To the Editor:
African Americans face a higher risk for chronic kidney disease and kidney failure compared with people of European ancestry. Part of this risk has been attributed to genetic factors. The presence of 2 risk alleles in the APOL1 gene, a genotype present in 13% of African Americans, is associated with 2-fold increased risk for end-stage kidney disease (ESKD).1 However, not everyone with the high-risk genotype progresses to ESKD. It has been suggested that a “second hit,” an exposure that increases the risk of the APOL1 high-risk genotype, is required for kidney function decline.2
Cardiovascular damage could play a role in precipitating kidney function decline. Cardiac troponin T, troponin I, and N-terminal pro–brain natriuretic peptide (NT-proBNP) are markers of cardiac damage that are used in diagnosing myocardial infarction and heart failure and are increasingly recognized as prognostic markers, even at subclinical levels.3, 4, 5 High-sensitivity cardiac troponin T (hs-cTnT) and NT-proBNP have been associated with ESKD.6 The APOL1 gene is widely expressed, including in the vasculature. A recent study7 suggested minimal association between APOL1 genotype and clinical cardiovascular disease; however, this has not been tested using the more sensitive markers of subclinical disease, such as hs-cTnT, high-sensitivity troponin I (hs-TnI), and NT-proBNP. Using data from African American participants in the Atherosclerosis Risk in Communities (ARIC) Study,8 we examined the associations of APOL1 genotypes with these cardiac markers and tested whether higher levels increased the risk of APOL1 associated with ESKD.
The ARIC Study is a prospective community-based cohort of adults aged 45 to 64 years that began in 1987. For this study, only African American participants without prevalent ESKD consenting to genotyping were included (N = 2,992). Hs-cTnT and NT-proBNP were assayed at study visit 2 (1990-1992), which was considered the baseline for our study, and visits 4 (1996-1998), 5 (2011-2013), and 6 (2016-2017; Item S1). We tested cross-sectional differences in cardiac markers by APOL1 genotype using Wilcoxon rank sum tests. For longitudinal change in cardiac marker levels, we used mixed models with random intercepts and slopes. For risk for ESKD, defined as entry into the US Renal Data System registry, we used Cox regression to estimate the risk associated with APOL1 by tertiles of hs-cTnT and NT-proBNP and as continuous log-transformed variables. Models were adjusted for age, sex, study center, and percentage of African ancestry. Analyses were repeated using ARIC Study visit 4 as baseline to evaluate hs-TnI, which was available only at visits 4 and 5. The ARIC Study has been approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board (IRB number: H.34.99.07.02.A1), and all participants provided informed consent.
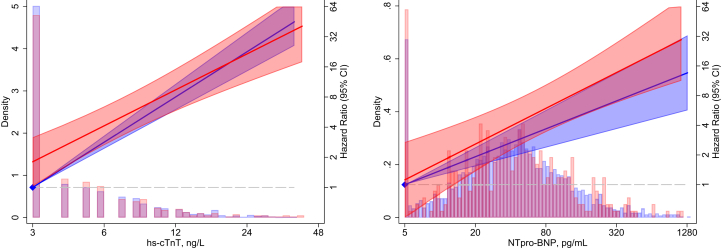
Mean age of the study population was 56 years, and mean estimated glomerular filtration rate was 104 mL/min/1.73 m2. There were no baseline differences in hs-cTnT or NT-proBNP levels by APOL1 risk status, but slightly higher cardiovascular disease and hypertension among the 399 (13%) participants with the APOL1 high-risk genotype (Table 1). There were no differences in change in hs-cTnT (7.7% vs 7.5% increase per year; P for interaction = 0.43) or change in NT-proBNP levels (7.9% vs 8.8% increase per year; P for interaction = 0.14) by APOL1 genotype. After visit 2, there were 165 ESKD events over a median follow-up of 24 years. hs-cTnT and NT-proBNP levels were both significant risk factors for ESKD. Risks for ESKD associated with the APOL1 high-risk genotype were not different across tertiles of hs-cTnT or NT-proBNP (interaction P > 0.05 for all comparisons; Table S2). Results were similar when cardiac markers were modeled continuously (interaction P > 0.05 for both; Fig 1).
Table 1.
