Abstract
Objective
Currently, little is known about the progression of an immune response against SARSCoV- 2 upon infection or sub-infection-exposure over time. We examined the serologic response in healthcare workers up to 12 weeks after a well-documented and contained outbreak and compared results with findings from earlier serologic testing in the same population.
Methods
This study followed 166 health care workers of the University Perinatal Care Center, Regensburg, Germany, for up to 12 weeks. 27 of the subjects had previously tested positive for the presence of SARS-CoV-2 by PCR testing and developed COVID-19. Serologic responses were tested with two independent commercially available test kits.
Results
77.8 % of COVID-19 study subjects developed a specific IgG-response over the course of the 12-week study, while none of the COVID-19 contact groups had a detectable IgG response. Amongst most COVID-19 patients the values of detectable IgG-responses significantly increased over time as confirmed with both tests, while that of positive IgA responses decreased. Between the number of reported symptoms and antibody responses in COVID-19 patients no correlation was found and no new cases of seroconversion were identified in asymptomatic coworkers with negative PCR during the outbreak.
Conclusions
Immune response after COVID-19 increases significantly over time but still approximately 22 % of COVID-19 patients did not mount a measurable serologic immune response within 60 days. Exposed co-workers did not develop any relevant antibody levels at all. We conclude that immunity after infection increases over time, but the antibody response does not develop reliably in all infected people.
Keywords: SARS-CoV-2, Corona virus, COVID-19, Health care workers, Immune response, SARS-CoV-2 antibodies
1. Introduction
Up to now (July 2020) around 10 million people worldwide have been identified as infected with SARS-CoV-2, of which nearly 500.000 succumbed to COVID-19 [1]. In addition to China, early outbreaks took place in South Korea, Japan, and Central Europe where by now, the spread of the virus could be contained with great efforts. However, COVID-19 had been declared a pandemic by the WHO on March 10, 2020 (World Health Organization, 2020) and many countries like the USA, Russia, Brazil, and India are still showing rising daily case numbers.
Health care workers are at exceptionally high risk of infection as they work on the frontline of this pandemic [2]. Perinatal centers seem to be an underestimated hotspot due to the increased presence of young, often paucisymptomatic patients, high aerosol exposure in the delivery room, and a multidisciplinary care team that requires the close proximity of staff members from multiple hospital departments such as anesthesiologists, midwifes, obstetricians, nurses and others [3].
In a well described outbreak at our University perinatal center in Regensburg, Germany, a total of 36 staff members were confirmed virus RNA-positive by reverse transcription, followed by real-time (RT)-PCR and 34 developed COVID-19 [4]. Our previously reported observations and initial antibody testing showed that 2–3 weeks after the initial PCR-based screening only a limited number of staff members affected by COVID-19 had developed relevant antibody responses (48.4 %). At this time point, very few staff members who were in contact with diseased co-workers but tested negative in the PCR-test, showed any antibody response which was limited to IgA (8.2 %) and in one case, to borderline elevated IgG [6]. The objective of this follow up study was now to investigate the progression of the immune response approximately 12 weeks after the outbreak in our hospital staff of COVID-19 affected and contact persons.
2. Methods
2.1. Study design and participants recruitment
The study was designed as a prospective cross-sectional study with optional longitudinal analysis, focused on immune response to SARS-CoV-2 in health care workers approximately 12 weeks after a COVID-19 outbreak in a large University children's and maternity hospital. Details of the outbreak have been reported elsewhere [4]. Briefly, extensive RT-PCR testing was performed on hospital employees (n = 379) initially and after the first serological test was commercially available, all employees were offered a voluntary participation in a SARS-CoV-2 serological test.
All study subjects were categorized into four groups according to their state of infection or exposure to SARS-CoV-2 positive individuals as previously reported [6]: The categories were briefly: (a) “COVID-19 patients”: individuals with at least one positive RT-PCR SARS-CoV-2 test; (b) “Close contact”, (c) “Moderate contact”, and (d) “No contact” (meaning health care workers not belonging to the groups mentioned before but working in the hospital during the outbreak; b, c and d: “Covid-19 contact group”).
