Abstract
This study investigated the survivals of two pathogens (Escherichia coli O157:H7 and Staphylococcus aureus) in different adhered forms on glass fiber filters (GFFs) at 43 and 68% relative humidity (RH). Efficacies of chemical sanitizers at reducing pathogenic biofilms on GFFs were also evaluated. Inoculated GFFs were incubated at 28 °C in TSB (type I), on TSA (type II), or on TSA under 100% RH (type III) to produce biofilms. When GFFs were incubated at 43 or 68% RH for 7 days, type III biofilms were less than 2 log10 CFU/filter reduction whereas type I and type II biofilms were 4–6 log10 CFU/filter reduction. Additionally, type III biofilms were highly resistant to sanitizing treatment compared than other biofilms (type I and II). Therefore, the method to produce biofilms used in this study could be used to produce highly resistance pathogenic biofilms in the laboratory for related experiments.
Keywords: Biofilm, Glass fiber filter, Adhered form, Chemical sanitizer, Relative humidity
Introduction
Microorganisms are widely present in our surroundings as biofilms. Biofilms is a community of microorganisms surrounded in hydrated extracellular polymeric substances (EPSs) that attach to surfaces (Decho and Gutierrez, 2017). Biofilm formation is dependent upon many factors, including microbial species, surface structure, nutrients, environment, temperature, and relative humidity (RH) (Phillips, 2016). In addition, biofilm formation properties of pathogens and their resistance differ significantly in response to different surface materials (Giaouris et al., 2014). For example, more pathogenic bacterial cells attach to hydrophobic surfaces (paper and plastics) than to hydrophilic surfaces (stainless steel and glass) (Jo et al., 2010). Also, Escherichia coli O157:H7 produced significantly high levels of biofilm on wooden compared than on stainless-steel, plastic, and glass surfaces (Bang et al., 2014). Rose et al. (2003) reported that two strains of Yersinia pestis exhibited significantly greater survival capabilities on paper than other tested surfaces.
Pathogenic biofilm contaminated on the food contact surface can act as a major cause of cross-contamination of pathogenic bacteria to food (Giaouris et al., 2014). Beside biofilms are strongly tolerant against environmental stresses including desiccation and treatment with sanitizers and antimicrobial compounds, compared to planktonic cells (Phillips, 2016). Therefore, pathogens can survive sanitizing treatment in food industry when they form biofilms, ultimately contributing to foodborne illnesses through cross-contamination (Bang et al., 2014). Accordingly, identification of effective methods for controlling pathogenic biofilms on material surfaces in the food industry is critical. There were many researches that have investigated physical or chemical control methods to eliminate pathogenic biofilms on material surfaces including ultrasound, ultraviolet (UV) irradiation, peracetic acid, chlorine, hydrogen peroxide, ozone, and so on (Bae et al., 2012; Park et al., 2012). However, the tolerance of pathogenic biofilms or adhered cells on material surfaces to various control methods can differ depending on the adhered form or type of biofilm produced under various conditions. Indeed, pathogenic biofilms produced in hydric environment (100% RH) were highly resistant on the survivals at dry conditions compared than biofilms produced in broth, which was a commonly used method for producing biofilms in the laboratory in previous studies (Bae et al., 2012). Aerosolized sanitizer was less effective for reducing pathogenic biofilms produced under 100% RH compared than biofilms formed in laboratory broth on polyvinyl chloride and stainless steel (Park et al., 2012). Based on these results, biofilms formed under hydrophilic conditions, such as by immersion in laboratory broth, may not possess greater resistance than that of adhered cells or planktonic cells, as expected. Therefore, the effects of sanitizing methods on the inactivation of biofilms formed on material surfaces may have been overestimated in previous studies, as these studies evaluated the effects of sanitizing methods on biofilms produced on material surfaces using the immersion method in laboratory broth (Nguyen and Yuk, 2013; Oliveira et al., 2009). In fact, as food processing environments are known to possess high humidity, biofilms present in real systems may be more resistant to various stresses than are biofilms tested in laboratories. The relative humidity for processing environment range represent 4–75% or over 75% in relatively dry to wet food production environments (Vogel et al., 2010). Therefore, it is necessary to perform laboratory tests using highly resistant forms of biofilms for the evaluation of sanitizing methods to inactivate bacterial biofilms on the surface of materials. Bacteria can form highly resistant biofilms on rough surfaces, microstructures, or filamentous structures than they can on smooth or even surfaces of materials, as they can produce biofilms possessing tightly packed forms or structures. In support of this, a previous study used glass fiber filters (GFFs) to produce a highly resistant form of biofilm, and this allowed for the investigation of the effectiveness of bactericide solutions on the inhibition of bacterial biofilms (Lebert et al., 2007).
Given these previous findings, in this study, we manufactured two pathogenic biofilms (E. coli O157:H7 and S. aureus) on GFFs produced by different methods, including immersing in broth and placing on agar without or with incubation under humid conditions (100% RH), and we tested their survivals at dry conditions (43% and 68% RH) and against treatments with various chemical sanitizers to determine a means to produce highly resistant forms of pathogenic biofilm in the laboratory. For sanitizing treatments, five chemical sanitizers including alcohol-, chlorine-, hydrogen peroxide-, iodophor-, and quaternary ammonium-based sanitizers were evaluated to determine an effective sanitizing method to control pathogenic biofilms.
Materials and methods
Bacterial cell preparation
Each three strains of E. coli O157:H7 (ATCC 43889, ATCC 43890, and ATCC 35150) and S. aureus (ATCC 12600, ATCC 49444, and ATCC 12692) from Chung-Ang University (Anseong-si, Korea) were used in this study. Cultures stored at − 80 °C in tryptic soy agar (TSB; Difco Laboratories, Detroit, MI, USA) with 20% glycerol were cultured on tryptic soy agar (TSA; Difco) at 37 °C for 24 h. Next, colonies on TSA were cultured in TSB at 37 °C for 24 h. Cell pellets were harvested by centrifugation at 13,000×g for 3 min at 4 °C, washed and resuspended in phosphate-buffered saline (PBS; pH 7.2). Cell suspension (ca. 105−6 CFU/mL) were prepared by dilution with PBS and used in further experiments.
