Abstract
In patients with advanced-stage chronic kidney disease (CKD), progressive kidney function decline leads to increased risk for hyperkalemia (serum potassium > 5.0 or >5.5 mEq/L). Medications such as renin-angiotensin-aldosterone system inhibitors pose an additional hyperkalemia risk, especially in patients with CKD. When hyperkalemia develops, clinicians often recommend a diet that is lower in potassium content. This review discusses the barriers to adherence to a low-potassium diet and the impact of dietary restrictions on adverse clinical outcomes. Accumulating evidence indicates that a diet that incorporates potassium-rich foods has multiple health benefits, which may also be attributable to the other vitamin, mineral, and fiber content of potassium-rich foods. These benefits include blood pressure reductions and reduced risks for cardiovascular disease and stroke. High-potassium foods may also prevent CKD progression and reduce mortality risk in patients with CKD. Adjunctive treatment with the newer potassium-binding agents, patiromer and sodium zirconium cyclosilicate, may allow for optimal renin-angiotensin-aldosterone system inhibitor therapy in patients with CKD and hyperkalemia, potentially making it possible for patients with CKD and hyperkalemia to liberalize their diet. This may allow them the health benefits of a high-potassium diet without the increased risk for hyperkalemia, although further studies are needed.
Index Words: Chronic kidney disease, hyperkalemia, plant-based diet, potassium, potassium-binder, patiromer, renin-angiotensin-aldosterone system inhibitor, sodium zirconium cyclosilicate
Introduction
The kidney is the major organ responsible for regulating potassium absorption and excretion in the body.1,2 In patients with advanced chronic kidney disease (CKD), the progressive decline in kidney function contributes to the development of hyperkalemia, the definition of which varies between institutional guidelines and clinical studies and is generally considered to be a serum potassium level > 5.0 or > 5.5 mEq/L.1,3,4 Hyperkalemia risk increases as CKD progresses,3 with a reported prevalence of up to 10% in those with CKD without kidney replacement therapy, 16% in patients with kidney failure receiving hemodialysis, and 11% in those receiving continuous ambulatory peritoneal dialysis.4
Hyperkalemia is associated with increased all-cause mortality risk, especially among patients with CKD, heart failure, or diabetes.5 Both hyper- and hypokalemia are associated with adverse clinical outcomes, with a U-shaped association observed between serum potassium level and risk for mortality or major adverse cardiac events in patients with CKD.6 Interestingly, newer data suggest that patients with advanced CKD may have increased tolerance to elevated serum potassium levels. Specifically, data suggest that 90-day mortality rates with out-of-range plasma potassium concentrations (normal range, 3.5-5.0 mEq/L) are reduced among patients with stages 4-5 CKD versus those with stages 1-2 CKD.7
Dietary potassium restriction is often recommended to prevent and treat hyperkalemia in patients with CKD.8,9 However, evidence to support stringent reductions in dietary sources of potassium are lacking for some patients. Accumulating evidence suggests that a high-potassium diet, which includes foods that are high in potassium as well as other vitamins, minerals, and fiber, is associated with several health benefits, including prevention of CKD progression, improvements in blood pressure (BP) and bone health, and decreased risks for cardiovascular disease (CVD) and coronary artery disease (CAD), diabetes, kidney stone formation, and stroke.10, 11, 12, 13, 14 Moreover, many potassium-rich foods, including fresh fruits and vegetables, grains, and legumes, are considered “heart-healthy” (Table 1).15
Table 1.
Summary of Heart-Healthy Foods That Are Restricted by a Low-Potassium Diet
| Recommended Food Groups | Examples of Heart-Healthy Foods | Restricted by Low-Potassium Diet? |
|---|---|---|
| Fruits and vegetables | Green vegetables (eg, spinach, kale, collard greens, broccoli, asparagus) | Yes (except kale) |
| Berries (eg, strawberries, blueberries, blackberries, raspberries) | No | |
| Avocadoes | Yes | |
| Potatoes and sweet potatoes | Yes | |
| Carrots | Yes (if raw) | |
| Peppers | No | |
| Pumpkin and squash | Yes | |
| Tomatoes | Yes | |
| Cantaloupes and papaya | Yes | |
| Citrus fruits (eg, oranges, grapefruits) | Yes | |
| Apples | No | |
| Garlic | No | |
| Soybeans | Yes | |
| Whole grains | Whole wheat | Yes (bran/bran products) |
| Brown rice | ||
| Oats | ||
| Rye | ||
| Barley | ||
| Buckwheat | ||
| Quinoa | ||
| Low-fat dairy products | Fat-free or low-fat milk, yoghurt, and cheese | Yes |
| Skinless poultry and fish | Prepared without added saturated or trans fat | Yes |
| Fish high in omega-3 | ||
| Nuts and legumes | Walnuts | Yes |
| Almonds | Yes | |
| Seeds (eg, chia, flaxseed, hemp) | Yes | |
| Dried beans and lentils | Yes | |
| Nontropical vegetable oils | Extra-virgin olive oil | No |
The Dietary Approaches to Stop Hypertension (DASH) diet, which is high in fruits and vegetables, low-fat dairy, lean meats, fish, poultry, whole grains, nuts, seeds, and legumes and low in saturated fat and sugar, lowers BP, particularly when combined with a reduced sodium intake.16,17 Adherence to the DASH diet is also associated with reduced risks for incident CVD, CAD, stroke, and diabetes12 and lower mortality risk from CVD, CAD, or stroke.10 In addition, the DASH diet has been associated with significant reductions in markers of bone turnover, indicating potential beneficial effects on bone health.14
There are data suggesting that high-potassium intake increases the risk for hyperkalemia in some patients with CKD, and current recommendations and guidelines severely restrict dietary potassium in those patients with CKD, leading to a mismatch between overall health recommendations regarding the benefits of a plant-based/high-potassium diet and the type of diet recommended in patients with CKD. This review discusses dietary potassium restriction in patients with CKD and summarizes the barriers to patient adherence and the impact on adverse clinical outcomes associated with dietary potassium restriction. This review also re-examines the evidence supporting a plant-based diet in some patients with CKD and alternative approaches to risk reduction for recurrent hyperkalemia, including the use of newer potassium-binding agents.
