Abstract
The emerging pandemic of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) presents an unprecedented challenge for healthcare systems globally. The clinical course of COVID-19 and its ability to rapidly create widespread infection has major implications, warranting vigorous infection prevention and control measures. As the confirmed number of cases has surpassed 5.6 million worldwide and continues to grow, the potential severity of the disease and its deadly complications requires urgent development of novel therapeutic agents to both prevent and treat COVID-19. Although vaccines and specific drug therapies have yet to be discovered, ongoing research and clinical trials are being conducted to investigate the efficacy of repurposed drugs for treating COVID-19. In the present review, the drug candidates that have been suggested to treat COVID-19 will be discussed. These include anti-viral agents (remdesivir, ribavirin, lopinavir-ritonavir, favipiravir, chloroquine, hydroxychloroquine, oseltamivir, umifenovir), immunomodulatory agents (tocilizumab, interferons, plasma transfusions), and adjunctive agents (azithromycin, corticosteroids), among other miscellaneous agents. The mechanisms of action and further pharmacological properties will be explored, with a particular focus on the evidence-based safety and efficacy of each agent.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Drug targets, Clinical trials, Mechanism of action
1. Prevalence
The coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 was initially contracted in Wuhan, China, in December 2019 and has since spread globally. On March 11, 2020, the World Health Organization (WHO) declared COVID-19 as a pandemic. COVID-19 is a rapidly developing crisis that is having a detrimental impact on available resources, with cases continuing to increase daily. As of May 26, 2020, there are 5.6 million reported cases and 350,000 deaths in over 200 countries. Global efforts have been directed towards containing and reducing further spread of the virus.
2. Epidemiology
As of May 26, 2020, there are 1,843,581 confirmed cases of COVID-19 in Europe and over 168,308 deaths, which represents 9.1% of all confirmed cases. The first COVID-19 case in Europe was reported in France on January 25, 2020. Following this, the number of cases increased drastically in many European countries, particularly in Russia, United Kingdom, Spain, and Italy (“Coronavirus cases in Europe, by country 2020 | Statista,“). The World Health Organization (WHO) began to consider Europe as the epicentre of the pandemic on March 13, 2020, as the number of new cases surpassed those in China (“World Health Organization COVID-19 Update March 13, 2020,“). The pandemic has resulted in varying degrees of healthcare system burden, specifically intensive care units, with some countries experiencing significant barriers to treating positive patients in a timely manner (“Epidemiological summary of COVID-19 cases in Canada - Canada.ca,“).
3. History of coronaviruses
Coronaviruses belong to a large family of viruses called Coronaviridae. Several members of this family continually circulate among the human population and commonly target the upper-respiratory system, resulting in mild to moderate symptoms, such as the common cold. Conversely, some coronaviruses are capable of causing more severe illness, which may result in death. Severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) are transmitted from animal to human hosts and are known to cause severe illness in humans. The mortality rate of SARS-CoV and MERS-CoV are 10% and 27%, respectively (Chen et al., 2020b, Chen et al., 2020a). SARS-CoV was first identified in Asia in February of 2003, and quickly resulted in a global outbreak that was associated with 8098 cases and 774 deaths (CDC, 2017). SARS-CoV spreads either through close contact with SARS-infected individuals or by direct contact with the respiratory secretions and body fluids of an infected individual. It was understood that infected individuals were not contagious until the onset of symptoms. As such, contact tracing during the SARS-CoV outbreak was highly effective. The severity of symptoms made it easier to identify and contain the virus. The transmission of MERS-CoV is similar to that of SARS-CoV, but MERS-CoV is not spread as readily as SARS-CoV. The outbreak of MERS-CoV occurred in 2012, with the majority of cases in the Middle East. In comparison to SARS-CoV and MERS-CoV, COVID-19 appears to be much more contagious but less lethal, as the majority of patients present with mild symptoms and a good prognosis. The resulting high transmissibility of COVID-19 poses a unique challenge for public health officials to effectively contact trace and resolve the ongoing crisis.
4. Transmission
COVID-19 has the ability to spread through respiratory droplets during close contact due to its predominance in the upper respiratory tract. It is possible to acquire COVID-19 when in close proximity to an infected individual who coughs, sneezes, or even talks. Following the initial exposure, it may take up to 14 days before an individual develops symptoms. The median time from exposure to symptom onset has been reported to be four to five days (CDC, 2020). Additionally, over 80% of infected individuals are asymptomatic or have mild symptoms (Wu et al., 2020). Asymptomatic and pre-symptomatic individuals are capable of unknowingly spreading the virus, although the risk of transmission is the highest in symptomatic patients due to viral shedding (CDC, 2020). As the majority of mild or symptomatic cases often go unreported, it is difficult for communities to contain high risk areas. Management guidelines have recommended frequent hand washing, avoidance of direct contact, as well as stay-at-home and physical distancing orders to help slow the spread of the virus.
5. Clinical presentation
COVID-19 has a wide spectrum of clinical presentations. The most commonly reported symptoms of COVID-19 include fever, dry cough, headaches, weakness, and shortness of breath (Statistics Canada, 2020). Other non-specific symptoms include sore throat, dysgeusia, poor appetite, nasal congestion, and diarrhea (Guzik et al., 2020). Although most infections are self-limited, approximately 15% of infected individuals develop respiratory symptoms that require supplemental oxygen. Moreover, an additional 5% require advanced ventilator support due to hypoxemic respiratory failure, acute respiratory distress syndrome (ARDS), and multiorgan failure (Wang et al., 2020a, Wang et al., 2020b). While symptoms of COVID-19 are predominantly respiratory, direct or indirect involvement of other organ systems is common, such as neurologic symptoms and cardiac damage (Guzik et al., 2020; Wu et al., 2020). Furthermore, individuals with pre-existing comorbidities such as cardiovascular dysfunction, respiratory disease, or diabetes may experience more severe symptoms of COVID-19. Significant respiratory symptoms ensue that may lead to ARDS and death (Wu et al., 2020).
6. Susceptible populations
Although all humans are capable of contracting COVID-19, select populations are at higher risk of developing more severe outcomes. It is estimated that 13.4% of patients ages 80 and older will die of COVID-19, compared to 1.25% and 0.3% of patients in their 50s and 40s, respectively (Verity et al., 2020). Increased susceptibility may be due to comorbidities and a weakened immune system, permitting faster progression of viral infection (Rothan and Byrareddy, 2020). With one of the oldest populations in Europe, Italy has seen the largest number of outbreaks in long-term care facilities and nursing homes. In Italy, COVID-19 patients 60 years or older make up 95.4% of deaths related to COVID-19 (“Italy: coronavirus deaths by age | Statista,” 2020). Individuals with underlying medical conditions such as cardiovascular disease, hypertension, diabetes, chronic respiratory diseases, cancer, and chronic kidney disease, are also at increased risk of fatal outcomes. Immunocompromised hosts, either from pre-existing conditions or medical treatments (e.g. HIV or chemotherapy), have a reduced ability to resolve viral infections, making these patients more vulnerable to severe consequences of COVID-19. It is important to note that severe illness attributable to COVID-19 can occur in people with no known risk factors, albeit at a lower rate.