Characteristics of Study Participants
| Variable | Overall | 0 or 1 APOL1 Allele | 2 APOL1 Alleles | P |
|---|---|---|---|---|
| N | 2,992 | 2,593 | 399 | |
| hs-cTnT, ng/L | 5.0 (1.9) | 4.9 (1.9) | 5.1 (2.0) | |
| hs-cTnT, ng/L | 4.0 [3.0-7.0] | 4.0 [3.0-7.0] | 4.0 [3.0-7.0] | 0.42 |
| NT-proBNP, pg/mL | 41.2 (3.3) | 41.1 (3.3) | 41.3 (3.4) | |
| NT-proBNP, pg/mL | 40.5 [19.7-82.1] | 40.5 [19.7-81.7] | 39.9 [19.2-85.4] | 0.89 |
| Age, y | 56.1 (5.8) | 56.2 (5.8) | 55.6 (5.6) | 0.05 |
| Female sex | 1,907 (63.7%) | 1,648 (63.6%) | 259 (64.9%) | 0.60 |
| Percentage African ancestry | 82.5 (10.0) | 82.0 (10.3) | 85.3 (7.8) | <0.001 |
| Current smoker | 763 (25.6%) | 644 (25.0%) | 119 (30.0%) | |
| Former smoker | 866 (29.1%) | 756 (29.3%) | 110 (27.7%) | |
| Never smoker | 1,348 (45.3%) | 1,180 (45.7%) | 168 (42.3%) | 0.10 |
| BMI, kg/m2 | 30.0 (6.3) | 30.0 (6.2) | 30.3 (6.4) | 0.36 |
| Systolic blood pressure, mm Hg | 126.5 (20.5) | 126.5 (20.4) | 126.7 (21.3) | 0.88 |
| Cardiovascular disease | 354 (12.1%) | 292 (11.5%) | 62 (16.0%) | 0.01 |
| Hypertension | 1,631 (54.9%) | 1,393 (54.1%) | 238 (59.9%) | 0.03 |
| Diabetes | 739 (24.9%) | 638 (24.8%) | 101 (25.4%) | 0.78 |
| eGFR, mL/min/1.73 m2 | 104.1 (19.7) | 104.2 (19.5) | 103.6 (21.0) | 0.60 |
| Incident ESKD events | 165 (5.5%) | 133 (5.1%) | 32 (8.0%) | 0.02 |
| Time to ESKD, y | 23.9 [15.2-26.1] | 24.0 [15.0-26.1] | 23.5 [16.3-26.1] | 0.58 |
Note: Values are shown as number (percentage) for female sex, smoking, hypertension, cardiovascular disease, and diabetes; geometric mean (geometric SD) and median [interquartile range] for hs-cTnT and NT-proBNP and mean (SD) for the rest. Geometric mean and SD are calculated on a multiplicative scale and better represent the central value and spread in highly skewed data. eGFR is from the Chronic Kidney Disease Epidemiology Collaboration equation. Cardiovascular disease is defined as history of stroke, coronary artery disease, or heart failure.
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; hs-cTnT, high-sensitivity cardiac troponin T; ESKD, end-stage kidney disease; NT-proBNP, N-terminal pro–brain natriuretic peptide; SD, standard deviation.
Figure 1.
Hazard ratios (95% confidence intervals [CIs]) for end-stage kidney disease by APOL1 status and high-sensitivity cardiac troponin T (hs-cTnT) and N-terminal pro–brain natriuretic peptide (NT-proBNP) measures at baseline. Hazards are adjusted for age, sex, Atherosclerosis Risk in Communities Study center, and percentage of African ancestry. Values less than the minimum level of detection were imputed with the minimum. Hazards are relative to the minimum level of detection: 3 ng/L for hs-cTnT and 5 pg/ml for NT-proBNP. APOL1 low risk is in blue and high risk is in red.
When visit 4 was used as a baseline, results were consistent. hs-TnI at visit 4 and subsequent change in hs-TnI levels did not differ by APOL1 genotype. After visit 4, there were 86 ESKD events occurring over a median follow-up of 19 years (Table S1). Risk for ESKD increased with higher hs-TnI levels, but the risk associated with APOL1 did not vary significantly by hs-TnI level (Table S2; Fig S1). Results were similar after accounting for the competing risk for death using the model of Fine and Gray (Table S3).