Preparation of GFFs
Type A/E GFFs (Pall Corporation, Mexico; 25 mm diameter, 1270 μm thickness, and 1 μm pore size) were used in this study. GFFs were sterilized before use.
Formation of biofilm on GFFs
GFFs were transferred into sterilized Petri dishes and inoculated with each pathogen using 0.5 mL of each pathogenic suspension of three-strain mixture by depositing droplets. Inoculated GFFs were dried for 2 h at 22 °C in a laminar flow biosafety hood to facilitate cell attachment. And then, inoculated GFFs were placed in 50 mL tubes containing TSB (30 mL) and incubated at 28 °C. Alternatively, the same inoculated GFFs were placed on TSA in a desiccator containing distilled water to produce humid condition (100% RH) and incubated at 28 °C. Each set of for 2 h was removed from TSB or TSA after 0, 1, 3, or 5 days using sterile forceps, washed with PBS, and dried in a hood for 2 h. Following incubation, the numbers of pathogen on GFFs were enumerated as described below.
Preparation of different adhered forms on GFFs
In this study, adhered cells or three different types were prepared on the surfaces of GFFs by of biofilm by biofilm formation stages (bacterial attachment, microcolony formation, and bacterial biofilm maturation). These included adhered cells, biofilms produced in TSB (type I; bacterial biofilm maturation stage), biofilms produced on TSA (type II; microcolony formation stage), and biofilms produced on TSA under 100% RH (type III; bacterial biofilm maturation stage). Culture cocktails (0.5 mL) of the two pathogens were prepared as described above and inoculated onto the GFF. Following adherence of each pathogen onto GFFs, inoculated GFFs used as adhered cells following drying in a hood for 2 h. Inoculated GFFs were incubated in 50 mL tubes containing TSB (30 mL) at 28 °C for 3 days. Following incubation, GFFs were washed with PBS and dried for 2 h, and these GFFs were used as type I biofilms (produced in TSB). The same inoculated and dried GFFs described above were alternatively placed on TSA and then incubated at 28 °C for 1 day. Following drying for 2 h, these GFFs were considered as type II biofilms (produced on TSA). Additionally, the same inoculated and dried GFFs described above were incubated on TSA in a desiccator containing distilled water to produce humid condition (100% RH) at 28 °C for 3 days. 1 day. Following drying for 2 h, these GFFs were considered as type III biofilms (produced on TSA under 100% RH condition).
Survivals of pathogens in different adhered forms on GFFs
Prepared GFFs were transferred to sterile Petri dishes and then stored under 43% or 68% RH to investigate bacterial survivals in biofilm on GFFs at dry condition. The two RH conditions (43% and 68% RH) were prepared in a desiccator containing 200 mL of potassium carbonate (Samchun Pure Chemical Co., Ltd., Gyeonggi-do, Korea) or lithium acetate (Samchun Pure Chemical Co., Ltd., Gyeonggi-do, Korea), respectively. Survived numbers of pathogens on GFFs in the desiccator were enumerated during incubation for 7 days at 28 °C.
Treatment with chemical sanitizers
For treatment with chemical sanitizers, four types of adhered cell or biofilms on GFFs were treated with distilled water (control) or commercial sanitizers including alcohol-based (70% ethanol, Fisher Scientific Korea, Seoul, Korea; 99.9% ethyl alcohol, 0.02% water), chlorine-based (Clean Shot, Chemical Leader Co., Seoul, Korea), hydrogen peroxide-based (P3-oxonia active, Deasung CNS Co., Korea; 22% hydrogen peroxide, 6% acetic acid, 4% peroxy acetic acid, 0.6% bisphosphoric acid), iodophor-based (Mikroklene DF, Ecolab Inc., USA; 6.5% phosphoric acid, 1.8% 2-butoxy-ethanol, 12.8% polyalkylene colichol butoxy monoether, 2% iodophor, 1.9% sodium iodide), and quaternary ammonium-based sanitizers (Quat Plus, 3 M, USA; di-n-alkyl dimethylammonium chloride, alkyl dimethyl benzyl ammonium chloride, 6% alkyl dimethyl ethylbenzyl ammonium chloride, ethyl alcohol, sequestering agent). All chemicals were prepared using distilled water according to the manufacturer’s instruction before each experiment. GFFs were immersed in 30 mL of each sanitizer or distilled water at 22 °C for 10 min as treatments or control, respectively.
Bacterial quantification
For quantification of the tolerance of pathogens on the surfaces of GFFs, GFFs in a stomacher bag (3 M) stomached for 90 s (Bagmixer 400; Interscience, France) with 20 mL D/E neutralizing broth (Difco). After serial dilution in 0.2% peptone water (PW; Difco), samples were surface plated onto TSA and plates were incubated at 37 °C for 24 h. The number of colonies on TSA were enumerated.
FE-SEM observation
The micro-structures of pathogenic biofilms on GFFs (adhered cells or biofilms) were analyzed using Field emission-scanning electron microscopy (FE-SEM; SIGMA; Carl Zeiss, UK). GFFs were fixed in 2% glutaraldehyde for 4 h and washed twice with 0.05 M sodium cacodylate buffer (pH 7.2). Next, GFFs were immersed in 1% osmium tetroxide in 0.05 M sodium cacodylate buffer for 2 h. Fixed GFFs were dehydrated in graded ethanol series (30, 50, 70, and 100%). Following dehydration, GFFs dried, sputter coated with gold were used for FE-SEM analysis.
Curve fitting
The Weibull distribution was used for fitting the survival curves of pathogens in GFFs: S(t) = e −(t/a)n where t is the storage days, and a and n are the scale and shape parameters, respectively (Buzrul and Alpas, 2007). The n parameter accounts for the upward concave curve (n < 1), a linear survival curve (n = 1), and downward concave curve (n > 1) (Fernandez et al., 2009). The least-squares criterion in Graph Pad PRISM (Graph Pad Software, San Diego, CA, USA) was utilized for determining the a and n values. The model fitting performances were statistically analyzed using R-square values in order to ensure the model suitabilities.
