Abstract
A combination of sous-vide (SV) and enzymatic treatment (one commercial, Neutrase (NE), and two fruit-extracted enzymes obtained from kiwifruit and pineapple, KE and PE, respectively) was applied to pork fore shanks prepared at different temperatures (45, 60, 70, and 100 °C) for 0.5, 4 or 8 h, and the properties of pork were compared. For the hardness, SV itself resulted in a 27% decrease; however, a significant softening effect could be obtained by the addition of enzymes (38–60%). The KE treatment appeared to be more effective (~ 60%) than either the PE or the NE treatment. During SV, both the L* and b* values of the samples generally increased while the a* value decreased. Among the samples, the lowest hardness was obtained for the sample treated with SV-KE at 70 °C for 8 h, and the lowest total microbial count, lowest pH and the least amount of color change were also observed for the sample.
Keywords: Pork fore shank, Hardness, Sous-vide, Fruit-extracted enzyme, Microstructure
Introduction
When purchasing meat, tenderness is an important factor, especially for elderly people. The succulence related to tenderness comes from melting of the intramuscular fat content of meat (Osório et al., 2009), such that lower fat content sections of pork such as the fore shank and behind shank, which are relatively tough, are non-preferred. Because it is difficult to make these sections more tender by standard cooking methods, they can be harder to chew after cooking, especially for elderly people.
Techniques to improve the tenderness of meat can be classified on the basis of the mode of the process into three main categories: physical, chemical, and enzymatic (Bekhit et al., 2014). One of the most common methods to improve the edibility of non-preferred meats is by marinating in seasonings, including salt. With the addition of salt, the thick filaments in raw meat may be partially depolymerized into myosin molecules, which upon heating, may aggregate to form a gel, and water can also be tightly held within this gel fraction (Offer and Knight, 1988). The other approach to meat tenderization is the addition of proteolytic enzymes. Plant extract proteases are the most commonly used enzymes commercially, particularly papain (papaya extract), bromelain (pineapple extract), and ficin (fig extract). Other proteases that have been studied for use include actinidin (kiwifruit extract) and zingiber (ginger extract) (Ha et al., 2012).
Low-temperature vacuum processing (sous-vide, SV) involves vacuum packing raw or partially cooked food in plastic pouches, pasteurizing in hot water, and then rapidly cooling followed by cold storage (Creed and Reeve, 1998). Vacuum packaging not only prevents food oxidation and bacterial growth but also diminishes the loss of mass from the meat that occurs during cooking due to water evaporation or separation, such that the texture becomes softer in addition to the increased yield after cooking (Peck and Stringer, 1996). Earlier studies have reported SV cooking of pork loins (Diaz et al., 2008), whole muscle beef (Grigioni et al., 2008), and lamb loins (Roldan et al., 2015).
SV cooking is carried out at approximately 50–70 °C, and this cooking temperature is within the active temperature range of most fruit tenderizing enzymes (20–80 °C) (Suh et al., 1992). There has been research on the effects of SV or fruit-extracted enzymes on meat tenderization; however, there have been no reports regarding their combined effect. Therefore, this study was carried out to investigate the synergistic effect of SV coupled with a natural fruit-extracted crude enzyme on meat tenderization. For the crude enzyme solution, two extracts from kiwifruit and pineapple, which are known to have a higher proteolytic activity than pear or fig enzymes (Kim et al., 2011), and one commercial protease were chosen, and the quality characteristics, including texture, color and microorganismal levels, of the meat were examined. The properties of the SV-cooked samples were also compared with those of samples cooked by a conventional method (at 100 °C).
Materials and methods
Materials
Pineapple (Philippine Dole), kiwifruit (Zespri, Chile), and pork fore shank were purchased from a local market in Seoul. For each treatment, the pork fore shank was cut to a uniform size (10 × 5 × 1 cm) for each treatment and stored at 5 °C for 1 day. The commercial protease, Neutrase, was purchased from Novozymes (Bagsnard, Denmark). Casein, phosphate buffered saline, trichloroacetic acid, sodium carbonate, and Folin reagent were purchased from Sigma-Aldrich (Sigma-Aldrich Co., St. Louis, Mo., USA), and 99% DL-tyrosine was purchased from Acros (Acros Co., NJ, USA).
Crude enzyme extraction
The proteolytic enzymes of the fruits were extracted by the method reported by Cho et al. (1994). After the skin of the fruit was peeled away from the pulp, the edible portion was finely ground, homogenized with twice the amount of a 0.1 M sodium phosphate buffer (pH 7.0) and centrifuged at 5500 rpm for 20 min. The supernatant was used as the crude enzyme solution.
Enzyme activity measurement
The activity of the enzyme was measured by slight modification of the method reported by Minami et al. (1971). One milliliter of the extracted enzyme solution was added to a 0.6% casein solution (5 mL) and shaken for 10 min in a 37 °C water bath. Five milliliters of a 0.44 M trichloroacetic acid (TCA) solution was added and then the sample was stored in a constant temperature bath for approximately 30 min. After filtration (Whatman filter paper, # 40), the filtrate (2 mL) was added to a 0.55 M Na2CO3 solution (5 mL) and Folin reagent (1 mL) and shaken at 37 °C. After 30 min of reaction, the absorbance was measured by a UV spectrophotometer (UV-1601, Shimadzu, Kyoto, Japan) at 660 nm. The enzyme activity was expressed as the rate of a reaction catalyzed by a given amount of enzyme. The unit of enzyme activity is the international unit (IU), which is the enzyme activity that converts 1 μmol of substrate per unit of time (1 min). In this study, standard curves (y = 1.1894x + 0.0581, R2 = 1) were obtained based on the tyrosine content, and enzyme activity was calculated by the following formula.
E = tyrosine-converted content of the experimental group, B = tyrosine conversion of the control, F = dilution of the enzyme solution.
Determination of the temperature stability on the enzyme
Casein (0.6%) was dissolved in a 0.1 M sodium phosphate buffer solution, and the pH was adjusted to 7.0. The enzyme solution was incubated at 30-80 °C, and the enzyme activity was measured at 10 °C intervals. The concentration of the enzyme was determined by a preliminary experiment with Neutrase to confirm the change in concentration. Each enzyme was diluted to 1 unit/mL with distilled water and 1 mL was analyzed for each measurement.
