other through the cytokines they release. Th-1 cells are proinflammatory and produce IL-2, IL-12 and IFN-γ, the latter activating macrophages and Cytotoxic T-Lymphocytes(30). The Th-2 cells release IL-4, IL-5 and IL-10 and function to destroy infected and injured cells. Naïve CD8+ helper cells are recruited by DCs with an important role played by the chemokine-chemokine receptor pair XCL1-XCR1 which may also form a ‘feed-forward loop between the CD8+T cells and the DCs’. Recruitment of CD8+ lymphocytes is also regulated by IL-2 and chemokines released by the CD4+ Helper T-lymphocytes. One of the downstream targets of IL-2 signalling in promotion of CD8+ recruitment is the MAPK molecular pathway(31). It has been shown in coronavirus infections that IL-10 production may be promoted by strong T-Cell Receptors-MAPK signalling. This is significant as IL-10 is a cytokine that ‘prevent immunopathology during viral infection without affecting the kinetics of viral clearance(32).
Fig. 5.
Mechanism of Placental Extract mediated immunosurveillance.
CD8+ Helper T-lymphocytes are also referred to as cytotoxic T-lymphocytes (CTLs) have three mechanisms in the event of infections. First they secrete cytokines primarily TNF-α and IFN-γ which have anti-viral effects. Second they release, selectively along the immune synapse, cytotoxic granules containing perforin and granzymes which enter the infected cell, shut down production of viral proteins and cause apoptosis of cells. After killing one cell, these CTLs can move to target other infection/diseased cells, thus multiplying their effectivity. Third, they express Fas-L on the cell surface and cause trimerization of Fas molecules on the target cell surface, activating the caspase cascade(33). Caspase 1 cleaves the pro-IL-1β released by DCs to affect inflammation. These cells release of large amounts of pro-inflammatory cytokines (IFN-α, IFN-γ, IL-1β, IL-6, IL-12, IL-18, IL-33, TNF-α, TGFβ, etc.) and chemokines (CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10, etc.)in addition to the IL-10(13,16). The humoral response in adaptive immunity involves the release of IgA and IgG by the activated B Lymphocytes or Plasma cells as described above. The IgA are neutralizing antibodies. The IgG are responsible for antibody dependent cellular cytotoxicity (ADCC) wherein the NK cells recognise the injured cells coated by the IgG antibodies and destroy them. NK cells can be activated by IFN-γ, IL-2, IL-12, and TNF to amplify the lysis of infected cell(26,27). Thus, innate immunity in early stages and the adaptive immunity in later stages work in tandem in the event of viral infection. Cytokines of different types play a vital role in cell-to-cell communication, signalling pathways and recruitment as well as differentiation of immune cells. The key role played by these cytokines makes therapies targeted at these in the event of any viral infections (including novel coronavirus 2019) a highly attractive potential option.
1.4. Symptoms of COVID-19 infection
Symptoms of CoVID-19 infection are mostly non-specific and the disease presentation can range from asymptomatic to severe pneumonia and death [34].Symptoms of COVID-19 may appear 2–14 days after exposure and can includefever, cough, fatigue, sputum production, headache, haemoptysis, diarrhoea and dyspnoea(16,35).Difficulty in breathing is associated with progressive hypoxemia not fully corrected by mechanical ventilation [36]. Like all viral pneumonias, the risk of secondary bacterial infections can be a complication in patients worsening prognosis. Elderly patients and patients with co-morbidities, immunocompromised or immunosuppressed have been seen to have worse prognosis compared to others.In elderly patients, COVID-19 infection results in lower respiratory tract followed to fatal pneumonia. Vulnerable groups especially the elderly are more susceptible to developing lower respiratory tract infections and fatal pneumonia(37,38).In a study from China, patients with severe disease were older (than non-severe patients) by a median of 7 years and those needing ICU and mechanical ventilation were older by median 17 years with higher prevalence of hypertension of 35.8% as against 13.7% in those not needing ICU/ventilation(39). A study from Wuhan reported median age of non-survivors as 71 years with increased prevalence of comorbidities like hypertension (58.4%), Diabetes (21.8%), etc. [40]. In the second week of infection, patients observed progressive hypoxemia, difficulty in breathing and ARDS. Additionally, chances of secondary bacterial infections are inevitable which leads to secondary bacterial pneumonia.
1.5. Diagnosis of COVID-19
Diagnosis of CoVID-19 depends on a high index of suspicion in patients with respiratory symptoms, history of contact with confirmed cases, history of international travel and health care workers. Investigations like X-Ray Chest, HRCT Chest and sputum examination need to be accompanied by antigen detection using nasopharyngeal or oropharyngeal swabs. A study in China showed that the SARS-CoV-2 viral RNA were detected in symptomatic and asymptomatic contacts of confirmed case, with weakly detected RNAdetected in swabs of asymptomatic contact even after 11 days from index contact(41). This demonstrates the need for physical distancing and hygiene in the fight against the infection. Molecular approach based on RT-qPCR is the most common, effective and straightforward method for detecting pathogenic viruses in respiratory secretions and blood. Antibody detection can be used as screening investigation as the antibodies are detected. Corman VM described two step RT-qPCR assays (TaqMan-based fluorescence signal) to detectCOVID-19 viral genome separately(42) (see Table 1 ).
Table 1.
Primers and probes, real-time RT-PCR for SARS-CoV-2 virus(42).
| Assay/use | Oligonucleotide | Sequencea | Concentrationb |
|---|---|---|---|
| RdRP gene | RdRp_SARSr-P2 | FAM-CAGGTGGAACCTCATCAGGAGATGC-BBQ | Specific for 2019-nCoV, will not detect SARS-CoV. |
| Use 100 nM per reaction and mix with P1 | |||
| RdRP_SARSr-P1 | FAM-CCAGGTGGWACRTCATCMGGTGATGC-BBQ | Pan Sarbeco-Probe will detect 2019-nCoV, SARS-CoV and bat-SARS-related CoVs. | |
| Use 100 nM per reaction and mix with P2 | |||
| RdRp_SARSr-R | CARATGTTAAASACACTATTAGCATA | Use 800 nM per reaction | |
| E gene | E_Sarbeco_F | ACAGGTACGTTAATAGTTAATAGCGT | Use 400 nm per reaction |
| E_Sarbeco_P1 | FAM-ACACTAGCCATCCTTACTGCGCTTCG-BBQ | Use 200 nm per reaction | |
| E_Sarbeco_R | ATATTGCAGCAGTACGCACACA | Use 400 nm per reaction | |
| N gene | N_Sarbeco_F | CACATTGGCACCCGCAATC | Use 600 nm per reaction |
| N_Sarbeco_P | FAM-ACTTCCTCAAGGAACAACATTGCCA-BBQ | Use 200 nm per reaction | |
| N_Sarbeco_R | GAGGAACGAGAAGAGGCTTG | Use 800 nm per reaction |
W is A/T; R is G/A; M is A/C; S is G/C. FAM: 6-carboxyfluorescein; BBQ: blackberry quencher.
Optimised concentrations are given in nanomol per litre (nM) based on the final reaction mix, e.g. 1.5 μL of a 10 μM primer stock solution per 25 μL total reaction volume yields a final concentration of 600 nM as indicated in the table.
RT-qPCR technique has few challenges including certain biological safety hazardretention, false positive resultsand long waiting time.Many clinicians proposed CT scans as more sensitive auxiliary diagnostic method detection of COVID-19. Patient with a high clinical suspicion of COVID-19 infection with negative RT-qPCR screening, it is recommended to use a combination repeated RT-qPCR tests and chest CT scan [42].Currently, ELISA kits for IgM/IgG response of COVID-19 have been developed and pre-tested by some companies and have shown higher detection rates. The sensitivity of COVID-19 N-based IgG ELISA (94.7%) is significantly higher than that of COVID-19 S- based IgG ELISA (58.9%) [43].Sensitivity of COVID-19 IgG/IgM ELISA kits remains a major challenge and need to validate the assay on larger sample size.ELISA kits for antibody detection against the virus are available for both IgG and IgM. However, antibodies are absent in the initial stages of the disease as the body develops an immunological response to the pathogen. Hence the tests are likely to be negative even in the presence of early infection. The USFDA does not recommend using the antibody tests as the “sole basis to diagnose COVID-19 but instead as information about whether a person may have been exposed”. In a letter to health care workers, the FDA recommends that the test could be used to identify those exposed to the infection or recovered from it to as they could serve as potential donors for manufacture of convalescent plasma.
1.6. Current treatment options
Current management of the infection is based on previous experience with the MERS or SARS epidemics and the China experiences at Wuhan. Supportive management directed to alleviating symptoms and treat pathologies is accompanied in many countries with use of anti-viral and anti-inflammatory agents. At last count, the International Clinical Trials Registry Platform of the WHO had 1135 registered clinical trials related to studies on treatment options for COVID-19 infection. Currently anecdotal and case series reports reveal drug repositioning efforts with anti-virals like Remdesivir (tried for Ebola)and Lopinavir- Ritonavir (approved anti-retroviral drug) and anti-inflammatory drugs likeChloroquine (approved for malaria) andHydroxychloroquine (approved for Rheumatoid Arthritis), all of which are undergoing clinical trials for the new indication. Favipiravir is another anti-viral approved for influenza in Japan and beneficially tried in Ebola infection that is also being tried in COVID-19 infections. Anti-inflammatory agents with targets like IL-6, VEGF and other cytokines are being studied in several trials viz. Sarilumab, Tocilizumab, Eculizumab (anti-VEGF). Human convalescent plasma is the intervention being used in multiple clinical trials registered on the ICRR platform. Plasma from recovered patients (convalescent plasma) is a form of adaptive immunotherapy that was used in previous corona-virus infections such as SARS, MERS and the 2009H1N1 pandemic. A study in China was reported that has used convalescent plasma severe cases with beneficial effects on symptomatology and oxygenation(44). Vaccines are under development with China, USA and UK at the forefront. Recombinant n-COV vaccines with Adenovirus type 5 vector, intradermal prophylactic caccine (Phase 1, Inovio Pharmaceuticals, China), Recombinant chimeric COVIC-19 epitope DC vaccine (Shenzhen Third People's Hospital, China), bacTRL-Spike Vaccine for Prevention of COVID-19 (Phase 1.Symvivo Corporation, China), COVID-19 vaccine ChAdOx1nCoV-19 (Phase I/II, Oxford, UK), Recombinant Novel Coronavirus Vaccine (Adenovirus Vector) (Phase II, Insitute of Biotechnology, Academy of Military Medical Sciences, PLA of China), COVID-19 aAPC Vaccine (Shenzhen Geno-Immune Medical Institute, Phase I, China) and Recombinant Novel Coronavirus (2019-nCOV) Vaccine (Adenovirus Vector) (Phase II, Jiangsu Provincial Center for Disease Control and Prevention, China) are some trials that are eagerly awaited(45).
