Abstract
A case who revealed the longest duration of viral shedding (67 days) in current reports, presented complicated characteristic on the relapse of COVID-19 due to the inconsistent performance of chest radiography and SARS-CoV-2-RNA detection after discharge. Lopinavir-interferon α2b boosted ribavirin following with lopinavir boosted budesonide might be a potent treatment for viral clearance.
Keywords: Coronavirus disease 2019, Prognosis, Recovered patients, SARS-CoV-2 re-positive, Viral shedding
Introduction
The outbreak of Corona Virus Disease 2019 (COVID-19) from Wuhan is currently arousing great concern in the medical community as the virus is spreading rapidly around the world.1 Nowadays, many thousands of hospitalized patients have recovered from COVID-19 and have been discharged, but multiple recovered cases have presented positive for SARS-CoV-2 during post-discharge surveillance,2 , 3 which suggests that a portion of discharged patients still may be virus carriers. However, little attention has been paid to such patients. Here, we present the clinical dynamics of a case who was hospitalized three times due to the positive detection of SARS-CoV-2 RNA and radiographic progression during post-discharged surveillance.
Methods
The case was admitted to Xixi Hospital of Hangzhou (a designated hospital of Zhejiang province, China) on February 18, 2020, and was confirmed as COVID-19 by a positive real-time reverse transcriptase-polymerase chain reaction (RT-PCR) test of SARS-CoV-2 RNA, which was conducted according to the methods described previously.4 During the period of discharge surveillance, two consecutively RT-PCR tests were conducted on each respiratory sample. The RT-PCR test kits were recommended by Zhejiang Center for Disease Control and Prevention.
Before released from the hospital, this patient met all of the discharge criteria.5 The case was quarantined at a community isolation center for two weeks and evaluated with RT-PCR tests for SARS-CoV-2 RNA at days 7 and 14 after discharge, to determine if the case could be released from isolation ultimately.
This study was approved by the Institutional Review Board of Xixi Hospital. All data were anonymized to comply with the provisions of personal data protection legislation. Due to the retrospective nature of this study and the fact that only historical medical data were collected, written informed consent was not required.
Results
The case was a 57-years-old female with a moderate type of COVID-19. Her earliest symptoms were fever (37.5 °C), cough, and yellow sputum. The first chest X-ray showed ground-glass opacification and patchy consolidation. The incubation period of the case was 24 days, which was estimated based on her history of travel or potential exposure. She had no other potential exposure history except for the traveling from Hangzhou to Ningbo by train on January 17, 2020.
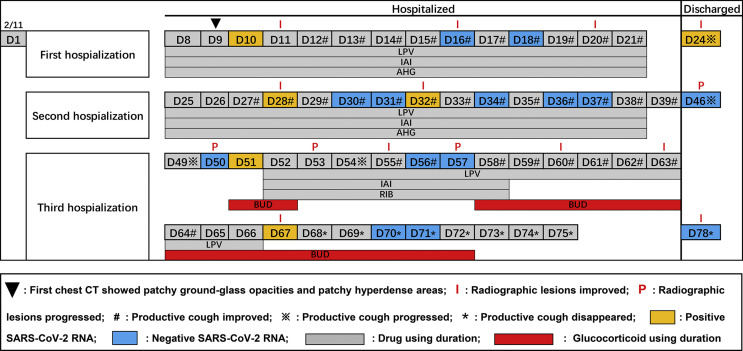
The day of symptom onset was defined as Day 1 (Fig. 1 ). The clinical symptoms of cough and sputum presented an inseparable and lingering characteristic during the observation period. The time from symptom onset to disappearance was 68 days.
Figure 1.
Nasopharyngeal swab virus tests and chest CT findings in COVID-19 patient from symptom onset to post-discharge, China, February–April 2020. legend: LPV: lopinavir; IAI: interferon α2b atomization inhalation; AHG: arbidol hydrochloride granules; RIB: ribavirin; BUD: budesonide.
