Abstract
肺癌是全球发生率最高和死亡人数最多的恶性肿瘤。近些年肺癌的治疗取得重大突破,但是随着病情的进展,患者不可避免地出现耐药,药物疗效大大降低。P21蛋白是一种在肿瘤中发挥双重作用的蛋白,既能调控细胞周期、诱导细胞凋亡、抑制细胞增殖,也能保护细胞抵抗凋亡,在肿瘤细胞耐药中发挥重要作用。本文就P21与肺癌耐药及其相关研究进行综述,为临床肺癌的个体化治疗与克服肺癌耐药提供新的思路。
Keywords: 肺肿瘤, P21, 耐药
Abstract
Lung cancer is the most common malignant tumor in the world with the highest incidence of deaths. In recent years, the treatment of lung cancer has made a significant breakthrough. However, as the tumor progresses, lung cancer cells inevitably acquire resistance and the efficacy of the treatment are greatly reduced. P21 protein plays a dual role in tumors, which not only regulates the cell cycle, induces apoptosis, inhibits cell proliferation, but also protects cells against apoptosis and promotes tumor cell resistance. This article reviews the research on P21 and lung cancer resistance, to provide new ideas for individualized treatment of lung cancer and overcoming lung cancer resistance.
Keywords: Lung neoplasms, P21, Drug resistance
根据2018年全球癌症统计数据[1]显示,2018年全球肺癌新增病例数占总癌症病例数的11.6%,其中肺癌死亡人数占癌症总死亡人数的18.4%,是全球人类发生率最高和导致癌症死亡的主要原因。手术是早期肺癌患者的主要治疗手段,但是由于肺癌患者早期症状不明显,发病隐匿,大部分患者确诊时往往已是中晚期。放疗、化疗、靶向治疗和免疫治疗是中晚期肺癌患者的主要治疗方法,靶向治疗是近十几年来肺癌治疗取得的巨大突破,免疫治疗是近几年肺癌治疗的新方法。尽管这些不同的治疗方法有较好的疗效,能有效缓解患者痛苦并提高生存期,但是随着病情的进展,患者在用药一段时间后仍不可避免地出现获得性耐药,获得性耐药是导致临床肺癌患者治疗失败和癌症治疗最难以克服的问题之一。因此,深入探讨肺癌耐药机制、逆转肺癌耐药是研究肺癌的难点和热点。
P21又叫P21Waf1/Cip1或CDKN1A(cyclin-dependent kinase inhibitor 1A),最先被el-Deiry等[2]运用差减杂交技术发现,它处于野生型p53的下游,是由野生型p53活化片段基因1(wild-type p53-activated fragment, WAF1)编码的大小为21 kDa的蛋白质。el-Deiry等[2]发现p53能直接诱导p21的表达并且它可能是P53发挥抑癌作用的中介。与此同时,Harper等[3]利用酵母双杂交系统发现了能编码21 kDa的细胞周期依赖性激酶(cyclin-dependent protein, CDK)相互作用蛋白的基因,并将其命名为细胞周期依赖性激酶相互作用蛋白1(CDK interaction protein 1, CIP1);该蛋白通过与CDKs紧密结合,从而在细胞周期中发挥负性调控作用。然而,P21还在保护细胞抵抗凋亡方面发挥着重要作用,因此P21在肿瘤的发生、发展以及肿瘤药物治疗的敏感性中也发挥着重要作用[4]。本文就P21与肺癌耐药及其相关研究进行综述。
1. P21蛋白的生物学功能
P21最先作为细胞周期负性调节因子被发现,与P53构成细胞周期检查点,当DNA发生损伤时,P21被激活使细胞周期阻滞直至DNA修复完成,因此它在诱导细胞衰老、凋亡、促进DNA修复、维持基因组的稳定性等方面起重要作用[5-7]。然而,P21也能促进肿瘤细胞侵袭、抵抗凋亡,在肿瘤细胞耐药方面发挥着不可忽视的作用[8, 9]。
2. P21的调控
2.1. P53依赖途径
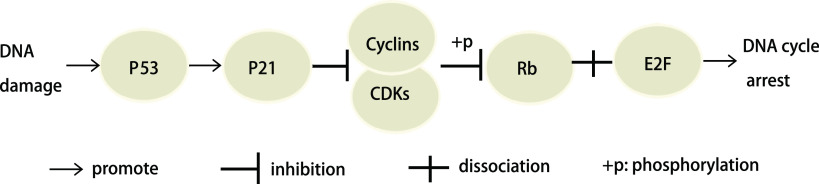
p53是p21的主要转录调控因子,P21最初被认为是一种肿瘤抑制因子,是DNA损伤诱导的P53依赖细胞周期阻滞的主要介质。P21可以与P53构成细胞周期G1期检查点,如图 1所示,当DNA损伤或在其他应激条件下,p53被激活,直接与p21启动子结合而激活其表达,P21蛋白通过与CDK4、CDK6/cyclin D或CDK2/cyclin E结合,从而抑制细胞周期蛋白依赖激酶活性,使视网膜细胞瘤(retinoblastoma, Rb)蛋白不能被磷酸化,从而可以与转录因子E2F牢固结合而抑制E2F的活性,阻断DNA复制,使细胞周期阻滞直至修复完成,这样能减少受损DNA的复制和积累,阻止异常细胞的增殖以维持基因的保真度从而发挥抑癌作用[10]。P21主要通过阻滞细胞周期发挥抑癌作用,大部分研究表明,抑制P21表达能促进肿瘤的发生发展[11-14],诱导P21表达能抑制肿瘤细胞的增殖[15]。
1.