Influence of Dietary Potassium Intake on Serum Potassium
The diet is a primary source of potassium, with the highest potassium content found in fruits, vegetables, and meats.3 Current dietary guidelines recommend potassium intake of 3,400 mg/d in adult men and 2,600 mg/d in adult women with normal kidney function.18 These recommendations take into account the health and cardioprotective benefits of a high-potassium diet.11
Potassium content varies widely among different foods and is dependent on the method of food preparation, its carbohydrate content, and whether it is combined with other foods that influence potassium homeostasis. This presents a challenge when determining the relative potassium exposure from the diet. Additionally, potassium content does not always directly equate to the bioavailability of potassium from food, which may differ based on its preparation.19,20 For example, the potassium content of fresh green beans is reduced by 15% with soaking, 33% with cooking, and 46% with soaking and cooking.20
The contribution of dietary intake to potassium balance may vary based on the carbohydrate content of the food. Although steady-state serum potassium concentrations are maintained by renal potassium excretion,2 high-potassium foods that also contain high carbohydrate levels initially promote insulin secretion, which facilitates potassium uptake into cells and minimizes the increase in serum potassium levels.21,22 In contrast, high-potassium foods that are low in carbohydrates, such as meat, may result in greater increases in serum potassium levels despite having similar potassium content to high-carbohydrate foods. The processing of foods may also influence potassium content because there are hidden sources of potassium due to food additives and products such as low-sodium salt substitutes.23 Therefore, this review does not include a table of potassium content of various foods because such tables may be misleading because they present an incomplete picture of actual dietary potassium exposure. A recent meta-analysis indicated that restriction of dietary potassium significantly reduces serum potassium levels in patients with CKD; however, this effect was driven by the results of 1 randomized trial, in which dietary potassium intake was strictly controlled with the sole source of nutrition provided by a manufactured liquid diet, which may not be attainable under normal dietary conditions.24
Dietary potassium intake can be assessed by recording meal intake using a food diary or questionnaire, dietary recall, or measurement of urinary potassium excretion.11,25, 26, 27, 28, 29 However, diaries and recall rely on patients reporting all consumed food and may be subject to inaccuracies. Urinary potassium excretion is generally considered to be a better indicator of potassium intake, although other variables should also be measured to increase its validity.
Studies using urinary potassium excretion to estimate dietary potassium intake have shown conflicting results regarding the association between urinary potassium excretion and CKD progression.25, 26, 27, 28 A population-based study in the Netherlands25 and a Korean study in patients with CKD26 both demonstrated higher risk for CKD development or progression with low urinary potassium excretion. In contrast, the Chronic Renal Insufficiency Cohort (CRIC) Study in US patients with CKD found that increased urinary potassium excretion was associated with an increased risk for CKD progression,27 and the Modification of Diet in Renal Disease Study in patients with stages 2-4 CKD showed no association between urinary potassium excretion and kidney failure (defined as initiation of dialysis or kidney transplantation).28
Potential Benefits of a Plant-Based Diet
CKD Incidence or Progression
Evidence suggests that a plant-based diet, which includes consumption of high-potassium foods, may prevent the development of CKD in individuals with relatively preserved or normal kidney function (Table 2).30, 31, 32, 33 For example, adherence to a healthy plant-based diet (fruits, vegetables, whole grains, nuts, and legumes) was associated with lower risk for developing CKD,30,31 whereas a higher animal-based protein intake was associated with increased risk for kidney hyperfiltration and rapid kidney function decline.34 Similarly, a Mediterranean-style diet (ie, high in fruits and vegetables) was associated with lower risk for incident loss of kidney function (estimated glomerular filtration rate [eGFR] < 60 mL/min per 1.73 m2).32 Although red meat intake is strongly associated with increased risk for kidney failure in the general population, substituting 1 daily serving of red meat with another source of protein was shown to reduce the risk for kidney failure by 62% (for poultry), 50% (for soy, legumes, or eggs), or 49% (for fish).33 A meta-analysis of dietary studies in individuals with normal kidney function found that healthy dietary patterns (ie, rich in fruit, vegetables, whole grains, legumes, nuts, and fish and low in sodium, sugar-sweetened drinks, and red and processed meats) were associated with lower odds of incident CKD and albuminuria.35
Table 2.