7. Mechanisms of disease
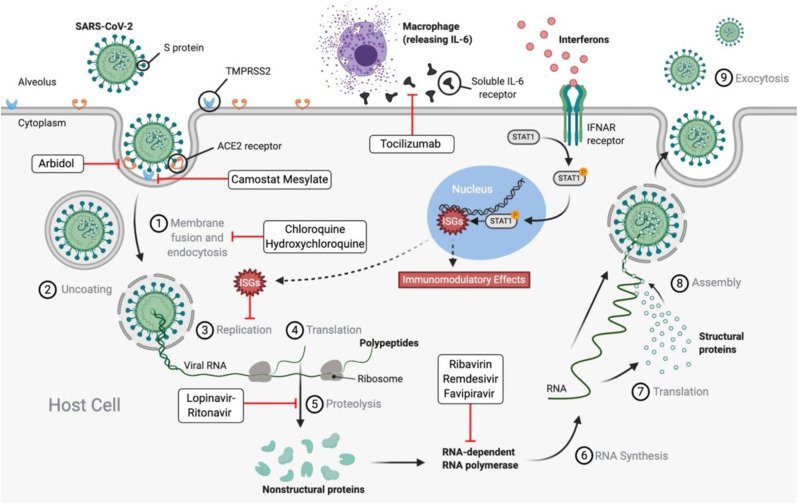
Understanding the mechanism of transmissibility and pathogenesis of SARS-CoV-2 allows researchers to identify targets for novel therapeutic agents to prevent or treat the disease. SARS-CoV-2 is a single-stranded RNA-enveloped virus (Sanders et al., 2020). Its entry into host cells is dependent on binding of its structural spike (S) protein to host cell receptors and S-protein priming via host cell proteases (Hoffmann et al., 2020). The primary target is human lung epithelial cells (Rothan and Byrareddy, 2020). SARS-CoV-2 binds to angiotensin converting enzyme 2 (ACE2) receptors on the surface of human cells through its S-protein and, following this initial binding, 2 transmembrane serine protease (TMPRSS2) primes the S-protein, facilitating viral entry into the cell through endosomes (Fig. 1 .) (Guzik et al., 2020; Hoffmann et al., 2020). Once the virus has entered the human cell, it is capable of hijacking the host cell's machinery to undergo viral replication (Wu et al., 2020). The binding of S-proteins to ACE2 receptors is a critical step required for viral entry and is a potential target for COVID-19 pharmacotherapy that is being studied vigourously (Guo et al., 2020). Additionally, sequencing of the viral genome of SARS-CoV-2 has created opportunity for diagnostic testing, with hopes of developing effective preventive and therapeutic strategies (Sanders et al., 2020). Researchers have discovered that the genome of SARS-CoV-2 is 76.6% similar to SARS-CoV (Wu et al., 2020). Although similar, subtle genetic differences may translate to significant differences in infectivity and severity.
Fig. 1.
Mechanisms of Severe Acute Respiratory Syndrome Coronavirus 2 infection cycle and various drug candidates for treatment of COVID-19. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; S protein: Spike protein; TMPRSS2: 2 transmembrane serine protease; ACE2: Angiotensin converting enzyme 2; IL-6: Interleukin 6; RNA: Ribonucleic acid; IFNAR: Interferon-α/β receptor; ISGs: Interferon-stimulated genes; P: Phosphorus; STAT1: Signal transducer and activator of transcription 1.
7.1. Cytokine storm
Cytokine storm is an aberrant host immune response characterized by high concentrations of pro-inflammatory cytokines and chemokines, such as tumour necrosis factor-α (TNF-α) and various interleukins (IL), including IL-1 and IL-6 (Levi et al., 2020). TNF- α and IL-1 suppress endogenous anticoagulant pathways, while IL-6 aids in coagulation activation and thrombin generation (Levi et al., 2020). The excessive release of cytokines results in excessive inflammation, contributing to the severity and pathogenesis of COVID-19.
There have been reports of cytokine storm associated hypercoagulopathy in patients with severe COVID-19. Characteristic findings include increased D-dimer concentration, prolonged prothrombin time, increased fibrin degradation products, and thrombocytopenia. Cohort studies have shown a 31% incidence of venous and arterial thrombotic complications, with the most common being potentially life-threatening pulmonary embolisms (Helms et al., 2020; Klok et al., 2020; Levi et al., 2020). Although the pathogenesis of COVID-19-associated hypercoagulability is still unknown, systemic inflammation and hypoxia secondary to COVID-19 may increase inflammatory cytokine levels and subsequent coagulation pathway activation (“Antithrombotic Therapy Coronavirus Disease COVID-19,” CDC, 2020).
8. Pharmacotherapy
Identifying a drug that slows or kills SARS-CoV-2 requires a multi-factorial approach. Successfully implemented pharmacotherapy has the potential to save severely ill COVID-19 patients and ease the burden of the pandemic on healthcare systems. Prophylactic treatment has been suggested, particularity to frontline workers and those at higher risk of susceptibility (Kupferschmidt, 2020). As the detrimental consequences of COVID-19 continue to impact nations globally, the need for a safe and effective treatment is paramount. Currently, there is no vaccine or specific therapeutic drug to treat COVID-19, others than supportive care. Pharmacotherapy has been aimed at alleviating symptoms, combined with various attempts to prevent the spread and complications of COVID-19. At present, repurposing of available medications has been the standard of care for treatment of SARS-CoV-2 patients (Kupferschmidt, 2020). This includes unapproved agents that have demonstrated in vitro activity against SARS-CoV and MERS-CoV. Furthermore, many clinical trials are rapidly underway to develop potential therapeutic agents and vaccines (Antithrombotic Therapy, 2020; Coronavirus, 2020; Epidemiological, 2020; Lexicomp for Dentistry, 2020; Zhang et al., 2020).
8.1. Anti-viral agents
8.1.1. Remdesivir
Remdesivir was first developed during the peak of the Ebola virus outbreak in 2016, and has been shown to be the most promising therapy in treating COVID-19 (Ko et al., 2020; Sanders et al., 2020). It is a broad-spectrum anti-viral agent that acts as an inhibitor of RNA-dependent RNA polymerase, an enzyme needed for viral replication (Fig. 1.) (Kupferschmidt, 2020). Although Remdesivir failed in clinical trials for treatment of Ebola in 2014, it is understood to be a safe drug. Similar to the doses used in the clinical trials to treat Ebola, remdesivir is administered as a 200 mg loading dose on day 1, followed by a daily 100 mg IV dose for nine days (Table 2).
Table 2.
Dosing regimens of potential pharmacological agents for treatment of COVID-19.
| Drug | Administration | Dosage | Approved indication(s) |
|---|---|---|---|
| Remdesivir | IV | 10 day administration; day 1 200 mg QD loading dose, followed by 100 mg QD | None |
| Ribavirin | Oral | 500 mg BID or TID in combination with IFN-α or lopinavir/ritonavir | RSV infection, hepatitis C, bunyavirus, herpesvirus, adenovirus, poxvirus, and some viral hemorrhagic fevers |
|
Lopinavir-ritonavir (Kaletra) |
Oral | 400mg/100 mg BID for up to 14 days | HIV |
| Favipiravir | Oral | 600 mg BID | Influenza A and B, Ebola virus, Norovirus |
|
Chloroquine (Aralen) |
Oral | 500 mg orally QD or BID for 5–10 days | Systemic lupus erythematosus (SLW), rheumatoid arthritis (RA), malaria |
|
Hydroxychloroquine (Plaquenil) |
Oral | Day 1 400 mg BID, followed by 200 mg BID for 5–10 days Alternative: 200 mg TID for 10 days or 400 mg QD for 5 days |
Systemic lupus erythematosus (SLW), rheumatoid arthritis (RA), malaria |
| Oseltamivir | Oral | 75 mg QD | Influenza A and B |
| Umifenovir (Arbidol) | Oral | 200 mg TID for 7–14 days | Influenza A and B |
Note. There are currently no approved doses for treatment of COVID-19. Doses listed are for the approved indication(s) or clinical trials (“Lexicomp for Dentistry,” 2020, “Table 2b Characteristics of Potential Antiviral Agents | Coronavirus Disease COVID-19,” 2020; Sanders et al., 2020; Yousefi et al., 2020).