Sous-vide (SV) treatment
Pork fore shank (100 g) either with needle-injected fruit-extracted crude enzyme (1 mL) or without enzyme (1 mL of distilled water was added instead of the enzyme) was sealed and packed by a vacuum packing machine (FR-B100WB, CSE Co., Gyeonggi-Do, Korea) using vacuum packing film (polyethylene + LLDP + nylon, 200 × 140 mm). To minimize the initial temperature change, the samples were immersed in a water bath (5510E-DTH, Bransonic, Danbury, CT, USA) preheated to 80 °C for 1 s. The conditions were set to 45 ± 2, 60 ± 2 and 70 ± 2 °C based on the optimum temperature of the enzyme used, and kept at the temperature for 0.5, 4 and 8 h. For comparison, 100 °C treatment by a general cooking method was also applied.
As shown in Table 1, this study examined the combined effect of SV and enzyme treatment at different temperature on the pH, harness, chromaticity, and total viable count of pork fore shank across three conditions of reaction time. The total number of treatment was 48. Variables included; (1) SV only, no enzyme treatment (SV, CON); (2) SV with commercial enzyme, Neutrase (SV + NE); (3) SV with kiwifruit extracted enzyme (SV + KE); and (4) SV with pineapple extracted enzyme (SV + PE) at each time–temperature condition. After cooking, the packed sample was immediately placed in a pan filled with cold water and cooled to below 3 °C within 90 min. Three times per each treatment were repeated.
Table 1.
Hardness characteristics of the pork fore shank samples prepared under different SV cooking conditions (mean ± SE, N/m2)
| Temperature | Reaction time (h) | ||
|---|---|---|---|
| 0.5 | 4 | 8 | |
| RT | |||
| Raw | 186,300 | ||
| 45 °C | |||
| SV | 164,300 ± 20,666fAB | 168,800 ± 11,000efA | 154,200 ± 1,500efAB |
| SV-NE | 145,700 ± 2,733fAB | 126,400 ± 2,333gB | 138,200 ± 1,133fgAB |
| SV-KE | 145,900 ± 2,633fAB | 127,700 ± 2,900gAB | 123,900 ± 3,966gB |
| SV-PE | 191,000 ± 11,000efA | 113,000 ± 1,633gB | 125,000 ± 4,000gB |
| 60 °C | |||
| SV | 386,900 ± 9,877abcA | 341,500 ± 4,833cdBC | 318,200 ± 3,566bcBC |
| SV-NE | 358,600 ± 12,888abcdAB | 275,500 ± 8,766dCD | 302,400 ± 3,500bcdCD |
| SV-KE | 289,200 ± 27,000defCD | 231,200 ± 3,800deE | 243,600 ± 3,000deE |
| SV-PE | 322,000 ± 22,333cdeBC | 286,500 ± 14,000dCD | 268,900 ± 2,733bcdDE |
| 70 °C | |||
| SV | 444,800 ± 12,666abA | 325,300 ± 5,733bcCD | 284,300 ± 1,166dF |
| SV-NE | 374,400 ± 20,433bcBC | 215,900 ± 3,700deH | 272,400 ± 4,000bcdFG |
| SV-KE | 309,100 ± 1,366cdEF | 199,800 ± 7,000deH | 176,300 ± 17,333eI |
| SV-PE | 311,600 ± 1,400cdEF | 248,100 ± 20,133dGH | 216,100 ± 4,000deG |
| 100 °C | |||
| SV | 516,400 ± 14,000aA | 420,200 ± 14,733aBC | 383,900 ± 14,366abCD |
| SV-NE | 446,900 ± 15,666abB | 402,000 ± 10,733abCD | 384,300 ± 14,633abCD |
| SV-KE | 417,500 ± 17,000bBC | 329,700 ± 7,100bcdDE | 318,300 ± 7,733bcE |
| SV-PE | 412,200 ± 16,066bBC | 348,300 ± 7,800bcdDE | 342,400 ± 8,233bcE |
SV means sous vide cooking, N means neutrase, K means kiwifruit, P means pineapple, E means enzyme. Values A–Iat same temperature and a–gat same treatment time followed by different superscripts are significantly different at the P < 0.05 level
Hardness analysis
After the SV treatment, the samples were cut into 1 × 1 × 1 cm cubes, and the hardness of the samples was measured using a texture analyzer (TA-XT2, Stable Micro Systems, Surrey, UK) in TPA mode with a 50% distance. For the measurement, a cylinder ebonite probe (10 mm dia.) with pre/test/post speeds of 2.0, 2.0, and 2.0 mm/s, respectively, was used. Each test was reported as the mean value of 10 repetitions.
Chromaticity measurement
Lightness (L*), redness (a*) and yellowness (b*) were measured using a color difference colorimeter with D65 standard CIE Illuminant, 8 mm aperture, and 2° standard observer (CR-300, Minolta Co., Ltd, Osaka, Japan). The measurement was repeated three times per sample, and the average values were calculated. The calibration plates that were used were L* = 96.60, a* = 0.24, and b* = 1.97.
pH measurement
Distilled water (8 mL) was added to each treated sample (2 g), which was then mixed with a homogenizer (IKA T 25 digital Ultra-Turrax, IKA Ltd., Staufen, Germany) for 2 min and then cooled to room temperature. The pH of the sample was measured using a pH meter (SevenEasy S20, Mettler Toledo Inc., Schwerzenbach, Switzerland), which was calibrated intermittently at ambient temperature using pH buffers 4.01 and 6.86 (Thermo Scientific™ Orion™, Waltham, MA, USA). The analysis was repeated three times, and the mean value was calculated.
Microbiological analysis
To measure the total viable and coliform counts, ten grams of each sample and an 0.85% sodium chloride solution (100 mL) were homogenized for 2 min with a homogenizer (IKA T 25 digital Ultra-turrax, IKA Ltd., Staufen, Germany), and then tenfold diluted for analysis. Total viable cells were counted on standard agar plates (Difco, Detroit, MI, USA) after 45 h incubation in an incubator at 37 °C. The coliform count was performed on petri films (3 M Petrifilm™ Coliform Count Plates, 3 M, St. Paul, MN, USA) after incubation at 37 °C for 24 h, and then expressed as colony forming units (CFU/g). The analysis was performed using the methods suggested the ministry of food and drug safety method (Ministry of Food and Drug Safety, 2020) by triplicate.