1.7. Placental extracts: biological drug
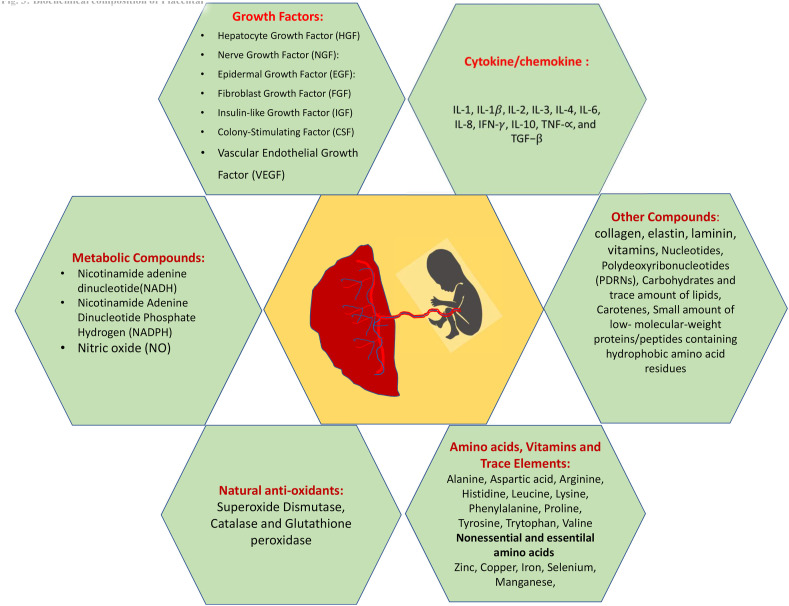
The placenta is a large lobular temporary organ that develops upon implantation of the blastocyst into the endometrium. This vascular organ is attached to the uterine wall and forms a conduit for maternal blood to provide oxygen, glucose and biologically active substances for the growth and development of the fetus. The placenta contains a wide range of biologically active components such as growth factors, Cytokine/chemokine, natural Metabolic and other compounds, anti-oxidants, Amino acids, Vitamins and Trace Elements(Fig. 3 ).Importantly, the placenta is capable of producing all substances found in any organ of the body [46]. The placenta is considered to be bio-waste after delivery and this treasure of bioactive compounds many a time goes waste.Use of human placenta has been a part of folk remedy in Oriental countries and there is substantial experience of over a century in the application of the placental extract (PE)for experimental and clinical practice.In Japan, the hydrolysate of human placenta, which contains therapeutic compounds, has long been refined as human placental extract (HPE). In India, several studies have been made on the application of PE as wound healing material [47]. Aqueous extract of human placenta shows accelerated cellular metabolism, absorption of exudates, effects debridement, antibacterial activity and stimulates tissue regeneration processes [48]. Various methods have been standardized for preparation of PE for clinical use(49) and therapeutic efficacy of PE was studied for variety of pathologies, including inflammatory diseases, immunomodulation, anti-inflammatory, cellular proliferation and tissue regeneration, osteoarthritis, anti-ageing, chronic pain, ischemic brain damage, liver damage, etc. [[50], [51], [52], [53], [54], [55], [56]]. Widespread clinical usage was unfortunately not accompanied by systematized basic research and clinical trials and the use of PE received no scientific follow-up. It is reasonable to expect that compilation of available data of clinical application of PE will shed additional light on the use of PE as an anti-viral therapy for COVID-19 infection.
Fig. 3.
Biochemical composition of placenta.Therapeutic advantages of placental extract are attributed to their composition and properties. Different growth factors, cytokines/chemokines, metabolic and other trace elements, and natural antioxidants have anti-inflammatory, antibacterial, antiviral properties that are supportive of activation of monocytes and macrophages. These factors enhance the migration, proliferation and survival of macrophages and attracts monocytes and stimulates macrophages to release angiogenic and lymphangiogenic factors. Placental biochemical composition has direct consequence on the inflammatory reaction because, by binding to VEGF on monocytes, activates TNF-α and IL-6 production via a calcineurin-dependent pathway. These immunoglobulin therapies can assist to treat patients with the virus infection.
1.8. Anti-inflammatory and immunoregulatory properties of placental extracts
The Placenta, is considered an ideal example of allograft, and it plays an important role of immunomodulation to maintain an environment conducive to foetal development. The placentalmesenchymal stem cells (pMSCs) play a critical immunomodulatory role on the maternal immune system through expression of pMSCs express human leukocyte antigen (HLA)-G which is known to inhibit T cell function and proliferation [[57], [58], [59]]. Specifically, pMSC-mediated immunomodulation operates through a synergy of cell contact-dependent mechanisms and soluble factors that induce changes of monocytes/macrophages, dendritic cells, T cells, B cells, and natural killer cells [[60], [61], [62], [63]].MSC-secreted Interleukin 1 Receptor Antagonist (IL1-RA) can promote the polarization of macrophages toward the type 2 phenotype which inhibit the differentiation into the type 1 phenotype and dendritic cells [[64], [65], [66]]. Anti-inflammatory monocytes secrete high levels of IL-10 and have decreased levels of IL-12p70, TNF-a, and IL-17 expression—a process that is mediated by MSC-produced IL-6 and hepatocyte growth factor (HGF) [64].MSCssecrete IL-6 and HGF, that induce monocyte to produce IL-10 which suppresses monocyte differentiation into Dendritic Cells and other cell types(67). Soluble factors produced by MSCsinclude indoleamine 2, 3-dioxygenase, prostaglandin E2, TGF-β1, IL-6 and nitric oxide suppress NK-cell proliferation and cytotoxicity and impair T cell activation and proliferation [[68], [69], [70]].Fitzgerald W et al. studied mechanisms of placenta function and the role of extracellular vesicles (EVs) in pregnancy. Placental villous explants also produced large amounts of the pro-inflammatory cytokines IL-6, IL-8, GRO-α, IP-10, and MCP-1 as well as CRP and TRAIL. They also found that placental villi and amnion continuously produced growth factors(angiogenin, fibronectin, galectin-1,ICAM-1, IGFBP1, IL-1Ra, IL-27, PAPP-A, serpin E1), angiogenic factors(VEGFR1,andVEGFR2), anti-angiogenic factors (uPA, and uPAR), and hormones(hCG and PGE2). Additionally, Placental extracts also depot of natural anti-oxidants such as superoxide dismutase, catalase and glutathione peroxidase which neutralize free radicals, preventing cellular death and ultimately, halt disease development [71].Kim SY et al. studied the immunoregulatory effects of placental extract on mouse model of allergic contact dermatitis. Administration of placental extract reduced numbers of CD4+ T cells in peripheral blood, decrease of tissue-infiltrating lymphocytes, and preferential production of Th2-type cytokines(72).
1.9. Antiviral activities of placental extracts
The placenta acts as a immunological barrier between the mother and fetus and protects the developing fetus from the transmission of viruses.The aqueous extract of human placenta is a depot of growth factors, chemokines and cytokine which execute antiviral activity in coordination with innate and humoral immunity. Interferons (IFNs) are a large family of cytokines defined by their ability to confer resistance to viral infections and providing rapid and broad protection against a wide variety of invading pathogens. Placental trophoblast derived interferons (INF) and TNF-, have been shown to impair virus replication and activity [73,74]. There is growing evidence that IFNs are constitutively released from human trophoblasts and play essential roles in protection against viral infection(75,76).Production of type I IFNs control infection systemically, and type III IFNs (IFN-λs) control infection locally at barrier surfaces by placental trophoblasts.
Human mid-gestation placentas showed potent antiviral activity against RNA and DNA viruses, including teratogenic viruses such as Zika virus (ZIKV), rubella virus (RuV), human cytomegalovirus (hCMV), varicella zoster virus (VZV), and herpesvirus (HSV-1) [77].The placenta reveals a variety of mechanisms to limit HIV-1 replication. Placental macrophages (Hofbauer cells) are key mediator factors. Hofbauer cells express elevated concentrations of regulatory cytokines, which inhibit HIV-1 replication, and possess intrinsic antiviral properties. Yet viral-induced activation with maternal HCMV may override this protection to facilitate in-utero. IFNs plays unique role to counter pathogen invasion at mucosal sites and they stimulate pathogen clearance while controlling inflammation to maintain barrier integrity(78,79).
These novel placental factor (PF) which is a small, heat- and pH-stable molecule with broad activity against different strains of HIV-1, and does not share identity with any other known cytokines. Study suggest that placental derived novel PF exabit an antiviral properties that protects the fetus during gestation [80]. Ouyang Y et al., studied extracellular vesicles (EVs) derived from human trophoblasts. EVs contain a distinct repertoire of proteins found to suppress the replication of a wide range of diverse viruses and exhibit the highest antiviral activity(81).
1.10. Potential role of placental growth factors as anti-viral drugs
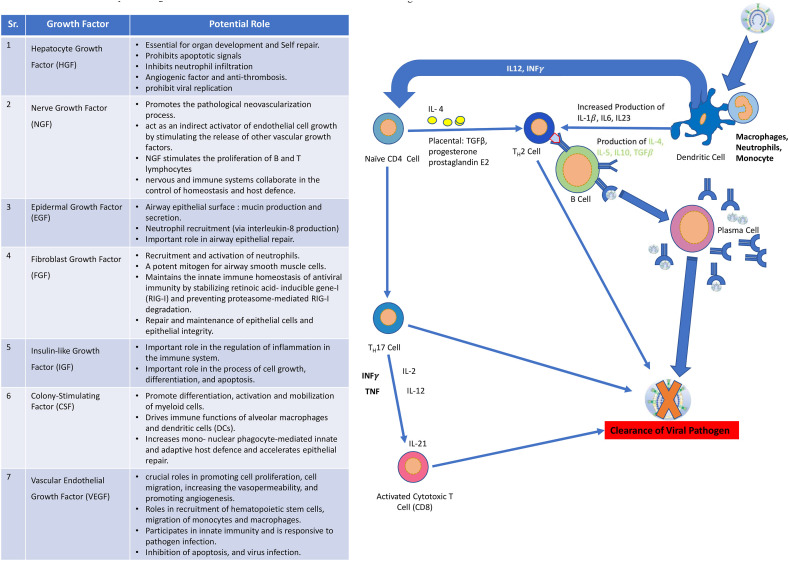
Placental growth factors are expressed in greatest quantities under normal conditions within villous cytotrophoblastic tissue and the syncytiotrophoblast which forms the barrier between maternal and foetal blood from sixth month of gestation. Placental growth factor concentrations peak during the third trimester, thus ample amounts of growth factors can be isolated for clinical use.Placental growth factors have mitogenic, angiogenic, and immunoregulatory properties, enhancing cellular survival, and involved in host defensive mechanism. Placental growth factors, chemokines and cytokines play important role in adapting host to newer infections. Important role of placental growth factors in host defence are presented in Table 2 .