The first randomized, placebo-controlled clinical trial by the National Institute of Allergy and Infectious Diseases (NIAID) demonstrated a significantly faster recovery time of 11 days (31% improvement) for 1000 COVID-19 patients taking remdesivir, compared to 15 days in the placebo arm. However, there was no significant difference identified in the number of deaths between participants who received remdesivir versus those who did not. The mortality rate was 8% for patients receiving remdesivir compared to 11.6% in the control group (“Adaptive COVID-19 Treatment Trial (ACTT) ClinicalTrials.gov,” 2020, “NIH Clinical Trial Shows Remdesivir Accelerates Recovery from Advanced COVID-19,” 2020; Ledford, 2020). Gilead Sciences of Foster City, California, the developers of remdesivir, released the results of a randomized, multicenter phase III clinical study on April 29, 2020 that evaluated the safety, efficacy, and optimal treatment duration of remdesivir to treat severe COVID-19. Researchers employed a 5-day dosing regimen compared to a 10-day regimen. Data revealed that patients receiving a 10-day course had similar clinical improvement compared to patients receiving the 5-day treatment course (OR = 0.75). This trial had an “open label” study design, meaning a placebo was not in place (Ledford, 2020). A second study by Gilead is evaluating remdesivir administration compared with standard of care, with results expected at the end of May (“Remdesivir Clinical Trials,” 2020). A randomized, double-blind, placebo-controlled multicentre phase III trial was conducted in China to evaluate the efficacy of remdesivir (“A Trial of Remdesivir in Adults With Severe COVID-19 ClinicalTrials.gov,” 2020; Ko et al., 2020). Severely ill COVID-19 patients (n = 237) were enrolled, and 158 were administered remdesivir while 79 were given placebo. Clinical improvements were defined as time to improvement (Wang et al., 2020b, Wang et al., 2020a). Statistically significant clinical improvements were not observed for patients taking remdesivir. The trial was ended prematurely due to lack of patient enrollment, as China's new case rate has dropped significantly.
Despite conflicting clinical results, the US Food and Drug Administration (FDA) approved an ‘emergency use authorization’ for hospital intravenous administration of Remdesivir to patients with severe COVID-19 on May 1, 2020 (Ledford, 2020).
Various clinical trials have reported serious adverse effects following administration of remdesivir, such as hepatoxicity (Table 1 ) (“Lexicomp for Dentistry,“). Additionally, over 10% of patients experienced nausea and acute respiratory failure in the Gilead clinical trial.
Table 1.
Review of proposed pharmacological agents to treat COVID-19 (“Lexicomp for Dentistry,” 2020, “UpToDate,” 2020.; Sallard et al., 2020; Sanders et al., 2020; Uno, 2020; Yousefi et al., 2020).
| Drug | Mechanism of action | Adverse drug reactions | Drug interactions |
|---|---|---|---|
| ANTI-VIRAL AGENTS | |||
| Remdesivir | RNA-dependent RNA polymerase inhibitor | Gastrointestinal disturbances (nausea, vomiting), aminotransferase elevations, infusion related reaction (hypotension, diaphoresis, shivering) |
|
| Ribavirin | RNA-dependent RNA polymerase inhibitor | Hemolytic anemia (may lead to death in cardiac patients), alopecia, abdominal pain, anemia, hyperbilirubinemia, arthralgia |
|
| Lopinavir-ritonavir (Kaletra) | 3CL protease inhibitor | Gastrointestinal disturbances (nausea, vomiting, diarrhea), transaminase elevations, increased bleeding, hyperlipidemia, hyperglycemia, insulin resistance, QT prolongation, possible risk of renal dysfunction |
|
| Favipiravir | RNA-dependent RNA polymerase inhibitor | Gastrointestinal disturbances (nausea, vomiting diarrhea), hyperuricemia, elevated transaminases, decreased neutrophil count |
|
| Chloroquine (Aralen) | Viral entry inhibitor | Gastrointestinal disturbances (nausea, vomiting, diarrhea), headache, anorexia, bitter taste, QT prolongation, Torsades de Pointes, arrhythmia, agranulocytosis, seizures rare renal toxicity |
|
| Hydroxychloroquine (Plaquenil) | Viral entry inhibitor | Gastrointestinal disturbances (nausea, vomiting, diarrhea), QT prolongation, hypoglycemia, neuropsychiatric effects, agranulocytosis, seizures, retinopathy |
|
| Oseltamivir | Neuraminidase inhibitor | Gastrointestinal disturbances (nausea, vomiting), headache, arrhythmia, hepatitis, anaphylaxis |
|
| Umifenovir (Arbidol) | Spike protein/ACE2 membrane fusion inhibitor | Gastrointestinal disturbances, allergic reaction, elevated transaminases |
|
| IMMUNOMODULATORY AGENTS | |||
| Tocilizumab (Actemra) | IL-6 inhibitor | Infusion reactions, GI perforation, increased neutrophils, decreased platelets, neutropenia, elevated ALT, increased lipids |
|
|
Interferons (ex. IFNα and IFNβ) |
Activate interferon-stimulated genes (ISGs):
|
Malaise, fatigue, fever |
|
| ADJUNCTIVE AGENTS | |||
| Azithromycin | Antibacterial; used in combination with hydroxychloroquine for synergistic antiviral effect | Gastrointestinal disturbances, rash, QT prolongation, hepatotoxicity |
|
|
Corticosteroids (ex. Methylprednisolone) |
Cytokine gene expression inhibitor | Adrenal suppression, osteoporosis, hypercholesterolemia, hyperglycemia, hypertension |
|
| MISCELLANEOUS AGENTS | |||
| Camostat mesylate | Serine protease inhibitor | Oedema, urticaria, elevated peripheral blood eosinophilia | Minimal information available due to studies published in Japanese |
| ACE inhibitor/ARB | ACE inhibitor; inhibit formation of angiotensin II ARB; angiotensin II receptor antagonist |
ACE inhibitor; dry cough, hyperkalemia, hypotension, dizziness, orthostatic hypotension, acute renal failure, skin rash, dysgeusia ARB; hypotension, dizziness, fatigue, orthostatic hypotension |
ACE inhibitor; Non-steroidal anti-inflammatory drugs (NSAIDs), diuretics, allopurinol, α-blockers, agents that act to increase serum potassium ARB; Non-steroidal anti-inflammatory drugs (NSAIDs) |
Note. The resulting adverse drug reactions and drug interactions are not exhaustive. See Lexicomp for complete list.
8.1.2. Ribavirin
Ribavirin is a guanine analogue that inhibits viral RNA-dependent RNA polymerase ( Fig. 1 .). Its limited ability to establish a definitive therapeutic benefit during the 2003 SARS-CoV and 2012 MERS-CoV outbreaks have led to its lower levels of clinical testing during COVID-19 (Khalili et al., 2020). In vitro data is available that investigated the effects of ribavirin on COVID-19. Unfortunately, these results have yielded inconclusive benefits (Bhimraj et al., 2020). The half-maximal effective concentration (EC50) of ribavirin was found to be significantly higher than remdesivir and chloroquine (EC50= 109.50μM, EC50=0.77μM, EC50=1.13μM, respectively). The researchers concluded a decreased in vitro potency of ribavirin compared to its comparative therapeutic agents (Wang et al., 2020b, Wang et al., 2020a). Furthermore, clinical studies of ribavirin for treatment of SARS-CoV have shown dose-dependent adverse drug reactions, including hematologic and liver toxicity (Table 1) (Sanders et al., 2020). This inconclusive research suggests that ribavirin has limited value in serving as a therapeutic agent against COVID-19. If used, combination therapy, such as with interferon-α or lopinavir-ritonavir, may provide improved clinical efficacy (Sanders et al., 2020; Yousefi et al., 2020; Zhong et al., 2020).