Internal structure measurement
Raw or SV treated sample with/without enzyme at 70 °C for 8 h was frozen in a deep-freezer (− 80 °C, NF-140SF, NIHON Freezer, Tokyo, Japan) for 1 day and then freeze-dried (PVTFD 10R, Ilshin Lab Co., Ltd. Yangju, Korea). The each dried sample was sectioned, cut into thin sheets on carbon-coated copper grids, plated with platinum and subjected to environmental scanning electron microscopy (ESEM, XL-30FEG, FEI Co., Eindhoven, Netherlands) at an acceleration voltage of 15 kV and 1000× magnification.
Statistical analysis
In experimental design, the fixed effects of three conditions of temperature, four types of treatment and three points of reaction time were explored. For each combination of fixed factors, at least three measurements were conducted with pork fore shank which were randomly selected. The data of repeated measurements for each experimental condition were averaged for analysis. For the experiment, at least 144 (48 × 3) pieces of pork fore shank were used, and the measured data for each combination of three factors were collected. The statistical analysis was performed using one-way ANOVA with SPSS (Statistical Package for Social Sciences, Version 20.0, SPSS Inc., Chicago, IL, USA). For the items with significant differences between groups, Duncan’s multiple range test was used to test for significant differences at the P < 0.05 level.
Results and discussion
Determination of enzyme activity and thermostability
Crude enzymes extracted from pineapple (PE), kiwifruit (KE), and a commercial protease, Neutrase (NE), were subjected to measurement of their protease activity based on casein. Casein products are the most common substrates used in characterization of proteases and for determining the proteolytic activity of the proteases (Bekhit et al., 2014). As a result, the protease activity of the enzymes extracted from fruit was lower (1.54 and 1.11 U/mL for PE and KE, respectively) than that of NE (17.86 U/mL). However, the total activities of the fruit-extracted enzymes, based on their protein contents, were calculated as 30.03 and 23.31 U for KE and PE, respectively (data not shown).
The temperature-dependent stability of the crude enzymes extracted from the fruits (KE and PE) and NE are shown in Fig. 1. These fruit-derived proteolytic enzymes are known to maintain their high activity at temperatures as high as 60–70 °C because of their thermal resistance (Greenberg, 1955). The optimal temperature for the enzyme (actinidin) activity extracted from kiwifruit has been reported to be approximately 58–62 °C (Yamaguchi et al., 1982), and bromelain, which belongs to the cysteine plant proteinases that are known to attack myofibrillar proteins, has an optimum temperature range of 60–70 °C (Suh et al., 1992). In our experimental results, the temperature for the highest activity was 70 °C for PE and 60 °C for KE, supporting the above results, and both enzyme extracts were relatively stable at 50–70 °C with activities above approximately 60%, and the activity of KE was higher than that of PE, supporting the results reported by Soda et al. (1987). Similar to the fruit extracted enzymes, NE also showed its highest activity at 60 °C. Based on the fact that the KE or PE showed thermostability, a synergistic tenderizing effect could be expected by applying these enzymes to meat during SV cooking at 60–70 °C.
Fig. 1.
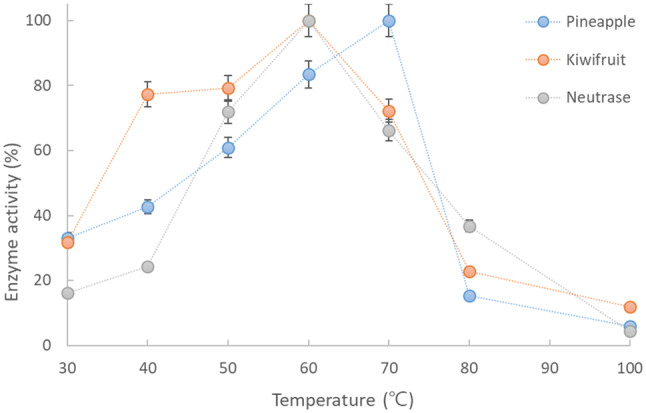
Effect of temperature on the relative activity of the crude proteases extracted from fruits (pineapple and kiwifruit) and a commercial enzyme (Neutrase)
Hardness properties
The hardness values (N/m2) of the pork fore shanks prepared with/without enzymes during SV are shown in Table 1. Changes in meat tenderness during cooking are associated with heat-induced alteration of the myofibrillar proteins and connective tissue. Heating solubilizes the connective tissue leading to meat tenderization, while denaturation of the myofibrillar proteins leads to meat toughening (Laakkonen et al., 1970). In this experiment, the hardness value decreased from 186,300 N/m2 (raw fore shank) to 164,300 (N/m2) by SV cooking at 45 °C for 0.5 h, and the value was mostly maintained (164,300–154,200 N/m2) for the 8 h treatment, showing an approximately 12-17% decrease. For the SV sample without enzymes, as the temperature increased, the hardness also increased; however, it somewhat decreased as the treatment time increased. The greatest softening effect of SV itself was observed when treated at 70 °C for 8 h, showing an approximately 36% hardness reduction, when compared to that treated at 70 °C for 0.5 h (444,800 vs. 284,300 N/m2). Longer cooking times seem to lead to higher collagen solubilization, which in turn would cause greater formation of gelatin and lower meat toughness. At 45 °C, although the hardness of the SV sample with enzymes was slightly lower than that of the SV without enzyme, however the enzymatic activity is weak at that temperature, it is difficult to expect a substantial softening effect from the enzymes.