Table 2.
Potential role of placental growth factors in host defense.
| Sr. | Growth Factor | Potential Role |
|---|---|---|
| 1. | Hepatocyte Growth Factor (HGF) | *Essential for organ development and Self |
| Repair | ||
| *Prohibits apoptotic signals | ||
| *Inhibits neutrophil infiltration | ||
| *Angiogenic factor and anti-thrombosis | ||
| *prohibit viral replication | ||
| 2. | Nerve Growth Factor (NGF) | *Promotes the pathological neovascularization process. |
| *act as an indirect activator of endothelial cell growth by stimulating the release of other vascular growth factors. | ||
| *NGF stimulates the proliferation of B and T lymphocytes. | ||
| *nervous and immune systems collaborate in the control of homeostasis and host defence. | ||
| 3. | Epidermal Growth Factor (EGF) | *Airway epithelial surface: mucin production |
| and secretion. | ||
| *Neutrophil recruitment (via interleukin-8 | ||
| production) | ||
| *Important role in airway epithelial repair. | ||
| 4. | Fibroblast Growth Factor (FGF) | *Recruitment and activation of neutrophils. |
| *A potent mitogen for airway smooth muscle cells. | ||
| *Maintains the innate immune homeostasis of antiviral immunity by stabilizing retinoic acid- inducible gene-I (RIG-I) and preventing proteasome-mediated RIG-I degradation. | ||
| *Repair and maintenance of epithelial cells and epithelial integrity. | ||
| 5. | Insulin-like Growth Factor (IGF) | *Important role in the regulation of inflammation in the immune system. |
| *Important role in the process of cell growth, differentiation, and apoptosis. | ||
| 6. | Colony-Stimulating Factor (CSF) | *Promote differentiation, activation and mobilization of myeloid cells. |
| *Drives immune functions of alveolar macrophages and dendritic cells (DCs). | ||
| *Increases mono- nuclear phagocyte-mediated innate and adaptive host defence and accelerates epithelial repair. | ||
| 7. | Vascular Endothelial Growth Factor (VEGF) | *crucial roles in promoting cell proliferation, cell migration, increasing the vasopermeability, and promoting angiogenesis. |
| *Roles in recruitment of hematopoietic stem cells, migration of monocytes and macrophages. | ||
| *Participates in innate immunity and is responsive to pathogen infection. | ||
| *Inhibition of apoptosis, and virus infection. |
2. The hepatocyte growth factor (HGF)
HGF is anmitogenic acidic protein expressed strongly in the villous syncytium, extravillous trophoblast, and amnionic epithelium, and, to a lesser degree in endothelial cells and villous mesenchyme [82]. HGFcan function as anti-apoptotic and anti-inflammatory which will have protective effects on epithelial and non-epithelial organs. HGF also plays important role inhematopoiesis that promotes the proliferation and colony formation of hematopoietic progenitors [83]. During viral infections, neutrophils, eosinophils and macrophages accelerate local or systemic inflammation resulting in tissue injury. Upon inflammatory stress, mesenchymal cells synthesize HGF that inhibitsneutrophil infiltration via the down-regulation of adhesion molecules (such as ICAM-1/E-selectin) on the endothelial cell surface [84]. It has been hypothesized that reduced HGF production in elderly patients can be corrected using the placental HGF, an intervention that would be of great use in management of aspiration pneumonia, a common respiratory ailment in this age group due to impairments of swallowing and of the cough reflex(85). Shigemura, N et al. administeredHGF cDNA to emphysematous rats and reported extensive pulmonary vascularization and increased proliferation of alveolar epithelial cells, which, in turn, improved exercise tolerance and gas exchange [86].Thus HGF has the potential to improve acute and chronic inflammatory disorders via its regenerative, anti-apoptotic, and anti-fibrotic effects.
2.1. Nerve growth factor (NGF)
NGFis a neurotrophic peptide biomolecule that supports the growth, survival and differentiation of both developing and mature neurons in central and peripheral nervous system. NGF is produced and utilized by several cell types, including structural (epithelial cells, fibroblasts/myofibroblasts, endothelial cells, smooth muscle cells and hepatocytes), accessory (glial cells, astrocytes and Muller cells) and immune (antigen presenting cells, lymphocytes, granulocytes, mast cells and eosinophils) cells. It is recognized as a pleiotropic factor. The NGF is a potent angiogenic factor with receptors on endothelial and vascular smooth muscle cells. Activation of these receptors indirectly activates endothelial cell growth by stimulating release of vascular growth factors [87].NGF is considered as potent angiogenic factor. Endothelial cells, vascular smooth muscle cells have NGF receptor and upon activation it indirectly activate endothelial cell growth by stimulating the release of other vascular growth factors [[88], [89], [90]].NGFalso modulates immune functions by stimulating the proliferation of B and T cells and the production of IgM, IgA (a1) and IgG4 antibodies [91]. NGFactivates interleukin-2 receptors on human peripheral blood mononuclear cells and promotes the growth and proliferation of human hematopietic cell(92). NGFis also involved in chemotaxis, viability and functional properties of human polymorphonuclear neutrophils and differentiation of thymic stromal non-lymphoid cells [93]. NGF can induce the shape of platelets and act as an autocrine survival factor for memory B cell [94,95]. It is been found that NGF stimulate connective tissue mast cells and daily injections of NGF in neonatal rats resulted in robust connective tissue mast cell hyperplasia in several peripheral tissues [96,97].
Thus, NGF carry different biological functions within and outside the nervous system. NGFinfluenced sympathetic, parasympathetic and sensory nervous system regulates immunity by antigen processing and presentation, Th1/Th2 balance, immunoglobulin production and antigen-specific responses. Importantly, NGF facilitate the collaboration between nervous and immune systems and maintains homeostasis and host defence.
2.2. Epidermal growth factor (EGF)
This was the first ligand of the EGF Receptor which is one of the superfamily of transmembrane receptors with intrinsic Receptor Tyrosine Kinase (RTK) activity. The EGF Receptors are one of 58 RTKs through which cells receive information from the external milieu and integrate them with intracellular responses.Epidermal growth factor (EGF) receptor (EGFR), also known as ErbB1/HER1, is the prototype of the EGFR family that also includes ErbB2/HER2/Neu, ErbB3/HER3, and ErbB4/HER(98,99). EGF is a functionally versatile polypeptide that plays an important role in regulating cell growth, survival, migration, apoptosis, proliferation, and differentiation. It leads to autophosphorylation of receptor tyrosine kinase (RTK) and subsequent activation of Ras/mitogen-activated protein kinases (Ras/MAPK), phosphatidylinositol 3-kinase/AKT (PI3K/AKT), phospholipase C-γ/protein kinase C (PLC-γ/PKC), and STATS signal pathways, to promote intestinal development, regulate tight junction protein expression, reduce cell autophagy, inhibit apoptosis induced by oxidative stress, and reduce the colonization of the intestinal epithelium by entero-pathogens(100).It has been linked to various cancers like small cell lung cancer, glioblastoma, head and neck cancer, breast cancer, pancreatic cancer, metastatic colorectal carcinoma through the EGFR upregulation that is commonly seen in them(99). EGF is implicated in the morphogenesis of teeth, brain, reproductive tracts, skin, gastrointestinal tracts, in cardiovascular differentiation and function, epithelial regeneration, and corneal epithelia(101). With respect to airways, deposition of pathogens or noxious particles on the epithelium generates an innate immune response through the EGF receptor. The EGR receptor signalling pathway produces various responses like mucin production and secretion, neutrophil recruitment (vis IL-8 production and epithelial wound healing in the lung tissue(102). These responses through EGFR signalling also respond to mechanical stress of the respiratory epithelium. Sequential actions following EGFR signalling in mechanically stressed epithelium involves endogenous nucleotides, G-protein and non-G-Protein coupled receptors leading to IL-8 production and macrophage-release of TGF-α(103,104). .
Air way epithelium is an important barrier against invading microorganisms, and epithelial innate immune mechanisms through EGFR signalling provide important pathways for repairing wounded epitheliumvia mechanical stimulation. The mechanical stress, endogenous nucleotides, such as ATP and UTP, are released into the extracellular space from mechanically stressed epithelium, stimulating epithelial cell proliferation [103]. These nucleotides bind to specific G-protein-coupled receptors (GPCR) and to non-GPCRs and stimulate IL-8 production in airway epithelial cells [104]. Activated EGFR signalling activate macrophage to produce TGF and contribute in the epithelial repair process [105].
The airway epithelium is the first site of contact with inhaled viral particles. Inhaled viral particles depositing on the airway epithelial surface activate EGFR signalling pathways. EGFR activation results in the production of mucins to assist in the clearance and IL-8 recruits neutrophils, and also stimulates epithelial repair(106).
2.3. Fibroblast growth factor (FGF)
Basic fibroblast growth factor (bFGF or FGF2) is a potent mitogen for many cell types, including airway smooth muscle cells, fibroblasts, and endothelial cells [107]. FGF2 can be released from inflammatory cells including T lymphocytes, eosinophil, mast cells, macrophages, and myeloid dendritic cells [[108], [109], [110]]. FGF carry out multiple biological processes by signalling through FGF receptors, including tumor angiogenesis, embryonic development, differentiation, proliferation, migration, and injury repair [[111], [112], [113]].FGF2 generate innate immune response by stabilizing retinoic acid-inducible gene-I (RIG-I) and preventing proteasome-mediated RIG-I degradation(114). Wang W et al. explored the role of FGF2 in host defence against influenza-A viral (IAV) infection using mouse model(115). Results, indicated that FGF2 plays a pivotal role in IAV-induced lung injury, and recombinant FGF2 protein administration markedly reduces mortality and the severity of lung injury. The underlying mechanisms were neutrophil activation and recruitment via the PI3K-Akt-NFκBsignalling pathway. Endothelial cell stress triggered by inflammatory mediators such as IL-25, IL-1β, TNF-α, prostaglandin E2 (PGE2) and IFN-α/IL-2 result in release of endothelial FGF2which might result in angiogenic response [116].In turn,FGF2 stimulate endothelial cells to produce various pro-inflammatory factors and chemo attractants, including IL-6, TNF-α, and monocyte chemo attractant protein 1 (MCP-1) [117].Thus, FGF functions as immunomodulatory factor by inducing the secretion of pro inflammatory factors in airway diseases. Role of FGF2 in modulating the function of airway cells in remodelling, inflammation, and lung function could provide potential alternative options for patients that are unresponsive to current anti-inflammatory treatments being used in COVID-19.
