Abstract
The fungal pathogen, Leptosphaeria maculans causes a severe and economically important disease to Brassica crops globally, well-known as blackleg. Besides, the anti-oxidative defense response of glucosinolates to fungal pathogens is widely established. Despite notable importance of glucosinolates in blackleg disease resistance the association of glucosinolate pathway genes in glucosinolate mediated defense response after L. maculans infection remains incompletely understood. The current study was designed to identify glucosinolate-biosynthesis specific genes among the eight selected candidates induced by L. maculans and associated alterations in glucosinolate profiles to explore their roles in blackleg resistance at the seedling stage of cabbage plants. The defense responses of four cabbage inbred lines, two resistant and two susceptible, were investigated using two L. maculans isolates, 03-02s and 00-100s. Pathogen-induced glucosinolate accumulation dynamically changed from two days after inoculation to four days after inoculation. In general, glucosinolate biosynthetic genes were induced at 24 h after inoculation and glucosinolate accumulation enhanced at two days after inoculation. An increase in either aliphatic (GIB, GRA) or indolic (GBS and MGBS) glucosinolates was associated with seedling resistance of cabbage. Pearson correlation showed the enhanced accumulation of MGBS, GBS, GIB, GIV and GRA after the inoculation of fungal isolates was associated with expression of specific genes. Principal component analysis separated two resistant cabbage lines—BN4098 and BN4303 from two susceptible cabbage lines—BN4059 and BN4072 for variable coefficients of disease scores, glucosinolate accumulation and expression levels of genes. Enhanced MGBS content against both fungal isolates, contributing to seedling resistance in two interactions—BN4098 × 03-02s and BN4303 × 00-100s and enhanced GBS content only in BN4098 × 03-02s interaction. Aliphatic GRA took part in resistance of BN4098 × 00-100s interaction whereas aliphatic GIB took part is resistance of BN4098 × 03-02s interaction. Aliphatic GIV accumulated upon BN4098 × 03-02s interaction but GSL-OH-Bol033373 and CYP81F2-Bol026044 showed enhanced expression in BN4303 × 03-02s interaction. The association between the selected candidate genes, corresponding glucosinolates, and seedling resistance broaden the horizon of glucosinolate conciliated defense against L. maculans in cabbage seedlings.
Keywords: blackleg disease, Leptosphaeria maculans, glucosinolates, seedling resistance, cabbage, expression analysis
Introduction
Blackleg is a devastating disease of Brassica crops that causes nearly one billion dollar crop losses every year, globally (Howlett, 2004; Fitt et al., 2006; Fitt et al., 2008). Leptosphaeria maculans, a hemi-biotrophic fungal pathogen, causes blackleg disease. Upon severe infestation of this pathogen, often complete loss of oilseed canola and rapeseed (Brassica napus) is reported (Li et al., 2003; Rouxel et al., 2003; Sprague et al., 2006). Blackleg disease is equally devastating to several subspecies of B. oleracea, including cabbage (Humpherson-Jones, 1985; Rico et al., 2001; Dilmaghani et al., 2010; Dilmaghani et al., 2013; Piliponyte-Dzikiene et al., 2015). Notably, this disease became epidemic in cabbage in Wisconsin, USA about a century ago (Henderson, 1918). Developing sustainable genetic resistance is the most suitable way of protecting Brassica germplasm from the devastation of blackleg disease despite the availability of chemical control methods (Del Rio and Ruud, 2013; Fraser et al., 2016; Koh et al., 2016; Potter et al., 2016).
In plants, a few secondary metabolites offer general resistance to insects and pathogens other than R-gene mediated race-specific resistance (Wink, 1988; Giamoustaris and Mithen, 1997; Tierens et al., 2001; Brader et al., 2006; Lattanzio et al., 2006; Evivie et al., 2019). Glucosinolates (GSLs) are the renowned secondary metabolites produced in Brassica crop plants which have pivotal importance in resistance of plants against pathogens and insects through their anti-oxidative properties (Hogge et al., 1988; Mithen et al., 1995; Benderoth et al., 2006; Hopkins et al., 2009). The Brassicaceae family members produce aliphatic, inodolic and aromatic glucosinolates (Fahey et al., 2001; Mithen, 2001; Bekaert et al., 2012). Breakdown products of these sulfur- and nitrogen-containing glucosinolates are primarily isothiocyanates and sulforaphane. These compounds upon the hydrolysis of endogenous myrosinase enzymes (β-thioglucoside glucohydrolases) become anti-oxidative and anti-fungal in plants (Chew, 1988; Giamoustaris and Mithen, 1995; Manici et al., 1997; Agerbirk et al., 1998; Brader et al., 2001; Tierens et al., 2001; Barth and Jander, 2006; Stotz et al., 2011; Calmes et al., 2015).
Despite general importance of glucosinolates against fungal pathogen, specific role and association between glucosinolate contents and resistance of the B. oleracea subspecies to various fungal pathogens remain obscure. A few studies, in nineties, reported no strong correlation between glucosinolates in different Brassica species and resistance to L. maculans (Mithen and Magrath, 1992; Sexton et al., 1999). Moreover, a negative association between glucosinolate contents and Alternaria infection in B. napus was reported (Doughty et al., 1991; Giamoustaris and Mithen, 1997). By contrast, Li et al. (1999a) in B. napus, reported a positive correlation between indolic glucosinolates induced upon the infection of Sclerotinia sclerotiorum and resistance of plants. In cabbage, inoculation of both resistant and susceptible plants with S. sclerotiorum and Mycosphaerella brassicicola revealed that both aliphatic and indolic glucosinolates raise in contents in the resistant lines (Abuyusuf et al., 2018a; Abuyusuf et al., 2018b). Furthermore, a recent study described possible mechanisms of overcoming glucosinolate–myrosinase–isothiocyanate defense system of Phoma lingam and Verticillium dahliae as these fungi species may degrade some primary glucosinolates to a less toxic level (Rahimi and Siamak, 2020). The apparent contradictions in literature may reflect the fungal lifestyles—e.g., necrotrophes and biotrophes (Sanchez-Vallet et al., 2010), type of hosts (Buxdorf et al., 2013), genetic identity of hosts—e.g., homozygous and heterozygous plant populations. In addition, against the L. maculans, majority of the earlier studies explored a relationship between total glucosinolates, rather than individual glucosinolate components, and resistance of plants (Mithen and Magrath, 1992; Giamoustaris and Mithen, 1997; Sexton et al., 1999; Kliebenstein et al., 2002).
By contrast, comparatively recent studies showed that upon infection of the hemibiotrophic fungus L. maculans in B. rapa both aliphatic and indolic glucosinolate compounds were induced (Abdel-Farid et al., 2010; Robin et al., 2017a). Similarly, in Brassicaceae family members, induced indolic glucosinolates exhibited association with resistance to both biotrophs and necrotrophs besides hemibiotrophs (Bednarek et al., 2009; Hiruma et al., 2013). Further, induced contents of 4-methoxy-glucobrassicin (MGBS) upon infection of fungal pathogens indicated that glucosinolate–myrosinase system has vital role in plant’s defense. In Arabidopsis, the expression of CYP81F2 gene, activated by myrosinase PEN2, was found to regulate the accumulation of 4-methoxy-glucobrassicin (Bednarek et al., 2009).
In our previous study with adult cabbage plants, L. maculans infection induced glucosinolate-biosynthesis genes in cabbage, with concomitant changes in individual glucosinolate contents. In resistant lines, both aliphatic and indolic glucosinolates are associated with resistance, with aliphatic glucoiberverin (GIV) and glucoerucin (GER) and indolic glucobrassicin (GBS) and MGBS glucosinolates particularly important (Robin et al., 2017a). This study also reported that resistance response of cabbage inbred lines differs between seedling stage and adult stage. Despite general belief that accumulation of glucosinolate may vary dynamically at different time intervals due to plant–microbe interactions, majority of the previous studies measured glucosinolate accumulation and the expression of glucosinolate biosynthesis genes at single time-point after inoculation of L. maculans at the seedling stage. The present study was, therefore, explored the expression profile of glucosinolate biosynthesis genes at three different time-points and measured glucosinolate accumulation at two different time-points after the inoculation of two isolates of L. maculans at the seedling stage. This study also explored a tri-angular association among expression levels of glucosinolate biosynthesis genes, accumulation of glucosinolate compounds and resistance response of plants to draw a conclusion. The results of the present study shed-light the existing contradictions in role of glucosinolates in glucosinolate-myrosinase system during L. maculans–host plants interaction.
Materials and Methods
Plant Materials and Growth Conditions
Seeds of four cabbage inbred lines were sown in 32-celled trays in a plant culture room. Two cabbage lines—BN4098 and BN4303 were reported to be resistant and two other lines—BN4059 and BN4072 were susceptible at the seedling stage (Robin et al., 2017b). The soil mixture used for sowing seeds was composed of peat moss, coco peat, perlite, zeolite, and vermiculite. Seedlings were allowed to grow at 20 ± 2°C temperature, 16:8 h day: night, 65% relative humidity and at a light intensity of ca. 400 μmol m−2 s−1 (Yi et al., 2015). Florescent light bulbs were used as the light source.
L. maculans Isolates and Seedling Inoculation
Hypersensitivity response of two fungal isolates used to inoculate cabbage inbred lines including culturing technique and methods of spore preparation have been published previously (Robin et al., 2017b). Two isolates of L. maculans, 03-02s (AvrLm1-4-6-7-11-J1-S, AvrLep1-2-3) and 00-100s (AvrLm2-3-6-9-J1-S, AvrLep1-2), were collected from Agriculture and Agri-Foods (AAFC), Saskatoon, Canada. Fungal isolates were cultured in 20% V8 medium for 10 days for spore preparation. After 10 days, spore suspension was prepared by overflowing 10 ml of sterile distilled water to each plate and scraping with a sterile microscope slide. To remove the mycelia and other debris, the spore suspension was filtered using sterile Miracloth (EMD Millipore Corporation, USA). The filtered spore suspension was concentrated before preparing a spore solution of 2 × 107 spores ml−1 concentration through dilution. Seedlings were inoculated twice with the prepared spores—at 10 and 26 days age of the plants. At 10 days age of the seedlings, 10 µl spores was inoculated at the center of each cotyledon of a seedling. At 26 days age of the seedlings, approximately four wounds were created per cm2 leaf area in all true leaves including cotyledons and 10 µl spores was inoculated per wound. There were six replicates in each experiment for control, mock-treatment (wounded only) and inoculation with 03-02s and 00-100s isolates.
Collection and Preparation of Leaf Samples for Analysis
Two separate sets of plants, grown simultaneously at the same plant culture room in the same growing conditions, were inoculated at 26 days old plants—one set is to collect samples to estimate relative expression levels of glucosinolate biosynthesis genes and another set is to measure glucosinolate accumulation. Samples of three biological replicates were kept for each analysis. In this study, we infected 26 days old plants when cotyledons were still alive. We inoculated both cotyledons and true leaves at the time of inoculation. For HPLC analysis, a large amount of samples are required—about 10 g for each biological replicate (Yi et al., 2016). If we infect only cotyledons of the 7–10 days old seedlings we need hundreds of plants to obtain that amount of samples. For this reason we allowed cabbage seedlings to grow 26 days before inoculation, so that sufficient samples can be obtained from the infected plants for the HPLC analysis.