For the 0.5 h treatment, as the treatment temperature increased to 70 °C, the hardness of all samples gradually increased. As the cooking temperature increases, the length of the muscular fibers decreases, which could be due to the denaturation of myosin (40–60 °C) and actin (66–73 °C) and shrinking of the connective tissue (56–62 °C) (Cheng and Parrish, 1979). This denaturation of the meat proteins during the cooking process causes structural changes, resulting in lateral shrinkage of the muscle fibers and contraction of the connective tissue, increasing the hardness (Barbera and Tassone, 2006). At the traditional cooking temperature of 100 °C, the hardness values of the samples were approximately 3 times higher than those of the samples treated at 45 °C. Similar results were reported by Palka and Daun (1999), who found increased hardness in samples when studying the texture of the semitendinosus muscle in beef cooked with increasing temperatures. Specifically, the SV sample cooked at 100 °C showed a higher hardness value (516,400 N/m2) than the SV samples prepared with enzymes (412,200–446,900 N/m2).
Regarding the treatment temperature of 60 °C, when the treatment time increased from 0.5 to 4 h, all of the samples showed decreased hardness values; however, after 4 h, no significant changes were observed. The SV-KE treatment showed the lowest hardness value (243,600 N/m2) among the samples treated at 60 °C from 0.5 to 8 h, which might be related to the optimum temperature of KE. Because a large portion of the original muscle protease could be inactivated by heating to 70 °C, it is difficult to soften the meat by standard cooking at 70 °C (Diaz et al., 2008). In this experiment, however, a significant reduction in hardness was observed because of protein degradation by the added enzymes, which have optimal activities at approximately 70 °C, and this softening effect was noticeable for the 8 h treatment. The SV-KE treatment showed much lower hardness values than the SV-PE treatment (176,300 vs. 216,100 N/m2, respectively) at 70 °C. The SV-NE treatment also showed a lower hardness (374,400 N/m2) value similar to SV-KE or SV-PE (309,100 or 311,600 N/m2) at 70 °C for 0.5 h; however, the softening effect of SV-KE was the greatest among the SV-enzyme treated groups, even after 8 h. Based on the hardness value of the SV treatment at 70 °C, it was obvious that, although SV alone showed a decreased hardness after 8 h (approximately 36%), a synergistic softening effect could be obtained by the addition of an enzyme (approximately 39–60%), and KE appeared to be more effective (60% softening effect) than either PE or NE.
The highest hardness of the samples prepared under a general cooking temperature, 100 °C for 0.5 h, could be associated with a greater degree of shrinking of the myofibrillar proteins. Palka and Daun (1999) also reported that the hardness increased when meat was cooked at temperatures over 80 °C because of the denaturation of myosin and actin and the shrinking of the connective tissue. The hardness of the samples slightly decreased as the treatment time was increased to 4 h, although enzyme softening effects could not be expected much because of the loss of enzyme activity by heating. Therefore, this softening might be ascribed to collagen solubilization. The hardness, however, did not change when the treatment time was increased up to 8 h.
Color properties
The color properties of the pork fore shank samples prepared under different SV conditions are shown in Table 2. The SV sample generally showed a higher L* value than the SV with enzyme samples. At 45 °C, the samples treated for either 0.5 h or 4 h had the lowest L* value range (56.6–59.6 and 59.1–65.1, respectively), and the values generally increased as the reaction temperature was increased to 70 °C, showing ranges of 69.5–74.9 and 72.5–74.1, respectively. The increase in the L* value with temperature could be explained by the denaturation and aggregation of proteins increasing at higher temperatures. For cooking at 100 °C, however, the L* value slightly decreased. The L* value was also affected by the cooking time, and an increasing trend was observed as the time increased from 0.5 to 4 h at the same cooking temperature. The L* value increasing over time was also reported by del Pulgar et al. (2012). After 4 h, however, the L* value slightly decreased, except for that of samples treated at 60 °C.
Table 2.
Color values of pork fore shank samples prepared under different SV cooking conditions (mean ± SE)
| Reaction time | 0.5 h | 4 h | 8 h | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature | L* | a* | b* | L* | a* | b* | L* | a* | b* |
| 45 °C | |||||||||
| SV | 58.9 ± 0.3eC | 6.0 ± 0.1aB | 4.7 ± 0.1deCD | 65.1 ± 0.2dA | 6.2 ± 0.2abB | 6.5 ± 0.1efB | 62.9 ± 0.2fAB | 5.7 ± 0.1aC | 6.7 ± 0.2gB |
| SV-NE | 56.6 ± 0.3fD | 6.3 ± 0.2aB | 3.3 ± 0.1fD | 61.3 ± 0.3eB | 6.0 ± 0.3abB | 5.9 ± 0.3efC | 60.9 ± 0.2gB | 5.9 ± 0.2aC | 6.5 ± 0.2gB |
| SV-KE | 59.6 ± 0.2eC | 6.4 ± 0.2aB | 4.1 ± 0.2efD | 64.6 ± 0.1dA | 6.7 ± 0.2aA | 7.1 ± 0.5eB | 63.2 ± 0.2fAB | 6.6 ± 0.3aA | 8.6 ± 0.1fA |