P21阻滞细胞周期图
P21 involved in blocking the cell cycle
此外,P53不仅能直接激活P21的表达发挥抑癌作用,还能通过直接与其形成复合物而发挥作用。Kim等[16]的研究结果首次阐明P53和P21共同作用的抑癌机制:P53、P21能结合Mdm2及其底物锌指转录因子Slug蛋白,形成P53/P21/Mdm2/Slug复合物,促进Mdm2依赖的Slug泛素化导致其降解,由于Slug能增强肿瘤细胞的侵袭性,因此Slug泛素化过程可能有助于抑制肿瘤细胞侵袭。除此之外,P53、P21能分别与Bcl-2家族蛋白(例如Bcl-w、Bcl-XL)结合,通过形成P53/P21/Bcl-w复合物使Bax蛋白释放,促进肿瘤细胞凋亡,从而抑制肿瘤细胞的侵袭[17]。
2.2. P53非依赖途径
P21复杂的调控途径是使其在肿瘤中发挥双重作用的基础。P21还可以通过非P53依赖途径被调控,比如:c-Myc可以直接与P21的羧基端结合,影响P21与PCNA的相互作用,降低了P21介导的DNA合成抑制作用[18]。SP1蛋白在p21启动子的近端与启动子富含GC的模体结合,对p21转录也至关重要[19]。信号传导及转录激活蛋白(signal transducer and activator of transcription, STAT)能介导P21的转录、表达和核定位,从而抑制耐药乳腺癌细胞的增殖和侵袭[20]。
3. P21与肿瘤耐药
P21在肿瘤中具有双重的作用,它的作用主要取决于细胞状态及其在细胞内的定位。2017年Manu等[21]研究发现抑制异丙氨酸半胱氨酸羧甲基转移酶能促进P21表达,P21表达上调诱导细胞周期阻滞及激活促凋亡蛋白BNIP3(BCL2/adenovirus E1B 19 kDa interacting protein 3)诱导细胞凋亡,从而增加胰腺癌对药物治疗的敏感性。野生型p53的存在是P21发挥抑癌作用的关键;如果p53发生突变,P21过表达会解除复制许可,细胞增殖会增加,表现出一定耐药倾向。2016年Galanos等[22]在体内与体外实验的研究中发现,在p53突变的肿瘤中,长期诱导P21过表达的细胞中,部分肿瘤细胞“躲避”了P21诱导的周期阻滞和细胞衰老,重新进入细胞周期,而且这些细胞中出现广泛的DNA修复,具有更强的侵袭性,且对阿霉素、顺铂等药物有更高的耐受能力。这表明在p53突变后,P21过表达可使DNA修复出错和基因组不稳定而导致肿瘤细胞更强的侵袭性和耐药能力。因此突变型p53可能是p21由抑癌基因转化成致癌基因的关键因素。
目前普遍认为胞核P21主要作用于细胞周期,抑制细胞增殖和促进凋亡,而胞质P21能保护肿瘤细胞免受凋亡,与肿瘤细胞的耐药相关[23, 24]。2014年Huang等[25]研究发现胞质和胞核P21在胃癌的发展、分期、预后等中发挥相反的作用,胞核P21抑制而胞质P21促进细胞迁移和侵袭能力。2016年Xie等[26]研究发现在浸润性肥大细胞介导多西他赛耐药的前列腺癌细胞中,通过调节P38/P53信号通路,使P21的总表达水平及胞质水平均升高;再通过敲减P21能逆转其耐药。但文中未能阐明其机制。2018年Maiuthed等[27]证明了在5-氟尿嘧啶耐药的结直肠癌细胞中,胞质P21通过将促凋亡蛋白p-Chk2从细胞核中释放到胞质,使p-Chk2下游的促凋亡蛋白E2F1的激活减少,从而保护肿瘤细胞免受凋亡,介导结肠癌细胞对5-氟尿嘧啶耐药。
