Highlights
-
•
Targeted anti-cytokine agents, tocilizumab and anakinra, used to treat COVID-19 related cytokine storm, yield mixed results
-
•
Early identification of cytokine storm using laboratory abnormalities can detect patients prior to mechanical ventilation
-
•
Early identification and treatment of cytokine storm with anakinra and corticosteroids led to improved outcomes compared to initiating tocilizumab shortly after mechanical ventilation.
-
•
Early identification and treatment of cytokine storm may be more important than which anti-inflammatory treatment is chosen
-
•
Our results provide additional support for the use of corticosteroid treatment of COVID19 cytokine storm
Keywords: COVID-19, Cytokine storm, Anakinra, Tocilizumab, Corticosteroids
Abstract
Objective
To examine outcomes among patients who were treated with the targeted anti-cytokine agents, anakinra or tocilizumab, for COVID-19 -related cytokine storm (COVID19-CS).
Methods
We conducted a retrospective cohort study of all SARS-coV2-RNA-positive patients treated with tocilizumab or anakinra in Kaiser Permanente Southern California. Local experts developed and implemented criteria to define COVID19-CS. All variables were extracted from electronic health records.
Results
At tocilizumab initiation (n = 52), 50 (96.2%) were intubated, and only seven (13.5%) received concomitant corticosteroids. At anakinra initiation (n = 41), 23 (56.1%) were intubated, and all received concomitant corticosteroids. Fewer anakinra-treated patients died (n = 9, 22%) and more were extubated/never intubated (n = 26, 63.4%) compared to tocilizumab-treated patients (n = 24, 46.2% dead, n = 22, 42.3% extubated/never intubated). Patients who died had more severe sepsis and respiratory failure and met COVID-CS laboratory criteria longer (median = 3 days) compared to those extubated/never intubated (median = 1 day). After accounting for differences in disease severity at treatment initiation, this apparent superiority of anakinra over tocilizumab was no longer statistically significant (propensity score-adjusted hazards ratio 0.46, 95% confidence interval 0.18–1.20).
Conclusions
Prompt identification and treatment of COVID19-CS before intubation may be more important than the specific type of anti-inflammatory treatment. Randomized controlled trials of targeted anti-cytokine treatments and corticosteroids should report the duration of cytokine storm in addition to clinical severity at randomization.
Some patients with coronavirus disease 2019 (COVID-19) develop a life-threatening hyper-inflammatory state, commonly referred to as cytokine storm. Tocilizumab and other anti-interleukin-6 (anti-IL6) monoclonal antibodies have been proposed as potential treatment options for patients with COVID-19-related cytokine storm (COVID19-CS). A small case series (n = 21) from China reported near-complete resolution of acute respiratory distress syndrome (ARDS) and fevers within five days, when tocilizumab, combined with corticosteroids, was initiated in non-intubated patients (Xu et al., 2020). Although the World Health Organization, extrapolating from studies of other conditions, currently recommends against using corticosteroids for patients with COVID-19, a large randomized trial from the United Kingdom (UK) reportedly found that a 10-day course of dexamethasone reduced mortality by 35% among mechanically ventilated patients (University of Oxford, 2020).
Anakinra, a short-acting IL-1 receptor antagonist, is the preferred treatment for severe forms of cytokine storm for patients with underlying conditions other than COVID-19, but its use was not reported for COVID-19 patients in China. However, a more recent study from Italy (Cavalli et al., 2020) (n = 29) reported significantly improved respiratory function in 21 (72%) patients 21 days after initiating high-dose IV anakinra in patients with moderate-severe ARDS treated with continuous positive airway pressure (CPAP).
At Kaiser Permanente Southern California (KPSC), the treatment of COVID19-CS has evolved along with this limited evidence base. Initially, treatment options for cytokine storm included tocilizumab (without corticosteroids), but not anakinra. Subsequently, a shift in practice by clinicians at some of our medical centers aimed to identify early COVID19-CS through laboratory abnormalities in patients with increasing O2 requirements and to initiate combined treatment with anakinra and corticosteroids occurred. This was guided by prior institutional experience with treating macrophage activating syndrome (MAS) and hemophagocytic lymphohistiocytosis (HLH), which present with similar but not identical manifestations to COVID19-CS (Jordan et al., 2019).
In this paper, we describe the initial experience with tocilizumab and anakinra for the treatment of patients with COVID19-CS at 15 KPSC hospitals in southern California. Although treatments were not randomly assigned, the evolution in practices over time provided us with an opportunity to compare the different approaches. Our primary aim was to describe clinical outcomes among tocilizumab- or anakinra-treated COVID-19 patients and to examine whether differences in outcomes could be accounted for by COVID19-CS severity and/or duration at the time of treatment initiation.
