Abstract
Clinical manifestations of coronavirus disease 2019 in pregnant women, in contrast to previous outbreaks, seem to be similar to those of nonpregnant women. During severe acute respiratory syndrome (SARS), SARS influenza A, and Middle East respiratory syndrome outbreaks, an increased severity of disease among pregnant women was observed.
In some pregnant women, respiratory failure can occur and progress quickly to acute respiratory distress syndrome requiring extracorporeal membrane oxygenation (ECMO) as a rescue therapy. Despite a lack of current guidelines on the use of ECMO in pregnant or postpartum women, this support therapy is an effective salvage therapy for patients with cardiac and/or respiratory failure, and is associated with favorable maternal and fetal outcomes. Herein, the authors report a case of severe COVID-19 disease in a pregnant patient after urgent cesarean delivery, who was treated successfully with ECMO during the postpartum. Extracorporeal membrane oxygenation should be considered early when conventional therapy is ineffective, and it is essential to refer to ECMO expert centers.
Key Words: Extracorporeal membrane oxygenation (ECMO), ARDS, postpartum, COVID-19, pregnancy complication, maternal mortality
Introduction
A new viral disease, caused by a novel coronavirus 2, was identified at the end of 2019 in China. Since then, the disease has expanded rapidly worldwide and the World Health Organization has characterized the disease as coronavirus disease 2019 (COVID-19), declaring it a pandemic in March 2020.1 Although most COVID-19 patients are asymptomatic or mildly symptomatic, several patients can experience respiratory failure and quickly progress to acute respiratory distress syndrome (ARDS). Clinical manifestations of COVID-19 in pregnant women seem to be similar to those of nonpregnant individuals. Pregnancy does not appear to promote infection or worsen the clinical course.2, 3, 4 However, in some patients, severe or critical forms can occur, requiring advanced and rescue therapies such as extracorporeal membrane oxygenation (ECMO).
The fundamental role of ECMO is the transient support of cardiac and/or lung function in the management of potentially reversible effects, providing time necessary for recovery of cardiac and/or respiratory function. In the past decade, the use of ECMO therapy has increased significantly in adults, especially during the last outbreaks (influenza A[H1N1] ARDS in 2009 and Middle East respiratory syndrome [MERS] in 2012), and currently is considered a lifesaving treatment when conventional therapies fail.
The authors report a case of severe COVID-19 disease in a pregnant woman who underwent urgent caesarean delivery and required postpartum ECMO therapy.
Case Report
A previously healthy 31-year-old black woman (gravida 5, para 2) presented to the labor and delivery unit, at 31 weeks of gestation, with 1 week of dry cough and fever without dyspnea. Her medical history was remarkable for obesity (body mass index 31), heterozygous sickle cell disease, unoperated small restrictive perimembranous ventricular septal defect, and prior thyroidectomy. On examination, she was found to be febrile (39°C), with a normal respiratory rate and tachycardia. Breath sounds were normal and she had good oxygen saturation. Laboratory tests and chest radiograph were within normal limits. Obstetric examination and fetal ultrasonography were normal. A COVID-19 infection was suspected and a nasopharyngeal swab was obtained for the detection of severe acute respiratory syndrome coronavirus 2, which was reported as negative on the next day. An antibiotic therapy was started. After 3 days, her clinical conditions deteriorated rapidly, requiring oxygen supplementation and admission to the intensive care unit. Acute chest syndrome of sickle cell disease, cardiogenic pulmonary edema, or infective endocarditis secondary to perimembranous ventricular septal defect was the differential diagnosis.