P21是一种核蛋白,它能否在胞质大量累积取决于激酶在其不同位点的磷酸化,例如Akt能通过PI3K/Akt信号通路使P21磷酸化导致其在胞质中积累[28]。P21在不同情况下发挥相反的作用可能是由于P21不同的细胞内定位造成的。胞核P21能阻滞细胞周期、促进细胞凋亡,而胞质P21能通过降低凋亡蛋白活性或抑制凋亡信号通路,促进肿瘤细胞增殖,保护肿瘤细胞抵抗凋亡,其表达和细胞内定位有望作为肿瘤药物治疗敏感性的判断指标[24, 25, 28]。
4. P21与肺癌耐药
P21表达水平与肺癌细胞增殖:P21的表达水平及其在细胞内分布与其功能密切相关。2017年Li等[29]研究发现miR-1236-3p和miR-370-5p可促进P21的表达,使E-cadherin表达水平上调,同时抑制cyclin D1-CDK4/CDK6,从而诱导细胞周期阻滞、细胞衰老并抑制肺癌细胞的增殖、迁移和侵袭。2019年Guo等[30]研究发现A549细胞中抑制长非编码RNA DANCR的水平能促进P21的表达水平增加,使细胞周期阻滞在G1期,从而抑制肺癌细胞的增殖。这说明P21表达水平与肺癌细胞增殖呈负相关。然而,2018年Su等[31]研究发现敲减肺癌细胞的长非编码RNA MIR22HG后,P21表达水平升高且大部分位于细胞质,同时促进了肺癌细胞增殖。经过转染P21 siRNA处理后,肺癌细胞增殖减少。这些结果表明,P21水平升高促进了肺癌细胞增殖,它的水平与肺癌细胞增殖呈正相关。
P21与肺癌耐药:P21表达水平与肺癌化疗、靶向治疗、放疗的敏感性相关,主要通过调节细胞周期参与肺癌耐药。在不同的研究发现中,其作用和机制如表 1所示[32-43]。P21的表达水平下降介导耐药。顺铂是细胞周期非特异性药物,是肺癌化疗的常用药物。2013年Liu等[32]研究认为上调P21表达水平能使肺癌细胞周期阻滞,抑制细胞增殖,增加肺腺细胞对顺铂敏感性。有研究[33]发现miR-224靶向P21使其表达水平降低,介导A549细胞对顺铂耐药。在耐药细胞中过表达P21后,不仅使细胞周期阻滞在G1期/S期,还降低抗凋亡蛋白Bcl-2、Bcl-xL的表达,促进促凋亡蛋白Bax、Bak的表达,因而促进细胞凋亡从而逆转顺铂耐药。Feng等[34]研究表明在顺铂耐药的A549细胞中,过表达膜联蛋白A2可激活JNK/c-Jun信号通路抑制p53的表达,进而使p21等凋亡相关基因表达下降,抑制顺铂诱导细胞凋亡,从而增强细胞对顺铂的耐受性。P21不仅参与了肺癌细胞对顺铂产生耐药,也与其他药物化疗耐药相关。2016年Shan等[35]的研究发现在耐甲氨蝶呤的A549细胞中,过表达miR-200c能激活P53/P21信号通路来诱导细胞周期阻滞在G0期/G1期及诱导细胞凋亡,从而逆转耐药。在Noro等[36]的研究中,药物作用使p21启动子组蛋白乙酰化能诱导p21的表达来阻断Rb-E2F1通路,从而增加肺癌细胞对5-氟尿嘧啶的敏感性。这些结果提示,P21主要通过调控不同期的细胞周期阻滞介导肺癌细胞对化疗药物治疗的敏感性。
1.