8.1.3. Lopinavir-ritonavir (Kaletra)
Lopinavir-ritonavir is used as antiretroviral combination therapy to manage HIV positive patients. Lopinavir inhibits the HIV protease, an enzyme required for new viral assembly ( Fig. 1 .). Due to its poor oral bioavailability and extensive biotransformation, lopinavir is co-administered with ritonavir in order to prolong levels in the human body and enhance its exposure (Table 2 ) (Kupferschmidt, 2020; McKee et al., 2020).
Current available data suggests a limited role of lopinavir-ritonavir in treatment of COVID-19. In a randomized controlled, open-label trial conducted by Cao et al., there was no benefit observed in COVID-19 patients receiving lopinavir-ritonavir compared to the standard care. The lopinavir-ritonavir group demonstrated similar mortality rates (19.2%) compared to the standard of care group (25%) (Cao et al., 2020). Additionally, a single-center controlled trial conducted in China showed that lopinavir-ritonavir monotherapy did not improve clinical outcomes for hospitalized patients with mild to moderate COVID-19 compared to standard of care (Li et al., 2020).
The most common adverse effect of lopinavir-ritonavir includes gastrointestinal disturbance (up to 28%), most notably diarrhea and nausea. Hepatotoxicity (2–10%) has also been observed (Table 1) (Sanders et al., 2020; Wu et al., 2020). In order to improve drug tolerability, reducing the current doses (Table 2) from twice daily to once daily has been suggested (Baldelli, 2020). The addition of interferon β-1a to lopinavir-ritonavir has also been initiated in clinical trials (Kupferschmidt, 2020; McKee et al., 2020).
8.1.4. Favipiravir
Favipiravir was developed by Toyama Chemical in Japan in 2014. Favipiravir acts as a selective inhibitor of RNA-dependent RNA polymerase ( Fig. 1 .) (Coomes, 2020). It is approved in some countries to treat influenza, Ebola, and norovirus (McKee et al., 2020; Wu et al., 2020). Preliminary clinical results indicate that favipiravir shows significantly greater improvement in chest imaging in COVID-19 patients compared to lopinavir-ritonavir (91.4% improvement with favipiravir, 62.2% improvement with lopinavir-ritonavir). Faster viral clearance (4 days versus 11 days) and fewer adverse events (11.4% versus 55.6%) were also observed in patients administered favipiravir compared to those taking lopinavir-ritonavir (Cai et al., 2020). A prospective randomized clinical trial conducted in China supports these results, demonstrating a significantly greater recovery rate in non-critical COVID-19 patients receiving favipiravir compared to umifenovir (71.4% versus 55.9%). In COVID-19 patients receiving favipiravir, fever, cough, and respiratory problems were reduced. It is important to note that this effect was not significant among critically ill COVID-19 patients (C. Chen et al., 2020). These early results have led to further trials on the efficacy of favipiravir. Currently, favipiravir is being sent to 43 countries for clinical trial testing in COVID-19 patients.
8.1.5. Chloroquine (Aralen) and hydroxychloroquine (Plaquenil)
Chloroquine and hydroxychloroquine are indicated for treatment of inflammatory diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), as well as prevention and treatment of malaria (Sanders et al., 2020). These agents act to decrease the acidity in endosomes, inhibiting viral fusion and subsequent entry inside of the cell ( Fig. 1 .) (Guzik et al., 2020). The hydroxyl group found in hydroxychloroquine results in less toxicity than chloroquine, while maintaining similar anti-viral activity (Wu et al., 2020).
Early studies attracted widespread attention into the potential benefits of these pharmacological agents for treating COVID-19 patients, despite limited and inconclusive evidence. On March 28, 2020, the FDA issued the emergency use of chloroquine and hydroxychloroquine for patients hospitalized with COVID-19 (Magagnoli et al., 2020). However, the FDA has since revoked its emergency use, as more recent findings have concluded that chloroquine and hydroxychloroquine have no benefit for treatment of COVID-19. The FDA does not recommend using these agents in hospitalized COVID-19 patients (“FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems | FDA,” 2020). Previous findings of the suggested dosing regimens for chloroquine and hydroxychloroquine displayed in Table 2 have more recently shown to have no anti-viral effect against COVID-19 (“FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems | FDA,” 2020). A recent randomized clinical trial compared 1542 patients who were given hydroxychloroquine to 3132 patients that received usual care alone. Results showed no significant difference in mortality rate (25.7% versus 23.5%) after 28 days (“No clinical benefit from use of hydroxychloroquine in hospitalized patients with COVID-19 — RECOVERY Trial,” 2020). An observational study of hydroxychloroquine was conducted at a large medical center in New York City, consisting of 1376 patients. Hydroxychloroquine (600 mg BID on day 1, followed by 400 mg QD for a median of 5 days) was administered to 811 patients. In these patients, 25.1% of cases resulted in intubation or death, revealing no added risk nor benefit of hydroxychloroquine administration (Geleris et al., 2020).
The FDA has issued a safety warning regarding the use of chloroquine and hydroxychloroquine for COVID-19, as there have been reports of serious heart rhythm problems (“FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems | FDA,” 2020). Many participants receiving pharmacological treatment for COVID-19 have shown severe cardiac side effects, including QT prolongation, Torsade de Pointes, and arrhythmia (Table 1) (Asensio et al., 2020; Bhimraj et al., 2020; Kalil, 2020; Rosenberg et al., 2020; Sanders et al., 2020). This raises concerns as patients with cardiovascular comorbidities are already at higher risk of severe COVID-19 complications. This risk is then compounded by administration of potential COVID-19 therapeutic agents, increasing the risk of cardiac death (Kalil, 2020; Kupferschmidt, 2020). Additional side effects documented include hepatitis, acute pancreatitis, neutropenia, and anaphylaxis (Table 1) (Kalil, 2020).
8.1.6. Oseltamivir
Oseltamivir is a neuraminidase inhibitor approved for the treatment of influenza A and B (Sanders et al., 2020; Wu et al., 2020). Current research has indicated that oseltamivir is not effective for management of COVID-19, and is not recommended at this time (Yousefi et al., 2020).
8.1.7. Umifenovir (arbidol)
Umifenovir inhibits membrane fusion of the viral envelope by targeting the interaction between viral S-proteins and ACE2 receptors ( Fig. 1 .). In Russia and China, umifenovir is approved for prophylaxis and treatment of influenza A and B (Sanders et al., 2020; Wu et al., 2020). Furthermore, it has demonstrated in vitro broad-spectrum antiviral activity against the Ebola virus, hepatitis C virus, hepatitis B virus, Lassa virus, human herpesvirus 8, and poliovirus (McKee et al., 2020).