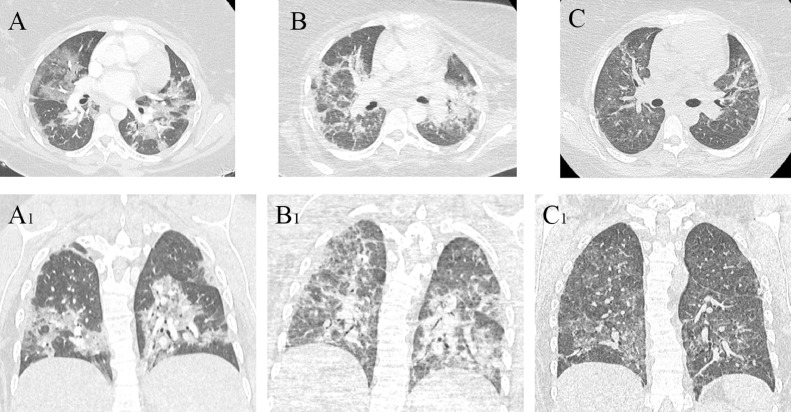
A transthoracic echocardiograph excluded cardiogenic pulmonary edema and infective endocarditis and demonstrated good biventricular function, no valvular abnormalities, and a small perimembranous interventricular defect with left-to-right shunt, with a gradient of 50 mmHg across the defect and normal pulmonary arterial pressure. A chest computed tomography scan (Fig. 1) ruled out the presence of pulmonary embolism, but showed bilateral extensive pulmonary involvement, with ground-glass opacities in the lower lobes and in the peripheral areas. These radiologic findings were found specific for COVID-19 infection, and a second positive nasopharyngeal swab confirmed this hypothesis. Antibiotic therapy was implemented and a prophylactic dose of anticoagulant was started by subcutaneous injection once a day of low-molecular-weight heparin (enoxaparin 4000 IU). Worsening of dyspnea, an increased necessity of oxygen, and a deterioration of arterial blood gas results (pH 7.3, PaO2 62 mmHg, PaCO2 45 mmHg, oxygen saturation as measured by pulse oximetry 90%), required the induction of fetal lung maturation with 24 mg of betamethasone (12 mg given intramuscularly 24 hours apart). Over the subsequent 24 hours, the patient was intubated with specific precautions to prevent contamination and was placed on mechanical ventilation. Urgent caesarean delivery was performed and a baby girl of 1680 g was born. Neither complications of the placenta nor the chorioamniotic membrane inflammation were reported. Three successive nasopharyngeal swab tests for the infant were done at 24 hours, 48 hours, and 1 week of age, which were negative.
After delivery, the patient was referred to the authors’ hospital. Despite optimization of mechanical ventilation, use of neuromuscular blockade, inhaled nitric oxide (80 ppm for the first 48 hours), and prone positioning to improve oxygenation for ARDS, all these advanced strategies were ineffective, with a ratio of arterial oxygen tension-to-fraction of inspired oxygen (PaO2/FIO2) lower than 70 mmHg. Cardiovascular status was unchanged and a week after the delivery, the decision was made to initiate venovenous ECMO (Oxygenator EOS, pump REVOLUTION, Livanova UK). A classic configuration with a double cannulation of the right internal jugular vein and the right femoral vein was established. The ECMO flow (about 5 L/min flow) was adjusted to maintain optimal oxygenation, and hemodynamic conditions remained satisfactory. After 7 days of ECMO support, the respiratory function improved to allow a successful weaning off ECMO therapy. The hemodynamic status was unchanged and she was extubated 1 week later, after 20 days of mechanical ventilation. The patient was transferred to the maternity ward 1 week later. On follow-up, the patient and the baby had gone home, the baby was in good health, and the mother had only moderate shortness of breath on effort.
Discussion
After the first cases in China, the COVID-19 pandemic has spread rapidly around the world. Although most COVID-19 patients are asymptomatic, the clinical manifestations of the disease can be most varied but are generally respiratory. An undefined proportion of patients can experience respiratory failure and quickly progress to ARDS. Severe acute respiratory syndrome coronavirus 2 is associated with higher mortality compared to the 2 recent outbreaks, influenza A(H1N1) ARDS and MERS, combined.5 This mortality is related to the direct and indirect effects of disease and to the lack of effective therapies.
It is well-known that pregnant women are predisposed particularly to respiratory pathogens and severe pneumonia compared to nonpregnant women, due to pregnancy-related immunoalterations and physiologic maternal adaptations to pregnancy, with subsequent higher maternal and fetal morbidity and mortality.6 Recent reports indicated that in contrast to the last outbreaks, SARS, influenza A(H1N1) ARDS and MERS, the clinical manifestations of COVID-19 in pregnant women seem to be similar to those in nonpregnant women. Pregnancy does not appear to promote infection or worsen the clinical course.2, 3, 4 However, as in nonpregnant cases, ARDS can occur, requiring advanced and rescue therapies such as ECMO.
To date, no drug has demonstrated a universally accepted effect against COVID-19, but a preliminary report (Randomised Evaluation of COVID-19 Therapy trial) suggested that the steroid dexamethasone can reduce mortality in COVID-19 patients.7 Another study showed that the administration of antenatal corticosteroids had a higher combined maternal and infant outcome compared with expectant management for women at high risk of preterm birth with COVID-19 infection.8 In the present patient, the administration of betamethasone may have played an important role in association with ECMO therapy.