Methods
We conducted a retrospective cohort study of all SARS-coV2-RNA-positive patients treated with at least 1 dose of tocilizumab between 3/1/2020−4/13/2020 or anakinra, 4/1–4/30/2020, at one of 15 KPSC hospitals in southern California. The dates vary because anakinra was not used in our hospitals for COVID19-CS in March. Outcomes and covariates were abstracted from the complete electronic health records (EHR) until the date of death or 30 days after the last dose.
Study population
We searched electronic databases to identify KPSC member patients treated with tocilizumab or anakinra and reviewed the complete EHR to confirm that these drugs were administered to treat COVID19-CS. COVID19-CS was defined clinically by increasing O2 requirements and bilateral infiltrates on chest X-ray or CT. Anakinra dosing and duration were guided on a per-patient basis by a team of experts in immunology and varied based on the severity of ARDS, laboratory abnormalities, and renal function. Anakinra use was defined as 1) five or more consecutive days of treatment regardless of daily dose (n = 35); or 2) at least one day of high-dose anakinra (100 mg SQ every 6 h; or every 12 h for those with renal failure) and discontinuation of anakinra due to death (n = 3), significant clinical improvement (n = 3) or adverse events (n = 0). Patients were excluded if they received tocilizumab or anakinra for other indications (n = 3), or if either drug was ordered but never administered (n = 3). Also, 13 anakinra-treated patients were excluded because the dose and/or duration of treatment were inadequate and not related to adverse events.
Setting
KPSC is a large pre-paid healthcare organization that provides comprehensive healthcare services to over 4.6 million members in Southern California. The membership of KPSC is representative of the general Southern California population (Koebnick et al., 2012). KPSC uses an integrated EHR system that includes all inpatient and outpatient encounters, laboratory and imaging tests, diagnoses and medications, and demographic and behavioral characteristics.
Standard Protocol Approvals, Registrations, and Patient Consents. The study protocol was approved by the KPSC institutional review board (#12396).
Data collection
Data were extracted by manually reviewing the EHR, including the onset of dyspnea and other COVID-19 symptoms, age, sex, comorbidities, smoking status, tocilizumab and anakinra use, other treatments rendered for COVID-19 (remdesivir, hydroxychloroquine, and/or corticosteroids), fever (≥100.4 F), hypotension requiring pressors, dates of admission, intubation, extubation, discharge and/or death. The following variables were abstracted from the EHR at three different periods, including the time of admission, the date of the first tocilizumab/anakinra dose and seven days post last tocilizumab or first anakinra dose (for those still intubated at the time): PaO2/FiO2 ratios (P/F ratio) calculated from first-morning blood gases; FiO2 requirements (for ventilated patients), liters of O2 on nasal cannula (NC); presence, onset and resolution of acute kidney injury (AKI, defined as a 2-fold increase in serum creatinine), acute kidney failure requiring hemodialysis and chest X-ray and computerized tomography (CT) radiology reports.
Beginning in April 2020, laboratory tests and absolute counts of lymphocytes and neutrophils obtained throughout the hospitalization were reviewed and classified according to modified HLH/MAS diagnostic criteria (Jordan et al., 2019). These COVID19-CS laboratory criteria are as follows: 1) ferritin >2000 ng/mL and one other abnormal inflammatory marker; or 2) ≥4 abnormal inflammatory markers, including C-reactive protein >70 mg/L; ferritin>700 ng/mL, d-dimer>1000 ng/mL, triglycerides >265 mg/dL, AST >59 u/L, LDH > 300IU/L, lymphopenia <800 cells/uL and neutrophilia >8000cells/uL.
Due to the relatively large number of missing lab test results at treatment initiation (n = 17), the criteria were adapted for the purposes of this study to include patients who had three abnormalities when four or fewer labs were measured. For patients who did not have five or more of these lab tests ordered on the same day, laboratory abnormalities occurring +/- 2 days apart were included. COVID19-CS was operationally defined based on a combination of these laboratory abnormalities and the clinical criterion of progressive worsening of respiratory status.
Outcomes of interest included treatment failure (death) and treatment response, defined as avoiding or being liberated from mechanical ventilation.
Statistical analyses
The primary purpose was to describe clinical outcomes among tocilizumab- or anakinra-treated COVID-19 patients and to examine whether differences in outcomes could be accounted for by COVID19-CS severity and/or duration at the time of treatment initiation (baseline). We also identified potential confounders of the relationship between treatment and outcomes by examining the crude association of baseline covariates with treatment failure and treatment response.