| SV-PE | 59.1 ± 0.1eC | 5.8 ± 0.2aC | 5.4 ± 0.1dC | 59.1 ± 0.2fC | 5.8 ± 0.2bC | 5.4 ± 0.4fC | 63.2 ± 0.1fAB | 5.7 ± 0.2aC | 8.1 ± 0.2fA |
| 60 °C | |||||||||
| SV | 68.4 ± 0.1bcC | 5.7 ± 0.2aB | 11.4 ± 0.2abC | 70.3 ± 0.4bcB | 3.1 ± 0.3cD | 10.5 ± 0.3dC | 69.0 ± 0.2cdB | 3.5 ± 0.2bD | 13.1 ± 0.2deAB |
| SV-NE | 66.6 ± 0.2cdD | 6.1 ± 0.2aA | 11.1 ± 0.2abC | 64.7 ± 0.2dE | 2.6 ± 0.2dE | 11.9 ± 0.2cB | 69.3 ± 0.1cB | 1.5 ± 0.3cF | 14.3 ± 0.2dA |
| SV-KE | 66.6 ± 0.5cdD | 5.5 ± 0.3aB | 12.0 ± 0.1abB | 68.0 ± 0.1cC | 4.9 ± 0.3bC | 12.3 ± 0.2cB | 74.0 ± 0.1aA | 5.7 ± 0.5aB | 13.0 ± 0.1deAB |
| SV-PE | 66.3 ± 0.2cdD | 5.5 ± 0.4aB | 11.9 ± 0.1abB | 69.1 ± 0.1cB | 2.9 ± 0.5cdE | 11.7 ± 0.1cB | 70.9 ± 0.1bB | 1.7 ± 0.1cF | 12.0 ± 0.2eB |
| 70 °C | |||||||||
| SV | 73.3 ± 0.3aAB | 0.1 ± 0.1dE | 10.9 ± 0.1bC | 73.2 ± 0.1aAB | − 0.5 ± 0.1fF | 14.8 ± 0.3bcB | 67.7 ± 0.2cdeD | 2.5 ± 0.2bcC | 17.7 ± 0.2bcA |
| SV-NE | 74.1 ± 0.1aA | 1.5 ± 0.3cD | 9.7 ± 0.2cCD | 72.5 ± 0.1abB | 0.2 ± 0.2eE | 15.5 ± 0.1bcB | 67.6 ± 0.2cdeD | 3.0 ± 0.0bcB | 15.7 ± 0.3cB |
| SV-KE | 74.9 ± 0.2aA | 2.6 ± 0.2bC | 10.1 ± 0.3cC | 72.9 ± 0.4abB | 2.0 ± 0.2dC | 16.0 ± 0.2bcB | 66.7 ± 0.2eD | 4.1 ± 0.2bA | 19.4 ± 0.2abA |
| SV-PE | 69.5 ± 0.3bC | 1.7 ± 0.1cD | 8.8 ± 0.3cD | 74.1 ± 0.1aA | 0.9 ± 0.3eE | 18.4 ± 0.2aA | 67.3 ± 0.1deD | 0.9 ± 0.1cE | 18.0 ± 0.1bA |
| 100 °C | |||||||||
| SV | 71.3 ± 0.2aA | 0.1 ± 0.1dD | 11.9 ± 0.1abG | 70.8 ± 0.1bcA | − 0.6 ± 0.2fE | 17.8 ± 0.1abE | 67.7 ± 0.2cdeB | − 0.8 ± 0.1dE | 21.7 ± 0.2aB |
| SV-NE | 67.8 ± 0.2cB | 0.3 ± 0.1dC | 12.2 ± 0.2abG | 70.3 ± 0.1bcA | 0.4 ± 0.1eC | 17.8 ± 0.3abE | 67.6 ± 0.1cdeB | − 1.5 ± 0.3dF | 19.7 ± 0.3abC |
| SV-KE | 64.9 ± 0.2cdD | 1.1 ± 0.2cB | 12.1 ± 0.2abG | 68.9 ± 0.1cBB | 1.9 ± 0.2dA | 16.6 ± 0.3abE | 66.7 ± 0.1eC | 0.8 ± 0.2cB | 23.4 ± 0.3aA |
| SV-PE | 65.5 ± 0.2cdCD | 1.0 ± 0.2cB | 14.8 ± 0.1aF | 71.1 ± 0.8bcA | 0.3 ± 0.1eCD | 18.1 ± 0.2aD | 67.3 ± 0.2deB | − 2.0 ± 0.3dG | 21.0 ± 0.4aB |
SV means sous vide cooking, N means neutrase, K means kiwifruit, P means pineapple, E means enzyme
Values A–Eat same temperature and a–gat same treatment time followed by different superscripts are significantly different at the P < 0.05 level
For the samples treated at 45 °C for 0.5 h, the a* value did not change substantially up to 60 °C and did not show a distinct trend with respect to the cooking time increasing from 0.5 to 8 h because the denaturing process of protein started at 60 °C (Geileskey et al., 1998). At moderate temperatures, the cooking time seemed not to greatly affect the reddish color of the cooked meat, even when cooked for a very long time. Similarly, myoglobin didn’t seem to be more intensely affected when cooked for 12 h at 60 °C (del Pulgar et al., 2012). However, with increasing temperature, a significant decrease in the a* value was observed, and a more distinct and continuous decrease was observed for the samples treated for 8 h. The value of a* is related to the concentration of myoglobin and the degree of myoglobin denaturation (Vaudagna et al., 2008). Although meat color is affected by the metmyoglobin formed by the oxidation of myoglobin, but several antioxidant components in plants added into meat can inhibit the formation of metmyoglobin (Sánchez-Escalante et al., 2003). For example, the a* values of ground pork meat increased with the addition of grape skin powder due to the inhibition of oxidation by antioxidants in the powder (Choi and Lee, 2016). In this experiment, the a* value of the SV-KE treatment was noticeably higher than that of the other samples at each condition, which might be due to strong antioxidant component, such as quercetin (Fiorentino et al., 2009) as well as vitamin or carotenoids in the kiwifruit extract, supporting the possibility suggested by Sánchez-Escalante et al. (2003).
The value for the yellow color (b*) tended to distinctly increase as both temperature and cooking time increased. In particular, the high b* values for samples prepared at 100 °C for 8 h seemed to be due to the generation of gravy from the meat upon browning. Consistent with the phenomena commonly observed during the cooking of meats, the L* and b* values tended to increase while the a* value decreased with increasing reaction temperature and time. Such color variations may be due to different developments of the Maillard reaction on the meat surface, combined with different extents of myoglobin denaturation (García-Segovia et al., 2007), which generates compounds that contribute to the final brown color of the product.
pH values
The pH values of the samples are presented in Table 3. The pH of the fresh pork fore shank used in this experiment was 5.66, which corresponds to 5.60 for sirloin (Shin et al., 2006). According to a study by James (1972), the pH in fresh meat should be 5.5–5.8, and gradually increases during storage until spoilage occurs at pH 8.0. The pH increase can be attributed to the generation of free amino acids by enzymes, changes in the buffering capacity of proteins, ammonia generation, and degradation of amino acids (Demeyer et al., 1979).