The widespread use of ECMO therapy is due on the one hand to the improved technology, making its use simpler and safe, and on the other hand to its extensive utilization during the outbreak of influenza A(H1N1) ARDS and MERS. The real benefit of ECMO in ARDS seems to be controversial in influenza A(H1N1) ARDS and more evident in MERS, with a clearly lower mortality in the latter.9 , 10 It is clear that ECMO may be helpful in selected patients, but the optimal timing of ECMO initiation still is debated. During pregnancy and the postpartum period, the use of ECMO for severe influenza A(H1N1) ARDS was associated with a 66% survival rate, while no data were available for MERS.11
Despite a lack of current guidelines on the use of ECMO in pregnant or postpartum patients, several observational studies have reported that ECMO is an effective salvage therapy for patients with cardiac and/or respiratory failure, and is associated with encouraging maternal and fetal outcomes, with a high rate of survival and low rate of major complications.12, 13, 14 The most common complication remains bleeding that contributes to significant maternal morbidity and mortality.11 , 14 The indication and type of ECMO (venovenous or venoarterial) seem to be unrelated to survival.12 , 14
The optimal timing for ECMO assistance is crucial, and outcomes may be related to its very early use. This is far more important in pregnancy, in which it is essential to limit maternal hypoxia, hypercarbia, and acidosis. Early referral to an ECMO expert center, with a dedicated ECMO team, also should be discussed, either directly or after rescue implementation by a mobile ECMO team. The case described confirmed the potential value of the ECMO in severe forms of ARDS and extreme levels of hypoxia in COVID-19 patients.
Conflicts of Interest
The authors declare that there are no conflict of interests.
Acknowledgment
The authors thank all anesthesiologists, intensive care physicians, cardiac surgeons, and the perfusionist team for their collaboration. (Fig. 1 )
Fig 1.
Computed tomography (CT) scan features at different interval. (A, A1) Axial and frontal view at admission to the intensive care unit, before induced delivery. (B, B1) Axial and frontal view after weaning off mechanical ventilation. (C, C1) Axial and frontal view after 1 month after discharge.
References
- 1.World Health Organization (WHO) 2020. Coronavirus disease (COVID-19) outbreak.https://www.who.int [Google Scholar]
- 2.Breslin N, Baptiste C, Gyamfi-Bannerman C, et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM2020:2:100118. [DOI] [PMC free article] [PubMed]
- 3.Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: A preliminary analysis. AJR Am J Roentgenol. 2020;215:127–132. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 5.Mahase E. Coronavirus: Covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 6.Chen YH, Keller J, Wang IT, et al. Pneumonia and pregnancy outcomes: A nationwide population-based study. Am J Obstet Gynecol. 2012;207 doi: 10.1016/j.ajog.2012.08.023. 288.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - Preliminary report [e-pub ahead of print] N Engl J Med. 2020 Jul 17 doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 8.Packer CH, Zhou CG, Hersh AR, et al. Antenatal corticosteroids for pregnant women at high risk of preterm delivery with COVID-19 infection: A decision analysis. Am J Perinatol. 2020;37:1015–1021. doi: 10.1055/s-0040-1713145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pham T, Combes A, Rozé H, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276–285. doi: 10.1164/rccm.201205-0815OC. [DOI] [PubMed] [Google Scholar]
- 10.Alshahrani MS, Sindi A, Alshamsi F, et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8:3. doi: 10.1186/s13613-017-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair P, Davies AR, Beca J, et al. Extracorporeal membrane oxygenation for severe ARDS in pregnant and postpartum women during the 2009 H1N1 pandemic. Intensive Care Med. 2011;37:648–654. doi: 10.1007/s00134-011-2138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JJY, Ong JA, Syn NL, et al. Extracorporeal membrane oxygenation in pregnant and postpartum women: A systematic review and meta-regression analysis. J Intensive Care Med2019:885066619892826. [DOI] [PubMed]
- 13.Moore SA, Dietl CA, Coleman DM. Extracorporeal life support during pregnancy. J Thorac Cardiovasc Surg. 2016;151:1154–1160. doi: 10.1016/j.jtcvs.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 14.Naoum EE, Chalupka A, Haft J, et al. Extracorporeal life support in pregnancy: A systematic review. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.016072. [DOI] [PMC free article] [PubMed] [Google Scholar]