Summary of Studies Investigating the Potential Benefits of a Plant-Based Diet in CKD Outcomes
| Study | Design (mean or median follow-up) | Population (n) | Parameter(s) Studied | CKD Outcome |
|---|---|---|---|---|
| ARIC Study30 | Prospective cohort (24 y) | Age 45-64 y, eGFR ≥ 60 mL/min/1.73 m2 at baseline (n = 14,686) | Overall plant-based diet, healthy plant-based diet, and less healthy plant-based diet | Lower risk for incident CKDa and slower annual eGFR decline with highest intake of overall and healthy plant-based diets |
| CRIC Study36 | Prospective cohort (4 y) | Age 21-74 y, eGFR 20–70 mL/min/1.73 m2 at baseline (n = 3,939) | Lifestyle factors: regular physical activity, BMI, nonsmoking, and healthy diet | No significant association between healthy diet and CKD progression |
| Jhee et al,31 2019 | Prospective cohort study (8.2 y) | Age 40-69 y, eGFR ≥ 60 mL/min/1/73 m2 at baseline (n = 9,229) | Daily consumption of fruit and vegetables (nonfermented or fermented) | Lower risk for incident CKDa with highest vs lowest intake of nonfermented vegetables, but no significant risk reduction with any level of intake of fermented vegetables or fruits |
| Northern Manhattan Study32 | Prospective cohort study (6.9 y) | Age > 40 y, eGFR ≥60 mL/min/1.73 m2 at baseline (n = 803) | MeDi score (< 5 vs ≥ 5)b | Lower odds of incident CKDa with MeDi score ≥ 5 vs < 5 |
| Singapore Chinese Health Study33 | Prospective cohort study (15.5 y) | Age 45-74 y, eGFR ≥ 15 mL/min/1.73 m2 (n = 60,198) | Red meat vs other protein sources | Dose-dependent increase in risk for ESKD with red meat intake; risk reduction when daily red meat replaced with other protein |
| Goraya et al,37 2014 | Randomized interventional study (3 y) | Age ≥ 18 y, nondiabetic stage 3 CKD (eGFR 30-50 mL/min/1.73 m2) with metabolic acidosis (n = 108) | Fruit + vegetables, oral NaHCO3, or usual care (no alkali therapy) | Reduced urine excretion of angiotensinogen and slower rates of eGFR decline with fruit + vegetables or NaHCO3 vs usual care |
| REGARDS Study38 | Prospective cohort study (6.4 y) | Age ≥ 45 y, eGFR < 60 mL/min/1.73 m2 (n = 6,009) | Dietary patterns: convenience, plant-based, sweets/fat, Southern, alcohol/salads | No significant association between risk for ESKD and convenience, plant-based, sweets/fat, or alcohol/salads dietary patterns |
| Satirapoj et al,39 2018 | Retrospective cohort study (1 y) | Age ≥ 18 y, nondialysis stages 3-4 CKD (eGFR 15-59 mL/min/1.73 m2) (n = 140) | Very low-protein diet + KA/EAA vs low-protein diet | Significantly slower rate of eGFR decline with very low-protein diet + KA/EAA vs low-protein diet |
Abbreviations: ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; CKD, chronic kidney disease; CRIC, Chronic Renal Insufficiency Cohort; eGFR, estimate glomerular filtration rate; ESKD, end-stage kidney disease; KA/EAA, ketoacid analogues of essential amino acids; MeDi, Mediterranean Diet; NaHCO3, sodium bicarbonate; REGARDS, Reasons for Geographic and Racial Differences in Stroke.
Defined as incident occurrence of eGFR < 60 mL/min/1.73 m2.
MeDi score ranged from 0 to 9, with higher scores representing closer similarity to a Mediterranean diet.
A balanced plant-based diet may also slow CKD progression among patients with CKD without kidney replacement therapy (Table 2).36, 37, 38, 39 In the CRIC Study, patients with mild to moderate CKD and a healthy lifestyle, including higher than median consumption of fruits and vegetables, regular physical activity, a normal body mass index, and a history of nonsmoking, had lower risk for CKD progression.36 In patients with stage 3 CKD and metabolic acidosis, a reduction in dietary acid by 50% through increased fruit and vegetable consumption or oral sodium bicarbonate administration for 3 years appeared to be kidney protective, with preserved eGFR and decreased urinary excretion of angiotensinogen compared with no alkali therapy.37 In patients with stages 3-4 CKD, a very low-protein diet with ketoacid supplementation may delay CKD progression compared with a low-protein diet alone.39 However, a US study of patients with CKD found that dietary patterns were not associated with increased risk for progression to kidney failure over time.38
Cardiovascular and Mortality Outcomes
Studies have indicated that a plant-based diet has potential benefits in cardiovascular and mortality outcomes (Table 3).36,38,40, 41, 42, 43, 44, 45 In the CRIC Study, risks for atherosclerotic events and all-cause mortality were reduced among patients with mild to moderate CKD with a healthy lifestyle.36 Similarly, another US study of patients with CKD found that a diet rich in fruits, vegetables, and fish appeared to lower the risk for mortality over time.38 Additional studies of patients undergoing maintenance dialysis showed beneficial effects of increased fruit and/or vegetable intake,40,41 which was associated with lower risk for all-cause mortality over 12 months40 and lower risks for noncardiovascular and all-cause mortality over 3 years.41 However, the risk for cardiovascular mortality was not significantly reduced by increased fruit and vegetable intake.41 In patients with CKD and metabolic acidosis, increased fruit and vegetable consumption over 5 years was associated with improvements in CVD risk factors, including body mass index, systolic BP, and low-density lipoprotein, lipoprotein(a), and serum vitamin K levels.42 A National Health and Nutrition Examination Survey study showed that a diet high in plant-based protein was associated with lower risk for mortality,43 and in a small study of patients with moderate CKD, a reduced-sodium DASH diet for 2 weeks led to improvements in nocturnal diastolic BP and was not associated with hyperkalemia. However, in the latter study, mean serum potassium levels showed a transient increase after 1 week of the DASH diet (change from baseline, +0.07 ± 0.01 mEq/L; P = 0.04) that was not significant after 2 weeks (+0.04 ± 0.07 mEq/L; P = 0.13).44 A meta-analysis of dietary intervention studies in patients with CKD concluded that further research was needed to confirm the beneficial effects of a plant-based diet on mortality and cardiovascular events.46 Further studies are also needed to establish the health benefits of a high-potassium diet in patients with CKD.
Table 3.