P21在肺癌耐药中的作用及相关机制
The role of P21 in lung cancer resistance and related mechanisms
| Type of cells | Type of resistance | Effect of P21 | Gene/Pathway interation | References |
| CDK: cyclin-dependent protein; RAD21: Rad21 homolog (S. pombe). | ||||
| A549 | Cisplatin | Sensitive | lnc RNA HOTAIR | [32] |
| A549 | Cisplatin | Sensitive | MiR-224 | [33] |
| A549 | Cisplatin | Sensitive | MiR-33p-3b | [43] |
| A549 | Cisplatin | Sensitive | Annexin A2, JNK/c-Jun, p53 | [34] |
| A549 | Methotrexate | Sensitive | miR-200c, P53 | [35] |
| PC9/f14 | 5-fluorouracil | Sensitive | Rb-E2F1, p53 | [36] |
| PC-9, H1299 | Gefitinib | Sensitive | Cyclins-CDKs | [37] |
| PC-9, H1975 | Gefitinib | Sensitive | Cyclins-CDKs | [39] |
| PC-9 | Gefitinib | Sensitive | P53 | [38] |
| A549 | Cisplatin | Resistance | miR-17, miR-92, RAD21 | [40] |
| A549 | cisplatin | Resistance | P53 | [41] |
| A549 | Chemoresistance | Resistance | Nrf-2 | [42] |
P21还影响肺癌细胞靶向治疗药物的敏感性。吉非替尼是一种表皮生长因子受体酪氨酸激酶抑制剂,能促进肿瘤细胞凋亡。2011年Zhao等[37]发现吉非替尼能通过P53非依赖途径诱导PC-9细胞中P21的表达,同时CDK2/4和cyclin D1/E表达水平降低,细胞周期阻滞在G1期,抑制细胞增殖。随后发现吉非替尼耐药的PC-9细胞中药物诱导P21的表达或直接使P21过表达均能增加细胞对吉非替尼的敏感性,这说明P21参与了PC-9细胞对吉非替尼的敏感性。此外,2015年Zhu等[38]研究发现,在吉非替尼耐药的PC-9细胞中同时使用白芦藜醇和吉非替尼,能激活P53/P21通路,诱导细胞周期阻滞在G2期/M期和细胞衰老,从而增强肺癌细胞对吉非替尼的敏感性。2017年Wang等[39]研究发现,与对吉非替尼敏感的PC-9细胞相比,耐药细胞中P21蛋白水平明显下降,通过诱导P21的表达,能降低CDK2/4和cyclin D1/E的活性或表达水平,导致细胞周期阻滞在G1期,从而逆转吉非替尼耐药。这说明升高P21表达水平可以增加耐药细胞对吉非替尼的敏感性。然而,也有相反观点,高表达P21能介导肺癌耐药。2015年Zhao等[40]发现,P21表达水平升高使细胞周期阻滞在G1期,抑制DNA的合成,再加上RAD21高表达增强了DNA的修复作用,抑制了顺铂介导的DNA损伤,从而对顺铂产生耐受。2018年Guo等[41]发现,A549细胞在低氧条件下能通过使P53表达上调从而激活P21的表达,使细胞周期阻滞在G1期/G0期,但此时却增加了A549细胞对顺铂的耐受,其潜在的原因可能是细胞周期阻滞,导致非增殖状态的细胞增加,减小了顺铂对A549细胞的作用。有研究[42]发现,Nrf-2可以通过非依赖P53途径直接与p21启动子结合,激活p21的表达以促进A549细胞在H2O2诱导的氧化应激下存活。由于肿瘤细胞的活性氧水平升高,同时抗氧化酶水平升高,使它们能抵抗细胞毒性化疗,因此认为Nrf-2/P21途径可能是介导肺癌细胞耐药的原因之一。
P21还在肺癌细胞对放疗的敏感性中扮演重要角色。P21表达下降或过多降解均会导致肺癌细胞耐辐射,上调P21的表达能增加非小细胞肺癌对放疗的敏感性[44, 45]。此外,研究[46]发现通过激活Akt/mTOR通路靶向P21能促进细胞增殖和诱导耐辐射。
5. 总结与展望
肺癌耐药机制复杂,大部分体内外实验表明,P21阳性表达是提示肺癌良好预后的标志,P21缺乏是导致肺癌细胞耐药、耐辐射的重要原因之一,其主要机制是P21诱导细胞周期阻滞、细胞衰老和凋亡,从而逆转耐药。但也有研究者[46]提出,在肺癌耐药细胞中P21表达升高使细胞周期阻滞从而介导耐药表型。不同的研究结果可能是由于不同实验室使用的细胞类型或细胞状态不一所致。
由于P21在肺癌增殖中的双重作用,P21的表达水平及其在细胞内的定位有望作为肺癌预后的指标,但是调控P21的基因较多,特异性并不高,需要联合其他指标。同时,P21有望作为逆转肺癌耐药和治疗的潜在靶点[47],结合p53的状态,在因P21缺乏而导致的耐药和预后不良相关的细胞中应用P21诱导药物,在P21过表达导致的耐药细胞中使用P21抑制剂有望作为肺癌治疗的新方法。我们所面临的挑战是如何能抑制P21介导的促癌和耐药活性,但又不影响其发挥抑癌活性。深入研究P21与肺癌耐药的关系,更好地理解P21在各种情况下的作用机制,有助于解决临床肺癌耐药问题,为临床逆转肺癌耐药和肺癌治疗提供新思路。
Funding Statement
本文受国家自然科学基金面上项目(No.81071853)资助
This study was supported by the grant from the National Natural Science Foundation of China (No.81071853)(to Yongjun LIU)
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.el-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 3.Harper JW, Adami GR, Wei N, et al. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 4.Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst) 2016;42:63–71. doi: 10.1016/j.dnarep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Kim YY, Jee HJ, Um JH, et al. Cooperation between p21 and Akt is required for p53-dependent cellular senescence. Aging Cell. 2017;16(5):1094–1103. doi: 10.1111/acel.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Pan K, Wang P, et al. HBP1-mediated regulation of p21 protein through the Mdm2/p53 and TCF4/EZH2 pathways and its impact on cell senescence and tumorigenesis. J Biol Chem. 2016;291(24):12688–12705. doi: 10.1074/jbc.M116.714147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barr AR, Cooper S, Heldt FS, et al. DNA damage during S-phase mediates the proliferation-quiescence decision in the subsequent G1 via p21 expression. Nat Commun. 