Details of symptoms, RT-PCR results and short-term immunoglobulin development (2–4 weeks) have already been reported [6]. All participants in the first assessment were invited to participate in the second assessment. As all data in both studies were fully anonymized and only accessible at an individual level to the participant, data from the first study could only be connected to the second study with the individual approval of the study participants and under the condition that the initial personal study log-in was still remembered by the participant. Therefore, longitudinal analyses were possible in 127 of the 166 participants of the second assessment. The study was approved by the Ethics Committee of the University of Regensburg (file-number: 20-1767-101).
2.2. SARS-CoV-2 testing
Specific antibody response to SARS-CoV-2 was now evaluated by the use of two different commercially available test kits - the Anti-SARS-CoV-2 IgG and IgA ELISA (EUROIMMUN AG, Lübeck, Germany; https://www.euroimmun.com) and the Elecsys Anti-SARS-CoV-2 (Roche Diagnostics, Rotkreuz, Switzerland; https://diagnostics.roche.com). Both tests were performed according to the manufacturer’s protocol. For EUROIMMUN kits, reagent wells were pre-coated with recombinant structural protein (S1 domain) of SARS-CoV-2 for the IgA and IgG assay as previously reported [6]. According to manufacturer’s recommendations, for the IgA and IgG assay an OD ratio of > 1.0 was considered positive.
The qualitative Elecsys Anti-SARS-CoV-2 Antibody immunoassay analyzer was used. The assay does not discriminate between the antibody type(s) present and can detect IgA, IgM, and IgG. The test is based on a recombinant nucleocapsid (N) antigen and has a threshold value of 1.0. Accordingly, all samples with a value < 1.0 were considered negative.
2.3. Statistical analyses
Characteristics of the study cohort are presented using descriptive statistics. Fisher’s exact test was used to examine group differences. For the comparison of the proportions of participants who showed antibody responses over time the McNemar test for paired samples was used. To analyze correlation between the two different antibody tests and for correlation of antibody test results with symptoms, Spearman's rank correlation coefficient or coefficient of determination was used, where applicable. All analyses were performed using SPSS 26 (IBM SPSS Statistics for Windows, USA)
3. Results
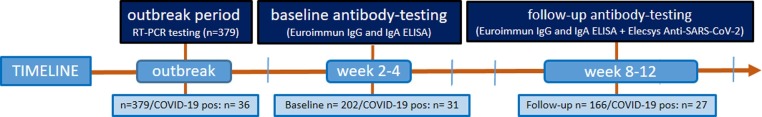
After a COVID-19 outbreak at the University children's and maternity hospital in Regensburg, Germany, serologic testing was performed 2–4 weeks after the outbreak as previously described [6] and now again 8–12 weeks after the outbreak in 166 individuals, including 27 COVID-19 patients who had participated also in the first testing (Fig. 1 ). Median time span between the first and second serologic tests was 38 days (range 29–47). Of the 166 participants in the follow up study, the longitudinal serological antibody course, symptoms and contact category could be analyzed for both timepoints in 127 subjects. The overall study participant number and the demographic characteristics of study participants with longitudinal follow up stratified for exposure groups are summarized in Table 1 .
Fig. 1.
Flow of participants throughout the study with time periods of serologic testing and applied tests including the respective numbers.
Table 1.
Characteristics of the study cohort.