The association between treatment with anakinra or tocilizumab (t0) and the time to death was graphically depicted using Kaplan-Meier survival curves. A propensity score (PS)-adjusted Cox regression model was employed to account for imbalances in multiple baseline characteristics at the time of treatment initiation. The predicted probability of starting anakinra (as opposed to tocilizumab) was modeled using multiple logistic regression. The following covariates were included: age (continuous); sex; comorbidities, diabetes (yes/no), hypertension (yes/no), obesity (body mass index ≥30, yes/no), ever smoker (yes/no), asthma or COPD (yes/no); other SARS-CoV-2 antiviral treatments, remdesivir (yes/no) or hydroxychloroquine (yes/no); time from symptom onset, the onset of dyspnea and admission to first-dose (in days); clinical measures of disease severity each modeled as a single yes/no variable, presence of fever, AKI, hypotension, lymphopenia, neutrophilia, lymphopenia, and neutrophilia or being intubated at baseline; duration of intubation (days, in tertiles; not intubated = 0) and duration of meeting COVID19-CS laboratory criteria (days, in tertiles) at baseline. The Cox regression model was then adjusted for the propensity score (PS) covariate derived from the logistic regression model. The robust sandwich covariance matrix estimate was used to account for patients who received tocilizumab and anakinra (n = 1).
The means and standard deviations of normally distributed variables were compared using 2-sample t-tests; for variables with non-normal distributions, the Wilcoxon rank-sum test was used; and for binary or categorical variables, we used Chi-square with the Fisher exact test. Statistical significance was set at p = 0.05. No adjustment for multiple comparisons was made. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Fifty-two patients received 1–4 doses of tocilizumab, and 41 received anakinra, a median of 14 days (IQR 9.5–17 days), and 13 days (IQR 10–18) after symptom onset, respectively. Most tocilizumab-treated patients received one dose (n = 26), 18 received two doses, seven received three doses, and one received four doses. The median duration of anakinra treatment was nine days (IQR 6–11), and the median cumulative dose was 1500 mg (IQR 1200–2400). All patients had bilateral infiltrates on chest X-ray or CT, and all non-intubated patients had increasing supplemental O2 requirements at the time of treatment initiation.
Table 1 shows the demographic, clinical, and laboratory characteristics of tocilizumab and anakinra-treated patients at treatment initiation. More tocilizumab-treated patients were males, but fewer had pre-existing hypertension or were obese compared to anakinra-treated patients. More tocilizumab-treated patients were intubated at the time of treatment initiation, but the duration of intubation and Pa02:FiO2 ratios among intubated patients treated with tocilizumab were similar compared to those treated with anakinra. Only 31 of 52 (59.6%) of tocilizumab-treated patients met full CS laboratory criteria at treatment initiation. Eight (15.4%) met clinical but not laboratory criteria for COVID19-CS, three (5.8%) patients had not been monitored for laboratory indicators of COVID19-CS, and ten (19.2%) had insufficient labs measured (<5) to fulfill the full CS laboratory criteria but did meet modified criteria (three abnormal labs). In contrast, 37 of 41 (90.2%) of anakinra-treated patients had been monitored (n = 37), and all but one met full COVID-CS laboratory criteria at treatment initiation. More tocilizumab-treated patients had fever, hypotension, AKI, neutrophilia, and the combination of lymphopenia and neutrophilia compared to anakinra-treated patients.
Table 1.
Clinical, Demographic Characteristics and Laboratory Abnormalities among COVID-19 Patients Treated with Anakinra or Tocilizumab.
| Anakinra | Tocilizumab | p-value | |
|---|---|---|---|
| n = 41 | n = 52 | ||
| Upon Admission | |||
| Age, mean (SD), yrs | 58.8 (12.7) | 59.8 (11.7) | 0.71 |
| Male Sex n, (%) | 28 (68.3) | 45 (86.5) | 0.03 |
| Sx Onset to admission, med (IQR), d | 8.0 (6.0−11.0) | 8.0 (5.5−11.0) | 0.86 |
| Onset of dyspnea to admission, med (IQR), d | 2.0 (0−5.0) | 1.0 (0−4.0) | 0.42 |
| Ever Smoker n, (%) | 15 (36.6) | 14 (26.9) | 0.32 |
| Comorbidities (n, %) | |||
| obesity (BMI > = 30) | 29 (70.7) | 26 (50.0) | 0.04 |
| diabetes | 20 (48.8) | 25 (48.1) | 0.95 |
| hypertension (HTN) | 29 (70.7) | 29 (55.8) | 0.14 |
| asthma/COPD | 4 (9.8) | 5 (9.6) | 1.00 |
| At Medication Initiation | |||
| Sx Onset to 1 st dose, median (IQR), d | 13.0 (10.0−18.0) | 14.0 (9.5−17.0) | 0.83 |
| Intubated, n (%) | 23 (56.1) | 50 (96.2) | <0.0001 |
| Intubation duration*, med (IQR), d | 3.0 (1.0−7.0) | 2.0 (1.0−4.0) | 0.34 |
| PaO2:FiO2 ratio | 0.60 | ||
| ≥200 | 5 (12.2) | 11 (21.2) | |
| 100- <200 | 19 (46.3) | 23 (44.2) | |
| <100 | 8 (19.5) | 13 (25.0) | |
| missing | 9 (22.0) | 5 (9.6) | |
| Fever (≥100.4 °F) | 16 (39.0) | 32 (61.5) | 0.03 |
| Hypotension (requiring pressors) | 13 (31.7) | 35 (67.3) | 0.0006 |
| AKI (≥2-fold increase in sCr) | 12 (29.3) | 24 (46.2) | 0.10 |
| Lymphopenia (<700 cells/μL) | 28 (68.3) | 36 (69.2) | 0.92 |
| Neutrophilia (>8000 cells/μL) | 23 (56.1) | 37 (71.2) | 0.13 |
| Lymphopenia and Neutrophilia | 16 (39.0) | 27 (51.9) | 0.22 |
| COVID-CS lab criteria met** | 40/41 (97.6) | 41/49 (83.7) | 0.04 |
| CS duration**, med (IQR), d | 3.0 (1.0−5.0) | 2.0 (0−4.0) | 0.04 |
*among patients intubated at first dose.