Table 3.
pH values of pork fore shank samples prepared under different SV cooking conditions (mean ± SE)
| Reaction time | Temperature | |||
|---|---|---|---|---|
| 45 °C | 60 °C | 70 °C | 100 °C | |
| 0.5 h | ||||
| SV | 5.57 ± 0.01bG | 5.66 ± 0.05abFG | 5.93 ± 0.01aCD | 6.00 ± 0.00nsA |
| SV-NE | 5.61 ± 0.01bG | 5.71 ± 0.07abFG | 5.95 ± 0.02aBC | 5.98 ± 0.01B |
| SV-KE | 5.35 ± 0.01eI | 5.62 ± 0.02bG | 5.77 ± 0.01cF | 5.90 ± 0.04D |
| SV-PE | 5.40 ± 0.01cdH | 5.60 ± 0.01bG | 5.83 ± 0.02bE | 5.96 ± 0.02BC |
| 4 h | ||||
| SV | 5.67 ± 0.01aE | 5.70 ± 0.01abE | 5.94 ± 0.01aC | 6.10 ± 0.00A |
| SV-NE | 5.70 ± 0.02aE | 5.68 ± 0.02abE | 5.93 ± 0.01aC | 6.06 ± 0.01bB |
| SV-KE | 5.46 ± 0.01cH | 5.55 ± 0.01cG | 5.76 ± 0.01cDE | 5.94 ± 0.00C |
| SV-PE | 5.56 ± 0.02bG | 5.62 ± 0.03abEF | 5.87 ± 0.01bD | 6.04 ± 0.01B |
| 8 h | ||||
| SV | 5.69 ± 0.03aC | 5.77 ± 0.01aBC | 5.93 ± 0.01aAB | 6.05 ± 0.01A |
| SV-NE | 5.71 ± 0.02aC | 5.77 ± 0.13aBC | 5.94 ± 0.01aAB | 6.02 ± 0.01A |
| SV-KE | 5.43 ± 0.01dD | 5.42 ± 0.08D | 5.71 ± 0.02cC | 6.04 ± 0.01A |
| SV-PE | 5.57 ± 0.02bD | 5.63 ± 0.05abD | 5.90 ± 0.02abB | 6.04 ± 0.02A |
SV means sous vide cooking, N means neutrase, K means kiwifruit, P means pineapple, E means enzyme
Values A–Iat same treatment time and a–eat same temperature followed by different superscripts are significantly different at the P < 0.05 level
The pH values of all samples used here were 5.35–6.10, which does not include in the optimum growth range (pH 6.8–7.2) of microorganisms; however, the values did include the range (pH 4.0–7.0) of potential-risk microorganisms as suggested by the National Restaurant Association (1992). When the pH was compared across treatment times (from 0.5 to 8 h) at each temperature, the values showed an increasing trend as the treatment time increased. All of the samples prepared with fruit-extracted enzymes (SV-KE or SV-PE treatments) showed lower pH values than those of the SV or SV-NE treatments, and this could be due to the fruit containing organic acids. The pH of the SV-KE treatment was maintained and even decreased when the cooking time was increased from 0.5 to 8 h, so the combination of SV and KE could be a good way for extending the shelf life.
Microbiological properties
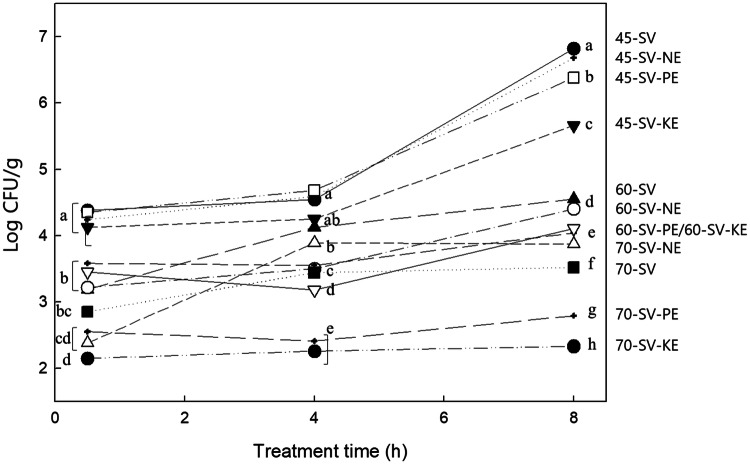
The microbial characteristics of the pork fore shank samples during SV treatment with different enzymes are shown in Fig. 2. The coliform bacteria were not observed in any samples in this experiment. The initial total viable cells of the pork fore shank were measured as 4.13 log CFU/g (data not shown), which was similar to that reported by Shin et al. (2006), but slightly higher than that of raw pork loin (3.00 log CFU/g) reported by Diaz et al. (2008). At 45 °C, the total microbial count of the SV samples considerably increased with increasing treatment times up to 8 h: 4.38 log CFU/g after 0.5 h, and 6.82 log CFU/g after 8 h; however, the count continuously decreased as the temperature was increased to 70 °C. This result was supported by the suggestion that a cooking temperature of 70 °C for 2 h effectively pasteurizes vacuum-packed cuts (Diaz et al., 2008).
Fig. 2.
Total viable count (CFU/cm2) on the surface of the pork fore shank samples prepared under different SV cooking conditions. SV means Sous Vide cooking; N means Neutrase; K means kiwifruit; P means Pineapple; E means enzyme
The SV-PE and SV-KE treated samples showed lower microbial counts when compared to the SV treatment alone; in particular, the SV-KE treatment showed the lowest count of all the samples at each treatment condition. For example, at 45 °C, while the total microbial count of the SV treatment at 8 h were 6.82 log CFU/g, the SV-KE treatment contained 5.66 log CFU/g, showing a difference of 1.16 log CFU/g. When cooked at 70 °C for 0.5 h and 8 h, the counts of the SV and SV-KE treatments were 2.85 versus 2.15 and 3.52 versus 2.33 log CFU/g, respectively. When cooked at 100 °C, almost no counts (below 10) were determined for all the samples (data not shown). Nottingham and Brown (1982) reported that spoilage begins when the microbial count of meat reaches 6–7 log CFU/g, and unbearable spoilage odor is generated at 8–9 log CFU/g. Ahn et al. (2014) also showed that the storage period for SV cooking was prolonged due to a lower amount of total bacteria in the microbial cells than in the control group, and this was due to the low possibility of cross-contamination by vacuum packaging. In our data, all samples prepared with SV cooking were in the safe zone in terms of their microbial counts, and the addition of KE showed a remarkable antibacterial effect.