Statistical analysis
The data was converted to log10 CFU/filter and averages values of three replicated experiments were evaluated in this study. D-values of pathogens were calculated from the linear regression of the log10 of bacterial surviving cells. The data was analyzed using the Statistical Analysis System (Version 9.4; SAS Institute, Cary, NC, USA) for analysis of variance (ANOVA) and Duncan’s multiple range to determine significantly differences (P ≤ 0.05) among treatment groups.
Results and discussion
Figure 1 represents pathogenic levels on the surfaces of GFFs in TSB or on TSA at 100% RH during incubation at 28 °C for 5 days. Initial counts of pathogens were approximately 4–5 log10 CFU/filter. Pathogen levels on the surface of GFFs increased significantly, reaching approximately 8–10 log10 CFU/filter after 1 day of incubation. These levels were maintained for 5 days of incubation. From these results, E. coli O157:H7 and S. aureus grew and formed biofilms on GFFs in TSB (hydrophilic condition) and at 100% RH (hydrophobic condition). Several studies have investigated the pathogenic survivals on the surface of materials under various RH conditions. Levels of C. sakazakii in biofilms formed in infant formula and M9 medium were maintained at 100% RH (Kim et al., 2008) Choi et al. (2011) studied the numbers of total aerobic bacteria recovered from spinach leaves stored at RHs of 43%, 85%, and 100% for up to 120 h, and they found that populations increased significantly when stored at 100% RH for 120 h. Other study (Iturriaga et al., 2007) also observed the levels of biofilm formation on a tomato surface were increased with the increase of RH over 10 days of storage. Through the results of these studies, we can conclude that hydrated environments, such as environments possessing 100% RH with nutrients, can enhance the numbers of bacteria and biofilm formation on material surfaces.
Fig. 1.
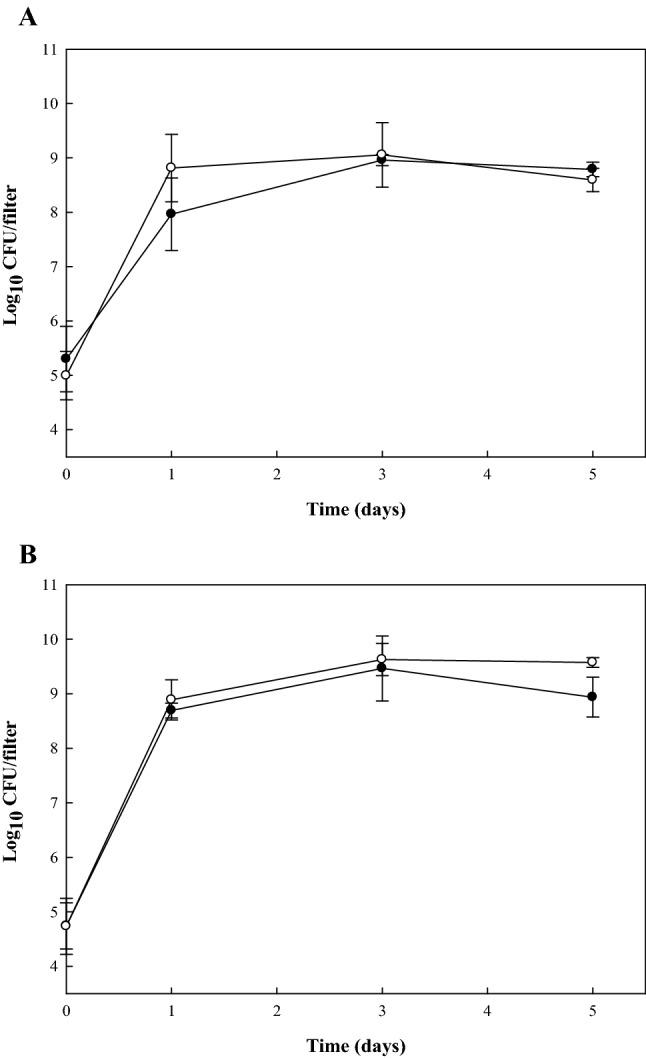
Growth (log10 CFU/filter) of Escherichia coli O157:H7 (filled circle) and Staphylococcus aureus (open circle) contaminating glass fiber filters as biofilms formed in TSB incubated at 28 °C for 5 days (A) or biofilms formed on TSA under 100% RH at 28 °C for 5 days (B)
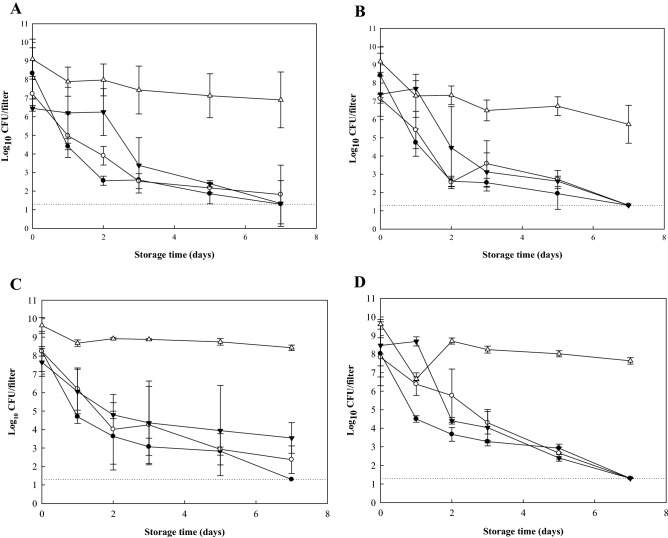
Next, we investigated the desiccation tolerance of pathogenic biofilms possessing different adhered forms on GFFs using various methods. Different forms of adhered cells on GFFs including (1) adhered cells, (2) biofilm produced in TSB (type I), (3) biofilm produced on TSA (type II), and (4) biofilm produced on TSA under 100% RH (type III) were tested in this study. Figure 2 shows the survival of E. coli O157:H7 and S. aureus on the surfaces of GFFs at 43% and 68% RH, respectively. Initial counts of E. coli O157:H7 on GFFs before storage at 43% and 68% RH were approximately 6–8 and 7–9 log10 CFU/filter, respectively (Fig. 2A and B), while those of S. aureus on GFFs before storage at 43% and 68% RH were approximately 7–9 and 8–10 log10 CFU/filter, respectively (Fig. 2C and D). Types I and II biofilms exhibited survival patterns similar to those of adhered cells, indicating similar levels of tolerance under dry conditions. Type III biofilms, however, showed high tolerance under dry conditions for both pathogens relative to that of other types of adhered cells on GFFs.