**excludes n = 3 with insufficient labs.
Abbreviations: SD = standard deviation; med = median; IQR = interquartile range; Sx = symptom; AKI = acute kidney injury; BMI = body mass index; COPD = chronic obstructive pulmonary disease; COVID19-CS = coronavirus 2019-related cytokine storm; μL = microliter; y = years; d = days, CS = cytokine storm; sCr = serum creatinine.
Concomitant corticosteroids were used in only seven tocilizumab-treated patients, although twelve others received rescue treatment with steroids later in their hospital course. In contrast, all anakinra-treated patients received concomitant corticosteroids. Of the 48 patients who received concomitant corticosteroids, only two patients in the anakinra-treated group received more than the maximum recommended daily dose (40 mg bid of methylprednisolone or equivalent doses of other corticosteroids). Remdesivir treatment was similar between groups (n = 20 tocilizumab, n = 16 anakinra) while fewer anakinra-treated patients received hydroxychloroquine (n = 34) compared to tocilizumab (n = 48).
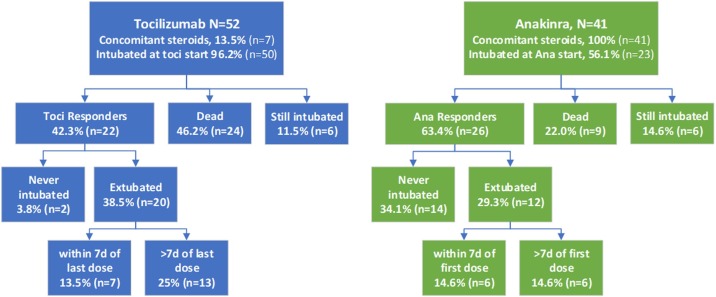
Figure 1 shows the outcomes among tocilizumab and anakinra-treated patients at the end of follow-up. The median follow-up time from the first dose to the end of the study among patients that survived was longer for tocilizumab (24 days, IQR 20.5–27.0) than anakinra (19 days, IQR 13.5–21.0). The risk of death was lower in the anakinra group (22.0%) than the tocilizumab group (46.2%), and the percentage of anakinra treatment responders was correspondingly higher (63.4% versus 43.2%). Among the 18 non-intubated patients at anakinra start, 14 never required intubation, three were subsequently intubated (one extubated and two still intubated), and one elderly man was not intubated in accordance with his family’s wishes and died. Of the 23 intubated patients at anakinra initiation, eleven (47.8%) were extubated at last follow-up compared to 20 (40%) of the 50 intubated patients at tocilizumab initiation.
Figure 1.
Clinical Outcomes among COVID-19 patients treated with Tocilizumab or Anakinra. Fewer tocilizumab (toci, in blue) showed significant clinical improvement (Toci Responders) compared to anakinra-treated patients (Ana Responders, in green). Among the 18 non-intubated patients at anakinra start, 14 never required intubation, three were subsequently intubated (one extubated and two still intubated), and one elderly man was not intubated in accordance with his family’s wishes and died. Among intubated patients, those that showed the type of rapid improvement (extubation within seven days, 7d) expected based on the treatment of non−COVID-related cytokine storm, is similar across treatment groups. COVID-19=coronavirus 2019.