2.4. Insulin-like growth factor (IGF)
IGF belongs to the insulin-like growth factor family, which includes growth hormone (GH), insulin-like growth factor II (IGF2), insulin-like growth factor 1 receptor (IGF1R), insulin-like growth factor II receptor (IGF2R), and insulin-like growth factor binding protein 1–6 (IGFBP1–6) [118]. IGF family plays an important role in the cellular growth, differentiation, and apoptosis [119,120]. IGF1 mainly functions by binding to IGF1R, a transmembrane protein composed of two α domains that binds to IGF1and activates two β domains [121]. The β domain has tyrosine kinase activity which promote the phosphorylation of the hepatocyte growth factor (HGF), docking protein insulin receptor substrate (IRS), vascular endothelial growth factor (VEGF), and growth factor receptor binding protein 2 (Grb2) [122]. IGF plays an important role in the regulation of inflammation. IGF1binds to the receptor and activates the PI3K/AKTsignalling pathway and induces Akt activation, which further activates the downstream IL-17-mediated inflammatory pathway [123].Asthmatic patients exhibit higher bronchial cell IGF1 mRNA expression than normal people and this was associated with fibrosis in epithelial cells [124]. IGF1is known to alleviate the inflammatory response by recruiting T regulatory cells to secrete IL-10 which is the anti-inflammatory cytokine [125]. Li G et al. studied the role of IGF1 in mediating inflammation and pathology during influenza infection. They found that IGF1 mRNA and protein increased after influenza virus infection. This overexpression of IGF1 aggravated cytokine expression, triggering the PI3K/AKT and MAPK signalling pathways to induce an inflammatory response(126).Rao P et al. studied role of IGFBP-3 in the pathogenesis of herpes stromal keratitis (HSK). Results showed an increased level of IGFBP-3 in HSK developing corneas and lack of IGFBP-3 resulted in the exacerbation of HSK which was associated with an increased number of leukocytes in infected corneas of IGFBP-3−/− than B6 mice. Thus depending upon the cellular microenvironment, IGFBP-3 can either have a protective or damaging effect in an ongoing inflammation [127].Very recently, a study by Fang J et al. demonstrated the fate and behaviour of muscle stem cells (MuSCs) during muscle repair and regeneration. Study revealed that MuSCs produce a large amount of insulin-like growth factor-2 (IGF-2) that results in macrophages maturation. Macrophages undergo oxidative phosphorylation and acquire anti-inflammatory properties(128).IGF family plays an important immune function in inflammatory lung injury and may provide a therapeutic target for humans in response to COVID-19 outbreak.Last year, researchers demonstrated the inhibitory effect of IGF-1 on phagocytosis by alveolar epithelial cells, with enhanced phagocytic activity noted on IGF-1 blockade in asthmatic mice. This supports potential use of IGF-1 as a target in inflammatory pulmonary conditions(129).
2.5. Colony-stimulating factor (CSF)
Initially believed to have relevance to haemopoietic system, CSFs are important modulators of immune response in human body. The Macrophage-CSF, produced by endothelial cells, fibroblasts, osteoblasts, smooth muscle, and macrophages is responsible for homoestatic maintenance of several myeloid lineages without changing their ‘activation’ state. Conversely, the Granulocyte-Macrophage CSF (GM-CSF) causes activation of monocytes/macrophages and also mediates their differentiation and maturation to effector forms like dendritic cells or iNKT Cells(130). Levels and expression of GM-CSF are increased during inflammatory responses and at sites of inflammation with proinflammatory cytokines inducing their expression and anti-inflammatory cytokines like IL-4, IFN- γ and IL-10 suppressing it.Increased mRNA expression and levels of pro-inflammatory cytokines like IL-23, IL-12p70,TNF, IL-1β, and IL-6 is seen monocytes/macrophages in vivo that are treated with GM-CSF, but high cytokine secretion by these ‘primed’ cells need the additional stimulus of lipopolysaccharide (LPS) [131]. However, cells primed by M-CSF are seen to produce IFN-, TGF-, and IL-10 in response to LPS Expression of GM‐CSF is induced by proinflammatory cytokines, such as IL‐1α, IL‐1β, TNF‐α, and IL‐12, whereas IL‐4, IFN‐γ, and IL‐10 suppress it. At sites of inflammation, GM‐CSF has proinflammatory effects through recruitment of myeloid cells and by enhancing their survival and activation([132], [133], [134]). Granulocyte/macrophage colony-stimulating factor (GM-CSF) and macrophage CSF (M-CSF) modulate differentiation and immune functions of macrophages.M-CSF and GM-CSF are cytokines that have the capability of aiding myeloid cell differentiation.CSFs are involved in proinflammatory cytokine response responsible for immunopathogenesis of several inflammatory or autoimmune diseases [132]. It maintains DC homeostasis in nonlymphoid tissue and modulates the development of inflammatory macrophages and monocytes [135]. Macrophages exposed to GM-CSF induce secretion of proinflammatory cytokines, such as IL-23, IL-12p70, TNF-, and IL-6 in response to LPS, whereas M–CSF– expose cells produced IFN-, TGF-, and IL-10 in re-sponse to LPS stimulation(132–134).
Dendritic cell (DC) homoeostasis in nonlymphoid tissue is maintained by GM-CSF. Dendritic or macrophages in response to bacterial infection or LPS stimulation secrete IL-23 and IL-12 resulting in NK and T cell activation leading to IFN- production. Production of IL-12 result inTh1-driven immune responses, whereas IL-23 enhances the proliferation of Th17 cells [25,136]. In late phase of sepsis, DCs secrete lesser amounts of IL-12 which are required for the T-Helper (Th)1 response. Use of GM-CSF and IFNγ in sepsis has been shown to restore IL-12 production by splenic DCs. Importantly, IL-10 is secreted by macrophages and monocytes to control inflammation after the infection is resolved [137].
Alothaimeen T et al. defined how GM-CSF and classical M–CSF–derived macrophages differ with regard to their ability to support the proliferation of antigen-specific CD8+T cells, as well as phenotypic and functional characteristics when responding to a virus infection. GM- CSF derived macrophages inducing a stronger proinflammatory cytokine response than M-CSF derived macrophages. While the M-CSF derived macrophages induce strong anti-inflammatory cytokine IL-10, indicating that these subsets have signature cytokines in response to virus infection and may regulate adaptive immunity eventually to different outcomes [138].Role of GM-CSF has been studied in acute states like sepsis and chronic inflammations like asthma, rheumatoid arthritis as well as in metastatic breast cancer. In the lung, GM- CSF play an important role in antimicrobial pulmonary host defence function [139].The balance of GM-CSF and M-CSF is of immense importance during states of inflammation. While further elucidation is required on the exact phenotype of patients who would benefit from CSFs and the time of administration in disease states, their role during inflammatory states though complex is well acknowledged. They thus provide a target for modulation of immune responses during infections. Thus, the balance of these cytokines after infection could be critical for clearing infections. Clinical benefit of intravenous injection of GM-CSF treatment in adult patients with severe sepsis and respiratory dysfunction were encouraging. GM-CSF treatment resulted in improved gas exchange and might play a homeostatic role(140). Therapeutic application of GM-CSF increases mononuclear phagocyte-mediated innate and adaptive host defense and accelerates epithelial repair processes during severe pneumonia. There is evidence that it might be a powerful antiviral therapy in pneumonia. Data suggest that GM-CSF compartment of Placental extract application seems to be most promising strategy for COVID-19 treatment.
2.6. Vascular endothelial growth factor (VEGF)
Placental Growth Factor (PlGF) is an angiogenic protein, belonging to the Vascular Endothelial Growth Factor (VEGF) family that is expressed in placenta. VEGF induces the proliferation, sprouting, and migration of endothelial cells and it regulates endothelial cell survival, and vascular permeability(141,142). A recent study describes the role of PlGF in cross-talk with the immune system, where PlGF was selectively secreted by activated TH17 subset of helper T-cells and stimulated neovascularization in vitro and in vivo. The study also postulated a role for PlGF in differentiation of TH17 cells and autoimmunity(143). The VEGF has high relevance to pulmonary integrity as it affects development and structural maintenance of the lung in addition to impacting the functions through its effect on Nitric Oxide and prostacyclins. Type II pneumocytes undergo differentiation under the influence of the VEGF stimulation. Upregulation has been also described in pulmonary pathological conditions such as acute lung injury, asthma, emphysema, chronic obstructive pulmonary disease and pulmonary hypertension. Overexpression of VEGF in murine lung was seen to induce an asthma-like phenotype with inflammation, remodelling, metaplasia, hyperplasia of mycytes and dendritic cells and augmented Th2 inflammation([144], [145], [146]). An age-dependent loss of the pro-survival effect of VEGF has been described on muscle, bone, vascular endothelialcells and progenitor cells. Mitochondrial dysfunction is a phenomenon underlying the process of aging, which has been reported to block VEGF expression and contribute to impaired angiogenesis. This phenomenon may extend to the lungs and could be a possible link between the increased mortality of elderly and severe lung infections as is seen in the COVID-19 pandemic too(147,148). Improving understanding of the role of VEGF has been obtained through animal studies. Kasahara et al. showed in rat-model that chronic treatment of rats with the VEGF receptor blocker SU5416 causes alveolar cell apoptosis–dependent emphysema within weeks [149].The baud et al. created a phenotype similar to bronchopulmonary dysplasia with alveolar simplification and loss of lung capillaries by VEGF blockade in new born rats. The model of irreversible lung injury showed reversal using Postnatal intratracheal adenovirus-mediated VEGF gene therapy with promotion of capillary formation, reduced vascular leak, preserved alveolar development and improved survival(150).Kumar, PA et al., studied the lung post-injury regeneration after H1N1 influenza-infected mice. lung regeneration started with endothelial proliferation, activation of distal airway stem cells, alveolar regeneration, and restoration of alveoar capillaries after H1N1 influenza infection(151).Ramasamy SK et al., elaborated the VEGF signalling cascade and involvement of other factors. VEGF and FGF signalling induced expression of MMP14 on endothelial cells, which led to the release of active EGF-like fragments from heparin-binding EGF-like growth factor (HB-EGF) and the laminin52 subunit. This led to the activation of EGFR in alveolar epithelial cells and bronchoalveolar stem cells (BASCs), proliferation of BASCs, and alveolar epithelium(152). Robust experimental and clinical evidence on role of VEGF in inflammatory and angiogenic responses are present in diseased lungs. The VEGF (PlGF) compartment of placental extracts will thus undoubtedly play a major role in function and integrity of alveolar epithelial cells, septaeandpulmonary capillaries in inflammatory responses due to CoVID-19 infection.Placental growth factor (PlGF) is a member of the vascular endothelial growth factor (VEGF) family found in placental extract. Angiogenesis is an important physiologic process which play important role in maintaining vasculature during wound healing and different diseases pathology. VEGF induces the proliferation, sprouting, and migration of endothelial cells and it regulates endothelial cell survival, and vascular permeability [142]. Bhandari V et al. demonstrated that the overexpression of VEGF in the murine lung induces an asthma-like phenotype with inflammation, parenchymal and vascular remodelling, oedema, mucus metaplasia, myocyte hyperplasia, AHR, dendritic cell (DC) hyperplasia and activation, enhanced respiratory antigen sensitization, and augmented Th2 inflammation. VEGF plays an important role in antigen-induced Th2 inflammation and IL-4 and -13 elaboration(147).Animal studies in the adult lung, (conditional genetic knockout or chronic pharmacological inhibition) demonstrated that vascular VEGFR2 is required for maintenance and repair of the lung [144,145].The expression of VEGF abundantly found in capillary endothelial cells which play an crucial role in maintaining the integrity of capillary beds. Report of Kasahara et al., showed that use of Fc-Anti VEGF blockade for VEGF signalling results in emphysema like phenotype within weeks [149].Thebaud B et al., reported that VEGF blockade decreases lung VEGF and VEGFR-2 expression in newborn rats and impairs alveolar development, leading to alveolar simplification and loss of lung capillaries, mimicking Bronchopulmonary dysplasia (BPD). This injury can be reversed using Postnatal intratracheal adenovirus-mediated VEGF gene therapy which improves survival rate, promotes lung capillary formation, reduces the vascular leakage and preserves alveolar development in this model of irreversible lung injury [150].Kumar, PA et al., studied the lung post-injury regeneration after H1N1 influenza-infected mice. lung regeneration started with endothelial proliferation, activation of distal airway stem cells, alveolar regeneration, and restoration of alveolarcapillaries after H1N1 influenza infection [151]. Ramasamy SK et al., elaborated the VEGF signalling cascade and involvement of other factors. VEGF and FGF signalling induced expression of MMP14 on endothelial cells, which led to the release of active EGF-like fragments from heparin-binding EGF-like growth factor (HB-EGF) and the laminin 52 subunit.