Fig. 2.
Numbers (log10 CFU/filter) of Escherichia coli O157:H7 (A and B) and Staphylococcus aureus (C and D) surviving on the surfaces of glass fiber filters as adhered cells (filled circle), biofilms formed at 28 °C for 3 days in TSB (type I) (filled inverted triangle), biofilms formed at 28 °C for 1 days on TSA (type II) (open circle), or biofilms formed at 28 °C for 3 days on TSA under 100% RH (type III) (open triangle) and stored at 43% (A and C) or 68% (B and D) RH for 7 days. Detection limit (dotted line) is 1.30 log CFU/filter
Table 1 shows the decimal reduction time and n-value index of E. coli O157:H7 and S. aureus survivals on GFFs when stored at 43% and 68% RH for 7 days. When GFFs were incubated at 28 °C for 7 days, levels of E. coli O157:H7 and S. aureus on GFFs were not significantly different between conditions of 43% and 68% RH (P > 0.05) based on D- and n-values. Populations of E. coli O157:H7 and S. aureus on GFFs produced under 100% RH (type III) were maintained at high levels (> 6 and > 8 log10 CFU/filter, respectively) for 7 days of storage, respectively, and therefore n-values were not determined for type III biofilms for both pathogens. D-values of type III biofilms were significantly higher than other adhered forms (P ≤ 0.05) for both pathogens. D-values of E. coli O157:H7 type III biofilms on GFFs were 2.73 and 3.56 days at 43% and 68% RH, respectively, while those of other adhered cells were less than 1.08 days. Additionally, D-values of S. aureus type III biofilms were 6.42 and 6.16 days at 43% and 68% RH, respectively, while those of other adhered cells were less than 1.59 days. Pathogenic biofilms produced under hydrated conditions on TSA under 100% RH (type III) exhibited the most tolerance against desiccation among the different adhered forms of pathogens on GFFs, including adhered cells or biofilms formed under hydrophilic conditions in TSB (type I). Another study also evaluated the tolerances of different forms of biofilms on material surfaces under dry conditions. In the study of Bae et al. (2012), biofilms of five pathogens including E. coli O157:H7, L. monocytogenes, S. Typhimurium, S. aureus, and C. sakazakii on stainless steel surfaces produced under 100% RH condition showed strong resistance on the survivals at dry environment compared to other adhered forms, including adhered cells and biofilms produced in TSB, which is consistent with the current results. For both pathogens, D-values among different adhered forms, with the exception of type III biofilms (P > 0.05) were not significantly different (P > 0.05). For type III biofilms, S. aureus exhibited smaller reductions than E. coli O157:H7 during storage for 7 days at 43% and 68% RH. Additionally, the resistance of E. coli O157:H7 and S. aureus biofilm on GFFs produced under 100% RH were much higher compared than those of biofilms on stainless steel under the same condition when the result of this study was compared with previous study (Bae et al., 2012). In particular, reduction of E. coli O157:H7 biofilms on stainless steel produced under 100% RH were ca. 4-5 log10 CFU/coupon after storage for 5 days at 68% and 43% RH (Bae et al., 2012), while reduction of E. coli O157:H7 biofilms on GFFs under same condition (RH 100%) were < 3 log10 CFU/filters after storage for 7 days at 68% and 43% RH. From these results, more resistant forms of pathogenic biofilms against dry conditions could be produced using GFFs in the laboratory relative to those produced using other materials such as stainless steel.
Table 1.