Internal structure by microscopy analysis
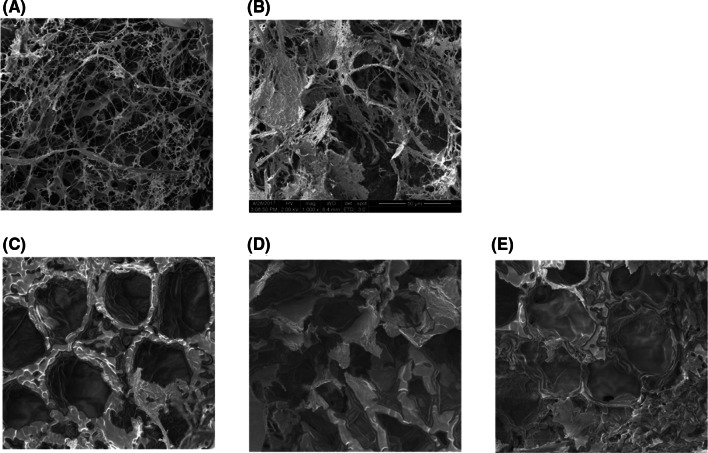
ESEM was performed to observe the microstructural changes of the pork fore shank during SV (Fig. 3). In Fig. 3A, the visible myofibers in the raw pork fore shank are shown to be twisted, similar to a thin thread. In Fig. 3B, obvious gaps between the fibers were observed after SV treatment at 70 °C for 8 h. In the combination of SV and enzyme treatment (Fig. 3C–E), some muscle fibers appeared to be soluble, and the structure became amorphous. The connective tissue dissolved at 70 °C, resulting in the formation of a gel that fills the spaces between the muscle fibers and fiber bundles, as reported by Roldan et al. (2013). This was consistent with the findings of Kim and Kim (1987), who reported that the dissolution of connective tissue occurred in the myofibrillar membrane and the myofiber lining during the enzymatic treatment of meat, resulting in amorphousness. When compared to Fig. 3C, more dissolution of the muscle fibers was observed in those samples treated with fruit-derived enzyme (Fig. 3D, E), and a more amorphous structure and a greater amount of fiber solution were observed in the sample treated with KE.
Fig. 3.
SEM micrographs of the pork loin prepared under several cooking conditions (×1000). (A) Raw pork loin, (B) 70 °C SV for 8 h without enzyme, (C) 70 °C SV for 8 h with Neutrase, (D) 70 °C SV for 8 h with crude pineapple enzyme and (E) 70 °C SV for 8 h with crude kiwifruit enzyme
Conclusively, when the effect of vacuum cooking (SV) with/without fruit-extracted enzymes was evaluated at different SV conditions, the tenderness, color and microstructure were affected by SV or SV with enzyme treatments based on increases in the cooking time and temperature. Especially for longer cooking time (8 h in this experiment) at 70 °C, SV alone showed a decrease in the hardness of the pork fore shank (approximately 27%); however, SV with enzymes had a synergistic tenderization effect (38–60%). Considering the optimum temperature of enzyme activity, the most effective conditions for pork tenderization were SV treatment with the kiwi-extract enzyme at 70 °C for 8 h. Under these conditions, the total microbial count as well as the hardness, pH and color change were the lowest.
Acknowledgements
This research was supported by a 2019 Research Grant (2019A000-0038) from Sangmyung University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jin-Hee Chang, Email: perijin2@naver.com.
Jung-Ah Han, Email: vividew@smu.ac.kr.
References
- Ahn JS, Kim SH, Kim NY. The sensory and physico-chemical of sous-vide cooking duck breast meat. J. Korean Soc. Food Sci. Nutr. 2014;27:990–998. doi: 10.9799/ksfan.2014.27.6.990. [DOI] [Google Scholar]
- Barbera S, Tassone S. Meat cooking shrinkage: measurement of a new meat quality parameter. Meat Sci. 2006;73:467–474. doi: 10.1016/j.meatsci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Bekhit AA, Hopkins DL, Geesink G, Bekhit AA, Franks P. Exogenous proteases for meat tenderization. Crit. Rev. Food Sci. Nutr. 2014;54:1012–1031. doi: 10.1080/10408398.2011.623247. [DOI] [PubMed] [Google Scholar]
- Cheng CS, Parrish FC. Heat-induced changes in myofibrillar proteins of bovine longissimus muscle. J. Food Sci. 1979;44:22–24. doi: 10.1111/j.1365-2621.1979.tb09995.x. [DOI] [Google Scholar]
- Cho SJ, Chung SH, Yang HC, Suh HJ, Lee H, Kang DH. Purification and characterization of a protease actinidin isolated from Cheju kiwifruit. Korean J. Food Nutr. 1994;7:87–94. [Google Scholar]
- Choi GW, Lee JW. Effect of grape skin on physicochemical and sensory characteristics of ground pork meat. Korean J. Food Cook. Sci. 2016;32:290–298. doi: 10.9724/kfcs.2016.32.3.290. [DOI] [Google Scholar]
- Creed PG, Reeve W. Principles and application of sous vide processed foods. In: Ghazala S, editor. Sous-vide and cook-chill processing for the food industry. Gaithersburg, MD: Aspen Publishers; 1998. pp. 57–88. [Google Scholar]
- Del Pulgar S, Gázquez A, Ruiz-Carrascal J. Physico-chemical, textural and structural characteristics of sous-vide cooked pork cheeks as affected by vacuum, cooking temperature, and cooking time. Meat Sci. 2012;90:828–835. doi: 10.1016/j.meatsci.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Demeyer DI, Vandekerckhove P, Moermans R. Compounds determining pH in dry sausage. Meat Sci. 1979;3:161–164. doi: 10.1016/0309-1740(79)90033-0. [DOI] [PubMed] [Google Scholar]
- Diaz P, Nieto G, Garrido MD, Banon S. Microbial, physico–chemical and sensory spoilage during the refrigerated storage of cooked pork loin processed by the sous vide method. Meat Sci. 2008;80:287–292. doi: 10.1016/j.meatsci.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Fiorentino A, D’Abrosca B, Pacifico S, Mastellone C, Scognamiglio M, Monaco P. Identification and assessment of antioxidant capacity of phytochemicals from kiwi fruits. J. Agric. Food Chem. 2009;57:4148–4155. doi: 10.1021/jf900210z. [DOI] [PubMed] [Google Scholar]
- García-Segovia P, Andrés-Bello A, Martínez-Monzó J. Effect of cooking method on mechanical properties, color and structure of beef muscle (M. pectoralis) J. Food Eng. 2007;80:813–821. doi: 10.1016/j.jfoodeng.2006.07.010. [DOI] [Google Scholar]