Relative expression levels of genes were measured at 6, 24 and 48 h (2 dpi, days post inoculation) after inoculation in control, mock-treated and L. maculans inoculated plants. By contrast, glucosinolate accumulation was measure at 2 and 4 dpi in control, mock-treated and L. maculans inoculated plants. The samples collected for HPLC analysis and real-time PCR were flash-frozen in liquid nitrogen and immediately stored at −80°C.
Primer Design for Expression Analysis of Glucosinolate Biosynthesis Genes
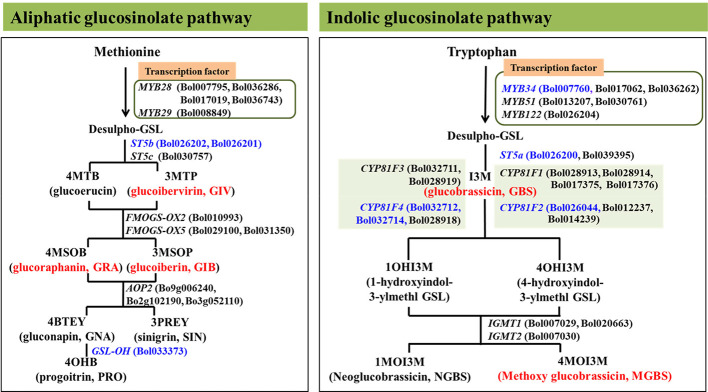
Eight genes involved in glucosinolate biosynthesis were selected for transcription analysis to determine how transcript levels are affected by pathogen inoculation (Table 1, Figure 1, Robin et al., 2017a). One of the gene encode indolic transcription factors, three are aliphatic biosynthesis genes and four are indolic biosynthesis genes (Robin et al., 2016; Yi et al., 2016). The efficiency of designed primers was tested according to Robin et al. (2016).
Table 1.
Primer sequences for glucosinolate-biosynthesis related genes used for relative expression analysis through qPCR in 26 days old plants inoculated with 03-02s and 00-100s isolates (Robin et al., 2016 and Yi et al., 2016).
| Gene Name | Accession Number | cDNA Size (bp) | Forward Primer Sequence | Reverse Primer Sequence | Product Size (bp) |
|---|---|---|---|---|---|
| Aliphatic biosynthesis-related genes | |||||
| ST5b | Bol026201 | 1,035 | CCGAGCCGTCAGAATTCAAG | GCTATGGCGAAAGTGAGAGC | 247 |
| Bol026202 | 1,035 | AAGCCTTGACTTTCGCCATC | ACTTCACAACTGAGTCCGGT | 204 | |
| GSL-OH | Bol033373 | 243 | GATTGTGCAAAAGGCTTGT | AGAGCATTAGGATTAGGAGGA | 188 |
| Indolic transcription factor-related gene | |||||
| MYB34 | Bol007760 | 843 | TGAAGGAGGATGGCGTACTC | CAGTTCGTCCCGCCAAATTA | 203 |
| Indolic biosynthesis-related genes | |||||
| ST5a | Bol026200 | 1,017 | GTCCGGTTGCAAGATGGTTT | CCTCTCCGGGTTCTCTTTGT | 214 |
| CYP81F4 | Bol032712 | 1,506 | CGGTGGAGGAGAAGGAGAAA | CTGACACATGGCTCGTAACG | 226 |
| Bol032714 | 960 | ACCCTGGTGAATACTTGCCA | GAAACACACTGAAGCAGAAC | 239 | |
| CYP81F2 | Bol026044 | 1,482 | TTCTCCCTACGTTACGGCTC | CTACGAACGGAGAGGAGTCC | 251 |
Figure 1.
Position of eight aliphatic and inodolic glucosinolate (GSL) biosynthetic genes analyzed in this study in glucosinolate biosynthesis pathway represented by blue colors. Red color represent the glucosinolates were altered in response to enhanced expression of the analyzed genes in this study (Robin et al., 2016; Robin et al., 2017a).
cDNA Synthesis and Real-Time Quantitative PCR Analysis
Total RNA of the collected samples was extracted using RNeasy mini kit, Catalog No. 74106, Qiagen, Valencia, CA, USA. PrimeScript-based kit (Takara Bio, Inc., Shiga, Japan) was used to synthesize cDNA from total RNA. iTaqTM SYBR® Green Super-mix was used with ROX (Bio-Rad, Hercules, CA, USA) to conduct quantitative RT-PCR (qPCR). A total reaction volume of 20 μl was prepared with PCR master mix 10 μl, ultra-pure water 7 μl, forward and reverse primers 2 μl and cDNA template (concentration of 60 ng μl−1) 1 μl for each reaction. PCR conditions were as follows: denaturation 10 min at 95°C, 40 cycles of denaturation 20 s at 95°C, annealing 20 s at 58°C, and amplification and elongation for 30 s at 72°C. Data were taped as fluorescence for each sample at the end of each of 40 cycles. Each biological replicate was repeated in three technical replicates. LightCycler96 software (Roche, Mannheim, Germany) was used for quantification cycle (Cq) analysis. Livak’s comparative 2−ΔΔCt method was used to quantify the relative transcription of each sample (Livak and Schmittgen, 2001). Three different actin genes were expressed in all inbred lines i.e. GenBank Accession Nos. AF044573 (Zhang et al., 2012), JQ435879 (Nawaz et al., 2014), and XM_013753106 (Lee et al., 2015), were used as a reference.
Measurements of Glucosinolate Contents
Desulfoglucosinolates were collected from three biological replicates of cotyledon samples for each of the control, mock-treated, and L. maculans-infected plants, via a modified HPLC protocol as previously described (Yi et al., 2015; Robin et al., 2016; Yi et al., 2016). Frozen samples stored at −80°C was treated with methyl alcohol and then ground to a fine powder. The powdered samples were preserved for 10 min at 70°C and then kept about 1 h at room temperature and centrifuged for 8 min at 10,000×g at 4°C to remove sediments (structural components and protein molecules). The supernatant fluid was carefully passed over an anion-exchange column. The action of centrifugation and anion-exchange chromatography was repeated two more times. The supernatant separated at the end of anion-exchange chromatography was treated as crude glucosinolate sample. For desulfation, this crude glucosinolates were used. In desulfation process, barium acetate (0.5 ml 50 mM) and lead acetate (0.5 ml 50 mM) were added with the crude glucosinolates and then centrifuged for 10 min at 2,000×g. The upper fluid was then passed through a pre-equilibrated (with 0.5 M sodium acetate) DEAE-Sephadex column. Desulfation was started by the addition of aryl sulfatase at 250 μl to the column and was permitted to run for 16 h. The desulfated glucosinolates were then eluted with the help of 1 ml distilled water. The eluted solutions were purified by centrifugation at 20,000×g for 4 min at 4°C and filtration by a PTFE filter (13 mm, 0.2 μm, Advantec, Pleasanton, CA, USA). The purified solutions were then allowed to HPLC analysis. Individual glucosinolate compounds were determined by a PDA 996 UV-visible detector (Waters) at of 229 nm wavelength. For quantification of the detected glucosinolates, a standard curve was used which was prepared from commercial sinigrin (SIN). Mass spectrometry analysis (HPLC/MS, Agilent 1,200 series, Agilent Technologies) was done to identify individual glucosinolate component (Yi et al., 2016; Abuyusuf et al., 2018a).
Statistical Analysis
A two-way analysis of variance was conducted to test the statistical significance among genotypes (cabbage lines), treatments and genotypes x treatments using Minitab 18 statistical software (Minitab Inc., State College, PA, USA). A posthoc Tukey’s pairwise comparison was conducted to explore statistical significance among treatment, genotypes and interactions. Test statistic, degrees of freedom, and p-values of statistical significance for relative expression of biosynthesis genes and glucosinolate contents are given in Tables S1 and S2, respectively.
Pearson correlation coefficient was estimated between glucosinolate content of each category—(aliphatic and indolic) and expression of biosynthesis genes under each category. Principal component analysis (PCA) was conducted taking disease scores of four genotypes, expression levels of genes and glucosinolate contents of control plus mock, 03-02s and 00-100s treatments at 2 days after inoculation (d2).
Results
Resistance of Selected Cabbage Lines to L. maculans Infection at Seedling Stage
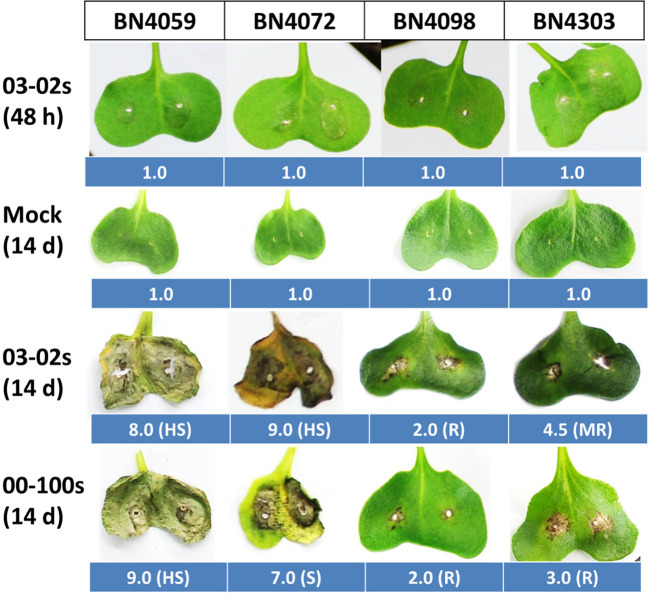
Inoculated cotyledon of four cabbage plants resulted in both hypersensitive and susceptible disease reactions. The cabbage line BN4098 showed complete resistance against both isolates, 03-02s and 00-100s, with the lowest median value of visual score (Table 2, Figure 2). BN4303 showed moderate resistance against isolate 03-02s and resistance against 00-100s (Table 2, Figure 2). Other two genotypes BN4059 and BN4072 showed susceptible disease reactions against both isolates with a high median score between 7 and 9 (Table 2, Figure 2).
Table 2.
Resistance scoring of four cabbage lines at seedling stage against two L. maculans isolates ‘03-02s’ and ‘00-100s’ 14-day post-inoculation.
| Line | L. maculans isolates | |||||
|---|---|---|---|---|---|---|
| 03-02s | 00-100s | |||||
| Score | Score range | Interaction | Score | Score range | Interaction | |
| BN4059 | 8.0 | 7—9 | HS | 9.0 | 8—9 | HS |
| BN4072 | 9.0 | 6—8 | HS | 7.0 | 6—8 | S |
| BN4098 | 2.0 | 1—3 | R | 2.0 | 2—3 | R |
| BN4303 | 4.5 | 3—5 | MR | 3.0 | 2—4 | R |
Each score is the median of ten observations. R, resistant; MR, moderately resistant; S, susceptible and HS, highly susceptible.
Figure 2.