| All groups | COVID-19 | COVID-19 contact group (all health care workers of the hospital with negative SARS-CoV-2-RT PCR) |
Difference between COVID-19 and all other groups, p (Fisher Exact, two-sided) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Close contact | moderate contact | no contact | |||||||||
| All at follow up (n) | 166 | 27 | 139 | ||||||||
| longitudinal follow up (n) | 127 | 25 | 22 | 39 | 41 | ||||||
| Female, N (%) | 109 | 85.8 % | 22 | 88.0 % | 15 | 68.2 % | 35 | 89.7 % | 37 | 90.2 % | n.s. |
| Male, N (%) | 18 | 14.2 % | 3 | 12.0 % | 7 | 31.8 % | 4 | 10.3 % | 4 | 9.8 % | n.s. |
| Age | |||||||||||
| 18−35 years, N (%) | 37 | 29.1 % | 10 | 40.0 % | 6 | 27.3 % | 10 | 25.6 % | 11 | 26.8 % | n.s. |
| 36−50 years, N (%) | 51 | 40.2 % | 8 | 32.0 % | 9 | 40.9 % | 18 | 46.2 % | 16 | 39.0 % | n.s. |
| 51−65 years, N (%) | 39 | 30.7 % | 7 | 28.0 % | 7 | 31.8 % | 11 | 28.2 % | 14 | 34.1 % | n.s. |
The given numbers refer to absolute case numbers. “All groups” describes all included study participants, “COVID-19“ describes those who suffered from COVID-19. The “COVID-19 contact group” includes all participating health care Workers (HCW) working in the hospital during the described outbreak with negative SARS-CoV-2-RT PCR. Included within this are the groups “close contact“ (unprotected contact with a distance of less than 2 m for 15 minutes or longer), “moderate contact“ (individuals with a negative PCR SARS-CoV-2 test who had moderate contact to a co-worker with COVID-19 or who had recently returned from a risk area) and “no contact“ (no significant contact with COVID-19-patients) [10]. The statistical significances of the differences between the groups were tested using the Fisher Exact Test and are enlisted on the right. “All at follow up” are all study participants at the time of the follow up measurement; “longitudinal follow up“ describes the HCW for whom the data from the follow up analysis was exactly relatable to the results from the baseline antibody-testing. “Female“, “Male“ and “Age“ give the gender- and age-distribution of the study participants within each enlisted group.
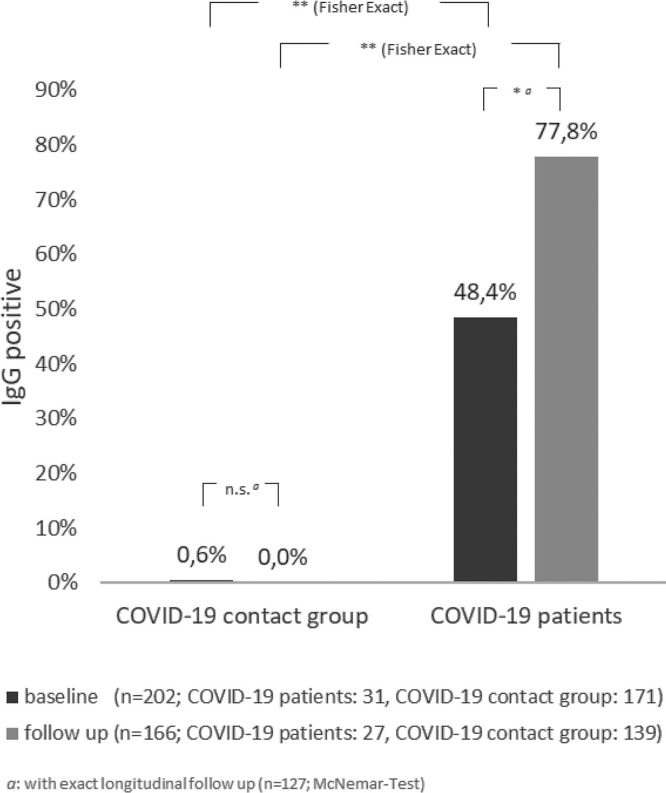
First, the development of IgG antibody responses between baseline and follow up serological test was investigated in COVID-19 patients and COVID-19 contact persons (Fig. 2 ). Only one subject of the COVID-19 contact group had relevant IgG antibody levels at baseline, and none at the follow up testing. A positive IgG response (>1.0) of COVID-19 patients at baseline was detected in 48.4 % of cases. This proportion showed a significant increase to 77.8 % (p < 0.05) at follow up. When analyzed on an individual level, a majority of COVID-19 cases (n = 18) showed an increase of IgG values over time (72 %) while only three individuals with initially positive IgG values (>1.0) showed decreased levels at follow up and two did neither (Fig. 3 ).
Fig. 2.
Development of anti-SARS-CoV-2-IgG antibody response over time in COVID-19 patients and COVID-19-contact groups.