Viral dynamics are shown in Fig. 1. During the first post-discharge surveillance, the detection of SARS-CoV-2 on nasopharyngeal swab was positive (Fig. 1, Day 24). For the duration of the second discharge surveillance, virus results were negative (Fig. 1, Day 46), but the CT imaging showed new lesions in the upper left lung (Fig. 2 a, Day 46). Then, positive virus RNA was detected on day 51. The same situation as day 46 came up on day 57, and positive virus RNA was detected on day 67 again. At the end of observation, the detection of SARS-CoV-2 on nasopharyngeal swab was negative (Fig, 1, Day 78). This patient did not contact any other persons with respiratory symptoms, and no person who contacted her was infected.
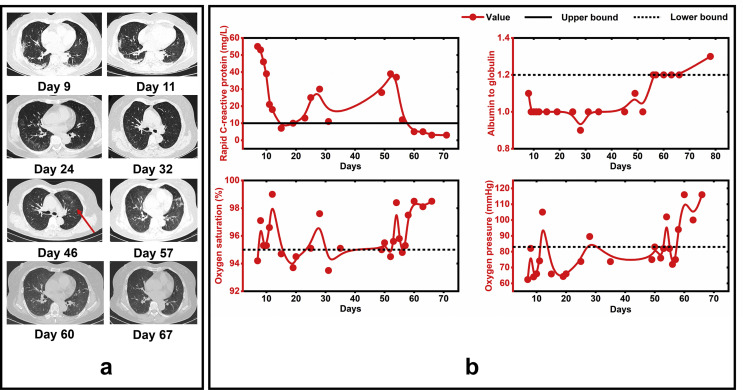
Figure 2.
Radiographic images and dynamic laboratory findings in COVID-19 patient from symptom onset to post-discharge, China, February–April 2020. Legend: Red arrow represents the new radiological lesions.
Lopinavir (LPV) and interferon α2b atomization inhalation (IAI) were both administered during each period of hospitalization (Fig. 1). Given the prolonged infection, arbidol hydrochloride granules (AHG) was changed to ribavirin (RIB) during the third hospitalization. The cumulative duration of AHG and RIB using was 28 and 7 days, respectively. The patient was treated with budesonide (BUD, a glucocorticoid with slight side effects) due to the deterioration of radiographic lesions in the upper left lung (Fig. 1, Days 50 and 57). The cumulative duration of BUD using was 17 days. The details of the drug dosage are shown in Supplementary Table S1.
The results of the chest CT are shown in Fig. 1. Overall, the radiographic lesions of this case improved on day 11, progressed significantly on day 46, then improved persistently since day 60, and all showed evident resorption before each discharge.
Dynamic laboratory results are shown in Fig. 2b. The level of rapid C-reactive protein (CRP), albumin to globulin (A/G), oxygen saturation, and oxygen pressure (PO2) turned into normal since days 60, 56, 57 and 58, respectively. IgG and IgM levels were tested on days 29, 46 and 51 (Supplementary Table S2). IgG levels plateaued after the first positive IgG measurement, and so is IgM after the first negative detection.
Discussion
This patient, who fulfilled discharge criteria, presented positive for SARS-CoV-2 RNA 6 days after her first discharge, which is consistent with a previous study reported by Lan et al.3 However, during the second discharge surveillance, the chest CT of this patient showed new diffuse lesions in the left lung but RT-PCR tests were negative (Fig. 1, Day 46). This phenomenon recurred on day 57 during the third hospitalization (Fig. 1). This finding is inconsistent with the previous study.6 Although false-negative SARS-CoV-2 RNA results could have occurred,7 reexamination of viral RNA on nasopharyngeal swab and subsequent examination results of RT-PCR testing and CT imaging (Fig. 1, Day 50) were consistent with that of previous (Fig. 1, Day 46), which decreased the possibility of false-results. It is difficult to find a reasonable explanation for this phenomenon. One possible explanation is that it is as a result of residual viruses in the lungs of discharged COVID-19 patients.8 Remaining viruses first cause pathological lung changes under conditions of weakened immunity, it will take some time for viruses to travel to the upper respiratory tract because of circulatory disturbance which resulted from low PO2 and lung dysfunction of this case. The later positive results of SARS-CoV-2 (Fig. 1, Days 51 and 67) were further evidence, which confirm this explanation from another perspective. These findings provide new insight into the process of COVID-19, the possibility that negative results of a nasopharyngeal swab might not synchronously reflect the presence of SARS-CoV-2 in lung tissue among patients recovered from COVID-19. This has significant implications for patient isolation decision making.