Cotyledon infection symptoms in four cabbage lines BN4059, BN4072, BN4098 and BN4303 at 14 days after inoculation in response to two isolates of Leptosphaeria maculans 03-02s and 00-100s. Center of each cotyledon was inoculated with 10 µl spores with a concentration of 2.0 × 107spores µl−1. Infected leaves were scored at 14 days after inoculation on a 1–9 visual scoring scale where a lower score represents less infection and a higher score represents severe infection. Values adjacent to each cotyledon represent median score of ten observations. BN4059 and BN4072 are the susceptible lines whereas BN4098 and BN4303 are the resistant lines (Robin et al., 2017b). R, resistant; MR, moderately resistant; S, susceptible and HS, highly susceptible.
Expression Changes in Aliphatic Glucosinolate Biosynthesis Genes in Cabbage Lines With L. maculans Inoculation
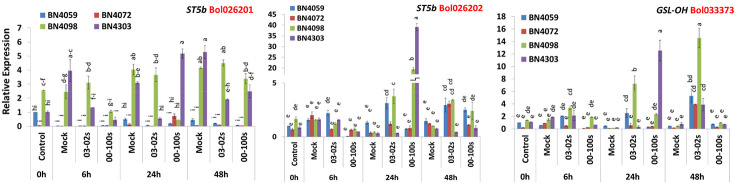
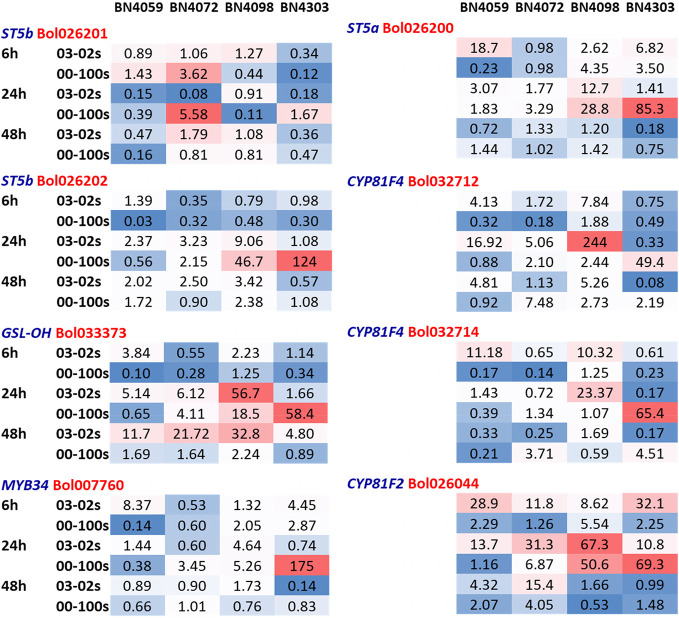
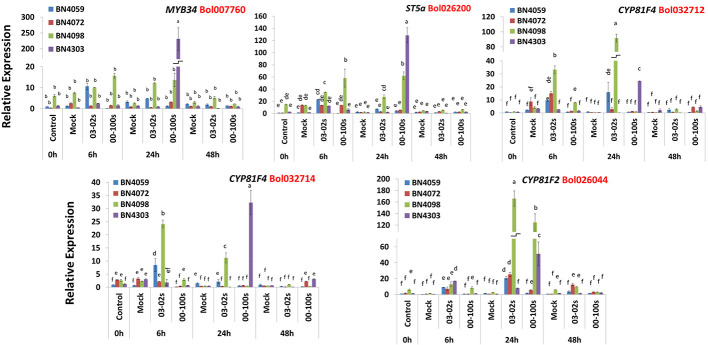
Three genes related to aliphatic glucosinolate biosynthesis—ST5b accessions Bol026201 and Bol026202, GSL-OH accession Bol033373 showed variable expression in four cabbage lines, time-points and treatments (Figure 3, Table S1). Expression level of Bol026201 gene was consistently lower in two susceptible lines BN4059 and BN4072 compared to two resistant lines BN4098 and BN4303 (Figure 3). The Bol026201 gene significantly exhibited 1.27-fold increase in expression and 0.18-fold decrease in expression in resistant line BN4303 at 24 h after inoculation with 00-100s and 03-02s isolates, respectively, compared to respective mock-treated plants (Figure 3). In the same line, this gene accounted for decreased expression by 0.36 and 0.47-fold at 48 h after inoculation with 03-02s and 00-100s, respectively, compared to mock-treated plants (Figures 3 and 4). The Bol026201 gene showed 0.11-fold decrease in expression in another resistant line BN4098 at 24 h after inoculation with 00-100s compared to mock-treated plants (Figures 3 and 4). The ST5b-Bol026202 gene showed 2.37-fold increase in expression in susceptible line BN4059 at 24 h after inoculation with 03-02s compared to mock-treated plants (Figures 3 and 4). This gene exhibited 9.06- and 46.7-fold increase in expression in resistant line BN4098 in response to 03-02s and 00-100s isolates, respectively, at 24 h after inoculation compared to respective mock-treated plants (Figures 3 and 4). Strikingly, the gene accounted for 124-fold increase in expression upon infection of 00-100s isolate in another resistant line BN4303 at 24 h after inoculation compared to mock-treated plants (Figures 3 and 4). ST5b-Bol026202 gene also showed 2.02-, 2.5- and 3.42-fold increase in expression at 48 h after inoculation with 03-02s isolate in cabbage lines BN4059, BN4072 and BN4098, respectively compared to respective mock-treated plants (Figures 3 and 4). GSL-OH accession Bol033373 showed 2.23-fold increase in expression in BN4098 at 6 h after inoculation with 03-02s isolate compared to mock-treated samples (Figures 3 and 4). This gene exhibited 5.4- and 56.7-fold upregulation in susceptible line BN4059 and resistant line BN4098, respectively; at 24 h after inoculation with 03-02s isolate compared to mock-treated samples (Figures 3 and 4). The Bol033373 gene also showed 11.7-, 21.7-, 32.8- and 4.8-fold increase in expression at 48 h after inoculation with 03-02s isolate in BN4059, BN4072, BN4098 and BN4303 cabbage lines, respectively compared to mock-treated samples (Figures 3 and 4).
Figure 3.
Expression levels of three aliphatic glucosinolate biosynthesis genes in leaf samples from cabbage lines. The lines BN4059 and BN4072 are susceptible and BN4098 and BN4303 are resistant to Leptosphaeria maculans (03-02s and 00-100s) inoculation at the seedling stage. Vertical bars represent standard error. Different letters indicate statistically significant differences between genotype × treatment combinations. Red letters represent gene accessions.
Figure 4.
Heatmap showing fold-changes in relative expression of glucosinolate biosynthesis genes compared to respective mock treatment at each timepoint-isolate combination. The cabbage lines BN4059 and BN4072 are susceptible and BN4098 and BN4303 are resistant to Leptosphaeria maculans (03-02s and 00-100s) inoculation at the seedling stage. ST5b and GSL-OH are aliphatic glucosinolate biosynthesis genes. MYB34 is an indolic transcription factor. ST5a and CYP81F are the indolic biosynthesis genes.
Expression Changes in Indolic Glucosinolate Biosynthesis Genes in Cabbage Lines With L. maculans Inoculation
Like aliphatic glucosinolate biosynthesis genes, the indolic glucosinolate biosynthesis genes also showed genotypic, treatment and genotype × treatment interaction (Table S1). The Bol007760 accession of MYB34 transcription factor exhibited 175-fold higher expression in resistant line BN4303 compared to mock-treated samples at 24 h after inoculation with 00-100s isolate compared to mock-treated plants (Figures 4 and 5). The ST5a-Bol026200 gene accounted for 18.7-fold increase in expression in susceptible line BN4059 at 6 h after inoculation with 03-02s isolate compared to mock-treated samples (Figures 4 and 5). In resistant line BN4098, this gene showed 2.62- and 4.35-fold enhanced expression compared to mock-treated plants at 6 h after inoculation with 03-02s and 00-100s isolates, respectively (Figures 4 and 5). In the same line, BN4098, the gene exhibited 12.7- and 28.8-fold increase in expression at 24 h after inoculation with 03-02s and 00-100s isolates, respectively compared to mock-treated samples (Figures 4 and 5). In another resistant line, BN4303 this gene showed 85.3-fold increase in expression at 24 h after inoculation with 00-100s isolate compared to respective mock-treated plants (Figures 4 and 5). CYP81F4-Bol032712, exhibited 7.84- and 1.88-fold increase in expression in response to 03-02s and 00-100s isolates, respectively, at 6 h after inoculation compared to mock-treated plants (Figures 4 and 5). At 24 h after inoculation with 03-02s isolate, the susceptible line BN4059 showed 16.9-fold increase in expression compared to mock-treated plants (Figures 4 and 5). This gene exhibited strikingly higher expression, 244-fold in resistant line BN4098 and 49.4-fold in another resistant line BN4303, at 24 h after inoculation with 03-02s and 00-100s isolates, respectively compared to mock-treated plants (Figures 4 and 5). CYP81F4- Bol032714 gene showed 11.2- and 10.3-fold increase in expression in BN4059 and BN4098 cabbage lines at 6 h after inoculation with 03-02s isolate compared to mock-treated samples (Figures 4 and 5). In resistant cabbage line BN4098, the gene accounted for 23.4-fold increase in expression at 24 h after inoculation with 03-02s isolate compared to mock-treated samples (Figures 4 and 5). In another resistant line BN4303, this gene showed 65.4-fold induced expression in response to 00-100s isolate compared to mock-treated plants (Figures 4 and 5). This gene also showed 3.71- and 4.51-fold increase in expression in BN4072 and BN4303 cabbage lines, respectively at 48 h after inoculation with 00-100s isolate compared to mock-treated plants (Figures 4 and 5). CYP81F2-Bol026044 gene showed 28.9-, 11.8-, 8.6- and 32.1-fold increase in expression in response to 03-02s isolate in cabbage lines BN4059, BN4072, BN4098 and BN4303, respectively, at 6 h after inoculation compared to mock-treated samples (Figures 4 and 5). In resistant line BN4098, this gene also showed 5.54-fold increase in expression at 6 h after inoculation with 00-100s isolate compared to mock-treated plants (Figures 4 and 5). This gene showed increase in expression at 24 h after infection in two resistant lines only. CYP81F2- Bol026044 accounted for 50.6- and 69.3-fold increase in expression in resistant lines BN4098 and BN4303, respectively at 24 h after inoculation with 00-100s isolate compared to mock-treated samples (Figures 4 and 5). This gene also showed 31.3- and 67.3-fold increase in expression at 24 h after inoculation with 03-02s isolate in BN4072 and BN4098 lines, respectively compared to mock-treated plants (Figures 4 and 5).
Figure 5.
Expression levels of three indolic glucosinolate biosynthesis genes in leaf samples from cabbage lines. The cabbage lines BN4059 and BN4072 are susceptible and BN4098 and BN4303 are resistant to Leptosphaeria maculans (03-02s and 00-100s) inoculation at the seedling stage. Vertical bars represent standard error. Different letters indicate statistically significant differences between genotype × treatment combinations. Red letters represent gene accessions.