Shown is the number of positive results in the Euroimmun IgG-testing at time points “baseline” (dark) and “follow up” (light) relative to all tests per group at the given time. The results are displayed separately for health care workers with initially positive PCR testing for SARS-CoV-2 (“COVID-19 patients”) and health care workers with negative PCR (“COVID-19 contact groups”). * p < 0.05; ** p < 0.01 (McNemar).
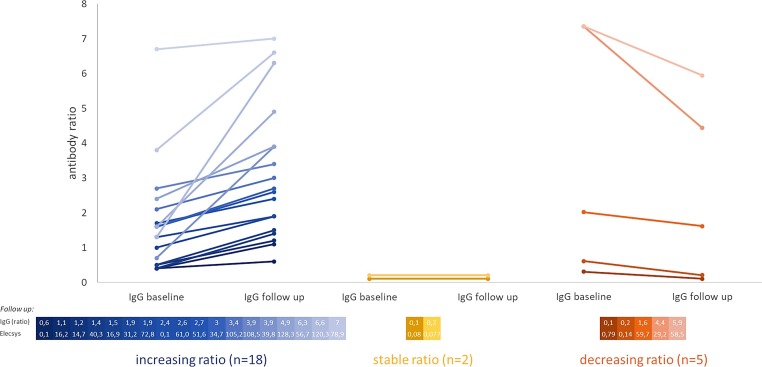
Fig. 3.
Shift of individual anti-SARS-CoV-2-IgG ratio from “baseline”- to “follow up”-antibody testing in COVID-19-patients.
Depicted is the individual anti-SARS-CoV-2-IgG antibody ratio (“antibody ratio”) for each study participant being initially positive in the SARS-CoV-2-PCR-testing at the time points “baseline” and “follow up”. The datapoints covering one individual are connected with colored lines; the colors represent an increasing (blue, left), stable (yellow, middle) and decreasing (red, right) antibody ratio.
The exact follow up-IgG antibody ratios (Euroimmun, “IgG (ratio)”) are shown paired with their respective results from the Elecsys® SARS-CoV-2-test (“Elecsys”) in the table below each chart. The colors of the result pairs are matching the respective results in the charts above them. The number of increasing ratios differs highly significantly (p < 0.01) from the stable and decreasing ratios, whereas comparing the stable and decreasing ratios gives no significant difference in their numbers.
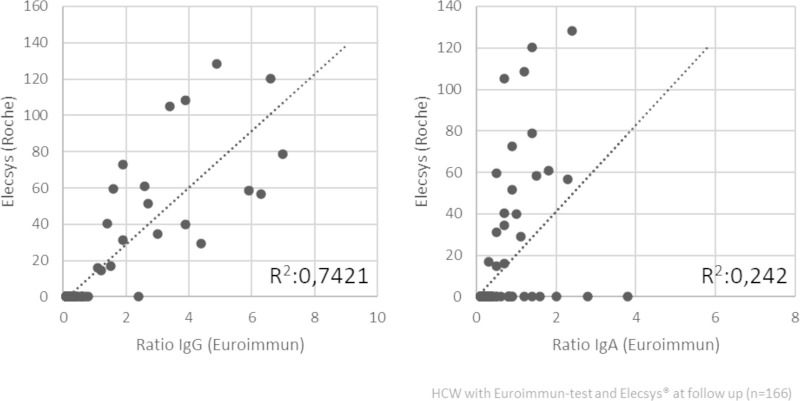
Individual values of both serological tests, the Euroimmun IgG ELISA and the Roche Elecsys assay, at follow up showed good correlation (Fig. 3) in the overall study population (R2 0.74). However, the correlation obtained for the Euroimmun IgA ELISA and the Roche Elecsys assay was low (R2 0.24) (Supplement Fig. 1).
As expected, sequential measurement of IgA antibodies showed a significant decrease from elevated IgA values in 76 % of subjects at baseline to 32 % of subjects (−44 %, p = 0.042) at follow up. Furthermore, IgA antibodies did not predict later IgG response, as COVID-19 patients who never had a detectable IgA-response, developed a specific IgG response in 40 % of cases at the later time point (data not shown).