2017;8:14728. doi: 10.1038/ncomms14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgakilas AG, Martin OA, Bonner WM. p21: a two-faced genome guardian. Trends Mol Med. 2017;23(4):310–319. doi: 10.1016/j.molmed.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Zlotorynski E. Cancer biology: The dark side of p21. Nat Rev Mol Cell Biol. 2016;17(8):461. doi: 10.1038/nrm.2016.90. [DOI] [PubMed] [Google Scholar]
- 10.Engeland K. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell Death Differ. 2018;25(1):114–132. doi: 10.1038/cdd.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin D, Lu X, Su J, et al. Long noncoding RNA AFAP1-AS1 predicts a poor prognosis and regulates non-small cell lung cancer cell proliferation by epigenetically repressing p21 expression. Mol Cancer. 2018;17(1):92. doi: 10.1186/s12943-018-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Ma Z, Feng L, et al. LncRNA MIR31HG targets HIF1A and P21 to facilitate head and neck cancer cell proliferation and tumorigenesis by promoting cell-cycle progression. Mol Cancer. 2018;17(1):162. doi: 10.1186/s12943-018-0916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y, Hu Z, Mangala LS, et al. MYC targeted long noncoding RNA DANCR promotes cancer in part by reducing p21 levels. Cancer Res. 2018;78(1):64–74. doi: 10.1158/0008-5472.CAN-17-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Q, Jin L, Wang S, et al. Long non-coding RNA UCA1 promotes gallbladder cancer progression by epigenetically repressing p21 and E-cadherin expression. Oncotarget. 2017;8(29):47957–47968. doi: 10.18632/oncotarget.18204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M, Zhang H, Zhang G, et al. Targeting TPX2 suppresses proliferation and promotes apoptosis via repression of the PI3k/AKT/P21 signaling pathway and activation of p53 pathway in breast cancer. Biochem Biophys Res Commun. 2018;507(1-4):74–82. doi: 10.1016/j.bbrc.2018.10.164. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Bae S, An S, et al. Cooperative actions of p21 WAF1 and p53 induce Slug protein degradation and suppress cell invasion. EMBO Rep. 2014;15(10):1062–1068. doi: 10.15252/embr.201438587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim EM, Jung CH, Kim J, et al. The p53/p21 complex regulates cancer cell invasion and apoptosis by targeting Bcl-2 family proteins. Cancer Res. 2017;77(11):3092–3100. doi: 10.1158/0008-5472.CAN-16-2098. [DOI] [PubMed] [Google Scholar]
- 18.Kitaura H, Shinshi M, Uchikoshi Y, et al. Reciprocal regulation via protein-protein interaction between c-Myc and p21(cip1/waf1/sdi1) in DNA replication and transcription. J Biol Chem. 2000;275(14):10477–10483. doi: 10.1074/jbc.275.14.10477. [DOI] [PubMed] [Google Scholar]
- 19.Karkhanis M, Park JI. Sp1 regulates Raf/MEK/ERK-induced p21(CIP1) transcription in TP53-mutated cancer cells. Cell Signal. 2015;27(3):479–486. doi: 10.1016/j.cellsig.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lui AJ, Geanes ES, Ogony J, et al. IFITM1 suppression blocks proliferation and invasion of aromatase inhibitor-resistant breast cancer in vivo by JAK/STAT-mediated induction of p21. Cancer Lett. 2017;399:29–43. doi: 10.1016/j.canlet.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manu KA, Chai TF, Teh JT, et al. Inhibition of isoprenylcysteine carboxylmethyltransferase induces cell-cycle arrest and apoptosis through p21 and p21-regulated BNIP3 induction in pancreatic cancer. Mol Cancer Ther. 2017;16(5):914–923. doi: 10.1158/1535-7163.MCT-16-0703. [DOI] [PubMed] [Google Scholar]