In a large well-characterized cohort of African Americans with preserved kidney function and long follow-up, we found no significant cross-sectional or longitudinal associations between APOL1 genotype and hs-cTnT, NT-proBNP, or hs-TnI levels. Consistent with previous studies,6 we found that cardiac marker levels were strongly associated with the development of ESKD. However, there were no significant differences in risk for ESKD associated with the APOL1 high-risk genotype at different levels of these markers. The relatively small number of events in our study may have limited our power. There were no visit 2 echocardiogram data to evaluate cardiorenal physiology. Our results suggest that APOL1 high-risk genotype is not a risk factor for subclinical cardiovascular disease and the second hit for APOL1-associated ESKD may not manifest through cardiac damage.
Article Information
Authors’ Full Names and Academic Degrees
Aditya L. Surapaneni, PhD, Shoshana H. Ballew, PhD, Josef Coresh, MD, PhD, Christie M. Ballantyne, MD, Elizabeth Selvin, PhD, Kunihiro Matsushita, MD, PhD, and Morgan E. Grams, MD, PhD.
Authors’ Contributions
Research idea and study design: AS, MEG; data acquisition: JC, CB, ES; data analysis/interpretation: AS, SHB, JC, ES, KM, MEG; statistical analysis: ALS; supervision or mentorship: MEG. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The ARIC Study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I; R01HL087641, R01HL086694; National Human Genome Research Institute contract U01HG004402; and NIH contract HHSN268200625226C. Funding for laboratory testing and biospecimen collection at ARIC Study visit 6 was supported by grant R01DK089174 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH. Genotyping services were provided by the Johns Hopkins University under federal contract number N01-HV-48195 from the NHLBI and the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the NIH to the Johns Hopkins University (contract no. N01-HG-65403). Drs Surapaneni, Coresh, and Grams also receive funding from NIDDK R01 DK108803. The funders of this study had no role in the study design; collection, analyses, and interpretation of data; writing of the report; or decision to submit the report for publication.
Financial Disclosure
Dr Ballantyne has received grant/research support from Abbott and Roche, has been a consultant for Abbott and Roche, and is named in a provisional patent (patent no. 61721475) entitled “Biomarkers to Improve Prediction of Heart Failure Risk” filed by Baylor College of Medicine and Roche. Dr Matsushita received research funding and personal fee from Kyowa Kirin, personal fee from Akebia, and nonfinancial support from Roche Diagnostics outside of the work. Dr Grams received travel support from DCI.
Acknowledgements
The authors thank the staff and participants of the ARIC Study for important contributions.
Disclaimer
Some of the data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Peer Review
Received November 25, 2019. Evaluated by 2 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form February 11, 2020.
Footnotes
Figure S1: Hazard ratios (95% CI) for ESKD by APOL1 status and hs-cTnT, NT-proBNP, and hs-TnI measures at baseline visit 4
Item S1: Genotyping and assay methods for cardiac markers.
Table S1: Characteristics of Study Participants at Visit 4 (1996-1998)
Table S2: Hazard Ratios of APOL1 High Risk by Tertile of hs-cTnT, NT-proBNP, and hs-TnI at Visits 2 and 4
Table S3: Subhazard Ratios of APOL1 High Risk by Tertile of hs-cTnT, NT-proBNP, and hs-TnI at Visits 2 and 4 Accounting for the Competing Risk of Death
Supplementary Material
Figure S1, Item S1, Tables S1-S3
References
- 1.Foster M.C., Coresh J., Fornage M. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484–1491. doi: 10.1681/ASN.2013010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman B.I., Skorecki K. Gene-gene and gene-environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol. 2014;9(11):2006–2013. doi: 10.2215/CJN.01330214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saunders J.T., Nambi V., de Lemos J.A. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123(13):1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myhre P.L., Claggett B., Ballantyne C.M. Association between circulating troponin concentrations, left ventricular systolic and diastolic functions, and incident heart failure in older adults. JAMA Cardiol. 2019;4(10):997–1006. doi: 10.1001/jamacardio.2019.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ndumele C.E., Matsushita K., Sang Y. NT-proBNP and heart failure risk among individuals with and without obesity: the ARIC Study. Circulation. 2016;133(7):631–638. doi: 10.1161/CIRCULATIONAHA.115.017298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Y., Matsushita K., Sang Y. Association of high-sensitivity cardiac troponin T and natriuretic peptide with incident ESRD: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2015;65(4):550–558. doi: 10.1053/j.ajkd.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grams M.E., Surapaneni A., Ballew S.H. APOL1 kidney-risk variants and cardiovascular disease: an individual participant data meta-analysis. J Am Soc Nephrol. 2019;30(10):2027–2036. doi: 10.1681/ASN.2019030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, Item S1, Tables S1-S3