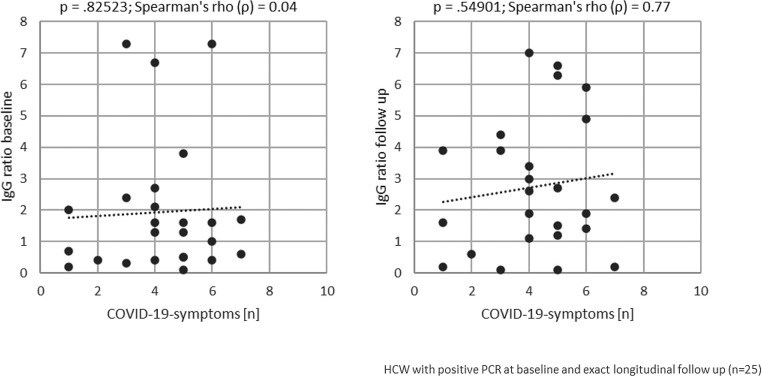
We further reasoned, that multiple symptoms in our group of moderately to mildly affected COVID-19 cases could indicate a more systemic disease and thus, may be associated with a higher likelihood to develop antibodies. Therefore, we correlated the number of reported symptoms with the strength of IgG antibody responses in the group of COVID-19 patients (Fig. 4 ). The correlation was positive, but not statistically significant.
Fig. 4.
Correlation of initially reported symptoms and anti-SARS-CoV-2-IgG ratios for COVID-19 patients. The number of ten of the most common symptoms seen with COVID-19 (“COVID-19 symptoms [n]”, runny nose, sore throat, headache, exhaustion/fatigue, muscle aches, anosmia, shortness of breath, coughing, fever, diarrhea and other [10] is correlated with its respective anti-SARS-CoV-2-IgG ratio (“IgG ratio”) at the time points “baseline” (left) and “follow up” (right) for each participating health care worker (“HCW”) initially suffering from COVID-19 with longitudinal follow up (n = 25). The p-value and the Spearman’s rho are depicted above each chart.
Finally, we investigated if hospital staff in contact with COVID-19 coworkers or those without any contact had acquired SARS-CoV-2 antibodies in the aftermath of the outbreak, which may have not been detectable at the early, first timepoint of the survey. However, neither with the Euroimmune IgA and IgG test nor with the Elecsys test seroconversion or indications for more recent infections could be detected in our study population.
4. Discussion
The detailed tracking of health care workers after a COVID-19 outbreak situation in our perinatal center confirms an increasing IgG based immune response after a COVID-19 infection over time but no silent seroconversion in hospital staff in contact with diseased coworkers.
Out of the 27 COVID-19 patients, 77.8 % developed a humoral IgG response after 8–12 weeks. The course of IgG responses were in line with other reports [5,6], however, our observation period was substantially longer. Therefore the main finding of the study is the significant rate of increasing detectable IgG levels from the first to the second test. Somehow concerning is the fact, that a relevant proportion of COVID-19 patients (22.2 %) did still not develop IgG antibody levels within our observation period. This results leave the possibility that patients without immune response might continue to be or again become susceptible to reinfection in the future [7].
Among the COVID-19 cases, the variability of values was wide: IgG values ranged from 0.1 to 7.3 using the Euroimmun IgG ELISA and the antibody values from the Elecsys ranged from 0.1 to 128.3. A few patients had particularly high IgG levels (Fig. 3). Further research is needed to examine whether high IgG levels are indicative of long-term immunity. A further point of interest regarding high IgG values after COVID-19 infection is the connection with the development of multisystem inflammatory disease, as it is currently being discussed in the literature [8]. Multisystem inflammatory disease is a rare complication of COVID-19 that typically affects children, but has recently been reported to also affect young adults [8,9].
Results of the Euroimmun IgG ELISA and the Roche immunoassay correlated well, while this was not the case for comparison of the IgA ELISA with the Elecsys immunoassay. These results are in line with other studies confirming good sensitivity and specificity of the IgG ELISA and the Elecsys Immunoassay [13,14]. Our longitudinal testing with the same assay (Euroimmun) allowed comparison of the two time points. The additional test with the Elecsys assay confirmed the results of seroconversion and further added to the sensitivity of early detection of humoral responses.