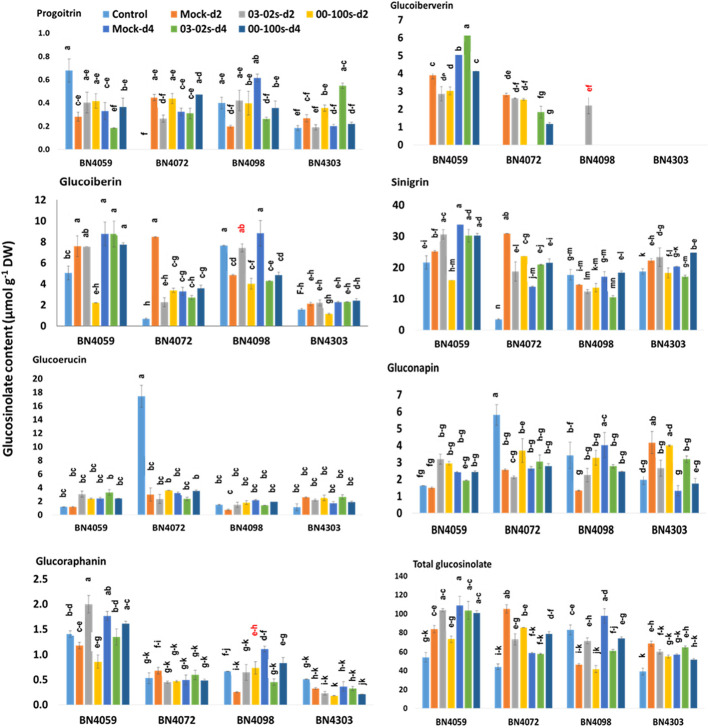
Glucosinolate Accumulation Differs in Cabbage Inbred Lines
In untreated plants, contents of aliphatic, indolic and total glucosinolates differed among four cabbage lines (Figures 6 and 7, Table S2). Constitutively, the susceptible cabbage line BN4059 recorded the highest levels of aliphatic progoitrin (PRO), glucoiberverin (GIV), sinigrin (SIN), glucoraphanin (GRA) and indolic neoglucobrassicin (NGBS) (Figures 6 and 7). Another susceptible line BN4072, estimated the highest contents of aliphatic glucoerucin (GER) and gluconapin (GNA) (Figure 6). By contrast, the resistant cabbage line BN4098 accumulated the highest levels of aliphatic glucoiberin (GIB), total glucosinolate and indolic glucobrassicin (GBS), 4-methoxyglucobrassicin (MGBS) and 4-hydroxy-glucobrassicin (HGBS) (Figures 6 and 7). The untreated control plants of cabbage line BN4072 detected no PRO, GIV and MGBS but this cabbage line accumulated 14-fold higher content of GER compared to average of other three lines (Figures 6 and 7). Two resistant lines BN4098 and BN4303 also detected no GIV in untreated control samples (Figure 6).
Figure 6.
Aliphatic and total glucosinolate contents of leaf samples from four cabbage lines altered due to Leptosphaeria maculans at day 2 (d2) and day 4 (d4) after inoculation. The cabbage lines BN4059 and BN4072 are susceptible and BN4098 and BN4303 are resistant to L. maculans (03-02s and 00-100s) inoculation at the seedling stage. The mean of three biological replicates is presented. Vertical bars represent standard error. Different letters indicate statistically significant differences between genotype × treatment combinations. Red letters indicate increased glucosinolate content in resistant lines in response to L. maculans infection compared to mock treatment.
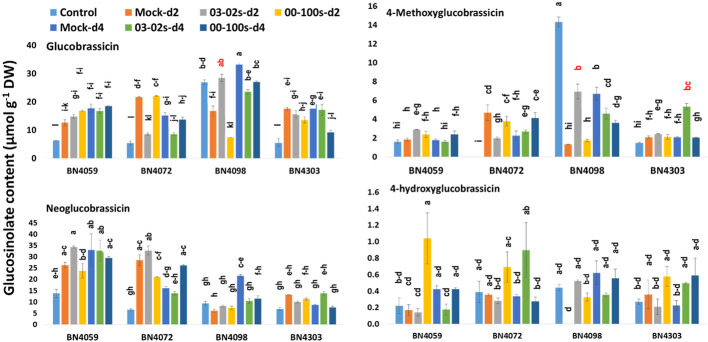
Figure 7.
Indolic glucosinolate contents of leaf samples from four cabbage lines altered due to Leptosphaeria maculans at day 2 (d2) and day 4 (d4) after inoculation. The cabbage lines BN4059 and BN4072 are susceptible and BN4098 and BN4303 are resistant to L. maculans (03-02s and 00-100s) inoculation at the seedling stage. The mean of three biological replicates is presented. Vertical bars represent standard error. Different letters indicate statistically significant differences between genotype × treatment combinations. Red letters indicate increased glucosinolate content in resistant lines in response to L. maculans infection compared to mock treatment.
Altered Glucosinolate Biosynthesis in Cabbage Lines With L. maculans Inoculation
Leaf inoculation of resistant and susceptible cabbage lines, with either of two L. maculans fungal isolates responsible for blackleg disease, altered accumulation of both total glucosinolate and individual glucosinolate components in the leaf samples. The content of aliphatic PRO decreased by 0.42-fold in resistant cabbage line BN4098 but that increased by 2.73-fold in another resistant line BN4303 compared to respective mock-treated samples at four days after inoculation (d4) with 03-02s isolate (Figure 6). The content of GIV showed inconsistent pattern of accumulation in four cabbage lines. The resistant line BN4303 detected GIV neither in treated nor in non-treated samples (Figure 6). Another resistant line BN4098 detected 2.2 µmol g−1 DW of GIV only in the treated plants at two days after inoculation (d2) with 03-02s isolate (Figure 6). In susceptible cabbage line BN4059, the content of GIV decreased by 0.77- and 0.78-fold at both d2 and d4, respectively, after inoculation with 00-100s isolate compared to mock-treated samples (Figure 6). In the same line, the content of GIV decreased by 0.73-fold at d2 but increased by 1.22-fold at d4 after inoculation with 03-02s isolate compared to mock-treated samples (Figure 6). In another susceptible line BN4072, the contents of GIV were 1.83 and 1.18 µmol g−1 DW at d4 after inoculation with 03-02s and 00-100s isolates, respectively, whereas both mock-treated plants at d4 and untreated control plants detected no GIV (Figure 6). The content of GIB in susceptible lines decreased by 0.29-, 0.4- and 0.4- fold in BN4059 × 00-100s, BN4072 × 03-02s and BN4072 × 00-100s interactions, respectively at d2 compared to mock-treated plants (Figure 6). In resistant line BN4098, content of GIB increased by 1.53-fold at d2 in response to 03-02s compared to mock-treated plants but that was decreased by 0.48- and 0.55-fold at d4 after inoculation with 03-02s and 00-100s isolates, respectively (Figure 6). The content of SIN decreased in two susceptible lines in response to fungal inoculation. In cabbage line BN4059, the content of SIN decreased by 0.63-fold at d2 in response to 00-100s compared to mock-treated plants (Figure 6). SIN content in cabbage line BN4072 decreased by 0.61- and 0.77-fold at d2 in response to 03-02s and 00-100s isolates, respectively, compared to mock-treated plants (Figure 6). The content of GRA was generally higher in susceptible line BN4059 at both d2 and d4 in response to both treatments (Figure 6). In cabbage line BN4059, the content of GRA increased by 1.7-fold at d2 after inoculation with 03-02s isolate compared to mock-treated plants (Figure 6). In resistant line BN4098, the content of GRA increased by 2.93-fold at d2 and decreased by 0.4-fold at d4 after inoculation with 00-100s and 03-02s isolates, respectively, compared to mock-treated plants (Figure 6).
Among the indolic glucosinolates, the content of GBS increased by 1.7-fold in resistant line BN4098 at d2 in response to 03-02s compared to mock-treated plants. In the same cabbage line, GBS accumulation decreased by 0.71- and 0.81-fold at d4 after inoculation with 03-02s and 00-100s, respectively, compared to mock-treated plants (Figure 7). In another resistant cabbage line BN4303, GBS accumulation decreased by 0.48-fold at d4 after inoculation with 00-100s compared to mock-treated plants (Figure 7). In susceptible cabbage line BN4072, the content of GBS decreased by 0.4- and 0.57-fold at d2 and d4, respectively, after inoculation with 03-02s isolate (Figure 7). The content of MGBS increased strikingly by 5.27-fold in resistant cabbage line BN4098 at d2 after inoculation with 03-02s isolate compared to mock-treated plants (Figure 7). In the same line, MGBS accumulation decreased by 0.69- and 0.54-fold at d4 after inoculation with 03-02s and 00-100s isolates, respectively, compared to mock-treated plants (Figure 7). MGBS accumulation also increased by 2.56-fold in another resistant cabbage line BN4303 at d4 after inoculation with 00-100s isolate (Figure 7). In the susceptible cabbage line BN4072, the content of MGBS decreased by 0.42-fold at d2 after inoculation with 03-02s isolate and increased by 1.84-fold in response to 00-100s isolate compared to mock-treated plants (Figure 7). Another indolic glucosinolate, NGBS showed increased accumulation by 1.63-fold at d4 after inoculation with 00-100s compared to mock-treated plants (Figure 7). NGBS accumulation decreased by 0.48- and 0.53-fold at d4 after inoculation with 03-02s and 00-100s, respectively compared to mock-treated plants (Figure 7). The content of HGBS showed increase in accumulation only in cabbage line BN4059 at d2 after inoculation with 03-02s isolate compared to mock-treated plants (Figure 7).
In brief, the contents of both aliphatic and indolic glucosinolates induced in two resistant cabbage inbred lines upon infection of two L. maculans isolates (Figures 6 and 7). Contents of GIB and GIV induced in BN4098 × 03-02s interaction whereas GRA was induced in BN4098 × 00-100s interaction (Figure 6, Table 3). MGBS accumulation induced in both BN4303 and BN4098 but the GBS accumulation induced only in BN4098 cabbage line (Table 3).
Table 3.
Association between enhanced expressions of glucosinolate (GSL) pathway genes, enhanced glucosinolate accumulation in resistance disease interactions.
| BN4098 × 03-02s(Resistant interaction) | BN4098 × 00-100s(Resistant interaction) | BN4303 × 03-02s(Moderately resistant interaction) | BN4303 × 00-100s(Resistant interaction) | ||
|---|---|---|---|---|---|
| Aliphatic | Gene | (ST5b-Bol026202)(9.06-fold, d1) (GSL-OH-Bol033373)(56.7-fold, d1) |
(ST5b-Bol026202)(46.7-fold, d1) (GSL-OH-Bol033373)(18.5-fold, d1) |
GSL-OH-Bol033373(4.8 fold, d2) | (ST5b-Bol026202)(124-fold, d1) (GSL-OH-Bol033373)(58.4-fold, d1) |
| GSL | GIB (1.53-fold, d2) GIV (2.2 µmol g−1 DW, d2) |
GRA (2.93-fold, d2) | |||
| Indolic | Gene |
ST5a-Bol026200(12.7-fold, d1) CYP81F4-Bol032712(244-fold, d1) CYP81F2-Bol026044(67.3-fold, d1) |
ST5a-Bol026200(28.8-fold, d1) CYP81F4-Bol032712(1.88-fold, 6h) CYP81F2-Bol026044(50.6-fold, d1) |
CYP81F2-Bol026044(32.1-fold, 6h) |
MYB34-Bol007760(175-fold, d1) ST5a-Bol026200(85.3-fold, d1) CYP81F4-Bol032712(49.4-fold, d1) CYP81F2-Bol026044(69.3-fold, d1) |
| GSL | GBS (1.7-fold, d2)MGBS (5.27-fold, d2) | MGBS (2.56-fold, d4) |
d1, d2 and d4; one, two and four days after inoculation. In general, transcript levels increased at d1 and glucosinolate accumulation induced at d2 in resistant lines upon L. maculans inoculation. Blue letters highlight fold-changes and red letter represent days after inoculation.