D-values (days)a and n-values (shape) of Escherichia coli O157:H7 and Staphylococcus aureus contaminated as biofilms formed on the surfaces of glass filters and stored under two different RHs for 7 days
| Strain | Adhered formb | D-value | Weibull distribution | ||||
|---|---|---|---|---|---|---|---|
| n-value | R2c | ||||||
| 43% RH | 68% RH | 43% RH | 68% RH | 43% RH | 68% RH | ||
| Escherichia coli O157:H7 | Adhered cell | 0.83 ± 0.14Bdae | 0.51 ± 0.36Ba | 0.31 ± 0.07Ba | 0.29 ± 0.11Ba | 0.99 ± 0.01 | 0.96 ± 0.02 |
| Biofilm (type I) | 0.94 ± 0.11Ba | 0.76 ± 0.13Ba | 0.69 ± 0.12Aa | 0.86 ± 0.26Aa | 0.89 ± 0.09 | 0.86 ± 0.02 | |
| Biofilm (type II) | 1.08 ± 0.10Ba | 0.98 ± 0.14Ba | 0.28 ± 0.06Ba | 0.44 ± 0.13Ba | 0.96 ± 0.03 | 0.84 ± 0.03 | |
| Biofilm (type III) | 2.73 ± 0.86Aa | 3.56 ± 1.72Aa | NDf | ND | ND | ND | |
| Staphylococcus aureus | Adhered cell | 0.87 ± 0.13Ba | 0.93 ± 0.20Ba | 0.39 ± 0.23Ba | 0.34 ± 0.10Ba | 0.97 ± 0.02 | 0.97 ± 0.02 |
| Biofilm (type I) | 1.59 ± 0.96Ba | 0.64 ± 0.06Ba | 0.52 ± 0.19Aa | 0.82 ± 0.24Aa | 0.89 ± 0.29 | 0.87 ± 0.03 | |
| Biofilm (type II) | 0.98 ± 0.38Ba | 0.80 ± 0.19Ba | 0.48 ± 0.23Aa | 0.83 ± 0.08Aa | 0.78 ± 0.09 | 0.95 ± 0.02 | |
| Biofilm (type III) | 6.42 ± 2.15Aa | 6.16 ± 2.54Aa | ND | ND | ND | ND | |
aData represent the means ± standard deviations of three measurements
bAdhered cells were prepared by drying GFFs for 2 h following inoculation; type I biofilms were formed by immersing inoculated GFFs in TSB for 3 days at 28 °C; type II biofilms were formed by cultivation on inoculated GFFs on TSA for 1 days at 28 °C; type III biofilms were formed by cultivation on inoculated GFFs on TSA under 100% RH for 3 days at 28 °C
cR2 values range from 0 to 1 and the closer the values are to the maximum value the better the fit of the model
dMeans with the same letter within a column for each pathogen are not significantly different (P > 0.05)
eMeans with the same letter within a row for D-value and n-value are not significantly different (P > 0.05)
fNot determined
Generally, it is accepted that bacterial biofilms are greater tolerance to treatment with sanitizers than planktonic cells (Cadena et al., 2019). The increased tolerance of biofilms to sanitizers may be explained by EPSs such as cellulose and curli (Phillips, 2016). The EPSs produced by attached bacteria may protect sessile cells from sanitizers therefore it is highly difficult to eliminate bacterial biofilms on food contact surfaces in food industry (Decho and Gutierrez, 2017). Treatments with various kinds of sanitizers such as iodophor (Ban and Kang, 2016), chlorine (Olmez and Temur, 2010), hydrogen peroxide (Choi et al., 2012), peracetic acid, quaternary ammonium compound (Nguyen and Yuk, 2013), ozonated water (Olmez and Temur, 2010), and sodium hypochlorite (Meira et al., 2012) have been investigated for reducing bacterial biofilms on material surfaces in published studies. Efficacies of chemical sanitizers on inhibiting biofilm on material surfaces could, however, differ depending on their adhered forms. To obtain accurate results for the identification of effective sanitizing methods that are able to eliminate pathogenic biofilms on material surfaces, sanitizing methods should be evaluated using highly resistant forms of biofilm. Therefore, in this study, various sanitizing methods were evaluated using different types of biofilms on GFF produced by the different methods described. Table 2 shows populations of E. coli O157:H7 and S. aureus on GFFs before and after treatment with five different chemical sanitizers. Initial counts of E. coli O157:H7 and S. aureus on GFFs were 8–10 and 9–10 log10 CFU/filter, respectively. Levels of E. coli O157:H7 and S. aureus on GFFs reduced by water treatment (control) were < 1 log10 CFU/filter, while chemical sanitizers were effective to reduce both pathogenic biofilms with the exception of iodophor-based sanitizers. Among tested sanitizers, alcohol-, chlorine-, hydrogen peroxide-, and quaternary ammonium-based sanitizers were effectively reduced adhered cells and biofilms of E. coli O157:H7on GFFs. Also, populations of S. aureus on GFFs after treatments with alcohol and chlorine-based sanitizers were significantly decreased (P ≤ 0.05). Reduction of pathogens by treatments with chemical sanitizers were significantly different depending on their adhered forms (P ≤ 0.05). For example, when alcohol-based sanitizer was used to treat different adhered forms of E. coli O157:H7 and S. aureus on GFFs, levels of these pathogens were 3.5, 5.4, 7.9, and 8.9 log10 CFU/filter for E. coli O157:H7 and 5.6, 5.2, 5.8, and 7.9 log10 CFU/filter for S. aureus after treatment for 10 min, respectively. Therefore, pathogenic biofilms showed similar results to those observed using adhered cells when biofilms on GFFs were produced in TSB for 3 days (type I). E. coli O157:H7 and S. aureus biofilms produced on TSA under 100% RH (type III), however, were highly resistant against sanitizing treatments. Given this, levels were maintained at high levels (> 8 and > 7 log10 CFU/filter), respectively, following treatment with alcohol-sanitizers, indicating that biofilms produced in hydrated environments exhibited enhanced tolerance to sanitizing treatments (Table 2). In particular, alcohol-based sanitizers were highly effective at reducing pathogenic adhered cells and biofilms on GFFs. Other studies also reported that treatment with alcohol were highly effective to reduce pathogenic biofilms compared to treatment with chlorine (Bae et al., 2009; Lee et al., 2007). Bae et al. (2012) reported that alcohol-based sanitizers are highly effective to inhibit adhered pathogens on stainless steel. Additionally, pathogenic biofilms formed under hydrophilic conditions, such as by immersion in laboratory broth, do not show differences in tolerance to treatment with chemical sanitizers when compared with those observed using adhered cells or planktonic cells (Bae et al., 2012; Park et al., 2012). Spoering and Lewis (2001) reported that Pseudomonas aeruginosa biofilms formed under hydrophilic conditions in Mueller–Hinton broth were not different from planktonic cells in stationary phase in their tolerance to antibiotics and biocides. It was also reported by Park et al. (2012), who found increased resistance of pathogenic biofilms on two coupons under 100% RH relative to that of biofilms produced by immersion in TSB at 25 °C for 6 days. These results support the observation that biofilms produced on TSA under 100% RH (type III) are highly resistant against sanitizers compared to biofilms formed under hydrophilic conditions, such as by immersion in laboratory broth. Additionally, several studies have found that the tolerance of pathogenic biofilms to sanitizer treatments could be various depending on the surface type of material. Salmonella biofilm on stainless steel were more susceptible to chlorine- and iodine-based sanitizers than those biofilms on plastic. Also, S. aureus biofilms on glass surfaces are more resistant to treatment with sanitizers than biofilm formed on stainless steel (Marques et al., 2007).