Summary of Studies Investigating the Potential Benefits of a Plant-Based Diet in Cardiovascular and Mortality Outcomes
| Study | Design (mean or median follow-up) | Population (n) | Parameter(s) Studied | CV and Mortality Outcomes |
|---|---|---|---|---|
| CRIC Study36 | Prospective cohort (4 y) | Age 21-74 y, eGFR 20-70 mL/min/1.73 m2 (n = 3,939) | Lifestyle factors: regular physical activity, BMI, nonsmoking, and healthy diet | Higher diet scores were not associated with reduced risk for atherosclerotic events or mortality |
| Diaz-Martinez et al,40 2019 | Prospective study (1 y) | HD patients (n = 77) | Dietary components | Mortality risk reduced 4-fold with intake of ≥2 fruit servings/d and 5-fold with ≥7 g/d cereal fiber |
| DIET-HD Study41 | Prospective cohort study (2.7 y) | HD patients (n = 9,757) | Dietary intake (fruits and vegetables) | Lower risk for all-cause mortality and CV and non-CV mortality with highest vs lowest intake of fruit and vegetables |
| Goraya et al,42 2019 | Randomized interventional study (5 y) | Age ≥ 18 y, nondiabetic CKD (30-59 mL/min/1.73 m2) with metabolic acidosis (n = 108) | Fruit + vegetables, oral NaHCO3, or usual care (no alkali therapy) | Better improvement in CV risk factors with fruit + vegetables than NaHCO3 or usual care |
| NHANES III Study43 | Observational study (8.4 ys) | Age ≥ 20 y, eGFR < 60 mL/min/1.73 m2 (n = 1,065) | Plant protein ratio | Mortality risk significantly reduced with each 33% increase in plant protein ratio |
| REGARDS Study38 | Prospective cohort study (6.4 y) | Age ≥ 45 y, CKD (< 60 mL/min/1.73 m2) (n = 6,009) | Dietary patterns: convenience, plant-based, sweets/fat, Southern, alcohol/salads | Lower risk for mortality with highest vs lowest consumption of plant-based diet |
| Tyson et al,44 2016 | Prospective pilot study (2 wk) | Age ≥ 18 y, eGFR 30-59.9 mL/min/1.73 m2 with hypertension (n = 11) | Reduced-sodium DASH diet | Significantly lower night-time DBP and improvements in lowering nocturnal SBP and DBP with DASH diet |
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; CRIC, Chronic Renal Insufficiency Cohort; CV, cardiovascular; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; DIET-HD, Dietary Intake, Death and Hospitalization in Adults With ESKD Treated With Hemodialysis; eGFR, estimated glomerular filtration rate; HD, hemodialysis; NaHCO3, sodium bicarbonate; NHANES, National Health and Nutrition Examination Survey; REGARDS, Reasons for Geographic and Racial Differences in Stroke; SBP, systolic blood pressure.
Dietary Potassium Restriction in Patients with CKD
Challenges of Dietary Potassium Restriction
As discussed, a low-potassium diet is currently recommended in patients with advanced CKD and hyperkalemia. Clinical guidelines recommend that patients with CKD and hyperkalemia receive individualized dietary advice regarding the need to restrict potassium intake, when indicated.8,9 A recent Kidney Disease: Improving Global Outcomes (KDIGO) consensus report recognized the validity of dietary potassium restriction as a strategy for managing acute hyperkalemia; however, this report also hypothesized that restriction of dietary potassium for the prevention of hyperkalemia in CKD may deprive patients of the benefits of a high-potassium diet.47 The KDIGO report acknowledged the lack of direct evidence supporting dietary potassium restriction in patients with CKD, although they found no evidence confirming the safety of increased potassium intake in those with advanced CKD.47 The KDIGO report recommended developing educational materials that include information regarding the potassium content of foods that promote a plant-based low-potassium diet, to be used when a reduction in high-potassium foods is clinically indicated.47
Most recommendations for a low-potassium diet assume similar bioavailability of the potassium in different foods.48 The data demonstrating the need for potassium restriction in patients with declining kidney function originated from a potassium balance study conducted in the 1940s, in which potassium tablet administration in individuals with normal kidney function resulted in a significant increase in serum potassium levels.49 Based on these findings and others, there is a long-held belief that potassium-containing foods increase serum potassium levels. However, there is limited evidence to suggest that plant-based diets are associated with increased risk for hyperkalemia in patients with CKD, with 1 study reporting hyperkalemia associated with a plant-based diet in a patient with type IV renal tubular acidosis.50 In an observational study of patients receiving long-term hemodialysis, an increase in dietary potassium intake was weakly correlated with predialysis serum potassium levels,29 whereas another study of hemodialysis patients found no correlation between serum potassium levels and absolute reported potassium intake or potassium density.51
As mentioned, hyperkalemia risk factors may differ among patients with advanced CKD because these individuals are potentially more tolerant of higher serum potassium levels than those with normal kidney function. It is hypothesized that patients with advanced CKD sense and maintain potassium homeostasis by different mechanisms, with the gastrointestinal tract potentially playing a role in potassium homeostasis in addition to the kidneys.52 Gastrointestinal potassium excretion may increase to compensate for reduced kidney function. For example, in healthy adults with a “normal” diet, ∼10% of potassium intake is actively secreted by the colon and excreted in feces,53 whereas fecal potassium excretion appears to be approximately 3-fold higher in patients with kidney failure.54 However, it is unclear whether this increase in fecal potassium excretion plays a clinically significant role in maintaining potassium homeostasis.53 Furthermore, there are no guidelines regarding how much dietary potassium restriction is needed to prevent hyperkalemia in patients with advanced CKD. This generally varies according to the patient’s age and comorbid conditions and further research is needed before recommendations can be made.
Adherence to Low-Potassium Diet
Patient adherence to a low-potassium diet requires individualized dietary regimens, skilled dietitians, and regular counseling, which may be available in hospitals and dialysis units but are less common in clinical practice.55 For example, outpatient nephrologists typically do not have the resources for this service.