Of this case, convalescence serological results for IgG and IgM maintained a relatively stable level since the first measurement of day 29, which suggests that serology testing could not help identify the presence of SARS-CoV-2 in patients recovered from COVID-19 during post-discharge surveillance.
In this report, the incubation period of this case was 24 days, which is longer than 5.2 days reported by Li et al.9 The long incubation period brings great difficulties and challenges for the initial control of COVID-19. The duration of viral RNA shedding after COVID-19 onset in this case lasted for 67 days, which is the longest duration of viral shedding as far as we know. Moreover, one RT-PCR test for SARS-CoV-2 RNA was positive (Fig. 1, Day 51) even after five-consecutively negative results, which suggests that current criteria for discharge and termination of isolation may need to be reconsidered.
Interestingly, most abnormal levels of CRP, A/G, and PO2, which indicates inflammation, liver impairment, and lung dysfunction, all turned into normal at around days 56–60 after symptom onset, and following with the disappearance of clinical symptoms on day 68 and sustained until the end of the observation. The exhilarating improvement of this case may benefit from the using of BUD and the treatment of a triple combination of antiviral therapy (LPV + IAI + RIB), which has been reported to be safe and highly effective in shortening the duration of virus shedding and alleviating symptoms in patients with moderate COVID-19.10
In conclusion, our study reported the dynamic features of a special case, who has the longest duration of viral shedding in current reports. Our report points out the relapse of COVID-19 presented complicated characteristics due to the inconsistent performance of chest CT imaging and SARS-CoV-2 RNA detection. Lopinavir-interferon α2b boosted ribavirin following with lopinavir boosted budesonide might be an effective treatment for viral clearance in COVID-19 patients with long duration of viral shedding. Longitudinal studies on a larger scale are strongly recommended for discharged patients to understand the prognosis of COVID-19 further.
Availability of data and materials
The data set used for this manuscript will be available from the corresponding author upon reasonable request.
Funding
This research was supported by grants from Hangzhou research project of 2019-coronavirus disease (20202013A01).
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
We thank all study participants and staff of all participating sites.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmii.2020.07.017.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Health Commission . China Daily Website; Beijing: 2020. Recovered patient re-hospitalized after positive test.http://ex.chinadaily.com.cn/exchange/partners/45/rss/channel/www/columns/2n8e04/stories/WS5e4fc442a31012821727963c.html Chinese. Available from: [Google Scholar]
- 3.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y. Positive RT-PCR test results in patients recovered from COVID-19. J Am Med Assoc. 2020;323(15):1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Health Commission of the People’s Republic of China . National Health Commission of the People’s Republic of China; Beijing: 2020. Diagnosis and treatment protocols of the novel coronavirus pneumonia (trial version 6)http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf Chinese. Available from: [Google Scholar]
- 6.Xing Y., Mo P., Xiao Y., Zhao O., Zhang Y., Wang F. Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China, January to February 2020. Euro Surveill. 2020;25(10):2000191. doi: 10.2807/1560-7917.ES.2020.25.10.2000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie X.A.-O., Zhong Z.A.-O., Zhao W.A.-O., Zheng C.A.-O., Wang F., Liu J.A.-O. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020:200343. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao X.H., He Z.C., Li T.Y., Zhang H.R., Wang Y.A.-O., Mou H. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020 doi: 10.1038/s41422-020-0318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199-1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial [published online ahead of print, 2020 May 8] Lancet. 2020;S0140–6736(20):31042–31044. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data set used for this manuscript will be available from the corresponding author upon reasonable request.