Table 2.
Population (log10 CFU/filter)a of Escherichia coli O157:H7 and Staphylococcus aureus as biofilms formed on the surfaces of glass filters before and after treatment with water or chemical sanitizers
| Strains | Adhered formb | None | Distilled water | Type of chemical sanitizer | ||||
|---|---|---|---|---|---|---|---|---|
| Alcohol-based | Chlorine-based | Hydrogen peroxide-based | Iodophor-based | Quaternary ammonium-based | ||||
| Escherichia coli O157:H7 | Adhered cell | 8.08 ± 1.08Ccad | 6.50 ± 0.17Db | 3.45 ± 0.62Cd | 4.78 ± 0.48Cc | 4.45 ± 0.15Cc | 7.82 ± 0.19Ca | 4.70 ± 0.35Bc |
| Biofilm (type I) | 10.03 ± 0.48Ba | 9.58 ± 0.10Cab | 5.42 ± 0.86Bcd | 4.54 ± 0.24Cd | 6.25 ± 1.65Bc | 8.45 ± 0.38Bb | 5.37 ± 0.78Bcd | |
| Biofilm (type II) | 10.26 ± 0.28ABa | 10.85 ± 0.49Aa | 7.89 ± 0.17Ac | 7.64 ± 0.92Bc | 7.59 ± 1.63ABc | 9.58 ± 0.16Aab | 8.76 ± 0.64Abc | |
| Biofilm (type III) | 10.76 ± 0.03Aa | 10.22 ± 0.38Bb | 8.88 ± 0.23Ad | 9.34 ± 0.34Acd | 9.40 ± 0.27Ac | 9.34 ± 0.28Acd | 9.12 ± 0.06Acd | |
| Staphylococcus aureus | Adhered cell | 9.12 ± 1.08ABa | 8.45 ± 0.31Bab | 5.60 ± 0.37Db | 5.22 ± 0.48Db | 6.48 ± 1.18Bcd | 8.82 ± 0.18Bab | 5.50 ± 1.11Bbc |
| Biofilm (type I) | 8.69 ± 0.15Ba | 8.83 ± 0.98Ba | 5.19 ± 0.47Bc | 5.71 ± 0.29Bbc | 6.44 ± 0.70Bb | 7.98 ± 0.41Ca | 8.18 ± 0.41ABa | |
| Biofilm (type II) | 9.17 ± 0.86ABa | 8.81 ± 0.11Ba | 5.81 ± 0.95Bb | 5.73 ± 2.41Bb | 7.70 ± 2.03ABab | 8.71 ± 0.29Ba | 7.76 ± 0.87Bab | |
| Biofilm (type III) | 10.36 ± 0.19Aa | 10.25 ± 0.32Aa | 7.88 ± 1.30Ba | 9.63 ± 0.20Aa | 9.81 ± 0.14Aa | 9.62 ± 0.40Aa | 9.32 ± 0.36Aa | |
aData represent the means ± standard deviations of three measurements
bAdhered cells were prepared by drying GFFs for 2 h following inoculation; type I biofilms were formed by immersing inoculated GFFs in TSB for 3 days at 28 °C; type II biofilms were formed by cultivation on inoculated GFFs on TSA for 1 days at 28 °C; type III biofilms were formed by cultivation on inoculated GFFs on TSA under 100% RH for 3 days at 28 °C
cMeans with the same letter within a column for each pathogen are not significantly different (P > 0.05)
dMeans with the same letter within a row for water and chemical sanitizer are not significantly different (P > 0.05)
Additionally, biofilm formation of pathogens and their resistance to survival under dry conditions or after treatment with sanitizers could differ depending on material types and surface structures. Generally, biofilms formed on hydrophobic surface such as paper, filter, and Teflon possessed higher resistance to dry conditions or sanitizers relative to resistance observed in biofilms formed on hydrophilic surfaces such as stainless steel and glass. Pan et al. (2006) reported that pathogenic biofilms on Teflon (hydrophobic) were more resistant to sanitizers than biofilms on stainless steel (hydrophilic). The structure of material surfaces could influence the resistance of pathogenic biofilms as well. A previous study indicated that biofilms formed on microstructures or filamentous structures using GFFs were highly resistant to treatment with bactericide solutions (Lebert et al., 2007). Indeed, treatment with alcohol-based sanitizers for 5 min reduced 5.73 and 6.71 log CFU/coupon of E. coli O157:H7 and S. aureus on stainless steel produced under 100% RH, respectively (Bae et al., 2012). In the present study, however, treatment with the same alcohol-based sanitizer for 10 min were 1.87 and 2.48 log10 CFU/filter of E. coli O157:H7 and S. aureus on GFFs produced under the same conditions (100% RH), respectively. From the results of this study, the tolerance of biofilms formed on GFFs under a hydrated environment (100% RH) was much higher than that of biofilms formed on stainless steel under the same condition (100% RH) or on GFFs in laboratory media. Therefore, the method for the formation of biofilms on GFFs under 100% RH could be used to produce highly resistant forms of pathogenic biofilms against environmental stresses, and these could in turn be applicable to the development of effective sanitizing methods for inhibiting and eliminating bacterial biofilms in laboratories.