Association Between Expression Levels of Pathway Genes and Accumulation of Glucosinolates Considering Resistance Response of Plants
Pearson correlation analysis revealed that ST5b-Bol026201 gene showed negative correlation with contents of aliphatic GRA, GIV and GER but ST5b-Bol026202 gene showed positive correlation with contents of GIB, GRA and GIV (Table 4A). Expression analysis revealed that ST5b-Bol026202 exhibited strikingly higher expression at 24 h after inoculation with 00-100s isolate in both resistant lines (46.7-fold in BN4098 and 124-fold in BN4303) compared to mock-treated samples (Figure 3). Simultaneously, the content of GRA, among these three aliphatic glucosinolates, increased by 2.93-fold in resistant line BN4098 at d2 after inoculation with 00-100s isolate (Figure 6). The other aliphatic gene GSL-OH accession Bol033373 showed positive correlation only with the GIB content (Table 4A). The expression levels of this GSL-OH gene was highly increased in the resistant line BN4098 by 56.7- and 32.8-fold at 24 h and 48 h, respectively, after inoculation with 03-02s isolate compared to mock-treated samples (Figure 3). Concurrently, aliphatic GIB accumulation was increased only in the resistant line BN4098 by 1.53-fold at d2 in response to 03-02s isolate compared to mock-treated samples (Figure 6).
Table 4.
Pearson correlation coefficient between glucosinolate contents (aliphatic and indolic) and relative expression level of genes.
| A) Aliphatic Glucosinolates | |||||
|---|---|---|---|---|---|
| ST5b-Bol026201 | ST5b-Bol026202 | GSL-OH-Bol033373 | |||
| Glucoiberin | 0.018 0.901 |
0.361 0.012 |
0.303 0.036 |
||
| Progoitrin | −0.044 0.766 |
0.237 0.104 |
0.125 0.397 |
||
| Glucoraphanin |
−0.383 0.007 |
0.380 0.008 |
0.133 0.369 |
||
| Sinigrin | −0.254 0.082 |
−0.038 0.797 |
−0.092 0.533 |
||
| Gluconapin | −0.020 0.893 |
−0.199 0.175 |
−0.160 0.279 |
||
| Glucoiberverin |
−0.506 <0.001 |
0.531 <0.001 |
0.207 0.158 |
||
| Glucoerucin |
−0.305 0.035 |
−0.219 0.134 |
−0.156 0.291 |
||
| B) Indolic Glucosinolates | |||||
| MYB34-Bol007760 | ST5a-Bol026200 | CYP81F4-Bol032712 | CYP81F4-Bol032714 | CYP81F2-Bol026044 | |
| 4-hydroxyglucobrassicin | 0.013 0.932 |
0.076 0.610 |
0.264 0.070 |
0.220 0.134 |
−0.043 0.771 |
| Glucobrassicin |
0.613 <0.001 |
0.483 0.001 |
0.304 0.036 |
0.083 0.577 |
0.270 0.063 |
| 4-Methoxyglucobrassicin |
0.783 <0.001 |
0.838 <0.001 |
0.079 0.594 |
0.276 0.058 |
0.319 0.027 |
| Neoglucobrassicin | −0.233 0.112 |
−0.287 0.048 |
0.004 0.980 |
−0.372 0.009 |
0.116 0.432 |
Bold letters represent significant correlation. Cell Contents: Pearson correlation and P-Value.
GBS accumulation was positively correlated with expression of MYB34-Bol007760, ST5a-Bol026200 and CYP81F4-Bol032712 (Table 4B). While the content of GBS increased by 1.7-fold in resistant line BN4098 at d2 after inoculation with 03-02s compared to mock-treated plants, the expression level of CYP81F4-Bol032712 gene increased by 244-fold in BN4098 × 03-02s at 24 h after inoculation (Figures 5 and 7). Expression levels of MYB34-Bol007760, ST5a-Bol026200 and CYP81F2-Bol026044 were positively associated with MGBS accumulation (Table 4B). The expression levels of MYB34-Bol007760, ST5a-Bol026200 and CYP81F2-Bol026044 genes increased by 175-, 85.3- and 69.3-fold, respectively, in resistant cabbage line BN4303 at 24 h after inoculation with 00-100s isolate compared to mock-treated plants (Figure 5). Expression of CYP81F2-Bol026044 gene also increased by 67.3-fold in another resistant line BN4098 at 24 h after inoculation with 03-02s isolate compared to mock-treated plants (Figure 5). Simultaneously, the content of MGBS increased by 5.27- and 2.56-fold in BN4098 × 03-02s interaction at d2 and BN4303 × 00-100s interaction at d4, respectively, compared to mock-treated plants (Figure 7). Expression levels of ST5a-Bol026200 and CYP81F4-Bol032714 were negatively correlated with contents of NGBS (Table 4B).
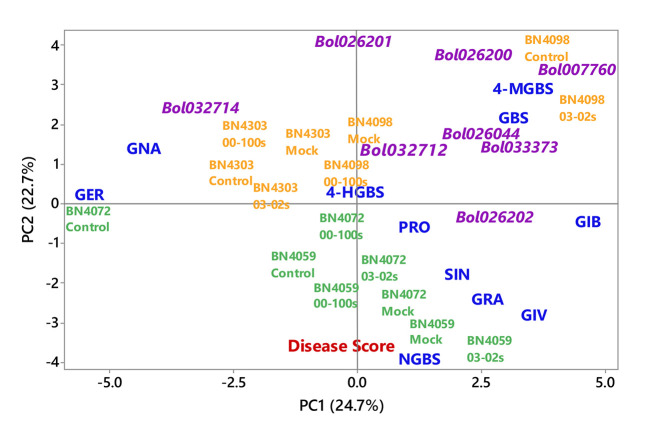
First two principal components, PC1 and PC2, explained 24.7 and 22.7% data variation, respectively. PC1 separated the contents of MGBS, GBS, GIB, GIV and GRA for their positive coefficients from the contents of GER and GNA for their negative coefficients (Figure 8). The enhanced accumulation of MGBS, GBS, GIB, GIV and GRA after the inoculation of fungal isolates was the feature of resistant cabbage lines (Figures 6 and 7). PC1 also separated the expression levels of MYB34-Bol007760, ST5a-Bol026200, CYP81F4-Bol032712, CYP81F2-Bol026044, GSL-OH-Bol033373 and ST5b-Bol026202 for their positive coefficients from the expression levels of ST5b-Bol026201 and CYP81F4-Bol032714 for their negative coefficients (Figure 8). Increased expression of MYB34-Bol007760, ST5a-Bol026200, CYP81F4-Bol032712, CYP81F2-Bol026044, GSL-OH- Bol033373 and ST5b-Bol026202 genes enhanced accumulation of indolic and aliphatic glucosinolates in the resistant cabbage lines BN4098 and BN4303 (Tables 4A, B, Figures 6 and 7). PC2 separated two susceptible lines BN4059 and BN4072 for their high sensitivity to blackleg disease (disease scores) from two resistant lines BN4098 and BN4303 for their comparatively lower disease scores (Figure 8).
Figure 8.
Biplot of individual glucosinolate contents of leaf samples (blue color), disease scoring index (red color) and relative expression of glucosinolate biosynthesis genes (magenta color) from four cabbage lines altered due to Leptosphaeria maculans inoculation at two days after inoculation. The cabbage lines BN4059 and BN4072 are susceptible (green color) and BN4098 and BN4303 are resistant (orange colour) to L. maculans (03-02s and 00-100s) inoculation at the seedling stage. Glucosinolate (GSL) components: GER, glucoerucin; GNA, gluconapin; GIB, glucoiberin; PRO, progoitrin; GRA, glucoraphanin; SIN, sinigrin; GIV, glucoiberverin; GBS, glucobrassicin; 4-MGBS, 4-methoxyglucobrassicin; NGBS, neoglucobrassicin; 4-HGBS, 4-hydroxyglucobrassicin.
Principal component analysis showed a strong association between induced glucosinolate accumulation, expression level of genes and blackleg disease response indicating that pathogen-induced glucosinolate accumulation has a strong influence on blackleg disease resistance in cabbage plants (Figure 8). Glucosinolate contents and expression levels of glucosinolate biosynthetic genes changed dramatically in response to two L. maculans isolates 03-02s and 00-100s in two resistant lines (Table 3). Alteration of glucosinolate accumulation and expression levels of biosynthesis genes occurred concomitantly in two resistant cabbage inbred lines BN4098 and BN4303 (Table 3). Most of the glucosinolate biosynthetic genes were highly transcribed at 24 h after inoculation of L. maculans isolates (Table 3). As a resultant effect the contents of glucosinolates were significantly changed in resistant lines at two days after inoculation, in most cases (Table 3). Only the contents of MGBS showed increase in accumulation at d4 after inoculation in BN4303 × 00-100s interaction (Figure 7).
Discussion
Glucosinolate Accumulation and Expression of Relevant Genes Were Associated With Blackleg Disease Resistance at the Seedling Stage
In our previous study we measured glucosinolate accumulation only at one time-point—at d4 after inoculation (Robin et al., 2017a) but here we have found that glucosinolate accumulation dynamically changed between two and four days after L. maculans infection (Figures 6 and 7). The selection of single time-point (d4) in Robin et al. (2017a) was based on some previous studies that looked at effect of exogenous application of methyl jasmonate and salicylic acid on glucosinolate accumulation (Ku et al., 2013a; Ku et al., 2013b; Yi et al., 2016). Induced glucosinolate accumulation at two days after inoculation of L. maculans indicated an immediate response of inoculated plants to tackle the disease. More interestingly, the major changes in pathogen-induced glucosinolate accumulation were followed by increasing transcript accumulation at the previous day (Table 3).
Moreover, an increase of a particular glucosinolate at a single time-point was not associated with resistance. In general, accumulation of individual glucosinolate components decreased in susceptible cabbage lines—BN4059 and BN4072 except a few cases (Figures 6 and 7). GIV in BN4059 × 03-02s at d4, GRA of in BN4059 × 03-02s at d2 and MGBS in BN4072 × 00-100s in d2 showed increase in contents in response to pathogen inoculation but their contents decreased in other time-points (Figures 6 and 7). This disparity in susceptible lines and pathogen-induced accumulation in resistant lines indicated that resistance of cabbage inbred lines to L. maculans follow genotype- and isolate-specific manner.