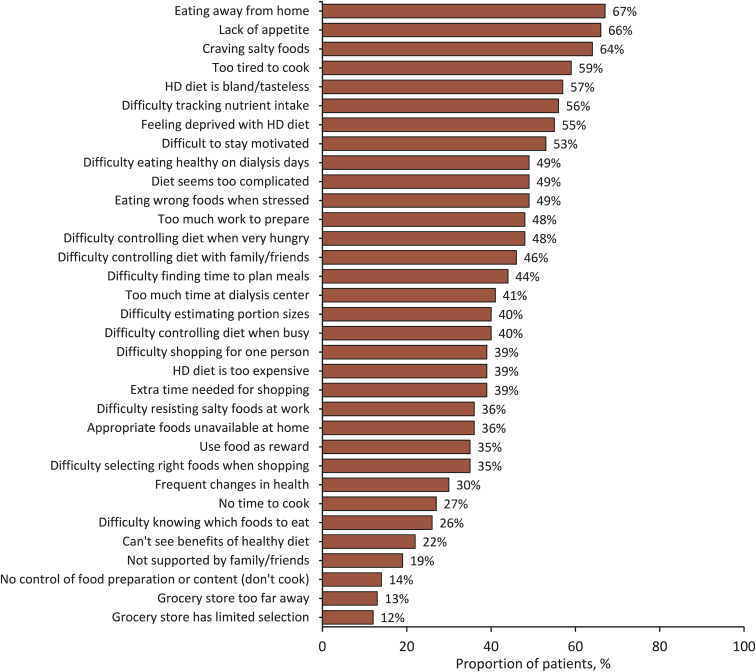
Dietary recommendations for patients receiving hemodialysis are considered to be among the most restrictive and may thus lead to reduced adherence in many patients,15 with nonadherence estimated at 25% to 86%.56 Patient-reported barriers to adherence include eating away from home, lack of appetite, craving salty foods, being too tired to cook, finding the diet bland and tasteless, difficulty tracking nutrient intake, feeling deprived, and lack of motivation to eat the right foods, each reported by more than half the participants (Fig 1).51 Onset of depression, stress, beliefs about loss of control, and the patient's level of social support also contribute to nonadherence.56, 57, 58 Adding a low-potassium diet to already restricted diets required in comorbid conditions such as diabetes and heart disease can also increase the burden of dietary requirements.59
Figure 1.
Patient-reported barriers to adherence to hemodialysis (HD) diet, as reported by St-Jules et al51 2016.
Nondietary Factors Affecting Serum Potassium
In addition to the effects of dietary intake, medications such as renin-angiotensin-aldosterone system (RAAS) inhibitors can also affect serum potassium levels. Hyperkalemia risk often represents a barrier to optimal RAAS-inhibitor treatment,60 which is recommended in patients with CKD.8 RAAS-inhibitor therapy is associated with reductions in the rate of CKD progression, BP, and the risks for CVD and all-cause mortality.61, 62, 63 However, because aldosterone regulates potassium excretion by the kidneys,22 RAAS-inhibitor use in patients with reduced kidney function can increase the risk for hyperkalemia.64 Physicians often reduce the dose or discontinue RAAS-inhibitor therapy entirely when hyperkalemia develops, which results in suboptimal RAAS-inhibitor therapy in patients with CKD.60,65 In addition, patients with CKD have an increased risk for developing metabolic acidosis as eGFR declines.42 Metabolic acidosis can cause a shift of potassium out of cells, thereby increasing serum potassium levels and contributing to hyperkalemia risk.1
Impact of Low-Potassium Diet on Clinical Outcomes
In patients with CKD, the National Kidney Foundation (NKF) currently recommends limiting consumption of foods that are high in potassium.66 However, this conflicts with the American Heart Association’s recommendations for a heart-healthy diet.67 Restriction of high-potassium fruits and vegetables and the resultant reduction in fiber intake may also lead to adverse clinical outcomes in patients receiving hemodialysis, including an increased risk for CVD.68,69 In a Taiwanese study of patients with CKD, the kidney function–related dietary pattern, which includes a high intake of preserved or processed foods and low intake of plant-based foods, was correlated with being overweight or obese and a greater risk for CVD and worsening CKD.70 An NKF study showed that female patients with kidney failure receiving maintenance dialysis had significantly lower intake of fruits and vegetables, fiber, potassium, sodium, and phosphorus and a lower daily intake of protein compared with baseline data from the Women's Health Initiative-Dietary Modification (WHI-DM) trial of postmenopausal women without CKD.71 In the WHI-DM trial, eligible participants consumed ≥32% of their total energy intake from fat;72 whereas women receiving dialysis consumed significantly greater.71
A low-potassium diet may also lead to other nutritional deficiencies, such as folic acid deficiency, in patients with advanced CKD. A study of patients with CKD found 3-fold higher incidence of folic acid deficiency among patients with stages 3-4 CKD on a potassium-restricted diet compared with those with stages 1-2 CKD on an unrestricted potassium diet.73 Severe folic acid deficiency is associated with an increased risk for some cancers and can contribute to CAD and hematologic and neurologic disorders.73
Current diet recommendations in patients with CKD may also contribute to the development of acidosis. Dietary acid load is determined by the intake of acid-inducing foods, such as animal-based protein, and base-inducing foods, such as fruits and vegetables.74 A higher dietary acid load leads to increased ammonia production in the kidney proximal tubule and increased metabolic acidosis, which is associated with increased risk for incident CKD in the general population74 and increased risk for progression in patients with CKD.75,76 A higher dietary acid load has been shown to lead to increased risk for progression to kidney failure, particularly in patients with albuminuria with albumin excretion ≥ 30 mg/g75 and is associated with a faster decline in eGFR.76 In contrast, potassium intake and correction of acidosis was not shown to be related to a decline in eGFR.76 In patients with CKD and mild metabolic acidosis, reduction in dietary acid by 50% with either oral sodium bicarbonate or base-inducing fruits and vegetables over 5 years improved plasma total carbon dioxide levels (a measure of metabolic acidosis) and attenuated decline in eGFR.42
A low-potassium diet may lead to poor nutritional status in patients with advanced-stage CKD due to limited food choices. Data suggest that poor nutritional status can lead to increased mortality risk.77 In a study of patients with stage 5 CKD without kidney replacement therapy, 44% of patients were malnourished, and these patients had higher levels of inflammatory markers and a greater prevalence of atherosclerotic CVD compared with well-nourished patients, indicating a close association between malnutrition, inflammation, and atherosclerosis.77 This may be the result of low amounts of fruits and vegetables in the diet and a lack of essential naturally occurring vitamins and minerals.
