To the Editor:
There is no standard practice for coronary artery disease (CAD) screening of asymptomatic patients before kidney transplantation. Available guidelines1, 2, 3 generally do not reflect the last 2 decades of cardiology literature demonstrating the lack of efficacy in preemptively screening and revascularizing asymptomatic patients without kidney disease.4,5 The only survey of American kidney transplantation programs addressing pretransplantation cardiac screening was more than 15 years ago. It reported that 8% and 18% of programs routinely engaged in cardiac screening of all or none of their wait-listed patients, respectively, while the rest screened only patients deemed to be “high risk.”6 We conducted a web-based survey of transplantation providers to study contemporary CAD screening practice patterns.
This work is a product of the American Society of Transplant (AST) Kidney-Pancreas Community of Practice (KPCOP) Cardiovascular Disease Workgroup. We administered a web-based survey consisting of 10 questions (Item S1). Item S2 contains the detailed methods. The Institutional Review Board of the Einstein Medical Center, Philadelphia, PA, approved the study (protocol number 5044EXE) and waived the need for informed consent given survey respondent deidentification.
A total of 477 KPCOP members received and 188 (39%) opened the e-mail. We received a response from 78 members, a response rate of 42% (of those who opened the e-mail) and 16% (of the total number surveyed). Fifty-five (71%) were transplant nephrologists and 9 (12%) were transplant surgeons. Forty-one (53%) practiced at centers performing more than 100 transplantations per year. All 11 United Network of Organ Sharing (UNOS) regions were represented. Of the guidelines followed (question 4), the most commonly selected was the 2012 American Heart Association/American College of Cardiology Foundation Scientific Statement (42%), followed by other (29%). Of the 19 respondents who selected other, 17 entered details: 12 (71%) indicated that a local protocol existed and 5 (29%) indicated that no particular protocol existed.
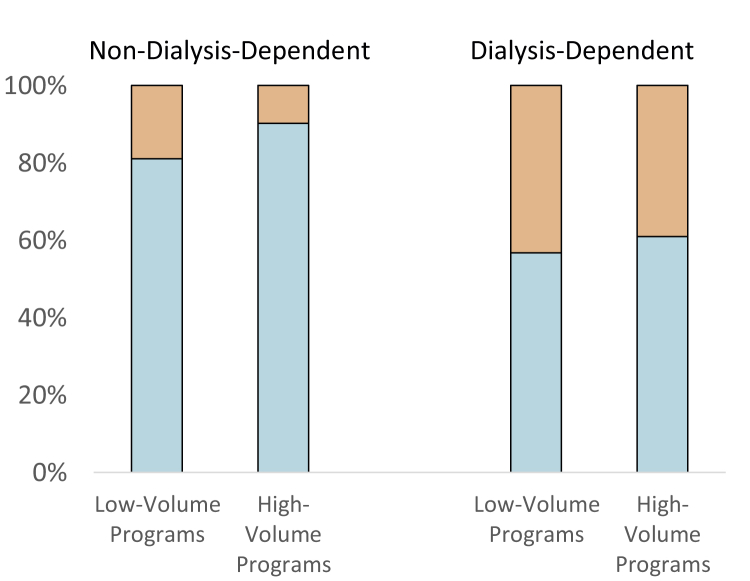
Regarding test modality (questions 5-6), respondents favored noninvasive over invasive testing in non–dialysis- (91%) and dialysis-dependent patients (74%). Dialysis-dependent patients were more likely to receive invasive testing compared with nondialysis patients (P < 0.001; Fig 1). Of the noninvasive modalities, myocardial perfusion scintigraphy and dobutamine stress echocardiography were the 2 most popular modalities in non–dialysis- (61% and 22%, respectively) and dialysis-dependent patients (49% and 28%, respectively). The responses did not differ by center volume or provider type.
Figure 1.
Invasive (orange) versus noninvasive (white) modality of choice for coronary artery disease screening in patients who are not or are receiving maintenance dialysis, in transplantation programs with 100 or fewer or more than 100 transplantations per year.
In our 3 case scenarios (questions 7-9), all involving asymptomatic patients with no risk factor other than age, most respondents selected aggressive evaluation or revascularization in the case of a mildly “positive” stress test (61%-68%; Item S3). In contrast, most (85%) respondents did not monitor asymptomatic patients for ischemia after kidney transplantation (question 10). The responses did not differ by center volume or provider type.
From the 40 free-text responses provided to the question on perceived barriers to pretransplantation CAD screening, we identified 7 themes. Three pertained to clinical and medical factors: (1) lack of clarity regarding the goal of CAD screening, (2) challenges in clinical decision making (population characteristics, diagnostic test performance, and absence of high-grade evidence), and (3) concern over contrast-induced nephropathy. Four pertained to systems factors: (1) health care delivery, especially related to health care fragmentation and access; (2) interprogram and provider variability; (3) perceived restraints posed by regulatory bodies; and (4) specific challenges of a clinical trial to investigate the area. Table 1 provides the themes by transplantation program volume and sample statements provided by the respondents.
Table 1.