Pathogen-Induced Glucosinolate Accumulation and Hypersensitivity Response to L. maculans Differ Between Seedling and Adult Stages of Cabbage Plants
In adult cabbage plants, the contents of aliphatic GER and indolic NGBS were induced in resistant cabbage lines in addition to GIV, GBS and MGBS but aliphatic GRA was not induced (Robin et al., 2017a). These results indicated that glucosinolate mediated resistance in cabbage plants differs between seedling and adult stages. In consistence with variable accumulation of individual glucosinolate compound between seedling and adult stages, the hypersensitivity response also differed. The cabbage inbred line BN4098 showed susceptibility reaction against 03-02s isolate and moderately resistance against 00-100s isolate at the adult stage but in this study this line showed complete resistance at the seedling stage (Figure 2, Robin et al., 2017a). Another resistant line BN4303 exhibited moderate resistance against 00-100s at the seedling stage but that interaction was complete resistance at the adult stage (Figure 2, Robin et al., 2017a). The disparity in hypersensitivity response between seedling stage and adult stage against blackleg disease is known for quite a long since these two stages have different genetic control (Ballinger and Salisbury, 1996; Larkan et al., 2016) and the resistance of adult plants may not be race-specific (Rimmer, 2006). Moreover, recent findings believe that hypersensitivity response of plants requires complex interaction between proteins related to innate immunity in plants and those related to glucosinolate biosynthesis (Nintemann et al., 2017).
Accumulation of GIV and Other Aliphatic Glucosinolates Differs in Resistant and Susceptible Lines
In 3-month old cabbage plants accumulation of GIV induced in BN4303 in response to both 03-02s and 00-100s isolates at d4 after inoculation and two resistant lines accumulated GIV in both treated and non-treated samples (Robin et al., 2017a). In this study, the content of GIV showed inconsistent pattern of accumulation among the tested cabbage inbred lines. No GIV was detected in two resistant plants, except BN4098 × 03-02s at d2, whereas two susceptible lines accumulated abundant GIV in both treated and non-treated plants (Figure 6). This results supported the fact that glucosinolate mediated resistance in cabbage plants differs between seedling and adult stages. In recent studies, resistant cabbage plants inoculated with S. sclerotiorum and M. brassicicola showed increased accumulation of GIV (Abuyusuf et al., 2018a; Abuyusuf et al., 2018b) indicating its importance in resistance against diverse fungal infection.
GRA is the aliphatic glucosinolate was not induced in any previous studies after inoculation with diverse fungal species including L. maculans, S. sclerotiorum, M. brassicicola and Albugo candida in Brassicaceae family (Singh et al., 2015; Robin et al., 2017a; Abuyusuf et al., 2018a; Abuyusuf et al., 2018b). Thus, this compound was induced only at the seedling stage rather than adult stage (Robin et al., 2017a). In resistant line BN4098, content of GIB increased by 1.53-fold at d2 upon infection of 03-02s compared to mock-treated plants but in all other combination it was decreased (Figure 6). GIB and PRO were induced significantly in leaves of broccoli and roots of Indian mustard, compared to the control, after inoculation with A. candida (Singh et al., 2015). SIN have a potential role in suppressing the growth of S. sclerotiorum fungus and reducing disease severity in kale (Madloo et al., 2019). These results indicated GIV and other aliphatic glucosinolates have vital role in plant-microbe interactions in Brassicaceae family.
Role of Indolic GBS and MGBS in Blackleg Disease Resistance at the Seedling Stage
Our results indicated that pathogen-induced MGBS accumulation can be anti-oxidative to both L. maculans isolates since accumulation of MGBS at the seedling stage was greatly induced by 5.27- and 2.56-fold in two resistant interactions in two different cabbage lines—BN4098 × 03-02s at d2 and BN4303 × 00-100s at d4, respectively (Table 3, Figure 7). In the sensitive cabbage line, the content of MGBS decreased (Figure 7) indicating that indolic MGBS has a pivotal role in blackleg resistance in B. oleracea.
The accumulation of GBS induced in BN4098 × 03-02s at d2 by 1.7-fold, similar to our previous study (Robin et al., 2017a). In the adult plants, however, the induced accumulation of GBS was observed in both resistant lines against 00-100s isolate that showing difference in pathogen-induced GBS accumulation in cabbage seedlings. Role of indolic glucosinolates in offering resistance against a number of fungal species is established (Li et al., 1999b; Robin et al., 2017a; Abuyusuf et al., 2018a; Abuyusuf et al., 2018b). In Chinese and European cultivars of B. napus, the accumulation of indolic glucosinolate was induced upon inoculation of S. sclerotiorum (Li et al., 1999b). Notably, indolic glucosinolate alone can provide resistance to both nectrotrophic and hemibiotrophic pathogens (Sanchez-Vallet et al., 2010; Hiruma et al., 2013; Frerigmann et al., 2016; Wu et al., 2016). Other than the anti-fungal properties, indolic GBS and aromatic glucosinolate inhibits infection by bacteria, e.g., infection by Xanthomonas campestris pv. campestris in kale (Madloo et al., 2019); protists, e.g., infection by Plasmodiophora brassicae in B. napus (Xu et al., 2018) and insects, e.g., infestation by Diamond backmoth in cabbage (Robin et al., 2017c).
Increased Transcription of ST5b-Bol026202 Gene Led to Increased Accumulation of GRA, GIB and GIV in Resistant Cabbage Line
ST5b is a sulfotransferase protein involved in aliphatic glucosinolate pathway. In our previous with adult cabbage plants, the expression level ST5b-Bol026202 gene was only positively associated the contents of GIV (Robin et al., 2017a) but at the seedling stage this gene was found positively correlated with accumulation of aliphatic GRA, GIB and GIV (Table 4A) and increase in the accumulation of these three glucosinolate compounds was associated with blackleg disease resistance. In response to M. brassicicola, cabbage seedlings at third leaf-stage showed positive association between expression of ST5b-Bol026202 gene and accumulation of GIB and GIV (Abuyusuf et al., 2018b). In another study, this gene showed positive correlation only with GIV accumulation upon infection of S. sclerotiorum in two months old cabbage plants (Abuyusuf et al., 2018a). These results indicated that pathogen-induced transcript accumulation of ST5b-Bol026202 gene is more pronounced at the seedling stage that alter glucosinolate accumulation.
MYB34 Likely Activated Higher GBS and MGBS Accumulation in Blackleg Resistant Cabbage Lines
MYB34-Bol007760 gene showed positive correlation both GBS and MGBS in our previous study with blackleg disease infection at the adult stage similar to this study (Robin et al., 2017a). This gene also showed positive correlation with both GBS and MGBS in response to Mycosphaerella brassicicola infection (Abuyusuf et al., 2018a). But under the infection of S. sclerotiorum this gene exhibited positive correlation with NGBS only, in two months old cabbage plants (Abuyusuf et al., 2018b). The biosynthesis of indolic glucosinolates was directly regulated by the MYB34 genes in Arabidopsis (Frerigmann and Gigolashvili, 2014) and B. oleracea (Yi et al., 2016; Robin et al., 2016). Moreover, MYB34, in combination with MYB122 and MYB51 in Arabidopsis, actively favour protection against Plectosphaerella cucumerina, where PEN2 (PENETRATION2) plays a key role in enhancing the pathogen-induced expression of specific biosynthesis genes (Frerigmann et al., 2016). In a previous study, this MYB34-Bol007760 gene was found to be induced upon elicitation with methyl jasmonate in cabbage lines, indicating pathogen-induced resistance might follow jasmonic acid signaling (Yi et al., 2016).
Accumulation of Indolic GBS in Blackleg Resistant Cabbage Lines Is Triggered by Increased Expression of ST5a-Bol026200 and CYP81F4-Bol032712 Genes
Expression levels of both ST5a-Bol026200 and CYP81F4-Bol032712 genes showed positive correlation with GBS accumulation at both seedling and adult stage of cabbage plants (Table 4B, Robin et al., 2017a). GBS, has antifungal reactions in plants, exhibited increased accumulation in the resistance line, as compared to mock-treated plants (Figure 7). GBS accumulation was associated with upregulation of CYP81F4 and CYP81F2 in S. sclerotiorum infection (Abuyusuf et al., 2018a). Similar association was also found between GBS levels and CYP81F2 upregulation in B. oleracea (Sotelo et al., 2016).
Increased Transcription of ST5a-Bol026200 and CYP81F2-Bol026044 Lead to Accumulation of MGBS and Is Associated Blackleg Resistance in Cabbage at the Seedlings Stage
At the 3-months old adult plants, increase in accumulation of MGBS upon L. maculans inoculation was associated with moderate resistance of BN4098 line against 00-100s isolate (Robin et al., 2017a) but in this study, pathogen-induced MGBS accumulation by 2–5 folds was associated with complete resistant of 28–30 days old cabbage seedlings of both resistant lines—BN4098 and BN4303 (Table 3, Figure 2). MGBS was also induced in B. napus after 5–8 days of infection of L. maculans (Wretblad and Dixelius, 2000). The pathogen-induced increase in MGBS was strongly associated with expression level of ST5a-Bol026200 gene and moderately associated with expression level of CYP81F2-Bol026044 (Table 4B). Transcript abundance of CYP81F2 increased in both cabbage and Arabidopsis upon infection of several other pathogens including Plectosphaerella cucumerina, Erysiphe pisi, and Blumeria graminis (Bednarek et al., 2009). Thus the results of our study is consistent with a previous report indicating that both CYP81F2 (Bol026044) and myrosinase PEN2, which hydrolyzes MGBS, simultaneously induce antifungal defense (Bednarek et al., 2009).
Conclusions
The GSL profiling and expression analysis of GSL-related genes in cabbage infected by L. maculans reveal that glucosinolate accumulation and relevant gene expression were directly associated with blackleg resistance at the seedling stage of cabbage. This study showed that both aliphatic and indolic glucosinolates take part in resistance to blackleg disease in a genotype- and isolate-specific manner. Enhanced MGBS content against both fungal isolates, contributing to seedling resistance in two interactions- BN4098 × 03-02s and BN4303 × 00-100s --- and enhanced GBS content contributed to the resistance of BN4098 x 00-100s interaction only. Aliphatic GRA took part in resistance of BN4098 × 00-100s interaction whereas aliphatic GIB took part is resistance of BN4098 × 03-02s interaction. Aliphatic GIV accumulated in BN4098 × 03-02s interaction but GSL-OH-Bol033373 and CYP81F2-Bol026044 showed enhanced expression in BN4303 × 03-02s interaction. The GSLs and the corresponding genes identified in this study could improve blackleg resistance in cabbage seedlings.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Author Contributions
I-SN, J-IP, and AR conceived of and designed the study. AR managed and inoculated the experimental plants, collected samples, prepared cDNA, performed the qPCR analysis, and prepared samples for HPLC. RL assisted with the cDNA preparation and sample preparation for HPLC analysis. AR and MA wrote the manuscript. AR critically revised the manuscript.