Outcomes have been reported from a single-center, retrospective cohort study of 16 COVID-19 patients who either received umifenovir and lopinavir-ritonavir or lopinavir-ritonavir monotherapy. After 14 days of administration, SARS-CoV-2 was undetectable by RT-PCR in 94% of the umifenovir-treated patients compared to 53% in the control group. The umifenovir experimental group also demonstrated chest improvement, evaluated by CT scans (69% compared to 29% in lopinavir-ritonavir monotherapy) (Deng et al., 2020). A similar retrospective study conducted in February 2020 in Changzhou and Wuhu, China, showed undetectable viral loads in all 16 patients taking umifenovir (200 mg TID), compared to a 44.1% viral load detection in patients given lopinavir-ritonavir monotherapy (400mg/100 mg BID) (Zhu et al., 2020). This suggests that umifenovir may be more efficacious than lopinavir-ritonavir in treating COVID-19. However, a retrospective study conducted by Lian et al. reported no improved outcomes with use of umifenovir in non-intensive care unit patients (Lian et al., 2020). The current data for umifenovir's role in COVID-19 treatment is inconclusive. Prospective, multicenter studies with larger sample sizes are needed to better determine its efficacy.
8.2. Immunomodulatory agents
COVID-19 induces the release of pro-inflammatory cytokines, primarily IL-1β and IL-6, which mediate lung and tissue inflammation, fever, and fibrosis. Many inflammatory diseases, including viral infections, have been shown to benefit from suppression of IL-1β and IL-6 (Conti et al., 2020). Recent studies have consistently found high levels of IL-6 and other pro-inflammatory cytokines in COVID-19 patients. Furthermore, high levels of IL-6 were found to be the main cause of cytokine storm (Zhang et al., 2020a, Zhang et al., 2020b; S. Zhang et al., 2020a, Zhang et al., 2020b). Suppression of these pro-inflammatory cytokines may provide a therapeutic effect for treatment of cytokine storm induced by COVID-19 (Conti et al., 2020).
8.2.1. Tocilizumab (Actemra)
Tocilizumab is a recombinant humanized anti-human IL-6 receptor monoclonal antibody that binds to the IL-6 receptor with high affinity ( Fig. 1 .). It is approved for treatment of cytokine release syndrome (CRS), rheumatic arthritis, and systemic juvenile idiopathic arthritis (Wu et al., 2020; C. Zhang et al., 2020a, Zhang et al., 2020b). At present, there is insufficient data to recommend either for or against its use in treating COVID-19. Retrospective studies have reported some efficacy in critically ill COVID-19 patients with significantly elevated levels of IL-6 (Luo et al., 2020; C. Zhang et al., 2020a, Zhang et al., 2020b). A preliminary observational study conducted by Xu et al. offers promising results for tocilizumab therapy. Of the 21 critically ill COVID-19 patients who received tocilizumab (4–8 mg/kg body weight, 400 mg through an intravenous drip to a maximum of 800 mg), all patients experienced a rapid normalization of body temperature and a remarkable improvement of respiratory function with no reported adverse drug reactions. Researchers confirmed that 20 of the 21 patients fully recovered and were discharged within two weeks following tocilizumab treatment (Xu et al., 2020). Motivated by these results, Genentech announced on March 23, 2020, that the FDA approved a randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of tocilizumab in hospitalized patients with severe COVID-19. It plans to enroll 330 participants globally, with initial results by early summer of 2020 (“Genentech: Press Releases | Monday, Mar 23, 2020,“; Salvi and Patankar, 2020).
8.2.2. Interferons
Type 1 interferons (IFN-1) are a group of cytokines with non-specific antiviral and immunomodulatory properties. They are comprised α and β subtypes, among others (ε, ω, κ) (Samuel, 2001). Interferons -α (IFNα) and -β (IFNβ) have been suggested as candidates in COVID-19 pharmacotherapy (Belhadi et al., 2020; Martinez, 2020; Sallard et al., 2020). Interferons bind to interferon-alpha/beta receptors (IFNAR) on the cell membrane, which phosphorylate STAT1 and other transcription factors. STAT1 translocates to the nucleus, where it activates interferon-stimulated genes (ISGs). Activated ISGs lead to immunomodulatory effects and interfere with viral replication (Fig. 1 ., Table 1) (Sallard et al., 2020). IFNα and IFNβ are commonly investigated as combination therapy with ribavirin and or lopinavir-ritonavir (Arabi et al., 2018; Sanders et al., 2020; Sheahan et al., 2020). It is difficult for researchers in these studies to determine if improvements are due to IFN-1 alone or due to the additional combination drugs. IFNα and IFNβ have differing degrees of coronavirus inhibition depending on potency. It has been reported that IFNβ has superior potency against coronaviruses compared to IFNα (Chan et al., 2013; Dong et al., 2020). Furthermore, IFNβ was suggested as the most appropriate IFN-1 subtype to treat COVID-19 in the early stages of infection. It has a protective effect in the lung by inducing secretion of anti-inflammatory adenosine and maintaining endothelial barriers (Sallard et al., 2020). However, in China, IFNα vapour (1 million U BID) in combination with ribavirin is recommended for COVID-19 patients (Dong et al., 2020; Lu et al., 2020). MERS-CoV and SARS-CoV are unresponsive to prophylactic IFN-1 administration, while SARS-CoV-2 has shown to be more sensitive (Menachery et al., 2014; Sheahan et al., 2020; Shen and Yang, 2020). This was confirmed by in vitro pre-treatment with INF-1 (Lokugamage et al., 2020). However, due to the lack of clinical trials investigating IFN-1 and the conflicting in vitro and animal studies, the use of interferons to treat COVID-19 is not currently recommended (Sanders et al., 2020; Totura and Bavari, 2019). In China, ongoing trials of IFN-1 in COVID-19 treatment is expected to reveal additional findings once published.
8.2.3. Plasma transfusions
Convalescent plasma has been widely used to improve the survival rate of patients during other coronavirus outbreaks, such as Influenza A, SARS-CoV, MERS-CoV, and Ebola virus (Rajendran et al., 2020; Rojas et al., 2020; Zhao and He, 2020). Some blood centers have begun collecting plasma from patients who have recovered from COVID-19, with hopes that their plasma contains antibodies that are able to resolve the virus in infected individuals. Canadian Blood Services is taking part in CONCOR, a national trial aimed at testing the safety and efficacy of using COVID-19 convalescent plasma for treatment. To be eligible for donation, individuals must be younger than 67 years old, have a previously confirmed positive laboratory test for COVID-19, and be symptom free and fully recovered from the virus for at least 28 days (“COVID-19 and convalescent plasma,” 2020). The therapeutic benefits of plasma transfusion therapy are suggested to be multifold, including the reduction of viremia, improvement of immune function in COVID-19 patients, and inhibition of cytokine storm formation (Brown and McCullough, 2020; Y. Chen et al., 2020). Although current data is limited, the available results are promising. Significant reductions in viral load, improvement of symptoms, and reduced mortality have all been demonstrated (Rajendran et al., 2020). In a Chinese pilot study, nine patients who received convalescent plasma all showed improved clinical symptoms within three days. Increased amount of neutralizing antibody was found, with undetectable viral load in seven patients previously with viremia. No severe adverse effects were reported (Duan et al., 2020). It is important to establish the timing of administration, as convalescent plasma may be most efficacious when used prophylactically or in the earlier stages of disease, shortly after symptom onset (Brown and McCullough, 2020; Zhao and He, 2020). Additional considerations include transfusion-related adverse effects, such as chills, fever, and anaphylactic reactions (Zhao and He, 2020). Based on the available data, the use of plasma transfusion therapy has shown promise for treating COVID-19. Further evidence is needed to prove its safety and efficacy.