- Geileskey A, King RD, Corte D, Pinto P, Ledward DA. The kinetics of cooked meat haemoprotein formation in meat and model systems. Meat Sci. 1998;48:189–199. doi: 10.1016/S0309-1740(97)00089-2. [DOI] [PubMed] [Google Scholar]
- Greenberg DM. Plant proteolytic enzymes. Methods Enzymol. 1955;2:54–64. [Google Scholar]
- Grigioni G, Langman L, Szerman N, Iruruet M, Vaudagna SR. Effect of whey protein concentrate and sodium chloride concentrations on the odour profile of sous vide cooked whole-muscle beef from Argentina. Meat Sci. 2008;79:568–575. doi: 10.1016/j.meatsci.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Ha M, Bekhit AEDA, Carne A, Hopkins DL. Characterisation of commercial papain, bromelain, actinidin and zingibain protease preparations and their activities toward meat proteins. Food Chem. 2012;134:95–105. doi: 10.1016/j.foodchem.2012.02.071. [DOI] [Google Scholar]
- James MJ. Mechanical and detection of microbial spoilage in meat at low temperature. J. Milk Food Technol. 1972;35:467–471. doi: 10.4315/0022-2747-35.8.467. [DOI] [Google Scholar]
- Kim JS, Kim JP. Studies on the digestion of beef by ficin treatment. Appl. Biol. Chem. 1987;30:210–218. [Google Scholar]
- Kim MH, Rho JH, Kim MJ. Stabilizing and optimizing properties of crude protease extracted from Korean figs. Korean J. Food Cook. Sci. 2011;27:29–37. doi: 10.9724/kfcs.2011.27.3.029. [DOI] [Google Scholar]
- Laakkonen E, Wellington GH, Sherbon JW. Low temperature, long time heating of bovine muscle 1. Changes in tenderness, water-binding capacity, pH and amount of water soluble components. J. Food Sci. 1970;35:175–177. doi: 10.1111/j.1365-2621.1970.tb12131.x. [DOI] [Google Scholar]
- Minami Y, Doi E, Hata T. Fractionation, purification and some properties of proteolytic enzymes from stem bromelain. Agric. Biol. Chem. 1971;35(9):1419–1430. doi: 10.1080/00021369.1971.10860086. [DOI] [Google Scholar]
- Ministry of Food and Drug Safety (2020). https://www.foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=11056. Accessed 02 May 2020
- National Restaurant Association (US), and Laventhol & Horwath. Restaurant industry operations report. National Restaurant Association. (1992)
- Nottingham PM, Brown MH. Microbiology of carcass meat. In: Meat Microbiology. Applied Science Publisher Ltd, pp. 13-66 (1982)
- Offer G, Knight P. The structural basis of water holding in meat. Part 1: General principles and water uptake in meat processing. Development in Meat Science-6 (RA Lawrie Ed.). Elsevier Science Publisher, London (1988)
- Osório JCS, Osório MTM, Sañudo C. Sensory characteristics of sheep meat. Rev. Bras. Zootecn. 2009;38:292–300. doi: 10.1590/S1516-35982009001300029. [DOI] [Google Scholar]
- Palka K, Daun H. Changes in texture, cooking losses, and myofibrillar structure of bovine M. semitendinosus during heating. Meat Sci. 1999;51:237–243. doi: 10.1016/S0309-1740(98)00119-3. [DOI] [PubMed] [Google Scholar]
- Peck MW, Stringer SC. Clostridium botulinum: Mild preservation techniques. Second European Symposium on Sousvide. April 10-12, Leuven, Belgium. Alma sous-vide Competence Centre, Brussels, Belgium. pp. 181-197 (1996)
- Roldan M, Antequera T, Martin A, Mayoral AI, Ruiz J. Effect of different temperature-time combinations on physicochemical, microbiological, textural and structural features of sous-vide cooked lamb loins. Meat Sci. 2013;93:572–578. doi: 10.1016/j.meatsci.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Roldan M, Antequera T, Hernandez A, Ruiz J. Physicochemical and microbiological changes during the refrigerated storage of lamb loins sous-vide cooked at different combinations of time and temperature. J. Med. Food. 2015;21:512–522. doi: 10.1177/1082013214552861. [DOI] [PubMed] [Google Scholar]
- Sánchez-Escalante A, Torrescano G, Djenane D, Beltran JA, Roncales P. Stabilisation of colour and odour of beef patties by using lycopene-rich tomato and peppers as a source of antioxidants. J. Sci. Food Agr. 2003;83:187–194. doi: 10.1002/jsfa.1298. [DOI] [Google Scholar]
- Shin HY, Ku KJ, Park SK, Song KB. Use of freshness indicator for determination of freshness and quality change of beef and pork during storage. Food Sci. Biotechnol. 2006;38:325–330. [Google Scholar]
- Soda I, Kaneko M, Sato T, Nakagawa H, Ogura N. Studies on utilization of kiwifruit, 2: Studies on utilization of kiwifruit (Actinidia chinensis) protease. J. Jpn. Soc. Food Sci. 1987;34:36–41. [Google Scholar]
- Suh HJ, Lee H, Cho HY, Yang HC. Purification and characterization of bromelain isolated from pineapple. Appl. Biol. Chem. 1992;35:300–307. [Google Scholar]
- Vaudagna SR, Pazos AA, Guidi SM, Sanchez G, Carp DJ, Gonzalez CB. Effect of salt addition on sous vide cooked whole beef muscles from Argentina. Meat Sci. 2008;79:470–482. doi: 10.1016/j.meatsci.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Yamashita Y, Takeda I, Kiso H. Proteolytic enzymes in green asparagus, kiwi fruit and miut: occurrence and partial characterization. Agric. Biol. Chem. 1982;46:1983–1986. [Google Scholar]