This led to the activation of EGFR in alveolar epithelial cells and bronchoalveolar stem cells (BASCs), proliferation of BASCs, and alveolar epithelium(151,153). Thus, given pivotal and specific role of VEGFRin vascular homeostasis, there is robust clinical evidence of direct involvement of VEGF in regeneration of disturbed vasculature in diseased lung. VEGFR compartment in the placental extract will undoubtedly facilitate the regeneration of alveolar epithelial cells, restoration of alveoar capillaries after CoVID-19 infection.
Placental Extract derived cytokine and chemokines:Probable protective mechanism against CoVID-19.
Human body get exposure to numerous pathogens daily, and majority of them are immediately tackled and cleared off the body. Pathogenic attach on the body is been taken care by immune system in two different defence systems: these are the innate and the adaptive immune systems. Innate immune system provides immediate attack on pathogen with broad specificity or prolonged protection with exquisite specificity. Cytokines are small functional proteins play an important role in initial immune response to infection and in adaptation of immune response to foreign or self-antigens. The tissue specific cells release cytokines as message to attract the help from immune system. The chemokines are a family of chemo attractant cytokines which are also released form variety of cells in response to viruses; stimulate cells that undergo changes in cell adhesiveness, and cytoskeleton, resulting in a directed migration of the cell. They mainly attract different leukocytes,and recruiting effector cells onto the sites of infection, initiate an inflammatory response. Probable protective mechanism and involvement of cytokine and chemokines for COVID-19 infection is presented in Fig. 5.The acute-phase inflammatory responses are initiated by viral exposure.The phagocytes, neutrophils, monocytes, and macrophages rapidly come in to action after exposure of virus. This exposure results in synthesis of macrophage derived cytokines to initiate the inflammation. These cytokines induce the adhesion of neutrophils, monocytes, macrophages and dendritic cells, to migration towards inflammatory cells. Macrophages phagocytize viral particles and act as an antigen presenting cell (APC) that can be recognized by antigen-specific T cells, activating production of IL-1. Macrophages produce of IL-1, IL-6, and TNF-α as a pyrogenic response while production of IL-10 results in TH2 activation, and production of IL-12 results in TH1 activation. NK cells can be activated by IFN-γ, IL-2, IL-12, and TNF to amplify the lysis of infected cell. The production of IFN-γ by NK cells may influence the CD4+ T cell response to infectious cells, and they differentiate into pro-inflammatory TH1 cells able to activate macrophages [26,27]. The DCs are considered to be the main antigen-presented cells which can migrate to any tissue and inspect the cellular appearance. They efficiently present antigens to T cells in lymphoid organs. The DCsexpress several Toll-like receptors (TLRs) the key players in the interactions between the innate and adaptive immune systems, and able to detect the presence of viral components on the infected cells. TLRs induce several chemokines such as IL-8 and the inflammatory cytokines IL-1β and IL-12(23).Placentation after pregnancy can be defined as temporary allogenic transplantation where maternal immune system and placenta collaboratively take a challenge to accept and facilitate fetal growth till birth. The maternal immune system is regulated by placental derived cytokines to protect the the fetus and to promote proper development. Tolerance to an fetusallo graft is achieved by an increase in TH2 cytokines including IL-4 and IL-10 and a decrease in TH1 cytokines such as IL-2 and IFN-γ. Placenta plays an central role in controlling pro-inflammatory phase during the early pregnancy and anti-inflammatory phase till complete development of fetus(154). Precursor T cells generated in the bone marrow and then migrate to prime in the thymus. T cells are having distinct populations and different functionality: the CD4+ T helper, or TH cells, and the CD8+cytotoxic cells. TH cells show four subsets: TH0, TH1, TH2, and TH17 effector cells and regulatory T cells (T regs). Antigen stimulation results in differentiation Naive T cells to intermediate state termed TH0. The decision as to whether the TH0 will develop into an inflammatory TH1 cell, a helper TH2 cell, or a TH17 cell depends on cytokine environment at the site of priming(24,25). Activation and adaptation of immune system depends on the cytokine signals of TH cell. Naïve T cells stimulated with TGF-ᵦand IL-6 results in differentiation in to TH17 cells. TH17 cells secrete important cytokines IL-17 family which has pleiotropic effect on the immune cells. IL-17 stimulates the production of inflammatory cytokines, such as IL-6, TNF-, IL-1, chemokines (CXCL1, CXCL3, CXCL5, CXCL6), and several growth factors G/GM-CSF, and VEGF [141].TH17 cell also produces other important effector molecules, such as IL-21, IL-22, IL-26, IL-6 and CCL20(28). Typical cytokines secreted by human TH1 are IFN-γ and TNF-β which activate macrophages and the cytotoxic lymphocytes, resulting in a cell-mediated immune response. Cytokines secreted by human TH2 are IL-4, IL-9, and IL-13 help to activate B cells, resulting in antibody production.TH1 cells inhibits TH2 cells through production of IFN-γ and IL-10 produced by TH2 cells inhibits production of IFN-γ by TH2 cells [155].IL-4 inhibits production of TH1 cells and differentiation of TH17 cells. Thus, the activation of the immune system is directed at the viral antigen. Cell-mediated response is generated for intracellular pathogens and antibody related response is generated for extracellular response of infected cell. Cytokines IL-2, IL-4, and IL-5, contribute to B cell proliferation during the active antiviral response, where IL-4 and IL-5 are the most potent inducers of antibody secretion by the B cell. Macrophages and T cells secrete IL-6, which is a growth factor for differentiated, antibody secreting B cells [156,157]. The next step towards anti-viral signalling for B cells is to develop into effector plasma cells clone the particular viral antigen. Plasma cells found in lymphoid organs and at the sites of immune responses. Effector cells generated in the mucosal immune system converted into memory cells and these clones can be reactivated when the same viral pathogen is encountered again. Thus long-lasting immunity can be achieved for secondary immune response [158].
3. Conclusions
The SARS-2 coronavirus and the resulting pandemic has taken the entire world by storm with a rapid spread and high morbidity and mortality that has challenged health care services in many countries. Large numbers of efforts are directed to finding management solutions for the disease and a cure for the virus. Regenerative medicine is one such field that has the potential to provide required answers. Clinical applications of placental extracts have been shown to be beneficial and there is now an acute need for researchers in the field to respond in a manner that satisfies the scientific temperament of this evidence-based era. This review aims to underline the immense possibilities available in this field and highlight some of the available scientific data that support further inquiry. Two predominant areas that can be targeted using placental extract are 1) Generating an early, protective immune response against SARS CoV-2 and 2) Moderating an immune overreaction such as cytokine storm that is the cause of complications and even death. It bears repetition that the placenta is an armoury with balance of inflammatory, anti-inflammatory, and immunomodulatory bioactive molecules that are potential weapons in the fight against novel, emerging infections. Well-designed, outcome-oriented clinical trials to test placental extract as an antiviral agent could provide the answers the world seeks.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this review.
Antigen-specific immune response is efficiently involved in clearing of the pathogens. Dendritic cells serve as a link between antigens and T cells by recognizing the pathogen in the form of antigenic determinants, process these antigens and present it to T cells. The exposure of phagocytes, neutrophils, monocytes, and macrophages result in the synthesis of cytokines to initiate the inflammation. Macrophages migrate toward the site of inflammation by chemotaxis and act as APC recognized by T cells and activates IL-1. Macrophages produce of IL-1, IL-6, and TNF-α while IL-10 and IL-12 results in TH2 and TH1 activation respectively. Activated NK cells amplify the lysis of infected cells. The production of IFN-γ by NK cells influences the CD4+ T cell response to infectious cells.