Funding
This study was supported by the Center for Horticultural Seed Development (Golden Seed Project no. 213007-05-4-SB510) of the Ministry of Agriculture, Food and Rural Affairs in the Republic of Korea (MAFRA).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Asia Seed Co., Ltd., Republic of Korea for providing B. oleracea seeds.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.01134/full#supplementary-material
References
- Abdel-Farid I. B., Jahangir M., Mustafa N. R., Van Dam N. M., Van den Hondel C. A., Kim H. K., et al. (2010). Glucosinolate profiling of Brassica rapa cultivars after infection by Leptosphaeria maculans and Fusarium oxysporum. Biochem. Syst. Ecol. 38, 612–620. 10.1016/j.bse.2010.07.008 [DOI] [Google Scholar]
- Abuyusuf M., Robin A. H. K., Lee J. H., Jung H. J., Kim H. T., Park J., II, et al. (2018. a). Glucosinolate Profiling and Expression Analysis of Glucosinolate Biosynthesis Genes Differentiate White Mold Resistant and Susceptible Cabbage Lines. Int. J. Mol. Sci. 19 (12), 4037. 10.3390/ijms19124037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuyusuf M., Robin A. H. K., Kim H. T., Islam M., Park J., II, Nou I. S. (2018. b). Altered glucosinolate profiles and expression of glucosinolate biosynthesis genes in ringspot-resistant and susceptible cabbage lines. Int. J. Mol. Sci. 19 (9), 2833. 10.3390/ijms19092833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agerbirk N., Olsen C. E., Sørensen H. (1998). Initial and final products, nitriles, and ascorbigens produced in myrosinase-catalyzed hydrolysis of indoleglucosinolates. J. Agric. Food Chem. 46, 1563–1571. 10.1021/jf9708498 [DOI] [Google Scholar]
- Ballinger D. J., Salisbury P. A. (1996). Seedling and adult plant evaluation of race variability in Leptosphaeria maculans on Brassica species in Australia. Aust. J. Exp. Agric. 36, 485–488. 10.1071/EA9960485 [DOI] [Google Scholar]
- Barth C., Jander G. (2006). Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant J. 46, 549–562. 10.1111/j.1365-313X.2006.02716.x [DOI] [PubMed] [Google Scholar]
- Bednarek P., Piślewska-Bednarek M., Svatoš A., Schneider B., Doubský J., Mansurova M., et al. (2009). A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323, 101–106. 10.1126/science.1163732 [DOI] [PubMed] [Google Scholar]
- Bekaert M., Edger P. P., Hudson C. M., Pires J. C., Conant G. C. (2012). Metabolic and evolutionary costs of herbivory defense: systems biology of glucosinolate synthesis. New Phytol. 196, 596–605. 10.1111/j.1469-8137.2012.04302.x [DOI] [PubMed] [Google Scholar]
- Benderoth M., Textor S., Windsor A. J., Mitchell-Olds T., Gershenzon J., Kroymann J. (2006). Positive selection driving diversification in plant secondary metabolism. Proc. Natl. Acad. Sci. U. S. A. 103, 9118–9123. 10.1073/pnas.0601738103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brader G., Tas É., Palva E. T. (2001). Jasmonate-dependent induction of indole glucosinolates in arabidopsis by culture filtrates of the nonspecific pathogen Erwinia carotovora. Plant Physiol. 126, 849–860. 10.1104/pp.126.2.849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brader G., Mikkelsen M. D., Halkier B. A., TapioPalva E. (2006). Altering glucosinolate profiles modulates disease resistance in plants. Plant J. 46, 758–767. 10.1111/j.1365-313X.2006.02743.x [DOI] [PubMed] [Google Scholar]
- Buxdorf K., Yaffe H., Barda O., Levy M. (2013). The effects of glucosinolates and their breakdown products on necrotrophic fungi. PLoS One 8, e70771. 10.1371/journal.pone.0070771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmes B., N’Guyen G., Dumur J., Brisach C. A., Campion C., Iacomi B., et al. (2015). Glucosinolate-derived isothiocyanates impact mitochondrial function in fungal cells and elicit an oxidative stress response necessary for growth recovery. Front. Plant Sci. 6:414. 10.3389/fpls.2015.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew F. S. (1988). “Biological effects of glucosinolates,” in Biologically Active Natural Products ACS Symposium Series (Washington, DC: American Chemical Society; ), 155–181. 10.1021/bk-1988-0380.ch012 [DOI] [Google Scholar]
- Del Rio L., Ruud S. (2013). In vitro sensitivity of Leptosphaeria maculans to azoxystrobin. Can. J. Plant Pathol. 36, 258. [Google Scholar]
- Dilmaghani A., Balesdent M. H., Rouxel T., Moreno-Rico O. (2010). First report of Leptosphaeria biglobosa (blackleg) on Brassica oleracea (cabbage) in Mexico. Plant Dis. 94, 791–791. 10.1094/PDIS-94-6-0791C [DOI] [PubMed] [Google Scholar]
- Dilmaghani A., Gout L., Moreno-Rico O., Dias J. S., Coudard L., Castillo-Torres N., et al. (2013). Clonal populations of Leptosphaeria maculans contaminating cabbage in Mexico. Plant Pathol. 62, 520–532. 10.1111/j.1365-3059.2012.02668.x [DOI] [Google Scholar]
- Doughty K. J., Porter A. J. R., Morton A. M., Kiddle G., Bock C. H., Wallsgrove R. (1991). Variation in the glucosinolate content of oilseed rape (Brassica napus L.) leaves. Ann. Appl. Biol. 118, 469–477. 10.1111/j.1744-7348.1991.tb05648.x [DOI] [Google Scholar]
- Evivie E. R., Ogwu M. C., Cang W., Xu R., Li J. (2019). Progress and prospects of glucosinolate pathogen resistance in some brassica plants. J. Appl. Nat. Sci. 11 (2), 556–567. 10.31018/jans.v11i2.2117 [DOI] [Google Scholar]
- Fahey J. W., Zalcmann A. T., Talalay P. (2001). The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 56, 5–51. 10.1016/S0031-9422(00)00316-2 [DOI] [PubMed] [Google Scholar]
- Fitt B. D., Brun H., Barbetti M. J., Rimmer S. R. (2006). “World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus),” in Sustainable Strategies for Managing Brassica napus (Oilseed Rape) Resistance to Leptosphaeria maculans (Phoma Stem Canker) (Versailles: Springer Netherlands; ), 3–15. [Google Scholar]
- Fitt B. D., Hu B. C., Li Z. Q., Liu S. Y., Lange R. M., Kharbanda P. D., et al. (2008). Strategies to prevent spread of Leptosphaeria maculans (phoma stem canker) onto oilseed rape crops in China; costs and benefits. Plant Pathol. 57, 652–664. 10.1111/j.1365-3059.2008.01841.x [DOI] [Google Scholar]
- Fraser M., Hwang S. F., Ahmed H. U., Akhavan A., Stammler G., Barton W., et al. (2016). Sensitivity of Leptosphaeria maculans to pyraclostrobin in Alberta, Canada. Can. J. Plant Sci. 97, 83–91. 10.1139/CJPS-2015-0382 [DOI] [Google Scholar]
- Frerigmann H., Gigolashvili T. (2014). MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol. Plant 7, 814–828. 10.1093/mp/ssu004 [DOI] [PubMed] [Google Scholar]
- Frerigmann H., Piślewska-Bednarek M., Sánchez-Vallet A., Molina A., Glawischnig E., Gigolashvili T., et al. (2016). Regulation of pathogen-triggered tryptophan metabolism in Arabidopsis thaliana by MYB transcription factors and indole glucosinolate conversion products. Mol. Plant 9, 682–695. 10.1016/j.molp.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Giamoustaris A., Mithen R. (1995). The effect of modifying the glucosinolate content of leaves of oilseed rape (Brassica napus ssp. oleifera) on its interaction with specialist and generalist pests. Ann. Appl. Biol. 126, 347–363. 10.1111/j.1744-7348.1995.tb05371.x [DOI] [Google Scholar]
- Giamoustaris A., Mithen R. (1997). Glucosinolates and disease resistance in oilseed rape (Brassica napus ssp. oleifera). Plant Pathol. 46, 271–275. 10.1046/j.1365-3059.1997.d01-222.x [DOI] [Google Scholar]
- Henderson M. P. (1918). The Black-leg Disease of Cabbage Caused by Phoma lingam (Tode) Desmaz Vol. 8 (Madison: University of Wisconsin–Madison; ), 379–431. [Google Scholar]
- Hiruma K., Fukunaga S., Bednarek P., Piślewska-Bednarek M., Watanabe S., Narusaka Y., et al. (2013). Glutathione and tryptophan metabolism are required for Arabidopsis immunity during the hypersensitive response to hemibiotrophs. Proc. Natl. Acad. Sci. U. S. A. 110, 9589–9594. 10.1073/pnas.1305745110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogge L. R., Reed D. W., Underhill E. W., Haughn G. W. (1988). HPLC separation of glucosinolates from leaves and seeds of Arabidopsis thaliana and their identification using thermospray liquid chramatography/mass spectrometry. J. Chromatogr. Sci. 26, 551–556. 10.1093/chromsci/26.11.551 [DOI] [Google Scholar]
- Hopkins R. J., van Dam N. M., van Loon J. J. (2009). Role of glucosinolates in insect-plant relationships and multitrophic interactions. Ann. Rev. Entomol. 54, 57–83. 10.1146/annurev.ento.54.110807.090623 [DOI] [PubMed] [Google Scholar]
- Howlett B. J. (2004). Current knowledge of the interaction between Brassica napus and Leptosphaeria maculans. Can. J. Plant Pathol. 26, 245–252. 10.1080/07060660409507141 [DOI] [Google Scholar]
- Humpherson-Jones F. M. (1985). The incidence of Alternaria spp. and Leptosphaeria maculans in commercial brassica seed in the United Kingdom. Plant Pathol. 34, 385–390. 10.1111/j.1365-3059.1985.tb01377.x [DOI] [Google Scholar]
- Kliebenstein D., Pedersen D., Barker B., Mitchell-Olds T. (2002). Comparative analysis of quantitative trait loci controlling glucosinolates, myrosinase and insect resistance in Arabidopsis thaliana. Genetics 161, 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh J. C., Barbulescu D. M., Salisbury P. A., Slater A. T. (2016). Pterostilbene is a potential candidate for control of blackleg in canola. PLoS One 11, e0156186. 10.1371/journal.pone.0156186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku K. M., Choi J. H., Kim H. S., Kushad M. M., Jeffery E. H., Juvik J. A. (2013. a). Methyl jasmonate and 1-methylcyclopropene treatment effects on quinone reductase inducing activity and post-harvest quality of broccoli. PLoS One 8 (10), e77127. 10.1371/journal.pone.0077127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku K. M., Choi J. H., Kushad M. M., Jeffery E. H., Juvik J. A. (2013. b). Pre-harvest methyl jasmonate treatment enhances cauliflower chemoprotective attributes without a loss in postharvest quality. Plant Foods Hum. Nutr. 68 (2), 113–117. 10.1007/s11130-013-0356-y [DOI] [PubMed] [Google Scholar]
- Larkan N. J., Raman H., Lydiate D. J., Robinson S. J., Yu F., Barbulescu D. M., et al. (2016). Multi-environment QTL studies suggest a role for cysteine-rich protein kinase genes in quantitative resistance to blackleg disease in Brassica napus. BMC Plant Biol. 16, 183. 10.1186/s12870-016-0877-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio V., Lattanzio V. M., Cardinali A. (2006). Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 661, 23–67. [Google Scholar]