Thematic Analysis of Individual Responses on Perceived Barriers to Coordinate Approach to Cardiovascular Screening of Kidney Transplant Candidates
| Theme | Responders | Volume ≤ 100 Responders | Volume > 100 Responders | Representative Quotes |
|---|---|---|---|---|
| Unclear goal of screening | 8 | 3 (38%) | 5 (62%) |
|
| Challenge in clinical decision making | 9 | 5 (56%) | 4 (44%) |
|
| Contrast and kidney function preservation | 8 | 3 (38%) | 5 (62%) |
|
| Health care delivery systems factors | 10 | 2 (20%) | 8 (80%) |
|
| Transplant program and provider variability | 9 | 5 (56%) | 4 (44%) |
|
| Regulatory restraints | 5 | 2 (40%) | 3 (60%) |
|
| Logistic challenges of a clinical trial | 7 | 4 (57%) | 3 (43%) |
|
Note: Each respondent (N = 40) may have responses that touch on multiple themes and the numbers therefore add up to more than 40.
Abbreviations: CAD, coronary artery disease; CKD, chronic kidney disease; CMS, Centers for Medicare & Medicaid Services; UNOS, United Network for Organ Sharing.
This survey represents both nephrologists and surgeons and all UNOS regions and is the first of its kind in the last 15 years.6 The responses to our case questions and free-text responses illustrate lack of clarity regarding the fundamental goal of CAD screening in this patient population. Our survey shows a predilection toward revascularization when asymptomatic patients have “positive” stress test results despite evidence that preemptive revascularization does not change perioperative or long-term mortality in patients undergoing elective vascular surgery.7 The ISCHEMIA-CKD trial results may therefore cause a paradigm shift in the field.8 Our respondents were aggressive with CAD diagnosis before but not after kidney transplantation (question 10). A priority for the kidney transplantation community should therefore be to clarify the objectives and goals of pretransplantation CAD screening, as well as the optimal screening and intervention strategy in the posttransplantation period.
A striking finding of our survey is the high proportion of responses addressing health care system factors over medical factors. Concerns involved fracturing of health delivery systems, concern over Centers for Medicare & Medicaid Services regulation and oversight, and program- and provider-level variability. They highlight the importance of incorporating systems-based practice into designing and testing interventions and of expanding the community beyond transplantation providers into cardiologists, policymakers, and administrators.
In summary, this survey informs the kidney transplantation community about common clinical practices in pretransplantation cardiovascular evaluation and highlights major knowledge gaps and discrepancies. We conclude that current practice in the United States favors aggressive CAD detection before but not after kidney transplantation. This practice is incongruous with the epidemiology of CAD in kidney failure and reflects confusion regarding the ultimate objective of pretransplantation cardiovascular testing. Health system factors appear to drive much of practice.
Article Information
Authors’ Full Names and Academic Degrees
Xingxing S. Cheng, MD, MS, Roy O. Mathew, MD, Ravi Parasuraman, MD, Ekamol Tantisattamo, MD, Swee-Ling Levea, MD, Rajan Kapoor, MD, Darshana M. Dadhania, MD, MS, and Janani Rangaswami, MD.
Authors’ Contributions
Research idea and study design: XSC, ROM, RP, ET, SLL, RK, DMD, JR; data acquisition: DMD, JR; data analysis and interpretation: XSC, SLL, RK, JR; statistical analysis: XSC. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This study was supported by the American Heart Association, award no. 19CDA34490021 (Dr Cheng). The funders have no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
This manuscript is a work product of the AST’s KPCOP Cardiovascular Disease Workgroup. We thank the members of the work group and AST staff members for their support; specifically, Muhammad Yaqub for input on the survey design and Nicole Bhave and Edgar Lerma for input on the discussion.
Peer Review
Received February 12, 2020. Evaluated by 1 external peer reviewer, with direct editorial input from the Editor-in-Chief. Accepted in revised form April 24, 2020.
Footnotes
Item S1: Survey and Summary Answers
Item S2: Detailed Methods
Item S3: Detailed Responses to Case Scenario Questions 7-9
Supplementary Material
Items S1-S3
References
- 1.K/DOQI Workgroup K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153. [PubMed] [Google Scholar]
- 2.Abbud-Filho M., Adams P.L., Alberu J. A report of the Lisbon Conference on the care of the kidney transplant recipient. Transplantation. 2007;83(8 suppl):S1–S22. doi: 10.1097/01.tp.0000260765.41275.e2. [DOI] [PubMed] [Google Scholar]
- 3.Lentine K.L., Costa S.P., Weir M.R. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2012;60(5):434–480. doi: 10.1016/j.jacc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Hart A., Weir M.R., Kasiske B.L. Cardiovascular risk assessment in kidney transplantation. Kidney Int. 2015;87(3):527–534. doi: 10.1038/ki.2014.335. [DOI] [PubMed] [Google Scholar]
- 5.Poldermans D., Schouten O., Vidakovic R. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V Pilot Study. J Am Coll Cardiol. 2007;49(17):1763–1769. doi: 10.1016/j.jacc.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 6.Danovitch G.M., Hariharan S., Pirsch J.D. Management of the waiting list for cadaveric kidney transplants: report of a survey and recommendations by the Clinical Practice Guidelines Committee of the American Society of Transplantation. J Am Soc Nephrol. 2002;13(2):528–535. doi: 10.1681/ASN.V132528. [DOI] [PubMed] [Google Scholar]
- 7.McFalls E.O., Ward H.B., Moritz T.E. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351(27):2795–2804. doi: 10.1056/NEJMoa041905. [DOI] [PubMed] [Google Scholar]
- 8.Bangalore S., Maron D.J., O'Brien S.M. Management of coronary disease in patients with advanced kidney disease. N Engl J Med. 2020;382:1608–1618. doi: 10.1056/NEJMoa1915925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Items S1-S3