References
- 1.Tyrrell D.A., Bynoe M.L. Cultivation of viruses from a high proportion of patients with colds. Lancet. 1966;19:76–77. doi: 10.1016/s0140-6736(66)92364-6. [DOI] [PubMed] [Google Scholar]
- 2.Almeida J.D., Tyrrell D.A. The morphology of three previously uncharacterized human respiratory viruses that grow in organ culture department of medical microbiology. J. Gen. Virol. 1967;1:175–178. doi: 10.1099/0022-1317-1-2-175. [DOI] [PubMed] [Google Scholar]
- 3.Tyrrell D.A., Almeida J.D., Cunningham C.H. Coronaviridae. Intervirology. 1975;5:76–82. doi: 10.1159/000149883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik Y.S., Sircar S., Bhat S., Sharun K., Dhama K., Dadar M. Emerging novel coronavirus ( 2019-nCoV )— current scenario , evolutionary perspective based on genome analysis and recent developments. Vet. Q. 2020;40:68–76. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J.F., Yuan S., Kok K., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission : a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu D., Wu T., Liu Q., Yang Z. International journal of infectious diseases the SARS-CoV-2 outbreak : what we know. Int. J. Infect. Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin Y., Cai L., Cheng Z., Cheng H., Deng T., Fan Y. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus ( 2019-nCoV ) infected pneumonia ( standard version ) Military Medical Research. 2020;7:1–23. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;10:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Report S. 2020. Coronavirus Disease 2019 ( COVID-19 ) 2019(April) [Google Scholar]
- 10.Boheemen S.V., Graaf M De, Lauber C., Bestebroer T.M., Raj V.S., Zaki M. Genomic Characterization of a Newly Discovered Coronavirus associated with acute respiratory distress syndrome in humans. mBio. 2012;3:1–9. doi: 10.1128/mBio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raj V.S., Mou H., Smits S.L., Dekkers D.H., Müller M.A., Dijkman R. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M., Weber K., Schroeder H., Mu S., Drosten M.A., Po C. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven article SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:1–10. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams A.E., Chambers R.C. The mercurial nature of neutrophils : still an enigma in ARDS ? 2014. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;306:217–230. doi: 10.1152/ajplung.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan , China : a single-centered , retrospective , observational study. Lancet Respir Med. 2020;2600:1–7. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cameron M.J., Bermejo-martin J.F., Danesh A., Muller M.P., Kelvin D.J. Human immunopathogenesis of severe acute respiratory syndrome ( SARS ) Virus Res. 2008;133:13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan , China. Lancet. 2020;6736:1–10. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tripathi S., White M.R., Hartshorn K.L. The amazing innate immune response to influenza A virus infection. Innate Immun. 2015;21:7398. doi: 10.1177/1753425913508992. [DOI] [PubMed] [Google Scholar]
- 18.Kreijtz J.H.C.M., Fouchier R.A.M., Rimmelzwaan G.F. Immune responses to influenza virus infection. Virus Res. 2011;162:19–30. doi: 10.1016/j.virusres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Conti P.1, Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., Kritas S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34:11–15. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 20.Wang L., Quan Y., Yue Y., Heng X., Che F. Interleukin-37 : a crucial cytokine with multiple roles in disease and potentially clinical therapy. Oncology letters. 2018;15:4711–4719. doi: 10.3892/ol.2018.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Favre L., Spertini F., Corthe B. Stimulating mucosal and systemic immune responses. J. Immunol. 2020;175:2793–2800. doi: 10.4049/jimmunol.175.5.2793. [DOI] [PubMed] [Google Scholar]
- 22.Hemmi H., Akira S. TLR signalling and the function of dendritic. CellsChem Immunol Allergy. 2005;86:120–135. doi: 10.1159/000086657. [DOI] [PubMed] [Google Scholar]
- 23.Bowie A., Kiss-toth E., Symons J.A., Smith G.L., Dower S.K., Neill L.A.J.O. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. Unit. States Am. 2000;97:10162–10167. doi: 10.1073/pnas.160027697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macrophages A. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cell. 2013;31:1980–1991. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- 25.Kao C., Oestreich K., Paley M. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat. Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janeway C.A., Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 27.Hunt K.J., Walsh B.M., Voegeli D., Roberts H.C. Inflammation in aging Part 1 : physiology and immunological mechanisms. Biol. Res. Nurs. 2010;11:245–252. doi: 10.1177/1099800409352237. [DOI] [PubMed] [Google Scholar]
- 28.Liang S.C., Tan X., Luxenberg D.P., Karim R., Dunussi-joannopoulos K., Collins M. Interleukin ( IL ) -22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. j of experimental medicine. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guglani L., Khader S.A. Th17 cytokines in mucosal immunity and inflammation. Curr. Opin. HIV AIDS. 2010;5:120–127. doi: 10.1097/COH.0b013e328335c2f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braciale T.J., Sun J., Kim T.S. Regulating the adaptive immune response to respiratory virus infection. Nat Publ Gr. 2012;12:295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malek T.R., Castro I. Review interleukin-2 receptor Signaling : at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang N., Bevan M.J. CD8 + T Cells : foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wissinger E, London IC. CD8+ T cells. Cells:8.
- 34.LeiJ, Li J., Li X., Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Images in radiology. 2020;18:295. doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren L.L., Wang Y.M., Wu Z.Q., Xiang Z.C., Guo L. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chinese Med J. 2020;133:1015–1024.. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan , China : a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Outcomes M. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Internal Medicine. 2020:1–10. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu Q. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Am. J. Roentgenol. 2020;215:1–6. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 39.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Disease 2019 in China. N. Engl. J. Med. 2020:1–13. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi Q., Zhao K., Yu J., Feng J., Zhao K., Zhang X. medRxiv; 2020. Clinical Characteristics of 101 Non-surviving Hospitalized Patients with COVID-19 — A Single Center , Retrospective Study. [Google Scholar]
- 41.Organi -W.H., January F., Control D., Control D., Seafood H., Market W. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020:1–3. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W. Detection of 2019 novel coronavirus ( 2019-nCoV ) byreal-time RT-PCR. Euro Surveill. 2020;25:1–8. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Us F.D.A. Important information on the use of serological (antibody) tests for COVID-19 - letter to health care providers. https://www.fda.gov/medical-devices/letters-health-care-providers/important-information-use-serological-antibody-tests-covid-19-letter
- 45.International clinical trials Registry Platform (ICTRP)Covid-19 trials. https://www.who.int/ictrp/en/ Accessed on 22nd April 2020 at.
- 46.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. Unit. States Am. 2020:1–7. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sponsor S. Overview of planned and ongoing clinical studies of vaccines for COVID-19 Version. vaccine till COVID. 2020;19:1–6. [Google Scholar]
- 48.Chakraborty P.D., Bhattacharyya D. Aqueous extract of human placenta as a therapeutic agent. In: Zheng J., editor. Recent Advances in Research on the Human Placenta. InTech; Rijeka, Croatia: 2012. pp. 77–92. [Google Scholar]
- 49.Shukla V.K., Rasheed M.A., Kumar M., Gupta S.K., Pandey S.S. A trial to determine the role of placental extract in the treatment of chronic non-healing wounds. J. Wound Care. 2004;13:177–179. doi: 10.12968/jowc.2004.13.5.26668. [DOI] [PubMed] [Google Scholar]
- 50.Hong J.W., Lee W.J., Hahn S.B., Kim B.J. The effect of human placenta extract in a wound healing model. Ann. Plast. Surg. 2010;65:96–100. doi: 10.1097/SAP.0b013e3181b0bb67. [DOI] [PubMed] [Google Scholar]
- 51.Pan S.Y., Chan M.K.S., Wong M.B.F., Klokol D., Chernykh V. Placental therapy : an insight to their biological and therapeutic properties. J Med Therap. 2017;1:1–6. [Google Scholar]
- 52.Chen S., Liu Y., Sytwu H. Immunologic regulation in Pregnancy : from mechanism to therapeutic strategy for immunomodulation. Clin. Dev. Immunol. 2012;2012:1–10. doi: 10.1155/2012/258391. D 258391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De D., Datta P., Bhattacharyya D. Analysis of free and bound NADPH in aqueous extract of human placenta used as wound healer. J. Chromatogr. 2009;877:2435–2442. doi: 10.1016/j.jchromb.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 54.Jung J., Lee H., Min J., Na K., Hwang S., Jin G. International Immunopharmacology Placenta extract promote liver regeneration in CCl 4 -injured liver rat model. Int. Immunopharm. 2011;11:976–984. doi: 10.1016/j.intimp.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Taylor P., Lee K., Kim T., Lee W., Hoon S., Lee S.Y. Anti-inflammatory and analgesic effects of human placenta extract. Nat. Prod. Res. 2011;25:1090–1100. doi: 10.1080/14786419.2010.489050. [DOI] [PubMed] [Google Scholar]
- 56.Park K.M., Cho T.H. Therapeutic effect of acupuncture point injection with placental extract in knee osteoarthritis. J Integr Med. 2017;15:135–141. doi: 10.1016/s2095-4964(17)60316-9. [DOI] [PubMed] [Google Scholar]
- 57.Marleau A.M., Mcdonald G., Koropatnick J., Chen C., Koos D. Reduction of tumorigenicity by placental extracts. Anticancer Res. 2012;32:1153–1162. [PubMed] [Google Scholar]
- 58.Kong M., Park S.B. Effect of human placental extract on health status in elderly Koreans. Evid. base Compl. Alternative Med. 2012:1–6. doi: 10.1155/2012/732915. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim M.J., Shin K.S., Jeon J.H., Lee D.R., Shim S.H., Kim J.K. Human chorionic-plate-derived mesenchymal stem cells and Wharton ’ s jelly-derived mesenchymal stem cells : a comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011;346:53–64. doi: 10.1007/s00441-011-1249-8. [DOI] [PubMed] [Google Scholar]
- 60.Joshi M., Patil P.B., He Z., Holgersson J., Sumitran-holgersson S., Joshi M. Fetal liver-derived mesenchymal stromal cells augment engraftment of transplanted hepatocytes Fetal liver-derived mesenchymal stromal cells augment engraftment. Cytotherapy. 2012;3249(14):657–669. doi: 10.3109/14653249.2012.663526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi J.H., Jung J., Na K., Cho K.J., Yoon T.K., Kim G.J. Effect of mesenchymal stem cells and extracts derived from the placenta on trophoblast invasion and immune responses. Stem Cell. Dev. 2014;23:132–145. doi: 10.1089/scd.2012.0674. [DOI] [PubMed] [Google Scholar]