Compared with patients who were still alive, the patients who died were slightly older, more likely to have pre-existing hypertension or diabetes; more likely to be intubated, have AKI, hypotension and more severe ARDS; and had a longer duration of COVID19-CS laboratory abnormalities including neutrophilia at the time of treatment initiation with either drug (Table 2 ). Those who died all had rising inflammatory markers consistent with worsening COVID19-CS at the time of death. Lymphopenia and/or neutrophilia resolved following treatment initiation in most patients who survived, but not in those who died.
Table 2.
Clinical, Demographic Characteristics and Laboratory Abnormalities among COVID-19 patients treated with Anakinra or Tocilizumab Stratified by Outcome.
| Never Intubated/Extubated | Dead | p-value* | Still Intubated | |
|---|---|---|---|---|
| n = 48 | n = 33 | n = 12 | ||
| Upon Admission | ||||
| Age, mean (SD), y | 58.1 (11.7) | 61.9 (11.4) | 0.15 | 57.4 (15.2) |
| Male Sex n, (%) | 37 (77.1) | 27 (81.8) | 0.61 | 9 (75.0) |
| Sx Onset to admission, med (IQR), d | 8.0 (6.0−11.0) | 8.0 (6.0−13.0) | 0.77 | 8.5 (5.0−10.0) |
| Ever Smoker n, (%) | 16 (33.3) | 8 (24.2) | 0.38 | 5 (41.7) |
| Comorbidities (n, %) | ||||
| obesity (BMI ≥ 30) | 29 (60.4) | 19 (57.6) | 0.80 | 7 (58.3) |
| diabetes | 19 (39.6) | 18 (54.5) | 0.18 | 8 (66.7) |
| hypertension | 27 (56.3) | 26 (78.8) | 0.04 | 5 (41.7) |
| asthma/COPD | 5 (10.4) | 2 (6.1) | 0.69 | 2 (16.7) |
| At Medication Initiation | ||||
| Intubated, n (%) | 31 (64.6) | 32 (97.0) | 0.0006 | 10 (83.3) |
| Intubation duration**, med (IQR), d | 2.0 (1.0−4.0) | 3.0 (1.0−6.0) | 0.23 | 3.0 (1.0−5.0) |
| PaO2:FiO2 ratio | 0.17 | 0 (0) | ||
| ≥200 | 10 (20.8) | 4 (12.1) | 2 (16.7) | |
| 100- <200 | 21 (43.8) | 16 (48.5) | 5 (41.7) | |
| <100 | 6 (12.5) | 10 (30.3) | 5 (41.7) | |
| missing | 11 (22.9) | 3 (9.1) | 0 (0) | |
| Fever (≥100.4 °F) | 24 (50.0) | 15 (45.5) | 0.69 | 9 (75.0) |
| Hypotension (requiring pressors) | 22 (45.8) | 23 (69.7) | 0.03 | 3 (25.0) |
| AKI (≥2-fold increase in sCr) | 13 (27.1) | 20 (60.6) | 0.0026 | 3 (25.0) |
| Lymphopenia (<700 cells/μL) | 32 (66.7) | 23 (69.7) | 0.77 | 9 (75.0) |
| Neutrophilia (>7700 cells/μL) | 28 (58.3) | 26 (78.8) | 0.06 | 6 (50.0) |
| Lymphopenia and Neutrophilia | 18 (37.5) | 20 (60.6) | 0.04 | 5 (41.7) |
| COVID19-CS lab criteria met*** | 39/45 (86.7) | 32/33 (97.0) | 0.23 | 10/12 (83.3) |
| CS duration***, med (IQR), d | 1.0 (0−4.0) | 3.0 (1.0−6.0) | 0.08 | 3.0 (0−3.0) |
| At Death or Last Follow-up | ||||
| Fever (≥100.4 °F) | 4 (8.3) | 13 (39.4) | 0.0007 | 4 (33.3) |
| Hypotension (requiring pressors) | 5 (10.4) | 18 (54.6) | <0.0001 | 6 (50.0) |
| AKI (≥2-fold increase in sCr) | 10 (20.8) | 29 (87.9) | <0.0001 | 3 (25.0) |
| Lymphopenia (<700 cells/μL)**** | 9 (18.8) | 17 (56.7) | 0.0005 | 7 (70.0) |
| Neutrophilia (>8000 cells/μL)**** | 16 (33.3) | 24 (80.0) | <0.0001 | 6 (60.0) |
| Lymphopenia and Neutrophilia**** | 4 (8.3) | 15 (50.0) | <0.0001 | 5 (50.0) |
| COVID19-CS lab criteria met***** | 11 (23.4) | 32 (100) | <0.0001 | 4 (40.0) |
*comparing extubated to dead patients, Chi-square or Fisher exact test and Wilcoxon rank-sum.