A low-potassium diet, with limited high-fiber foods, could contribute to changes of the intestinal microbiota in patients with CKD. The diversity of the intestinal microbiota is significantly reduced in patients with kidney failure and increased uremia.78 This altered microbiota is associated with increases in intestinal barrier permeability, oxidative stress, systemic inflammation, and fibrosis.79,80 A plant-based diet may reduce inflammation through increased intake of antioxidants and fiber, and an increase in fiber may also lead to an increase in commensal bacteria and reduction in uremic dysbiosis.79 In patients with CKD, an increase in total fiber intake of 10 g/d reduced the risk for inflammation by 38% and all-cause mortality by 19%.81 Similarly, a population-based study has shown that an increase in total fiber intake of 5 g/d can reduce the risk for incident CKD by 11%.82
Impact of Low-Potassium Diet on Health-Related Quality of Life
Studies have suggested that poor nutritional status as a result of dietary potassium restrictions may cause reduced health-related quality of life (QoL).83,84 A 5-year study of patients receiving maintenance hemodialysis found that higher malnutrition-inflammation scores were associated with lower scores on the health-related QoL 36-Item Short Form Health Survey (SF-36) across all physical and mental dimensions and significantly increased the risk for mortality.84 In another study of patients receiving hemodialysis, poor nutritional status (ie, high body fat percentage and low serum albumin or creatinine concentrations) was associated with worse health-related QoL scores on the mental and physical domains of the SF-36 questionnaire.83 In this study, lower mental health scores were correlated with increased mortality risk.83 A systematic review of qualitative studies in patients with CKD indicated that complex dietary and fluid restrictions are associated with worsening of patient QoL and had a negative impact on patients’ relationships.85 However, the development of hyperkalemia was associated with significantly reduced health-related QoL in patients with CKD, particularly those receiving maintenance dialysis.86,87
Adjunctive Treatment with Potassium Binders
Adjunctive oral potassium-binder therapy has a potential role in managing hyperkalemia in patients with CKD. Until recently, the only potassium binder available for hyperkalemia management was sodium polystyrene sulfonate; however, long-term sodium polystyrene sulfonate use is limited by the associated risk for gastrointestinal adverse events.88 Two newer potassium binders, patiromer and sodium zirconium cyclosilicate, are now available for hyperkalemia treatment in the United States and European Union. In clinical studies that included patients with CKD and those receiving RAAS-inhibitor therapy, patiromer and sodium zirconium cyclosilicate showed efficacy for the treatment of hyperkalemia.89, 90, 91, 92, 93, 94, 95 Of note, the sodium zirconium cyclosilicate studies did not impose dietary restrictions during potassium-binder therapy,89, 90, 91, 92 indicating that sodium zirconium cyclosilicate may remain effective in patients who consume a heart-healthy plant-based diet. Both potassium binders were well tolerated in these studies, with no serious gastrointestinal adverse events. Long-term studies have indicated that these newer potassium binders can safely and effectively reduce the risk for recurrent hyperkalemia over 12 months.92,94
Studies have indicated that the newer potassium binders may be used in hemodialysis patients with hyperkalemia. In patients with kidney failure receiving hemodialysis, sodium zirconium cyclosilicate on nondialysis days reduced the incidence of predialysis hyperkalemia, with treatment response (predialysis serum potassium levels of 4.0-5.0 mEq/L during ≥3 of 4 hemodialysis sessions) achieved in 41% of patients with sodium zirconium cyclosilicate versus 1% with placebo.96 In a real-world study of patients with kidney failure receiving hemodialysis, patiromer significantly reduced serum potassium levels during the three 30-day intervals following initiation, with an ∼50% relative reduction in the prevalence of severe hyperkalemia (potassium ≥ 6.0 mEq/L) after patiromer therapy initiation.97 An analysis of a phase 3 study showed that patiromer was associated with reductions in BP and aldosterone levels, as well as lowering serum potassium levels, in patients with CKD and hyperkalemia who were receiving RAAS-inhibitor therapy, including those with hypertension and type 2 diabetes.98 Preliminary findings from the AMBER (Spironolactone With Patiromer in the Treatment of Resistant Hypertension in Chronic Kidney Disease) trial indicated that patiromer administration may allow for more patients with advanced CKD and resistant hypertension to continue spironolactone therapy.99
Taken together, these data suggest that patiromer and sodium zirconium cyclosilicate are both viable adjunctive long-term therapy options that may allow for diet liberalization in patients with CKD and hyperkalemia. In addition, the newer potassium binders were safe and effective in patients receiving concomitant RAAS-inhibitor therapy. Therefore, patiromer and sodium zirconium cyclosilicate use may also enable clinicians to optimize RAAS-inhibitor therapy, providing patients with the associated cardiorenal benefits of RAAS inhibitors while minimizing the risk for recurrent hyperkalemia. Further research is needed to determine whether potassium binders in combination with a plant-based diet can improve RAAS-inhibitor use and reduce the risk for recurrent hyperkalemia among patients with CKD.