- 62.Wu Y., Hoogduijn M.J., Baan C.C., Korevaar S.S., Kuiper R De, Yan L. Adipose tissue-derived mesenchymal stem cells have a heterogenic cytokine secretion profile. Stem Cell. Int. 2017;2017:1–7. doi: 10.1155/2017/4960831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Transplant R., Village M., Therapeutics O., Trust F., Kingdom U, unit L. Immunomodulation by therapeutic mesenchymal stromal cells ( MSC ) is triggered through phagocytosis of MSC by monocytic cells. Stem Cell. 2018;36:602–615. doi: 10.1002/stem.2779. [DOI] [PubMed] [Google Scholar]
- 64.Luk F., Carreras-planella L., Korevaar S.S., Witte SFH De, State P., Hershey M.S. Inflammatory conditions dictate the effect of mesenchymal stem or stromal cells on B cell function. Front. Immunol. 2017;8:1–13. doi: 10.3389/fimmu.2017.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonçalves F.C., Luk F., Korevaar S.S., Bouzid R., Ana H., López-iglesias C. Membrane particles generated from mesenchymal stromal cells modulate immune responses by selective targeting of pro- inflammatory monocytes. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-12121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Melief S.M., Geutskens S.B., Fibbe W.E., Roelofs H. Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematol. 2013;98:1–8. doi: 10.3324/haematol.2012.078055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng G., Huang R., Qiu G., Ge M., Wang J., Shu Q. Mesenchymal stromal cell-derived extracellular vesicles : regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018;374:1–15. doi: 10.1007/s00441-018-2871-5. [DOI] [PubMed] [Google Scholar]
- 68.Melief S.M., Schrama C.L.M., Brugman M.H., Tiemessen M.M., Hoogduijn J., Fibbe W.E. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes towards anti- inflammatory macrophages. Stem cells Translational and clinical research. 2013;31:1980–1991. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- 69.Deng Y., Zhang Y., Ye L., Zhang T., Cheng J. Umbilical cord-derived mesenchymal stem cells instruct monocytes towards an IL10- producing phenotype by secreting IL6 and HGF. Nat Publ Gr. Scientific Reports. 2016;6:1–9. doi: 10.1038/srep37566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singer N.G., Caplan A.I. Mesenchymal stem Cells : mechanisms of inflammation. Annu. Rev. Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 71.Yañez R., Oviedo A., Bueren J.A., Lamana M.L. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp. Cell Res. 2010;316:3109–3123. doi: 10.1016/j.yexcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 72.Meisel R., Zibert A., Laryea M., Go U. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2 , 3-dioxygenase – mediated tryptophan degradation. Immunobiology. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 73.Gomez-lopez W.F.N., Romero R., Margolis L., Erez O., Kennedy E., National S. Extracellular vesicles generated by placental tissues ex vivo : a transport system for immune mediators and growth factors. Am. J. Reprod. Immunol. 2018 doi: 10.1111/aji.12860. 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Son Y., Park J., Sakoda Y., Zhao Y., Hisamichi K., Kaku T. Preventive and therapeutic potential of placental extract in contact hypersensitivity. Int. Immunopharm. 2010;10:1177–1184. doi: 10.1016/j.intimp.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franco G.R., Carvalho AF De, Kroon E.G., Lovagie S., Werenne J., Golgher R.R. Biological activities of a human amniotic membrane interferon. Placenta. 2000;1999:189–196. doi: 10.1053/plac.1998.0364. [DOI] [PubMed] [Google Scholar]
- 76.Wu F., Zhang F., Ding E., Ding Y. HIV-1 infection in early human placenta in vitro: effects of Tumor Necrosis Factor (TNF-α) and Interleukins IL-1 and IL-6. Nagoya J. Med. Sci. 2016;9:151–160. [Google Scholar]
- 77.Bayer A., Delorme-axford E., Sleigher C., Frey T.K., Trobaugh D.W., Klimstra W.B. Viruses implicated in perinatal infection. Am. J. Obstet. Gynecol. 2015;212:71. doi: 10.1016/j.ajog.2014.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andreakos E., Zanoni I., Galani I.E. Lambda interferons come to light: dual function cytokines mediating antiviral immunity and dam- age control. Curr. Opin. Immunol. 2019;56:67–75. doi: 10.1016/j.coi.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.BroggiA, Granucci F., Zanoni I. Type III interferons: balancing tissue tolerance and resistance to pathogen invasion. J. Exp. Med. 2020;217 doi: 10.1084/jem.20190295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corry J., Arora N., Good C.A., Sadovsky Y., Coyne C.B. Organotypic models of type III interferon-mediated protection from Zika virus infections at the maternal – fetal interface. Proc. Natl. Acad. Sci. Unit. States Am. 2017:2–7. doi: 10.1073/pnas.1707513114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bayer A., Lennemann N.J., Ouyang Y., Cherry S., Sadovsky Y., Coyne C.B. Type III interferons produced by human placental trophoblasts confer protection against Zika virus short article type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe. 2016:1–8. doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson Erica L., Chakraborty Rana HIV-1 at the placenta. Curr. Opin. Infect. Dis. 2016;29:248–255. doi: 10.1097/QCO.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 83.Ouyang Y., Bayer A., Chu T., Tyurin V., Kagan V., Morelli A.E. Isolation of human trophoblastic extracellular vesicles and characterization of their cargo and antiviral activity. Placenta. 2016;47:86–95. doi: 10.1016/j.placenta.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bolata F., HaberalaN, Tunalib N., Aslanc E., Bala N., Tuncera I. Expression of vascular endothelial growth factor (VEGF), hypoxia inducible factor 1 alpha (HIF-1α), and transforming growth factors β1 (TGFβ1) and β3 (TGFβ3) in gestational trophoblastic disease. Pathol. Res. Pract. 2010;206:19–23. doi: 10.1016/j.prp.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 85.Akamura B.T.N., Izuno S.M. Review the discovery of Hepatocyte Growth Factor ( HGF ) and its significance for cell biology. life sciences and clinical medicine. 2010;86:588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mizuno S., Nakamura T. Prevention of neutrophil extravasation by hepatocyte growth factor leads to attenuations of tubular apoptosis and renal dysfunction in mouse ischemic kidneys. Am. J. Pathol. 2005;166:1895–1905. doi: 10.1016/S0002-9440(10)62498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakajima D., Watanabe Y., Ohsumi A., Pipkin M., Chen M., Mordant P., Keshavjee S. Mesenchymal stromal cell therapy during ex vivo lung perfusion ameliorates ischemia-reperfusion injury in lung transplantation. J. Heart Lung Transplant. 2019;38:1214–1223. doi: 10.1016/j.healun.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 88.Shigemura N., Sawa Y., Mizuno S., Ono M. Amelioration of pulmonary emphysema by in vivo gene transfection with hepatocyte growth factor in rats. Circulation. 2005;111:1407–1414. doi: 10.1161/01.CIR.0000158433.89103.85. [DOI] [PubMed] [Google Scholar]
- 89.Bicknell B.A., Pujic Z., Feldner J., Vetter I., Goodhill G.J. Chemotactic responses of growing neurites to precisely controlled gradients of nerve growth factor. Sci. Data. 2018;5:1–7. doi: 10.1038/sdata.2018.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lambiase A., Micera A., Sgrulletta R., Bonini S., Bonini S. Nerve growth factor and the immune system: old and new concepts in the cross-talk between immune and resident cells during pathophysiological conditions. Curr. Opin. Allergy Clin. Immunol. 2004;4:425–430. doi: 10.1097/00130832-200410000-00015. [DOI] [PubMed] [Google Scholar]
- 91.Sone Y., Takatori S., Ochi E., Zamami Y., Matsuyama A. Nerve growth factor facilitates the innervation of perivascular nerves in tumor-derived neovasculature in the mouse cornea. Pharmacology. 2017;99:57–66. doi: 10.1159/000450582. [DOI] [PubMed] [Google Scholar]
- 92.MelincoviciCS, Bosca A.B., Moldovan S.M., Roman A.L. Mihu CM. 2018;59 1220–0522. [Google Scholar]
- 93.lijima . 2017. CD4T Cells Provide Antibody Access to Immunoprivileged Tissue. US 2017/0326214 A1. [Google Scholar]
- 94.Skaper S., Id O., Skaper S.D. 2017. Nerve Growth Factor: a Neuroimmune Crosstalk Mediator for All Seasons 1. 0–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barrow A.D., Colonna M. ScienceDirect Innate lymphoid cell sensing of tissue vitality. Curr. Opin. Immunol. 2019;56:82–93. doi: 10.1016/j.coi.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lutsenko G.V., Grechikhina M.V., Efremov M.A. Autocrine survival factors of a cytotoxic CTLL-2 cell line. 2018;12:239–246. [Google Scholar]
- 97.Aloe L., Rocco M.L., Balzamino B.O., Micera A. Nerve growth factor : role in growth , differentiation and controlling cancer cell development. J. Exp. Clin. Canc. Res. 2016:1–7. doi: 10.1186/s13046-016-0395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ribatti D. 2019. The Mast Cell. [Google Scholar]
- 99.Hart D.A. 2017. Targeting Mast Cells as a Viable Therapeutic Option in Endometriosis; pp. 76–83. [Google Scholar]
- 100.Chen J., Zeng F., Forrester S.J., Eguchi S., Zhang M., Harris R.C. Growth factor receptor in physiology and disease. Physiol. Rev. 2016;96:1025–1069. doi: 10.1152/physrev.00030.2015. [DOI] [PubMed] [Google Scholar]
- 101.Wee P., Wang Z. Epidermal growth factor receptor cell proliferation. Cancers. 2017;9:1–45. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang X., Liu H., Yang S., Li Z., Zhong J., Fang R. Epidermal growth factor and intestinal barrier function. Mediat. Inflamm. 2016:27–30. doi: 10.1155/2016/1927348. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Edwin F., Wiepz G.J., Singh R., Peet C.R., Chaturvedi D., Bertics J. A historical perspective of the EGF receptor and related systems. Methods Mol. Biol. 2006;327:1–24. doi: 10.1385/1-59745-012-x:1. [DOI] [PubMed] [Google Scholar]
- 104.Nadel J.A. Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. Eur. Respir. J. 2008;32:1068–1081. doi: 10.1183/09031936.00172007. [DOI] [PubMed] [Google Scholar]
- 105.Sedehizade F., Welte T., Reiser G., Sedehizade F., Welte T. Atp- GR, et al. ATP- and UTP-activated P2Y receptors differently regulate proliferation of human lung epithelial tumor cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;285:376–385. doi: 10.1152/ajplung.00447.2002. [DOI] [PubMed] [Google Scholar]
- 106.Bayer H., Myrtek D., Ferrari D., Sorichter S., Ziegenhagen M.W., Zissel G. The P2Y14 receptor of airway epithelial cells coupling to intracellular Ca 2 ؉ and IL-8 secretion. Am. J. Respir. Cell Mol. Biol. 2005;33:601–609. doi: 10.1165/rcmb.2005-0181OC. [DOI] [PubMed] [Google Scholar]
- 107.Kramann R., Dirocco D.P., Humphreys B.D. Understanding the origin , activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. J. Pathol. 2013;231:273–289. doi: 10.1002/path.4253. [DOI] [PubMed] [Google Scholar]
- 108.Stolarczyk M., Scholte B.J. The EGFR-ADAM17 Axis in chronic obstructive pulmonary disease and cystic fibrosis lung pathology. Mediat. Inflamm. 2018:201822. doi: 10.1155/2018/1067134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Redington A.E., Roche W.R., Madden J., Frew A.J., Djukanovic R., Holgate S.T. Basic fibroblast growth factor in asthma : measurement in bronchoalveolar lavage fluid basally and following allergen challenge. J. Allergy Clin. Immunol. 2001;107:384–387. doi: 10.1067/mai.2001.112268. [DOI] [PubMed] [Google Scholar]