**among patients intubated at treatment initiation.
***excludes 3 patients with missing labs.
****excludes 5 patients missing WBC labs (n = 3 dead, n = 2 still intubated).
*****excludes 4 patients missing labs at last follow-up (n = 1 extubated, n = 2 still intubated, n = 1 dead).
Abbreviations: SDstandard deviation; medmedian; IQRinterquartile range; Sxsymptom; BMIbody mass index; COPDchronic obstructive pulmonary disease; COVID19-CScoronavirus 2019-related cytokine storm; μLmicroliter; yyears; ddays, CS = cytokine storm; sCrserum creatinine.
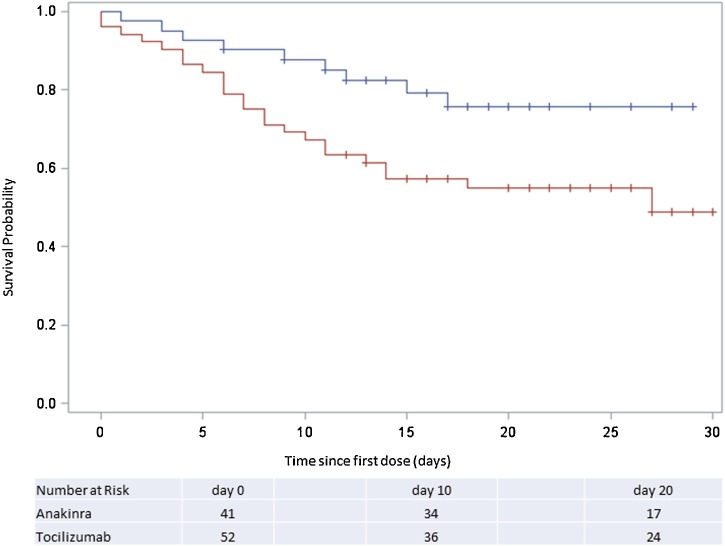
Unadjusted analysis indicated a survival advantage with anakinra compared to tocilizumab-treated patients (Figure 2 ), but after adjustment for multiple baseline imbalances, this difference did not reach statistical significance (PS-adjusted HR = 0.46, 95%CI = 0.18–1.20, p = 0.11).
Figure 2.
Survival Among Anakinra or Tocilizumab-treated COVID-19 Patients. Depicted is the Kaplan-Meier survival curve of patients treated with anakinra (n = 41, blue) or tocilizumab (n = 52, red line) up to 30 days after receiving their first dose. COVID-19=coronavirus 2019.
Discussion
Effective treatments for COVID-19 and COVID19-CS are needed urgently. In this study, we found that only 42.3% of tocilizumab-treated patients and 63.4% of those treated with anakinra responded favorably to treatment. Our health system’s initial experience with tocilizumab early in California’s COVID-19 outbreak was less favorable than that previously reported in the case series from China (Xu et al., 2020). This disappointing tocilizumab experience led to a shift in practice to identify COVID19-CS earlier in the disease course, ideally prior to intubation, through a combination of laboratory abnormalities and respiratory deterioration. We accomplished this by empowering a team of experts in immunology to guide the ordering and interpreting of laboratory tests and the subsequent treatment of COVID19-CS patients with corticosteroids and anakinra. This approach resulted in better outcomes compared to the early tocilizumab-treated patients, but our analyses suggest that this could be due to earlier identification and treatment of COVID19-CS, rather than superior efficacy of anakinra compared to tocilizumab. Also, concomitant treatment with corticosteroids may have contributed to the better response observed in the anakinra-treated group, as supported by the preliminary findings of the UK Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial (University of Oxford, 2020).
An exuberant hyper-inflammatory response to COVID-19 is increasingly recognized as a significant cause of morbidity and mortality in these patients (Mehta et al., 2020). While still not fully understood, COVID19-CS appears to start 8–10 days after onset of symptoms. It is characterized clinically by high fevers, dyspnea, hypoxemia and bilateral pulmonary infiltrates, and can progress rapidly to ARDS and multisystem organ failure with or without hypercoagulability and, ultimately, death.
In severe forms of cytokine storm due to MAS and HLH, the current standard of care includes prompt identification through a combination of laboratory abnormalities and symptoms followed by early treatment with anakinra alone or combined with corticosteroids (Halyabar et al., 2019). A rapid clinical response is expected, including resolution of fevers, hypotension, and improvement in inflammatory markers (Lee et al., 2014); if not, doses are escalated, often requiring treatment with continuous IV anakinra. In these patients, anakinra is preferred over tocilizumab because it targets IL-1, an upstream cytokine in CS. Also, it can be titrated based on CS severity and easily stopped should severe infection occur.