8.3. Adjunctive agents
8.3.1. Azithromycin
Azithromycin, a commonly used antibiotic, has been administered with hydroxychloroquine as a possible regimen to treat COVID-19. A multicentre retrospective cohort study of 1438 hospitalized patients tested the efficacy and adverse events of hydroxychloroquine and azithromycin taken together, compared to hydroxychloroquine alone, azithromycin alone, and a placebo control group. Results showed no significant difference in the experimental groups compared to the control group. Furthermore, it was suggested that the risk of cardiac arrest may be significantly higher in patients receiving both hydroxychloroquine and azithromycin (OR = 2.13) (Rosenberg et al., 2020). In a similar observational study, there was no evidence that combination of hydroxychloroquine and azithromycin decreased risk of death in COVID-19 patients, and risk of death was higher in patients taking hydroxychloroquine alone (Magagnoli et al., 2020). Due to the adverse cardiovascular effects observed in both hydroxychloroquine and azithromycin, clinical use of this combination requires baseline and follow-up ECG monitoring (Asensio et al., 2020; Bhimraj et al., 2020). A large observational study of nearly 15,000 hospitalized COVID-19 patients conducted by Mehra et al. found that administration of chloroquine or hydroxychloroquine with or without the addition of azithromycin was associated with an increased risk of in-hospital mortality (1 in 6 patients versus 1 in 11 patients in the control group). Researchers also observed an increased frequency of ventricular arrythmias in the experimental group (8%) compared to the control arm (0.3%) (Mehra et al., 2020). This study reveals a lack of benefit in treating COVID-19 patients with chloroquine or hydroxychloroquine either alone or in combination with azithromycin. Importantly, the study highlights the potential harms of administering these pharmacotherapies. There is an urgent need for data concluded from robust randomized clinical trials, as opposed to observational studies that have inherent bias and confounding variables (Geleris et al., 2020). Prospective randomized control trials of hydroxychloroquine with or without azithromycin are ongoing that will provide more definitive insight on its safety and efficacy in treating COVID-19 patients.
8.3.2. Corticosteroids
Corticosteroids, particularly methylprednisolone, has been suggested as an adjunctive agent in COVID-19 treatment. Corticosteroids have been widely used to treat severe pneumonia and prevent lung damage due their ability to suppress severe systemic inflammation (Yousefi et al., 2020; Zhou et al., 2020). There is limited data to support the use of corticosteroids as a therapeutic agent in treating COVID-19. Numerous observational studies and systematic reviews for viral pneumonias, including SARS-CoV and MERS-CoV, have shown inconclusive clinical evidence (Russell et al., 2020; Yang et al., 2020). Furthermore, early administration of high dose corticosteroids may be potentially harmful, resulting in delayed viral clearance and increased mortality risk (Zhou et al., 2020). High dose corticosteroids have also been associated with severe bacterial infection and hypokalemia (Table 1) (Yang et al., 2020). However, there is evidence that a specific subset of COVID-19 patients may benefit from corticosteroids. Low dose of corticosteroids may be therapeutic in patients with severe COVID-19 and other clinical indications, such as ARDS, sepsis, or septic shock (Zhou et al., 2020). The lack of direct evidence warrants caution when considering corticosteroid use for COVID-19 patients. Corticosteroid therapy should be evaluated on a case by case basis, with consideration of symptom severity, timing of intervention, dose, and duration of administration.
8.4. Miscellaneous agents
8.4.1. Camostat mesylate
Camostat mesylate, known as Foipan, was first developed in the 1980s in Japan for treatment of pancreatitis, oral squamous cell carcinoma, and dystrophic epidermolysis (McKee et al., 2020; Sanders et al., 2020). Camostat mesylate is a protease inhibitor, effective against trypsin, plasmin, kallikrein, and thrombin (Bittmann, 2020; Coote et al., 2009; Hoffmann et al., 2020). It also inhibits the serine protease TMPRSS2 (Hoffmann et al., 2020; Uno, 2020). Based on the current understanding of the SARS-CoV-2 infection mechanism, TMPRSS2 facilitates the activation of the viral S-protein and subsequent membrane fusion and entry (Hoffmann et al., 2020). This makes camostat mesylate a potential pharmacological agent to inhibit SARS-CoV-2 entry into host lung cells, preventing initial infection ( Fig. 1 .). Camostat mesylate is available as a crystalline solid that can be dissolved in organic solvents (Bittmann, 2020). Currently, there are no available recommendations for the use of camostat mesylate in COVID-19 patients. It has been suggested that camostat mesylate could be inhaled to directly target host lung cells (Bittmann, 2020). Further research is needed, although studies have been started at the University of Tokyo and University of Aarhus in Denmark.
8.4.2. Angiotensin converting enzyme (ACE) inhibitors, Angiotensin-2 receptor blockers (ARB)
Hoffman et al. found that SARS-CoV-2 utilizes the ACE2 receptor for host cell entry, making it a potential target for COVID-19 pharmacotherapy (Hoffmann et al., 2020). This has stimulated debate within the scientific community, as some postulate that blocking ACE2 receptors with ACE inhibitors and ARBs may lead to poorer outcomes by upregulating the receptors and increasing viral entry (Esler and Esler, 2020). Conflicting in vitro studies make it unclear whether these agents are therapeutic or harmful in COVID-19 patients (Sanders et al., 2020). However, a recent review concluded that many of the studies reporting an increase in ACE2 receptor expression use much higher doses of ACE inhibitors and ARBs compared to the therapeutic doses commonly administered to patients (Sriram and Insel, 2020). Data from human studies indicates that receptor expression is not increased with ACE inhibitor and ARB administration (Sriram and Insel, 2020). Further research is required to confirm the true effects in COVID-19 patients. Currently, it is recommended that patients with cardiovascular comorbidities continue taking ACE inhibitors and ARBs as prescribed (Gurwitz, 2020; “Patients taking ACE-i and ARBs who contract COVID-19 should continue treatment, unless otherwise advised by their physician | American Heart Association,” 2020; Rossi et al., 2020; Sriram and Insel, 2020).
9. Conclusion
The COVID-19 pandemic presents an immense challenge globally that has been met with a remarkable response through the rapid production of pharmacological research and clinical trials. The infection rates of COVID-19 are continually evolving, as are the potential therapeutic agents. At present, management is focused on case detection and monitoring, infection prevention, and supportive care. This review outlined COVID-19 epidemiology, clinical presentations, and mechanism of disease. Pharmacological agents such as anti-virals, immunomodulatory alternatives, miscellaneous agents, and vaccinations were discussed. There are currently no therapies that have been accepted as effective in treating COVID-19. Ongoing research continues to investigate the safety and efficacy of repurposed drugs, while SARS-CoV-2 vaccine trials are rapidly underway.
Credit Author Statement
Andrew Lombardi: Writing - original draft, Research and Writing - original draft preparation, tables and figures, Software. Aviv Ouanounou: Conceptualization, Methodology, preliminary research, Supervision, Writing - review & editing.
Declaration of competing interest
None.