Conclusions
Current clinical guidelines recommend limiting dietary potassium intake to prevent and treat hyperkalemia in patients with CKD. However, the traditional low-potassium diet is complex and can counteract the benefits of a plant-based diet, with only modest evidence to support the rationale for these dietary restrictions. Furthermore, reduction of fruit and vegetable intake due to their high-potassium content results in a less heart-healthy diet and may have adverse effects on the patient’s acid-base balance and intestinal microbiota. There is increasing evidence to support the multiple health benefits of a plant-based diet in patients with CKD, including improvements in BP and reductions in the risk for CVD, stroke, and CKD progression. Recent evidence suggests that adjunctive treatment with the newer potassium-binding agents may allow for diet liberalization and optimization of RAAS inhibitor therapy in patients with CKD and a history of hyperkalemia while minimizing the risk for recurrent hyperkalemia, although further studies are needed to confirm this.
Article Information
Authors’ Full Names and Academic Degrees
Deborah J. Clegg, PhD, Samuel A. Headley, PhD, and Michael J. Germain, MD.
Support
The development of this manuscript was supported by AstraZeneca. The authors had full editorial control of the manuscript and provided their final approval of all content.
Financial Disclosure
Dr Headley received research funding from Relypsa (the manufacturer of patiromer). Dr Germain received research funding from and is on speaker and advisory boards for AstraZeneca (the manufacturer of sodium zirconium cyclosilicate). Dr Clegg declares that she has no relevant financial interests.
Acknowledgements
The authors thank Sarah Greig, PhD, of inScience Communications (Auckland, New Zealand), and Meri Pozo, PhD, of inScience Communications (New York, NY), who provided editorial support funded by AstraZeneca.
Peer Review
Received March 24, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form April 24, 2020.
Footnotes
Complete author and article information provided before references.
References
- 1.DuBose T.D., Jr. Regulation of potassium homeostasis in CKD. Adv Chronic Kidney Dis. 2017;24(5):305–314. doi: 10.1053/j.ackd.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Palmer B.F. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015;10(6):1050–1060. doi: 10.2215/CJN.08580813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosano G.M.C., Tamargo J., Kjeldsen K.P. Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J Cardiovasc Pharmacother. 2018;4(3):180–188. doi: 10.1093/ehjcvp/pvy015. [DOI] [PubMed] [Google Scholar]
- 4.Belmar Vega L., Galabia E.R., Bada da Silva J. Epidemiology of hyperkalemia in chronic kidney disease. Nefrologia. 2019;39(3):277–286. doi: 10.1016/j.nefro.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Collins A.J., Pitt B., Reaven N. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213–221. doi: 10.1159/000479802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuland H., McEwan P., Evans M. Serum potassium as a predictor of adverse clinical outcomes in patients with chronic kidney disease: new risk equations using the UK clinical practice research datalink. BMC Nephrol. 2018;19(1):211. doi: 10.1186/s12882-018-1007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gasparini A., Evans M., Barany P. Plasma potassium ranges associated with mortality across stages of chronic kidney disease: the Stockholm CREAtinine Measurements (SCREAM) project. Nephrol Dial Transplant. 2019;34(9):1534–1541. doi: 10.1093/ndt/gfy249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.KDIGO KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):S1–S150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 9.KDOQI Chronic kidney disease: major recommendations. https://www.andeal.org/vault/pqnew95.pdf
- 10.Talaei M., Koh W.P., Yuan J.M., van Dam R.M. DASH dietary pattern, mediation by mineral intakes, and the risk of coronary artery disease and stroke mortality. J Am Heart Assoc. 2019;8(5) doi: 10.1161/JAHA.118.011054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aaron K.J., Sanders P.W. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013;88(9):987–995. doi: 10.1016/j.mayocp.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiavaroli L., Viguiliouk E., Nishi S.K. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11(2):338. doi: 10.3390/nu11020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferraro P.M., Taylor E.N., Gambaro G., Curhan G.C. Dietary and lifestyle risk factors associated with incident kidney stones in men and women. J Urol. 2017;198(4):858–863. doi: 10.1016/j.juro.2017.03.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin P.H., Ginty F., Appel L.J. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. 2003;133(10):3130–3136. doi: 10.1093/jn/133.10.3130. [DOI] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K., Tortorici A.R., Chen J.L. Dietary restrictions in dialysis patients: is there anything left to eat? Semin Dial. 2015;28(2):159–168. doi: 10.1111/sdi.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appel L.J., Moore T.J., Obarzanek E. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 17.Sacks F.M., Svetkey L.P., Vollmer W.M. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 18.The National Academies of Sciences Engineering and Medicine Dietary reference intakes for sodium and potassium. http://www.nationalacademies.org/hmd/Reports/2019/dietary-reference-intakes-sodium-potassium.aspx [PubMed]
- 19.Macdonald-Clarke C.J., Martin B.R., McCabe L.D. Bioavailability of potassium from potatoes and potassium gluconate: a randomized dose response trial. Am J Clin Nutr. 2016;104(2):346–353. doi: 10.3945/ajcn.115.127225. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Pineda M., Yague-Ruiz C., Caverni-Munoz A., Vercet-Tormo A. Reduction of potassium content of green bean pods and chard by culinary processing. Tools for chronic kidney disease. Nefrologia. 2016;36(4):427–432. doi: 10.1016/j.nefro.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo R.A., Felig P., Ferrannini E., Wahren J. Effect of graded doses of insulin on splanchnic and peripheral potassium metabolism in man. Am J Physiol. 1980;238(5):E421–E427. doi: 10.1152/ajpendo.1980.238.5.E421. [DOI] [PubMed] [Google Scholar]