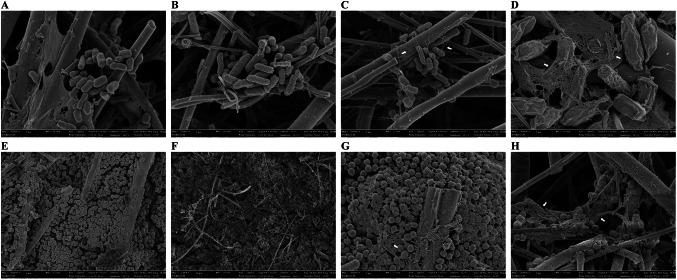
Figure 3 shows FE-SEM images of the four different adhered forms of E. coli O157:H7 and S. aureus biofilms on GFFs. Cells were surrounded under EPS within the formed biofilm, remaining adhered on the surface. Biofilms produced on the surfaces of GFFs on TSA showed an EPS formaton shielding the colonies (Fig. 3C and G), and slimy materials were also identified in the colonies for type III biofilms, which showed the most resistance to dry conditions and sanitizer treatment (Fig. 3D and H). Additionally, biofilm cells were observed as clump-like structures. This phenomenon was more prevalent in the biofilm produced under 100% RH on the surface of GFFs than it was in other adhered forms. Rodriguez et al. (2007) also observed that biofilms produced in this manner possess different structure and composition of EPS that could affect cell survival in various stress conditions than do biofilms produced under a highly hydrated environment. In this study, biofilms produced under 100% RH were more resistant in terms of survival on the GFF relative to biofilms produced in TSB. Therefore, the presence of a hydrated environment is an important factor to produce highly resistant biofilms on various material surfaces. Also, GFFs possessed a structure that promoted the formation of a fibrous or filamentary layer. Given this, the GFFs may provide a protective microenvironment for bacteria from desiccation. Tremoulet et al. (2002) observed that the E. coli O157:H7 cells on glass fibers and appeared encapsulated in polymeric matrix. Rose et al. (2003) also reported that paper coupons demonstrated an improved ability to support the bacteria cells relative to other surfaces.
Fig. 3.
Field-emission scanning electron microscopy (FE-SEM) micrographs of Escherichia coli O157:H7 (A–D) and Staphylococcus aureus (E–H) on the surfaces of glass fiber filters contaminated as adhered cells (A and E), type I biofilms formed by immersion of inoculated glass fiber filters in TSB for 3 days at 28 °C (B and F), type II biofilms formed by cultivation of inoculated glass fiber filters on TSA for 1 days at 28 °C (C and G), or type III biofilm formed by cultivation of inoculated glass fiber filters on TSA under 100% RH for 3 days at 28 °C (D and H). Magnification: (A–C and E–G), × 20,000; (D and H), × 30,000
In conclusion, biofilms formed on GFFs under hydrated conditions (100% RH) (type III biofilm) were highly resistant to dry conditions and chemical sanitizer treatment relative to other biofilms. Additionally, among the various chemical sanitizers, alcohol-based sanitizers were the most effective for eliminating all types of biofilms; however, efficacies of the sanitizers were very different depending on the types of biofilms produced under different conditions. There was 1.8 and 2.5 log10 CFU/filter reductions of E. coli O157:H7 and S. aureus in type III biofilms by treatments with alcohol-based sanitizer, respectively. These strong resistances exhibited by pathogenic biofilms produced on GFFs under 100% RH may be due to specific conditions such as GFF (filamentous structure) and hydrated conditions that result in the formation of biofilms that possess large amounts of EPS and slime around the cells. From the results of this study, the method of biofilm formation (biofilm produced under a highly hydrated environment on GFFs) could be used to produce highly resistant forms of pathogenic biofilms and could be applied to develop effective methods for eliminating bacterial biofilms on cooking equipment surfaces and other related environments.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korea government (MSIT) (NRF-2016R1A2B4014591) and the Chung-Ang University Excellent Student Scholarship in 2020.
Author contributions
Hana Song collected data and drafted the manuscript. Sun-Young Lee designed the study, interpreted the results and revised the manuscript.
Compliance with ethical standards
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hana Song, Email: gksk2512@naver.com.
Sun-Young Lee, Email: nina6026@cau.ac.kr.
References
- Bae YM, Baek SY, Lee SY. Resistance of pathogenic bacteria on the surface of stainless steel depending on attachment form and efficacy of chemical sanitizers. International Journal of Food Microbiology. 2012;153:465–473. doi: 10.1016/j.ijfoodmicro.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Bae YM, Heu SG, Lee SY. Inhibitory effect of dry-heat treatment and chemical sanitizers against foodborne pathogens contaminated on the surfaces of materials. J. Korean Soc. Food Sci. Nutr. 2009;38:1265–1270. doi: 10.3746/jkfn.2009.38.9.1265. [DOI] [Google Scholar]
- Ban GH, Kang DH. Effect of sanitizer combined with steam heating on the inactivation of foodborne pathogens in a biofilm on stainless steel. Food Microbiology. 2016;55:47–54. doi: 10.1016/j.fm.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Bang J, Hong A, Kim H, Beuchat R, Rhee MS, Kim Y, Ryu JH. Inactivation of Escherichia coli O157:H7 in biofilm on food-contact surfaces by sequential treatments of aqueous chlorine dioxide and drying. International Journal of Food Microbiology. 2014;191:129–134. doi: 10.1016/j.ijfoodmicro.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Buzrul S, Alpas H. Modeling inactivation kinetics of food borne pathogens at a constant temperature. LWT-Food Sci. Technol. 2007;40:632–637. doi: 10.1016/j.lwt.2006.02.019. [DOI] [Google Scholar]
- Cadena M, Kelman T, Marco ML, Pitesky M. Understanding antimicrobial resistance (AMR) profiles of Salmonella biofilm and planktonic bacteria challenged with disinfectants commonly used during poultry processing. Foods 8: 275, 10.3390/foods8070275 (2019) [DOI] [PMC free article] [PubMed]
- Choi NY, Baek SY, Yoon JH, Choi MR, Kang DH, Lee SY. Efficacy of aerosolized hydrogen peroxide-based sanitizer on the reduction of pathogenic bacteria on a stainless steel surface. Food Control. 2012;27:57–63. doi: 10.1016/j.foodcont.2012.02.027. [DOI] [Google Scholar]