References
- Adaptive COVID-19 Treatment trial (ACTT) - full text view - ClinicalTrials.gov. 2020. https://clinicaltrials.gov/ct2/show/NCT04280705 WWW Document, URL. (accessed 5.24.20)
- Antithrombotic Therapy Coronavirus disease COVID-19. 2020. https://covid19treatmentguidelines.nih.gov/antithrombotic-therapy/ WWW Document, URL. (accessed 5.23.20)
- Arabi Y.M., Alothman A., Balkhy H.H., Al-Dawood A., AlJohani S., Al Harbi S., Kojan S., Al Jeraisy M., Deeb A.M., Assiri A.M., Al-Hameed F., AlSaedi A., Mandourah Y., Almekhlafi G.A., Sherbeeni N.M., Elzein F.E., Memon J., Taha Y., Almotairi A., Maghrabi K.A., Qushmaq I., Al Bshabshe A., Kharaba A., Shalhoub S., Jose J., Fowler R.A., Hayden F.G., Hussein M.A., Martin G.S., Schoenfeld D.A., Walmsley S.L., Carson S., Harbi S. Al, Jeraisy M. Al, Muhaidib M. Al, Musharaf S., Anizi H. Al, Dael R., AlMazroa M., Asiri A., Memish Z.A., Ghazal S.S., Alfaraj S.H., Harthy A. Al, Sulaiman M. Al, Mady A., Ahmad A., Almekhlafi Ghaleb A., Muhammed R., Samirrai S. Al, Awad S., Cabal R.C., Onazi B. Al, Aljuhani M., Vince M., Enani M. Al, Alqurashi A., Alenezi F., Alkhani N., Thaqafi A., Oraabi O. Al, Rifai J., Elsamadisi P., Medhat S.H., Basher S.A.B., Abduldhaher M., Bajhamoum W., Alahsa S.S., Bashir S., Al-Dossary I., Al-Muhainy Dammam B., Khobar S.S. Al, Alshahrani M.S., Al Jabri A., Farid M., Alaidarous A., Alseraihi W., Shahada H., Taif J.S. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. 2018. Trials 19. [DOI] [PMC free article] [PubMed]
- Asensio E., Acunzo R., Uribe W., Saad E.B., Sáenz L.C. Recommendations for the measurement of the QT interval during the use of drugs for COVID-19 infection treatment. Updatable in accordance with the availability of new evidence. J. Intervent. Card Electrophysiol. 2020 doi: 10.1007/s10840-020-00765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldelli OUP accepted manuscript - Baldelli. J. Antimicrob. Chemother. 2020 doi: 10.1093/jac/dkaa190. [DOI] [Google Scholar]
- Belhadi D., Peiffer-Smadja N., Yazdanpanah Y., Mentré F., Laouénan C. A brief review of antiviral drugs evaluated in registered clinical trials for COVID-19. medRxiv. 2020 doi: 10.1101/2020.03.18.20038190. 2020.03.18.20038190. [DOI] [Google Scholar]
- Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Cheng V.C.-C., Edwards K.M., Gandhi R., Muller W.J., O'Horo J.C., Shoham S., Murad M.H., Mustafa R.A., Sultan S., Falck-Ytter Y. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittmann S. COVID 19: camostat and the role of serine protease entry inhibitor TMPRSS2. J. Regen. Biol. Med. 2020 doi: 10.37191/Mapsci-2582-385X-2(2)-020. [DOI] [Google Scholar]
- Brown B.L., McCullough J. Treatment for emerging viruses: convalescent plasma and COVID-19. Transfus. Apher. Sci. 2020 doi: 10.1016/j.transci.2020.102790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Qingxian, Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Qiue, Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang Jingli, Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li Huadong, Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li Hui, Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang Juan, Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC SARS | basics factsheet | CDC. 2017. https://www.cdc.gov/sars/about/fs-sars.html [WWW Document]. Centers Dis. Control Prev. URL. (accessed 5.23.20)
- CDC Management of patients with confirmed 2019-nCoV | CDC. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html [WWW Document]. Coronavirus Dis. 2019. URL. (accessed 5.23.20)
- Chan J.F.W., Chan K.H., Kao R.Y.T., To K.K.W., Zheng B.J., Li C.P.Y., Li P.T.W., Dai J., Mok F.K.Y., Chen H., Hayden F.G., Yuen K.Y. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J. Infect. 2013;67:606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Huang J., Cheng Z., Wu J., Chen S., Zhang Y., Chen B., Lu M., Luo Y., Zhang J., Yin P., Wang X. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.03.17.20037432. 2020.03.17.20037432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020 doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., Kritas S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- Coomes OUP accepted manuscript - Coomes. J. Antimicrob. Chemother. 2020 doi: 10.1093/jac/dkaa171. [DOI] [Google Scholar]
- Coote K., Atherton-Watson H.C., Sugar R., Young A., MacKenzie-Beevor A., Gosling M., Bhalay G., Bloomfield G., Dunstan A., Bridges R.J., Sabater J.R., Abraham W.M., Tully D., Pacoma R., Schumacher A., Harris J., Danahay H. Camostat attenuates airway epithelial sodium channel function in vivo through the inhibition of a channel-activating protease. J. Pharmacol. Exp. Therapeut. 2009;329:764–774. doi: 10.1124/jpet.108.148155. [DOI] [PubMed] [Google Scholar]
- Coronavirus Cases in Europe, by country 2020 | Statista. 2020. https://www.statista.com/statistics/1104837/coronavirus-cases-europe-by-country/ [WWW Document] (accessed 5.26.20)
- COVID-19 and convalescent plasma 2020. https://blood.ca/en/convalescentplasma [WWW Document] (accessed 5.24.20)
- Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H., Hong Z., Xia J. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Yong, Peng C., Yuan M., Huang J., Wang Z., Yu J., Gao X., Wang D., Yu X., Li L., Zhang J., Wu X., Li B., Yu Y., Chen W., Peng Y., Hu Yeqin, Lin L., Liu X., Huang S., Zhou Z., Zhang L., Wang Y., Zhang Z., Deng K., Xia Z., Gong Q., Zhang W., Zheng X., Liu Y., Yang H., Zhou D., Yu D., Hou J., Shi Z., Chen S., Chen Z., Zhang X., Yang X. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. medRxiv. 2020 doi: 10.1101/2020.03.16.20036145. 2020.03.16.20036145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epidemiological Summary of COVID-19 cases in Canada - Canada.ca. 2020. https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html [WWW Document] (accessed 5.24.20)
- Esler M., Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J. Hypertens. 2020;38:781–782. doi: 10.1097/HJH.0000000000002450. [DOI] [PubMed] [Google Scholar]
- FDA Cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems | FDA. 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or WWW Document. (accessed 7.23.20)
- Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D., Kubin C., Barr R.G., Sobieszczyk M.E., Schluger N.W. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genentech Press releases | Monday, mar 23, 2020. 2020. https://www.gene.com/media/press-releases/14843/2020-03-23/genentech-announces-fda-approval-of-clin [WWW Document] (accessed 5.24.20)
- Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K. Sen, Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- A n update on the status. Mil. Med. Res. 2020 doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik T., Mohiddin S., Dimarco A., Patel V., Savvatis K., Marelli-Berg F., Tomaszewski M., Maffia P., D'Acquisto F., Nicklin S., Marian A., Nosalski R., Murray E., Guzik B., Berry C., Touyz R., Kreutz R., Wang D., Bhella D., Sagliocco O., Crea F., Thomson E., McInnes I. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020;1–22 doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X. High risk of thrombosis in patients in severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06062-x. epub online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italy Coronavirus deaths by age | Statista. 2020. https://www.statista.com/statistics/1105061/coronavirus-deaths-by-region-in-italy/ [WWW Document] (accessed 5.26.20)
- Kalil A.C. Treating COVID-19 - off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA, J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.4742. [DOI] [PubMed] [Google Scholar]
- Khalili J.S., Zhu H., Mak N.S.A., Yan Y., Zhu Y. Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko W.C., Rolain J.M., Lee N.Y., Chen P.L., Huang C.T., Lee P.I., Hsueh P.R. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt K. WHO launches global megatrial of the four most promising coronavirus treatments. 2020. [WWW Document]. Science (80-. ) [DOI]