- Lee J., Yang K., Lee M., Kim S., Kim J., Lim S., et al. (2015). Differentiated cuticular wax content and expression patterns of cuticular wax biosynthetic genes in bloomed and bloomless broccoli (Brassica oleracea var. italica). Process Biochem. 50, 456–462. 10.1016/j.procbio.2014.12.012 [DOI] [Google Scholar]
- Li Y., Chen J., Bennett R., Kiddle G., Wallsgrove R., Huang Y. J., et al. (1999. a). “Breeding, inheritance, and biochemical studies on Brassica napus cv. Zhougyou 821: tolerance to Sclerotinia sclerotiorum (stem rot),” in Proceedings of the 10th International Rapeseed Congress, vol. 61 Ed. Wratten N. (Canberra: PA Salisbury; ). [Google Scholar]
- Li Y., Kiddle G., Bennett R. N., Wallsgrove R. M. (1999. b). Local and systemic changes in glucosinolates in Chinese and European cultivars of oilseed rape (Brassica napus L.) after inoculation with Sclerotinia sclerotiorum (stem rot). Ann. App. Biol. 134 (1), 45–58. 10.1111/j.1744-7348.1999.tb05234.x [DOI] [Google Scholar]
- Li H., Sivasithamparam K., Barbetti M. J. (2003). Breakdown of a Brassica rapa subsp. sylvestris single dominant blackleg resistance gene in B. napus rapeseed by Leptosphaeria maculans field isolates in Australia. Plant Dis. 87, 752–752. 10.1094/PDIS.2003.87.6.752A [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Madloo P., Lema M., Francisco M., Soengas P. (2019). Role of Major Glucosinolates in the Defense of Kale Against Sclerotinia sclerotiorum and Xanthomonas campestris pv. campestris. Phytopathology 109 (7), 1246–1256. 10.1094/PHYTO-09-18-0340-R [DOI] [PubMed] [Google Scholar]
- Manici L. M., Lazzeri L., Palmieri S. (1997). In vitrofungitoxic activity of some glucosinolates and their enzyme-derived products toward plant pathogenic fungi. J. Agric. Food Chem. 45, 2768–2773. 10.1021/jf9608635 [DOI] [Google Scholar]
- Mithen R. F., Magrath R. (1992). Glucosinolates and resistance to Leptosphaeria maculans in wild and cultivated Brassica species. Plant Breed. 108, 60–68. 10.1111/j.1439-0523.1992.tb00100.x [DOI] [Google Scholar]
- Mithen R., Raybould A. F., Giamoustaris A. (1995). Divergent selection for secondary metabolites between wild populations of Brassica oleracea and its implications for plant-herbivore interactions. Heredit. (Edinb). 75, 472–484. 10.1038/hdy.1995.164 [DOI] [Google Scholar]
- Mithen R. (2001). Glucosinolates–biochemistry, genetics and biological activity. Plant Growth Regul. 34, 91–103. 10.1023/A:1013330819778 [DOI] [Google Scholar]
- Nawaz I., Iqbal M., Hakvoort H. W., Bliek M., de Boer B., Schat H. (2014). Expression levels and promoter activities of candidate salt tolerance genes in halophytic and glycophytic Brassicaceae. Environ. Exp. Bot. 99, 59–66. 10.1016/j.envexpbot.2013.10.006 [DOI] [Google Scholar]
- Nintemann S. J., Vik D., Svozil J., Bak M., Baerenfaller K., Burow M., et al. (2017). Unravelling protein-protein interaction networks linked to aliphatic and indole glucosinolate biosynthetic pathways in Arabidopsis. Front. Plant Sci. 8:2028. 10.3389/fpls.2017.02028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piliponyte-Dzikiene A., Andriunaite E., Petraitiene E., Brazauskiene I., Statkeviciute G., Brazauskas G. (2015). Genetic diversity and occurrence of Leptosphaeria spp. on Brassica oleracea and B. napus in Lithuania. J. Plant Pathol. 97, 265–271. 10.4454/JPP.V97I2.027 [DOI] [Google Scholar]
- Potter T., Burton W., Edwards J., Wratten N., Mailer R., Salisbury P., et al. (2016). Assessing progress in breeding to improve grain yield, quality and blackleg (Leptosphaeria maculans) resistance in selected Australian canola cultivars, (1978–2012). Crop Pasture Sci. 67 (4), 308–316. 10.1071/CP15290 [DOI] [Google Scholar]
- Rahimi F., Siamak R. (2020). Overcoming Glucosinolate-Myrosinase-Isothiocyanate Defense System by Plant Pathogenic Fungi. Int. J. Secondary Metabolite 7 (1), 19–27. 10.21448/ijsm.697516 [DOI] [Google Scholar]
- Rico O. M., Trevi-o A. F., Ruiz J. L., Flores D. M., Cova S. R., Swartz G. S. (2001). Characterization and pathogenicity of isolates of Leptosphaeria maculans from Aguascalientes and Zacatecas, Mexico1. Can. J. Plant Pathol. 23, 270–278. 10.1080/07060660109506940 [DOI] [Google Scholar]
- Rimmer S. R. (2006). Resistance genes to Leptosphaeria maculans in Brassica napus. Can. J. Plant Pathol. 28, S288–S297. 10.1080/07060660609507386 [DOI] [Google Scholar]
- Robin A. H. K., Yi G. E., Laila R., Yang K., Park J., II, Kim H. R., et al. (2016). Expression profiling of glucosinolate biosynthetic genes in Brassica oleracea L. var. capitata inbred lines reveals their association with glucosinolate content. Molecules 21, 787. 10.3390/molecules21060787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin A. H. K., Yi G. E., Laila R., Hossain M. R., Park J., II, Kim H. R., et al. (2017. a). Leptosphaeria maculans alters glucosinolate profiles in blackleg disease–resistant and-susceptible cabbage lines. Front. Plant Sci. 8, 1769. 10.3389/fpls.2017.01769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin A. H. K., Larkan N. J., Laila R., Park J., II, Ahmed N. U., Borhan H., et al. (2017. b). Korean Brassica oleracea germplasm offers a novel source of qualitative resistance to blackleg disease. Eur. J. Plant Pathol. 149, 611–623. 10.1007/s10658-017-1210-0 [DOI] [Google Scholar]
- Robin A. H. K., Hossain M. R., Park J., II, Kim H. R., Nou I. S. (2017. c). Glucosinolate profiles in cabbage genotypes influence the preferential feeding of Diamondback moth (Plutella xylostella). Front. Plant Sci. 8:1244. 10.3389/fpls.2017.01244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouxel T., Penaud A., Pinochet X., Brun H., Gout L., Delourme R., et al. (2003). A 10-year survey of populations of Leptosphaeria maculans in France indicates a rapid adaptation towards the Rlm1 resistance gene of oilseed rape. Eur. J. Plant Pathol. 109, 871–881. 10.1023/A:1026189225466 [DOI] [Google Scholar]
- Sanchez-Vallet A., Ramos B., Bednarek P., López G., Piślewska-Bednarek M., Schulze-Lefert P., et al. (2010). Tryptophan-derived secondary metabolites in Arabidopsis thaliana confer non-host resistance to necrotrophic Plectosphaerella cucumerina fungi. Plant J. 63, 115–127. 10.1111/j.1365-313X.2010.04224.x [DOI] [PubMed] [Google Scholar]
- Sexton A. C., Kirkegaard J. A., Howlett B. J. (1999). Glucosinolates in Brassica juncea and resistance to Australian isolates of Leptosphaeria maculans, the blackleg fungus. Aust. Plant Pathol. 28, 95–102. 10.1071/AP99017 [DOI] [Google Scholar]
- Singh A., Guest D., Copeland L. (2015). Associations between glucosinolates, white rust, and plant defense activators in Brassica plants: A review. Int. J. Vegetable Sci. 21 (3), 297–313. 10.1080/19315260.2013.832465 [DOI] [Google Scholar]
- Sotelo T., Velasco P., Soengas P., Rodríguez V. M., Cartea M. E. (2016). Modification of leaf glucosinolate contents in Brassica oleracea by divergent selection and effect on expression of genes controlling glucosinolate pathway. Front. Plant Sci. 7:1012. 10.3389/fpls.2016.01012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague S. J., Balesdent M. H., Brun H., Hayden H. L., Marcroft S. J., Pinochet X., et al. (2006). Major gene resistance in Brassica napus (oilseed rape) is overcome by changes in virulence of populations of Leptosphaeria maculans in France and Australia. Eur. J. Plant Pathol. 114, 33–40. 10.1007/s10658-005-3683-5 [DOI] [Google Scholar]
- Stotz H. U., Sawada Y., Shimada Y., Hirai M. Y., Sasaki E., Krischke M., et al. (2011). Role of camalexin, indole glucosinolates, and side chain modification of glucosinolate-derived isothiocyanates in defense of Arabidopsis against Sclerotinia sclerotiorum. Plant J. 67, 81–93. 10.1111/j.1365-313X.2011.04578.x [DOI] [PubMed] [Google Scholar]
- Tierens K. F. J., Thomma B. P., Brouwer M., Schmidt J., Kistner K., Porzel A., et al. (2001). Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of Arabidopsis to microbial pathogens. Plant Physiol. 125, 1688–1699. 10.1104/pp.125.4.1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink M. (1988). Plant breeding: importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 75, 225–233. 10.1007/BF00303957 [DOI] [Google Scholar]
- Wretblad S., Dixelius C. (2000). B-genome derived resistance to Leptosphaeria maculans in near isogenic Brassica napus lines is independent of glucosinolate profile. Physiol. Plant. 110, 461–468. 10.1111/j.1399-3054.2000.1100406.x [DOI] [Google Scholar]
- Wu J., Zhao Q., Yang Q., Liu H., Li Q., Yi X., et al. (2016). Comparative transcriptomic analysis uncovers the complex genetic network for resistance to Sclerotinia sclerotiorum in Brassica napus. Sci. Rep. 6:19007. 10.1038/srep19007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Yang H., Ren L., Chen W., Liu L., Liu F., et al. (2018). Jasmonic acid-mediated aliphatic glucosinolate metabolism is involved in clubroot disease development in Brassica napus L. Front. Plant Sci. 9, 750. 10.3389/fpls.2018.00750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi G. E., Robin A. H. K., Yang K., Park J., II, Kang J. G., Yang T. J., et al. (2015). Identification and expression analysis of glucosinolate biosynthetic genes and estimation of glucosinolate contents in edible organs of Brassica oleracea subspecies. Molecules 20, 13089–13111. 10.3390/molecules200713089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi G.-E., Robin A., Yang K., Park J.-I., Hwang B., Nou I.-S. (2016). Exogenous methyl jasmonate and salicylic acid induce subspecies-specific patterns of glucosinolate accumulation and gene expression in Brassica oleracea L. Molecules 21, 1417. 10.3390/molecules21101417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Hu Z., Zhang Y., Li Y., Zhou S., Chen G. (2012). A putative functional MYB transcription factor induced by low temperature regulates anthocyanin biosynthesis in purple kale (Brassica oleracea var. acephala f. tricolor). Plant Cell Rep. 31, 281–289. 10.1007/s00299-011-1162-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.