As expected, the sequential study of IgA antibody levels showed that in the COVID-19 group, the rate of patients with detectable IgA antibodies decreased significantly over time. This finding supports the hypothesis already described by other authors, that IgA is not an adequate marker for the long-term course of an immunological response after COVID-19 infection and may only play a role in patients with mild symptoms, especially since our COVID-19 cohort was only affected by mild to moderate symptoms [10].
Further, also the number of experienced symptoms of the COVID-19 patient group did not correlate with immune response. None of our study participants experienced severe symptoms or symptoms correlated with a multisystem inflammatory disease. In this respect, the lack of correlation of the number of symptoms with IgG titers might be explained, but the low number of COVID-19 positive patients in our study population must also be taken into account.
We had initially speculated that exposure to colleagues with COVID-19 might lead to an antibody responses in some hospital staff dependent on the degree of exposure. Interestingly, our results showed no antibody responses in the contact group, rejecting the hypothesis of silent, antibody dependent immunization in this cohort. These results regarding the immune response of COVID-19 contact subjects are in line with the first reports on development of antibody response to COVID-19 [11,12]. In addition, the significant decrease in IgA levels over time in the contact group fits with reports of unspecific IgA reaction to common cold coronaviruses [10,13].
The fact that we did not identify additional seropositive cases in our study cohort 8–12 weeks after the outbreak also allows the conclusion that no new infections have occurred during the study period, although COVID-19 patients have been treated in our hospital throughout the study period. More importantly, this result underlines the effectiveness of the applied hygiene measures to slow the spread of the virus.
For our clinical work we can draw some encouraging results from this study. Firstly, we see that the immunological response of COVID-19 patients has increased significantly over time, and we can therefore assume an increase in immunity. Secondly, we found that none of our hospital staff developed COVID-19 after increased hygiene measures have been applied as described previously [5]. This finding confirms the conclusion, that maintaining service in an obstetric unit during an outbreak is feasible when personal protective equipment, workspace distancing and good hand hygiene are implemented [3,15,16].
The strengths of this observational serologic study are the close and detailed follow up in a population at high risk of COVID-19 infection, allowing correlation of exposure and symptoms of COVID-19 with serologic responses. A further strength is also the long monitoring period of our study with a follow up of up to 12 weeks. Limitations of our study are a recall bias on voluntary participation in the study and a follow up loss of participants. Further, the details of exposure to COVID-19 have been assessed at the time of the outbreak and are less reliable over time, due to possible exposure outside the hospital.
5. Conclusion
We conclude from our data that immune response after a COVID-19 outbreak increases significantly over time but still approximately 22 % of COVID-19 patients did not mount a measurable serologic immune response within 60 days after symptoms have occurred. Additionally, we can summarize that exposed co-workers did not develop any relevant IgG antibody levels over time. Maintaining clinical services using protective measures seems to be safe for employees and patients, even in an outbreak situation, if the necessary measures are taken fast and ferociously. Use of these protective measures need to be continued as neither immunity after infection nor herd immunity are reliable.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the University of Regensburg (file number: 20-1767-101). All hospital staff members participating provided written informed consent. The participants have the right to withdraw from the study at any time.
Consent for publication
Not applicable
Availability of data and materials
The datasets used and/or analyzed for this paper are available from the corresponding author on reasonable request.
Funding
None.
CRediT authorship contribution statement
Sara Fill Malfertheiner: Formal analysis, Investigation, Methodology, Writing - original draft. Susanne Brandstetter: Data curation, Formal analysis, Investigation, Methodology, Writing - original draft. Samra Roth: Investigation. Susanne Harner: Data curation, Investigation. Heike Buntrock-Döpke: Investigation, Project administration. Antoaneta A. Toncheva: Methodology. Natascha Borchers: Methodology. Rudolf Gruber: Formal analysis, Methodology. Andreas Ambrosch: Investigation, Methodology, Validation. Michael Kabesch: Conceptualization, Project administration, Resources, Supervision, Validation, Writing - original draft, Formal analysis. Sebastian Häusler: Formal analysis, Investigation, Methodology, Writing - original draft.