- 22.Galanos P, Vougas K, Walter D, et al. Chronic p53-independent p21 expression causes genomic instability by deregulating replication licensing. Nat Cell Biol. 2016;18(7):777–789. doi: 10.1038/ncb3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zohny SF, Al-Malki AL, Zamzami MA, et al. p21(Waf1/Cip1): its paradoxical effect in the regulation of breast cancer. Breast Cancer. 2019;26(2):131–137. doi: 10.1007/s12282-018-0913-1. [DOI] [PubMed] [Google Scholar]
- 24.Koster R, di Pietro A, Timmer-Bosscha H, et al. Cytoplasmic p21 expression levels determine cisplatin resistance in human testicular cancer. J Clin Invest. 2010;120(10):3594–3605. doi: 10.1172/JCI41939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Wang W, Chen Y, et al. The opposite prognostic significance of nuclear and cytoplasmic p21 expression in resectable gastric cancer patients. J Gastroenterol. 2014;49(11):1441–1452. doi: 10.1007/s00535-013-0900-4. [DOI] [PubMed] [Google Scholar]
- 26.Xie H, Li C, Dang Q, et al. Infiltrating mast cells increase prostate cancer chemotherapy and radiotherapy resistances via modulation of p38/p53/p21 and ATM signals. Oncotarget. 2016;7(2):1341–1353. doi: 10.18632/oncotarget.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maiuthed A, Ninsontia C, Erlenbach-Wuensch K, et al. Cytoplasmic p21 mediates 5-fluorouracil resistance by inhibiting pro-apoptotic Chk2. Cancers (Basel) 2018;10(10):373. doi: 10.3390/cancers10100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia X, Ma Q, Li X, et al. Cytoplasmic p21 is a potential predictor for cisplatin sensitivity in ovarian cancer. BMC Cancer. 2011;11:399. doi: 10.1186/1471-2407-11-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C, Ge Q, Liu J, et al. Effects of miR-1236-3p and miR-370-5p on activation of p21 in various tumors and its inhibition on the growth of lung cancer cells. Tumour Biol. 2017;39(6):1010428317710824. doi: 10.1177/1010428317710824. [DOI] [PubMed] [Google Scholar]
- 30.Guo L, Gu J, Hou S, et al. Long non-coding RNA DANCR promotes the progression of non-small-cell lung cancer by inhibiting p21 expression. Onco Targets Ther. 2019;12:135–146. doi: 10.2147/OTT.S186607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su W, Feng S, Chen X, et al. Silencing of long noncoding RNA MIR22HG triggers cell survival/death signaling via oncogenes YBX1, MET, and p21 in lung cancer. Cancer Res. 2018;78(12):3207–3219. doi: 10.1158/0008-5472.CAN-18-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Sun M, Lu K, et al. The long noncoding RNA HOTAIR contributes to cisplatin resistance of human lung adenocarcinoma cells via downregualtion of p21(WAF1/CIP1) expression. PLoS One. 2013;8(10):e77293. doi: 10.1371/journal.pone.0077293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Zhu LJ, Yang YC, et al. MiR-224 promotes the chemoresistance of human lung adenocarcinoma cells to cisplatin via regulating G(1)/S transition and apoptosis by targeting p21(WAF1/CIP1) Br J Cancer. 2014;111(2):339–354. doi: 10.1038/bjc.