Tocilizumab is not preferred in MAS/HLH because it increases the risk of bacterial infections, has a long half-life, and blunts CRP and ferritin levels in the absence of clinical response, which can lead to confusion and delays in escalating treatment (Halyabar et al., 2019). Tocilizumab is FDA-approved for treating a milder form of cytokine storm (renamed cytokine release syndrome) caused by T-cell inducing cancer therapies (CAR-T); it is used as an escalation therapy in these patients should corticosteroids fail. While it is unclear why tocilizumab was chosen over anakinra in the early COVID-19 outbreak, we speculate that it may be because IL-6, unlike IL-1, is relatively easy to measure in serum. This led to studies showing an association between high serum IL-6 levels and poor COVID-19 prognosis (Zhou et al., 2020) and a subsequent case series reporting the use of subcutaneous tocilizumab at doses similar to ours in combination with corticosteroids (Xu et al., 2020). It should be noted that IL-6 serum levels take three or more days to return in the US and thus are not useful in making treatment decisions in rapidly deteriorating patients.
The findings from the initial case series (Xu et al., 2020) differ from ours in that only two of 21 patients required mechanical ventilation, all patients were treated with concomitant corticosteroids, and the majority of patients showed rapid improvement (Xu et al., 2020). In contrast, the majority of our tocilizumab-treated patients required invasive ventilation, did not receive corticosteroids, and few showed rapid improvement, suggesting that tocilizumab alone is unlikely to be effective in later stages of COVID19-CS.
More recently, an Italian group reported findings using high dose IV tocilizumab (8 mg/kg, 2–3 doses) to treat 100 patients with respiratory failure from COVID19-CS in combination with high-dose dexamethasone (20 mg/day) in Italy (Toniati et al., 2020). This group (Toniati et al., 2020), similar to our study, incorporated laboratory abnormalities based on HLH to identify treatment candidates and identified 57 patients on the wards with worsening respiratory status, with the remaining 43 in the ICU on mechanical ventilation. Within ten days following tocilizumab initiation, 77 patients had improved, and 20 had died, ten of whom were on mechanical ventilation at treatment initiation, and three others had worsened. Fewer patients in this cohort (Toniati et al., 2020) appear to have neutrophilia at tocilizumab initiation (median = 6700, IQR 4900–9700) compared to our cohort (71.2% >8000), yet neutrophilia was a poor prognostic indicator in both studies. This suggests that one explanation for the improved outcomes reported (Toniati et al., 2020) compared to ours may be due to earlier identification of COVID19-CS. Other possible explanations for improved outcomes in mechanically ventilated COVID19-CS patients treated with tocilizumab are co-administration of high dose corticosteroids or higher IV doses of tocilizumab used. This latter explanation seems less likely as the patients who died or worsened had increasing IL-6 levels (Toniati et al., 2020).
We co-administered corticosteroids with anakinra despite the WHO's recommendations against corticosteroids because of the logic of treating cytokine storm; an aberration that involves multiple cytokines by targeting a single cytokine in critically ill patients is problematic. Corticosteroids have broad immunological effects that may dampen unique features of COVID19-CS, including neutrophilia (Barnes et al., 2020) and platelet activation. The recognition of some of these unique features of COVID19-CS is also why we adapted MAS/HLH criteria to include lymphopenia and neutrophilia and why we no longer screen for low levels of fibrinogen as an early indicator of COVID19-CS.
Neutrophilia appears to be a late finding in COVID19-CS, and, along with AKI and hypotension, was more common at treatment initiation in the tocilizumab group. These factors were associated with a lack of response to both tocilizumab and anakinra. Longer duration of COVID19-CS also appeared to be associated with poor prognosis, but this finding was not statistically significant. Early in the COVID-19 outbreak, clinicians identified potential tocilizumab candidates based on the development of severe respiratory failure. By assisting hospitalists and ICU physicians with monitoring and interpreting COVID19-CS labs, we were able to identify patients in cytokine storm often prior to intubation. Consequently, treating with anakinra and corticosteroids earlier may have contributed to improved outcomes.