- Ledford H. Hopes rise for coronavirus drug remdesivir. Nature. 2020:1–5. doi: 10.1038/d41586-020-01295-8. [DOI] [PubMed] [Google Scholar]
- Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020 doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexicomp for Dentistry 2020. http://online.lexi.com.myaccess.library.utoronto.ca/lco/action/home?siteid=9745 [WWW Document] (accessed 5.24.20)
- Li Y., Xie Z., Lin W., Cai W., Wen C., Guan Y., Mo X., Wang J., Wang Y., Peng P., Chen X., Hong W., Xiao G., Liu J., Zhang L., Hu F., Li F., Li F., Zhang F., Deng X., Li L. An exploratory randomized, controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI) medRxiv. 2020 doi: 10.1101/2020.03.19.20038984. 2020.03.19.20038984. [DOI] [Google Scholar]
- Lian N., Xie H., Lin S., Huang J., Zhao J., Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokugamage K.G., Hage A., Schindewolf C., Rajsbaum R., Menachery V.D. SARS-CoV-2 is sensitive to type I interferon pretreatment. bioRxiv. 2020;21:1–9. doi: 10.1101/2020.03.07.982264. [DOI] [Google Scholar]
- Lu C.C., Chen M.Y., Chang Y.L. Potential therapeutic agents against COVID-19: what we know so far. J. Chin. Med. Assoc. 2020 doi: 10.1097/JCMA.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magagnoli J., Narendran S., Pereira F., Cummings T., Hardin J.W., Sutton S.S., Ambati J. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. 2020 doi: 10.1101/2020.04.16.20065920. 2020.04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee D.L., Sternberg A., Stange U., Laufer S., Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol. Res. 2020:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. Articles Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Menachery V.D., Yount B.L., Josset L., Gralinski L.E., Scobey T., Agnihothram S., Katze M.G., Baric R.S. Attenuation and restoration of severe acute respiratory syndrome coronavirus mutant lacking 2’-O-methyltransferase activity. J. Virol. 2014;88:4251–4264. doi: 10.1128/jvi.03571-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Clinical Trial . National Institute of Allergy and Infectious Diseases; 2020. Shows Remdesivir Accelerates Recovery from Advanced COVID-19 | NIH.https://www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19 [WWW Document] (accessed 5.24.20) [Google Scholar]
- No clinical Benefit from use of hydroxychloroquine in hospitalised patients with COVID-19 — RECOVERY Trial. 2020. https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-on-hydroxychloroquine-5-june-2020-no-clinical-benefit-from-use-of-hydroxychloroquine-in-hospitalised-patients-with-co [WWW Document] (accessed 7.23.20)
- Patients taking ACE-i and ARBs who contract COVID-19 Should continue treatment, unless otherwise advised by their physician | American Heart Association. 2020. https://newsroom.heart.org/news/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise-advised-by-their-physician WWW Document, URL. (accessed 5.24.20)
- Rajendran K., Narayanasamy K., Rangarajan J., Rathinam J., Natarajan M., Ramachandran A. Convalescent plasma transfusion for the treatment of COVID-19: systematic review. J. Med. Virol. 2020 doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remdesivir Clinical Trials 2020. https://www.gilead.com/purpose/advancing-global-health/covid-19/remdesivir-clinical-trials [WWW Document] (accessed 5.24.20)
- Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., Rojas-Villarraga A., Ramírez-Santana C., Díaz-Coronado J.C., Manrique R., Mantilla R.D., Shoenfeld Y., Anaya J.M. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J., Weinberg P., Kirkwood J., Muse A., DeHovitz J., Blog D.S., Hutton B., Holtgrave D.R., Zucker H.A. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G.P., Sanga V., Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife. 2020;9 doi: 10.7554/eLife.57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell B., Moss C., Rigg A., Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallard E., Lescure F.X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi R., Patankar P. Emerging pharmacotherapies for COVID-19. Biomed. Pharmacother. 2020;128:110267. doi: 10.1016/j.biopha.2020.110267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001 doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA, J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11 doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K.L., Yang Y.H. Diagnosis and treatment of 2019 novel coronavirus infection in children: a pressing issue. World J. Pediatr. 2020 doi: 10.1007/s12519-020-00344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K., Insel P.A. Risks of ACE inhibitor and ARB usage in COVID‐19: evaluating the evidence. Clin. Pharmacol. Ther. cpt. 2020;1863 doi: 10.1002/cpt.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Canada . 2020. Epidemiological Summary of COVID-19 Cases in Canada - Canada.Ca. 2020-03-26. [Google Scholar]
- Totura A.L., Bavari S. Broad-spectrum coronavirus antiviral drug discovery. Expet Opin. Drug Discov. 2019 doi: 10.1080/17460441.2019.1581171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A Trial of Remdesivir . 2020. In Adults with Severe COVID-19 - Full Text View - ClinicalTrials.https://clinicaltrials.gov/ct2/show/NCT04257656 gov [WWW Document] (accessed 5.24.20) [Google Scholar]
- Uno Y. Camostat mesilate therapy for COVID-19. Intern. Emerg. Med. 2020;1–2 doi: 10.1007/s11739-020-02345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., Cuomo-Dannenburg G., Thompson H., Walker P.G.T., Fu H., Dighe A., Griffin J.T., Baguelin M., Bhatia S., Boonyasiri A., Cori A., Cucunubá Z., FitzJohn R., Gaythorpe K., Green W., Hamlet A., Hinsley W., Laydon D., Nedjati-Gilani G., Riley S., van Elsland S., Volz E., Wang H., Wang Y., Xi X., Donnelly C.A., Ghani A.C., Ferguson N.M. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. 2020;3099:1–9. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Yi, Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. COVID-19 Update March 13, 2020.https://www.rev.com/blog/transcripts/world-health-organization-covid-19-update-march-13-2020 [WWW Document] (accessed 5.26.20) [Google Scholar]
- Wu R., Wang L., Kuo H.-C.D., Shannar A., Peter R., Chou P.J., Li S., Hudlikar R., Liu X., Liu Z., Poiani G.J., Amorosa L., Brunetti L., Kong A.-N. An update on current therapeutic drugs treating COVID-19. Curr. Pharmacol. reports. 2020:1–15. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. chinaXiv. 2020;117:1–12. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Liu Jialong, Zhou Y., Zhao X., Zhao Q., Liu Jing. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi B., Valizadeh S., Ghaffari H., Vahedi A., Karbalaei M., Eslami M. A global treatments for coronaviruses including COVID-19. J. Cell. Physiol. 2020 doi: 10.1002/jcp.29785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Li L., Shen A., Chen Y., Qi Z. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin. Drug Invest. 2020 doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H., Wang Y., Zhang Z.-L., Liu Y.-X., Le K.-J., Cui M., Yu Y.-T., Gu Z.-C., Gao Y., Lin H.-W. Efficacy and safety of current therapeutic options for COVID-19 - lessons to be learnt from SARS and MERS epidemic: a systematic review and meta-analysis. Pharmacol. Res. 2020 doi: 10.1016/j.phrs.2020.104872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., He Y. Challenges of convalescent plasma therapy on COVID-19. J. Clin. Virol. 2020 doi: 10.1016/j.jcv.2020.104358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Liu Y., Tian D., Wang C., Wang S., Cheng J., Hu M., Fang M., Gao Y. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct. Target. Ther. 2020 doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Lu Z., Xu T., Chen C., Yang G., Zha T., Lu J., Xue Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]