Declaration of Competing Interest
All authors declare that they have no conflict or competing interests.
Acknowledgements
We are grateful to all staff members of our hospital who participated in the study and supported this research project. Moreover, we would like to thank Elke Fischer, Sarah Geitner, Silvia Gran, Patricia Schöberl, Elisa Valletta, and Christine Wolff for the collection and management of data for this study. We thank Jakob Niggel and Gabriel Zink from Datadesk.de for the donation of IT support in data managing and Barbara Schmidt from the virology laboratory of the University of Regensburg to provide the RT-PCR data from the outbreak phase.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jcv.2020.104575.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Organization WH . 2020. Coronavirus Disease (COVID-19) Situation Report – 162.https://www.who.int/docs/default-source/coronaviruse/20200630-covid-19-sitrep-162.pdf?sfvrsn=e00a5466_2 [updated 10:00 CEST, 30 June 2020. Available from: [Google Scholar]
- 2.Lombardi A., Consonni D., Carugno M., Bozzi G., Mangioni D., Muscatello A. Characteristics of 1573 healthcare workers who underwent nasopharyngeal swab testing for SARS-CoV-2 in Milan, Lombardy, Italy. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiefer M.K., McKiever M.E., Russo J.R., Ma’ayeh M., Gee S.E., Smith D.D. Exposure and seroconversion to severe acute respiratory syndrome coronavirus 2 among obstetrical healthcare providers following a contained outbreak. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabesch M., Roth S., Brandstetter S., Hausler S., Juraschko E., Weigl M. Successful containment of Covid-19 outbreak in a large maternity and perinatal center while continuing clinical service. Pediatr. Allergy Immunol. 2020 doi: 10.1111/pai.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor-Phillips S. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020;6 doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papachristodoulou E., Kakoullis L., Parperis K., Panos G. Long-term and herd immunity against SARS-CoV-2: implications from current and past knowledge. Pathog. Dis. 2020;78(3) doi: 10.1093/femspd/ftaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sokolovsky S., Soni P., Hoffman T., Kahn P., Scheers-Masters J. COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandstetter S., Roth S., Harner S., Buntrock-Dopke H., Toncheva A.A., Borchers N. Symptoms and immunoglobulin development in hospital staff exposed to a SARS-CoV-2 outbreak. Pediatr. Allergy Immunol. 2020 doi: 10.1111/pai.13278. 00:1–7. [DOI] [PubMed] [Google Scholar]
- 11.Guo L., Ren L., Yang S., Xiao M., Chang Yang F. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao A.T., Gao C., Zhang S. Profile of specific antibodies to SARS-CoV-2: the first report. J. Infect. 2020;81(1):147–178. doi: 10.1016/j.jinf.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egger M., Bundschuh C., Wiesinger K., Gabriel C., Clodi M., Mueller T. Comparison of the Elecsys(R) anti-SARS-CoV-2 immunoassay with the EDI enzyme linked immunosorbent assays for the detection of SARS-CoV-2 antibodies in human plasma. Clin. Chim. Acta. 2020;509:18–21. doi: 10.1016/j.cca.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer B., Torriani G., Yerly S., Mazza L., Calame A., Arm-Vernez I. Validation of a commercially available SARS-CoV-2 serological immunoassay. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pressler J., Fill Malfertheiner S., Kabesch M., Buntrock-Dopke H., Hausler S., Ambrosch A. Postnatal SARS-CoV-2 infection and immunological reaction: a prospective family cohort study. Pediatr. Allergy Immunol. 2020 doi: 10.1111/pai.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onwuzurike C., Meadows A.R., Nour N.M. Examining inequities associated with changes in obstetric and gynecologic care delivery during the coronavirus disease 2019 (COVID-19) pandemic. Obstet. Gynecol. 2020;136(1):37–41. doi: 10.1097/AOG.0000000000003933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed for this paper are available from the corresponding author on reasonable request.