The main limitation of this study is the possibility of unmeasured confounding that is present in all observational studies. In addition, we did not compare targeted therapy to treatment with corticosteroids alone or no anti-inflammatory treatments. Thus, we cannot exclude the possibility that neither anti-cytokine treatment is superior to corticosteroids alone, particularly in non-intubated patients. The small sample size is another limitation. While the apparent superiority of anakinra and corticosteroids over tocilizumab was not statistically significant at a point estimate of 54%, it is possible that the study was underpowered. The anakinra doses we used were also lower than those reported from Italy (Cavalli et al., 2020). Higher doses of anakinra may prove to be more effective than lower anakinra doses (Cavalli et al., 2020) or than anti-IL6 agents, specifically when administered early in COVID-CS prior to intubation. Another limitation is that only tocilizumab was used early in the outbreak, whereas a month later, both tocilizumab and anakinra were used, raising the possibility that secular trends in care may have contributed to the results. While we did our best to capture the other aspects of improved COVID-19 care (lab monitoring in non-ICU patients, the recognition that hyper-inflammation often corresponds with increasing O2 requirements and precedes intubation) and accounted for this in the statistical analysis; residual confounding from other improvements in care would further reduce differences in outcomes across treatment groups. Another possibility is that the stay-at-home orders in effect later in the study period may have led to an overall shift to milder disease in admitted patients. However, this does not seem to be the case in our study, as comorbidities associated with poor COVID-19 outcomes (Zhou et al., 2020) were more common in the anakinra-treated group. Lastly, the COVID19-CS lab criteria used in this study were adapted from the existing MAS/HLH criteria based on limited experience. The panel could likely be simplified, tailored to individuals, and a risk score developed. This should be addressed in future studies. Some studies have relied primarily on CRP (Xu et al., 2020) or CRP and ferritin (Cavalli et al., 2020) to identify hyper-inflammation and response to anti-inflammatory agents. This may be a reasonable approach for treatment with corticosteroids or anakinra, but other inflammatory markers would need to be monitored following treatment with anti-IL6 agents because they can blunt CRP and ferritin levels in the absence of a clinical response.
The strengths of this study are the importance of the question, the population-based sample, and the team-based approach to recognizing and treating COVID19-CS. In many clinical settings, tocilizumab, and, to a lesser extent, anakinra is being used to treat COVID-19 patients with very little information to guide patient selection, dosing, or monitoring treatment response.
Our most important finding is that COVID19-CS lab abnormalities may be the earliest signal to alert clinicians to initiate CS treatment before respiratory failure. Not measuring CS labs and delayed treatment, including corticosteroids, may have contributed to worse outcomes in tocilizumab-treated patients. Randomized controlled trials of targeted anti-cytokine treatments should report the duration of elevated COVID19-CS inflammatory markers in addition to clinical severity at randomization. Because of their general availability, low cost, and pleiotropic anti-inflammatory properties, corticosteroids should be compared with targeted anti-cytokine treatments. Studies to identify a parsimonious set of laboratory indicators of COVID-19-related hyper-inflammation are also urgently needed.
Funding
None.
Author contributions
Annette Langer-Gould conceptualized and designed the study, collected and interpreted the data, drafted and revised the manuscript for intellectual content, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Jessica B. Smith collected, analyzed, and interpreted the data and revised the manuscript for content.
Edlin G. Gonzales collected the data and revised the manuscript for content.
Rhina D. Castillo conceptualized and designed the study and revised the manuscript for content.
Judith G. Figueroa conceptualized and designed the study and revised the manuscript for content.
Anusha Ramanathan conceptualized and designed the study and revised the manuscript for content.
Bonnie H. Li analyzed and interpreted the data and revised the manuscript for content.
Michael K. Gould conceptualized and designed the study and revised the manuscript for content.
Conflicts of interests
Annette Langer-Gould currently serves as a voting member on the California Technology Assessment Forum, a core program of the Institute for Clinical and Economic Review (ICER). She has received sponsored and reimbursed travel from ICER. Michael K. Gould has received research support through his employer from Medial EarlySign to develop computer models of lung cancer risk and royalties from UpToDate to co-author topics on lung cancer diagnosis and staging. Jessica B. Smith, Edlin G. Gonzales, Rhina D. Castillo, Judith Garza Figueroa, Anusha Ramanathan, and Bonnie H. Li declare no conflicts of interest.
Acknowledgments
We would like to thank Luis M. Moreta-Sainz, MD, whose questions prompted these analyses, as well as David Silberstein MD and Robert M. Cooper MD, for many helpful discussions.
References
- Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., Dagna L. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halyabar O., Chang M.H., Schoettler M.L., Schwartz M.A., Baris E.H., Benson L.A., Henderson L.A. Calm in the midst of cytokine storm: a collaborative approach to the diagnosis and treatment of hemophagocytic lymphohistiocytosis and macrophage activation syndrome. Pediatr Rheumatol Online J. 2019;17(1):7. doi: 10.1186/s12969-019-0309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M.B., Allen C.E., Greenberg J., Henry M., Hermiston M.L., Kumar A. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: Recommendations from the North American Consortium for Histiocytosis (NACHO. Pediatr Blood Cancer. 2019;66(11):e27929. doi: 10.1002/pbc.27929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebnick C., Langer-Gould A.M., Gould M.K., Chao C.R., Iyer R.L., Smith N., Jacobsen S.J. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M., Mackall C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., Latronico N. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568. doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Oxford . 2020. RECOVERY: Randomised Evaluation of COVID-19 Therapy. Retrieved from https://www.recoverytrial.net/ (accessed 18 June 2020) [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]