- 110.Kearley J., Erjefalt J.S., Andersson C., Benjamin E., Jones C.P., Robichaud A. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am. J. Respir. Crit. Care Med. 2011;183:865–875. doi: 10.1164/rccm.200909-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Young H., Cho Y., Broide D.H. Allergen-induced coexpression of bFGF and TGF- ᵦ1 by macrophages in a mouse model of airway Remodeling : bFGF induces macrophage TGF- ᵦ 1 expression in vitro. Int. Arch. Allergy Immunol. 2010;155:12–22. doi: 10.1159/000317213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sozzani S., Rusnati M., Riboldi E., Mitola S., Presta M. Dendritic cell – endothelial cell cross-talk in angiogenesis. Trends Immunol. 2007;28 doi: 10.1016/j.it.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 113.Nugent M.A., Iozzo R.V. Fibroblast growth factor-2. Int. J. Biochem. Cell Biol. 2000;32:115–120. doi: 10.1016/s1357-2725(99)00123-5. [DOI] [PubMed] [Google Scholar]
- 114.Virag J.A.I., Rolle M.L., Reece J., Hardouin S., Feigl E.O., Murry C.E. Fibroblast growth factor-2 regulates myocardial infarct repair. Am. J. Pathol. 2007;171:1431–1440. doi: 10.2353/ajpath.2007.070003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu X., Luo D., Yang N., Alerts E. Cytosolic low molecular weight FGF2 orchestrates RIG-I − mediated innate immune response. J. Immunol. 2015:1–10. doi: 10.4049/jimmunol.1501503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang K., Lai C., Li T., Wang C., Wang W., Ni B. Basic fibroblast growth factor protects against influenza A virus-induced acute lung injury by recruiting neutrophils. Journal ofMolecular Cell Biology. 2018:1–13. doi: 10.1093/jmcb/mjx047(2017). 1–13 j. [DOI] [PubMed] [Google Scholar]
- 117.Wang W., Fan Y.Q., Lv Z., Yao X.J., Wang W., Huang K.W. Interleukin-25 promotes basic fibroblast growth factor expression by human endothelial cells through interaction with IL-17RB , but not Clinical & Experimental Allergy. Clin. Exp. Allergy. 2012;42:1604–1614. doi: 10.1111/j.1365-2222.2012.04062.x. [DOI] [PubMed] [Google Scholar]
- 118.Finetti F., Donnini S., Giachetti A., Morbidelli L., Ziche M. Prostaglandin E 2 primes the angiogenic switch via a synergic interaction with the fibroblast growth factor-2 pathway. Circ. Res. 2009:657–666. doi: 10.1161/CIRCRESAHA.109.203760. [DOI] [PubMed] [Google Scholar]
- 119.Leali D., Mitola S., Coltrini D., Camozzi M., Corsini M., Belleri M. A pro-inflammatory signature mediates FGF2-induced angiogenesis. J. Cell Mol. Med. 2009;13:2083–2108. doi: 10.1111/j.1582-4934.2008.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brahim S.J., Feldman D., Oh Y. Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr. Rev. 2009;30:417–437. doi: 10.1210/er.2008-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.El‐Shewy H.M., Luttrell L.M. Insulin and IGFs; 2009. Insulin‐Like Growth Factor‐2/Mannose‐6 Phosphate Receptors; pp. 667–697. [DOI] [PubMed] [Google Scholar]
- 122.Granata R., Trovato L., Garbarino G., Taliano M., Ponti R., Sala G. Dual effects of IGFBP-3 on endothelial cell apoptosis and survival: involvement of the sphingolipid signaling pathways. The FASEB Journal express. 2004;24:1–24. doi: 10.1096/fj.04-1618fje. [DOI] [PubMed] [Google Scholar]
- 123.Nordisk N., Lilly E., Siddle K., Niesler A., Soos M.A., Hospital A. Insulin action. Biochem. Soc. Trans. 2001;29:513–525. doi: 10.1042/bst0290513. [DOI] [PubMed] [Google Scholar]
- 124.Zhang X., Gureasko J., Shen K., Cole P.A., Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 125.Lee H., Kim S., Oh Y., Cho S.H., Schleimer R.P., Lee Y.C. Targeting insulin-like growth factor-I and insulin-like growth factor – binding protein-3 signaling pathways A novel therapeutic approach for asthma. Am. J. Respir. Cell Mol. Biol. 2014;50:667–677. doi: 10.1165/rcmb.2013-0397TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Z., Li W., Guo Q., Wang Y., Ma L., Zhang X. Insulin-like growth factor-1 signaling in lung development and inflammatory lung diseases. BioMed Res. Int. 2018;17–9 doi: 10.1155/2018/6057589. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Johannesson B., Sattler S., Semenova E., Pastore S., Kennedy-lydon T.M., Sampson R.D. Insulin-like growth factor-1 induces regulatory T cell-mediated suppression of allergic contact dermatitis in mice. Disease Models & Mechanisms. 2014:977–985. doi: 10.1242/dmm.015362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang R. Insulin-like growth factor 1 regulates acute inflammatory lung injury mediated by influenza virus infection. Front. Microbiol. 2019;1010:2541. doi: 10.3389/fmicb.2019.02541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rao P., Suvas P.K., Jerome A.D., Steinle J.J., Suvas S. Role of insulin-like growth factor binding protein-3 in the pathogenesis of herpes stromal keratitis. Invest. Ophthalmol. Vis. Sci. 2020;61:46. doi: 10.1167/iovs.61.2.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang S., Liu Z., Cao L., Hou P., Chen Y., Zhang Y. Skeletal muscle stem cells confer maturing macrophages anti-inflammatory properties through insulin-like growth. STEM CELLS Transl Med. 2020:1–13. doi: 10.1002/sctm.19-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mu M., Wu F., He J., Tang X.U., Ma H.U.A., Guo S. Insulin - like growth factor 1 inhibits phagocytosis of alveolar epithelial cells in asthmatic mice. Mol. Med. Rep. 2019;20:2381–2388. doi: 10.3892/mmr.2019.10456. [DOI] [PubMed] [Google Scholar]
- 132.Ushach I., Zlotnik A. Biological role of granulocyte macrophage colony-stimulating factor ( GM-CSF ) and macrophage colony-stimulating factor ( M-CSF ) on cells of the myeloid lineage. 2016;100:1–9. doi: 10.1189/jlb.3RU0316-144R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Borriello F., Iannone R., Di Somma S., Loffredo S., Scamardella E., Galdiero M.R., Varricchi G., Granata F., Portella G., Marone G. GM-CSF and IL-3 modulate human monocyte TNF-α production and renewal in in vitro models of trained immunity. Front. Immunol. 2017;7:1–12. doi: 10.3389/fimmu.2016.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hamilton J.A. Colony-stimulating factors in inflammation and autoimmunity. Immunology. 2008;8:11–17. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 135.Bailey M.J., Lacey D.C., Kok BVA De, Veith P.D., Reynolds E.C., Hamilton J.A. Extracellular proteomes of M-CSF ( CSF-1 ) and GM-CSF-dependent macrophages. Immunol. Cell Biol. 2010;89:283–293. doi: 10.1038/icb.2010.92. [DOI] [PubMed] [Google Scholar]
- 136.Fleetwood A.J., Lawrence T., Hamilton J.A., Cook A.D., Fleetwood A.J., Lawrence T. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor Activities : implications for CSF blockade in. J. Immunol. 2007;178:5245–5252. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- 137.Greter M., Helft J., Chow A., Hashimoto D., Mortha A., Agudo-cantero J. Article GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity. 2012;36:1031–1046. doi: 10.1016/j.immuni.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Khader S.A., Pearl J.E., Sakamoto K., Bell G.K., Jelley-gibbs D.M., Cooper A.M. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-γ responses if IL-12p70. J. Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 139.Chousterman B.G. Is there a role for hematopoietic growth factors during sepsis? Front. Immunol. 2018;9:1–11. doi: 10.3389/fimmu.2018.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alothaimeen T., Seaver K., Mulder R., Gee K., Basta S. 2020. Macrophages exhibit distinctive early immune response to lymphocytic choriomeningitis virus infection; pp. 1–12. 00. [DOI] [PubMed] [Google Scholar]
- 141.Chen G., Teitz-tennenbaum S., Neal L.M., Murdock B.J., Malachowski A.N., Anthony J. Local GM-CSF − dependent differentiation and activation of pulmonary dendritic cells and macrophages protect against progressive cryptococcal lung infection in mice. J. Immunol. 2016:1–12. doi: 10.4049/jimmunol.1501512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Presneill J.J., Harris T., Stewart A.G., Cade J.F., Wilson J.W. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. 2002;166:138–143. doi: 10.1164/rccm.2009005. [DOI] [PubMed] [Google Scholar]
- 143.Ribatti D. The discovery of the placental growth factor and its role in angiogenesis : a historical review. Angiogenesis. 2008:215–221. doi: 10.1007/s10456-008-9114-4. [DOI] [PubMed] [Google Scholar]
- 144.Tammela T., Enholm B., Alitalo K., Paavonen K. Vol. 65. 2005. The biology of vascular endothelial growth factors; pp. 550–563. [DOI] [PubMed] [Google Scholar]
- 145.Afzali B., Lombardi G., Lechler R.I., Lord G.M. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. J of translational immunology. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jang J.Y., Choi S.Y., Park I., Park D.Y., Choe K., Kim P. VEGFR 2 but not VEGFR 3 governs integrity and remodeling of thyroid angiofollicular unit in normal state and during goitrogenesis. EMBO Mol. Med. 2017;9:750–769. doi: 10.15252/emmm.201607341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kasahara Y., Tuder R.M., Cool C.D., Lynch D.A., Flores S.C., Voelkel N.F. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am. J. Respir. Crit. Care Med. 2001;163:737–744. doi: 10.1164/ajrccm.163.3.2002117. [DOI] [PubMed] [Google Scholar]
- 148.Voelkel N.F., Vandivier R.W., Tuder R.M., Norbert F., Vandivier R.W. Vascular endothelial growth factor in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:209–221. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- 149.Bhandari V., Choo-wing R., Chapoval S.P., Lee C.G., Tang C., Kim Y.K. Essential role of nitric oxide in VEGF-induced , asthma-like angiogenic , inflammatory , mucus , and physiologic responses in the lung. 2006;103:11021–11026. doi: 10.1073/pnas.0601057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ungvari Z., Tarantini S., Kiss T., Wren J.D., Giles C.B., Griffin C.T. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018;15:555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kasahara Y., Waltenberger J., Norbert F., Kasahara Y., Tuder R.M., Taraseviciene-stewart L. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema Find the latest version : and emphysema. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thébaud B., Ladha F., Michelakis E.D., Sawicka M., Thurston G., Eaton F. Vascular endothelial growth factor gene therapy increases survival , promotes lung angiogenesis , and prevents alveolar damage in hyperoxia-induced lung injury evidence that angiogenesis participates in alveolarization. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 153.Kumar P.A., Hu Y., Yamamoto Y., Hoe N.B., Wei T.S., Mu D. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ramasamy S.K., Kusumbe A.P., Adams R.H. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 2014;2:1–10. doi: 10.1016/j.tcb.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ding B., Nolan D.J., Guo P., Babazadeh A.O., Cao Z., Rosenwaks Z. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chatterjee P., Chiasson V.L., Bounds K.R., Mitchell B.M. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front. Immunol. 2014;5:1–7. doi: 10.3389/fimmu.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 158.Tough D.F. Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk. Lymphoma. 2009;45:257–264. doi: 10.1080/1042819031000149368. [DOI] [PubMed] [Google Scholar]