- Choi S, Bang J, Kim H, Beuchat LR, Ryu JH. Survival and colonization of Escherichia coli O157:H7 on spinach leaves as affected by inoculum level and carrier, temperature and relative humidity. Journal of Applied Microbiology. 2011;111:1465–1472. doi: 10.1111/j.1365-2672.2011.05175.x. [DOI] [PubMed] [Google Scholar]
- Decho AW, Gutierrez T. Microbial extracellular polymeric substances (EPSs) in ocean systems. Front. Microbiol. 10.3389/fmicb.2017.00922 (2017) [DOI] [PMC free article] [PubMed]
- Fernandez A, Alvarez-Ordonez A, Lopez M, Bernardo A. Effects of organic acids on thermal inactivation of acid and cold stressed Enterococcus faecium. Food Microbiology. 2009;26:497–503. doi: 10.1016/j.fm.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Giaouris E, Heir E, Hebraud M, Chorianopoulos N, Langsrud S, Moretro T, Habimana O, Desvaux M, Renier S, Nychas GJ. Attachment and biofilm formation by foodborne bacteria in meat processing environments: causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Science. 2014;97:298–309. doi: 10.1016/j.meatsci.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Iturriaga MH, Tamplin ML, Escartini EF. Colonization of tomatoes by Salmonella Montevideo is affected by relative humidity and storage temperature. J. Food Protect. 2007;70:30–34. doi: 10.4315/0362-028X-70.1.30. [DOI] [PubMed] [Google Scholar]
- Jo SH, Baek SB, Ha JH, Ha SD. Maturation and survival of Cronobacter biofilms on silicone, polycarbonate, and stainless steel after UV light and ethanol immersion treatments. J. Food Protect. 2010;73:952–956. doi: 10.4315/0362-028X-73.5.952. [DOI] [PubMed] [Google Scholar]
- Kim H, Bang J, Beuchat LR, Ryu JH. Fate of Enterobacter sakazakii attached to or in biofilms on stainless steel upon exposure to various temperatures or relative humidities. J. Food Protect. 2008;71:940–945. doi: 10.4315/0362-028X-71.5.940. [DOI] [PubMed] [Google Scholar]
- Lebert I, Lerory S, Talon R. Effect of industrial and natural biocides on spoilage, pathogenic and technological strains grown in biofilm. Food Microbiology. 2007;24:281–287. doi: 10.1016/j.fm.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Lee SY, Jung JH, Jin HH, Kim YH, Oh SW. Inhibitory effect of aerosolized commercial sanitizers against foodborne pathogens. J. Food Hyg. Saf. 2007;22:235–242. [Google Scholar]
- Marques SC, Rezende JGOS, Alves LAF, Silva BC, Alves E, Abreu LR, Piccoli RH. Formation of biofilms by Staphylococcus aureus on stainless steel and glass surfaces and its resistance to some selected chemical sanitizers. Braz. J. Microbiol. 2007;38:538–543. doi: 10.1590/S1517-83822007000300029. [DOI] [Google Scholar]
- Meira QGS, Barbosa IM, Athayde AJAA, Siqueira-Junior JP, Souza EL. Influence of temperature and surface kind on biofilm formation by Staphylococcus aureus from food-contact surfaces and sensitivity to sanitizers. Food Control. 2012;25:469–475. doi: 10.1016/j.foodcont.2011.11.030. [DOI] [Google Scholar]
- Nguyen HDN, Yuk H. Changes in resistance of Salmonella Typhimurium biofilms formed under various conditions to industrial sanitizers. Food Control. 2013;29:236–240. doi: 10.1016/j.foodcont.2012.06.006. [DOI] [Google Scholar]
- Oliveira MMM, Brugnera DF, Alves E, Piccoli RH. Biofilm formation by Listeria monocytogenes on stainless steel surface and biotransfer potential. Braz. J. Microbiol. 2009;41:97–106. doi: 10.1590/S1517-83822010000100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmez H, Temur SD. Effects of different sanitizing treatments on biofilms and attachment of Escherichia coli and Listeria monocytogenes on green leaf lettuce. LWT-Food Sci. Technol. 2010;43:964–970. doi: 10.1016/j.lwt.2010.02.005. [DOI] [Google Scholar]
- Pan Y, Breidt F, Kathariou S. Resistance of Listeria monocytogenes biofilms to sanitizing agents in a simulated food processing environment. Appl. Environ. Microb. 2006;72:7711–7717. doi: 10.1128/AEM.01065-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Cheon HL, Park KH, Chung MS, Choi SH, Ryu S, Kang DH. Inactivation of biofilm cells of foodborne pathogen by aerosolized sanitizers. International Journal of Food Microbiology. 2012;154:130–134. doi: 10.1016/j.ijfoodmicro.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Phillips CA. Bacterial biofilms in food processing environments: a review of recent developments in chemical and biological control. Int. J. Food Sci. Tech. 2016;51:1731–1743. doi: 10.1111/ijfs.13159. [DOI] [Google Scholar]
- Rodriguez A, Autio WR, Mclandsborugh LA. Effect of biofilm dryness on the transfer of Listeria monocytogenes biofilms grown on stainless steel to Bologna and Hard Salami. J. Food Protect. 2007;70:2480–2484. doi: 10.4315/0362-028X-70.11.2480. [DOI] [PubMed] [Google Scholar]
- Rose LJ, Donlan R, Banerjee SN, Arduino MJ. Survival of Yersinia pestis on environmental surfaces. Appl. Environ. Microb. 2003;69:2166–2171. doi: 10.1128/AEM.69.4.2166-2171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoering AL, Lewis K. Biofilms and Planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. Journal of Bacteriology. 2001;183:6746–6751. doi: 10.1128/JB.183.23.6746-6751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremoulet F, Duche O, Namane A, Martinie B, Labadie JC. A proteomic study of Escherichia coli O157:H7 NCTC 12900 cultivated in biofilm or in planktonic growth mode. FEMS Microbiology Letters. 2002;215:7–14. doi: 10.1016/S0378-1097(02)00879-0. [DOI] [PubMed] [Google Scholar]
- Vogel BF, Hansen LT, Mordhorst H, Gram L. The survival of Listeria monocytogenes during long term desiccation is facilitated by sodium chloride and organic material. International Journal of Food Microbiology. 2010;140:192–200. doi: 10.1016/j.ijfoodmicro.2010.03.035. [DOI] [PubMed] [Google Scholar]