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng X, Liu H, Zhang Z, et al. Annexin A2 contributes to cisplatin resistance by activation of JNK-p53 pathway in non-small cell lung cancer cells. J Exp Clin Cancer Res. 2017;36(1):123. doi: 10.1186/s13046-017-0594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shan W, Zhang X, Li M, et al. Over expression of miR-200c suppresses invasion and restores methotrexate sensitivity in lung cancer A549 cells. Gene. 2016;593(2):265–271. doi: 10.1016/j.gene.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 36.Noro R, Miyanaga A, Minegishi Y, et al. Histone deacetylase inhibitor enhances sensitivity of non-small-cell lung cancer cells to 5-FU/S-1 via down-regulation of thymidylate synthase expression and up-regulation of p21(waf1/cip1) expression. Cancer Sci. 2010;101(6):1424–1430. doi: 10.1111/j.1349-7006.2010.01559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao YF, Wang CR, Wu YM, et al. P21(waf1/cip1) is required for non-small cell lung cancer sensitive to gefitinib treatment. Biomed Pharmacother. 2011;65(3):151–156. doi: 10.1016/j.biopha.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y, He W, Gao X, et al. Resveratrol overcomes gefitinib resistance by increasing the intracellular gefitinib concentration and triggering apoptosis, autophagy and senescence in PC9/G NSCLC cells. Sci Rep. 2015;5:17730. doi: 10.1038/srep17730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Fei Z, Jiang H. Polyphyllin Ⅶ increases sensitivity to gefitinib by modulating the elevation of P21 in acquired gefitinib resistant non-small cell lung cancer. J Pharmacol Sci. 2017;134(3):190–196. doi: 10.1016/j.jphs.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Zhao J, Fu W, Liao H, et al. The regulatory and predictive functions of miR-17 and miR-92 families on cisplatin resistance of non-small cell lung cancer. BMC Cancer. 2015;15:731. doi: 10.1186/s12885-015-1713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo Q, Lan F, Yan X, et al. Hypoxia exposure induced cisplatin resistance partially via activating p53 and hypoxia inducible factor-1 alpha in non-small cell lung cancer A549 cells. Oncol Lett. 2018;16(1):801–808. doi: 10.3892/ol.2018.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jana S, Patra K, Jana J, et al. Nrf-2 transcriptionally activates P21(Cip/WAF1) and promotes A549 cell survival against oxidative stress induced by H2O2. Chem Biol Interact. 2018;285:59–68. doi: 10.1016/j.cbi.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 43.Xu S, Huang H, Chen YN, et al. DNA damage responsive miR-33b-3p promoted lung cancer cells survival and cisplatin resistance by targeting p21(WAF1/CIP1) Cell Cycle. 2016;15(21):2920–2930. doi: 10.1080/15384101.2016.1224043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biswas K, Sarkar S, Du K, et al. The E3 ligase CHIP mediates p21 degradation to maintain radioresistance. Mol Cancer Res. 2017;15(6):651–659. doi: 10.1158/1541-7786.MCR-16-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Wang Y, Mei H, et al. The BET bromodomain inhibitor JQ1 radiosensitizes non-small cell lung cancer cells by upregulating p21. Cancer Lett. 2017;391:141–151. doi: 10.1016/j.canlet.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 46.Tang Y, Cui Y, Li Z, et al. Radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells. J Exp Clin Cancer Res. 2016;35:7. doi: 10.1186/s13046-016-0285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parveen A, Akash MS, Rehman K, et al. Dual role of p21 in the progression of cancer and its treatment. Crit Rev Eukaryot Gene Expr. 2016;26(1):49–62. doi: 10.1615/CritRevEukaryotGeneExpr.v26.i1.60. [DOI] [PubMed] [Google Scholar]