- 22.Palmer B.F., Clegg D.J. Physiology and pathophysiology of potassium homeostasis: core curriculum 2019. Am J Kidney Dis. 2019;74(5):682–695. doi: 10.1053/j.ajkd.2019.03.427. [DOI] [PubMed] [Google Scholar]
- 23.Cupisti A., Kovesdy C.P., D'Alessandro C., Kalantar-Zadeh K. Dietary approach to recurrent or chronic hyperkalaemia in patients with decreased kidney function. Nutrients. 2018;10(3):261. doi: 10.3390/nu10030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris A., Krishnan N., Kimani P.K., Lycett D. Effect of dietary potassium restriction on serum potassium, disease progression, and mortality in chronic kidney disease: a systematic review and meta-analysis. J Ren Nutr. 2020;30(4):276–285. doi: 10.1053/j.jrn.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Kieneker L.M., Bakker S.J., de Boer R.A., Navis G.J., Gansevoort R.T., Joosten M.M. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease. Kidney Int. 2016;90(4):888–896. doi: 10.1016/j.kint.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Kim H.W., Park J.T., Yoo T.H. Urinary potassium excretion and progression of CKD. Clin J Am Soc Nephrol. 2019;14(3):330–340. doi: 10.2215/CJN.07820618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He J., Mills K.T., Appel L.J. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol. 2016;27(4):1202–1212. doi: 10.1681/ASN.2015010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonberg-Yoo A.K., Tighiouart H., Levey A.S., Beck G.J., Sarnak M.J. Urine potassium excretion, kidney failure, and mortality in CKD. Am J Kidney Dis. 2017;69(3):341–349. doi: 10.1053/j.ajkd.2016.03.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noori N., Kalantar-Zadeh K., Kovesdy C.P. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56(2):338–347. doi: 10.1053/j.ajkd.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim H., Caulfield L.E., Garcia-Larsen V. Plant-based diets and incident CKD and kidney function. Clin J Am Soc Nephrol. 2019;14(5):682–691. doi: 10.2215/CJN.12391018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jhee J.H., Kee Y.K., Park J.T. A diet rich in vegetables and fruit and incident CKD: a community-based prospective cohort study. Am J Kidney Dis. 2019;74(4):491–500. doi: 10.1053/j.ajkd.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Khatri M., Moon Y.P., Scarmeas N. The association between a Mediterranean-style diet and kidney function in the Northern Manhattan Study cohort. Clin J Am Soc Nephrol. 2014;9(11):1868–1875. doi: 10.2215/CJN.01080114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lew Q.J., Jafar T.H., Koh H.W. Red meat intake and risk of ESRD. J Am Soc Nephrol. 2017;28(1):304–312. doi: 10.1681/ASN.2016030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jhee J.H., Kee Y.K., Park S. High-protein diet with renal hyperfiltration is associated with rapid decline rate of renal function: a community-based prospective cohort study. Nephrol Dial Transplant. 2019;35(1):98–106. doi: 10.1093/ndt/gfz115. [DOI] [PubMed] [Google Scholar]
- 35.Bach K.E., Kelly J.T., Palmer S.C., Khalesi S., Strippoli G.F.M., Campbell K.L. Healthy dietary patterns and incidence of CKD: a meta-analysis of cohort studies. Clin J Am Soc Nephrol. 2019;14(10):1441–1449. doi: 10.2215/CJN.00530119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricardo A.C., Anderson C.A., Yang W. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2015;65(3):412–424. doi: 10.1053/j.ajkd.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goraya N., Simoni J., Jo C.H., Wesson D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014;86(5):1031–1038. doi: 10.1038/ki.2014.83. [DOI] [PubMed] [Google Scholar]
- 38.Gutierrez O.M., Muntner P., Rizk D.V. Dietary patterns and risk of death and progression to ESRD in individuals with CKD: a cohort study. Am J Kidney Dis. 2014;64(2):204–213. doi: 10.1053/j.ajkd.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satirapoj B., Vongwattana P., Supasyndh O. Very low protein diet plus ketoacid analogs of essential amino acids supplement to retard chronic kidney disease progression. Kidney Res Clin Pract. 2018;37(4):384–392. doi: 10.23876/j.krcp.18.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz-Martinez J., Martinez-Motta P., Delgado-Enciso I. Diet and all-cause mortality in hemodialysis patients. Curr Dev Nutr. 2019;3(suppl 1) nzz039.P18-010-19. [Google Scholar]
- 41.Saglimbene V.M., Wong G., Ruospo M. Fruit and vegetable intake and mortality in adults undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2019;14:250–260. doi: 10.2215/CJN.08580718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goraya N., Munoz-Maldonado Y., Simoni J., Wesson D.E. Fruit and vegetable treatment of chronic kidney disease-related metabolic acidosis reduces cardiovascular risk better than sodium bicarbonate. Am J Nephrol. 2019;49(6):438–448. doi: 10.1159/000500042. [DOI] [PubMed] [Google Scholar]
- 43.Chen X., Wei G., Jalili T. The associations of plant protein intake with all-cause mortality in CKD. Am J Kidney Dis. 2016;67(3):423–430. doi: 10.1053/j.ajkd.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyson C.C., Lin P.H., Corsino L. Short-term effects of the DASH diet in adults with moderate chronic kidney disease: a pilot feeding study. Clin Kidney J. 2016;9(4):592–598. doi: 10.1093/ckj/sfw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Headley S.A., Hutchinson J., Thompson B. The effect of a personalized multi-component lifestyle intervention program in stage 3 & 4 CKD patients [abstract 1646] Med Sci Sports Exerc. 2019;51(6):450. [Google Scholar]
- 46.Palmer S.C., Maggo J.K., Campbell K.L. Dietary interventions for adults with chronic kidney disease. Cochrane Database Syst Rev. 2017;4:CD011998. doi: 10.1002/14651858.CD011998.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clase C.M., Carrero J.J., Ellison D.H. Potassium homeostasis and management of dyskalaemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020;97(1):42–61. doi: 10.1016/j.kint.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 48.St-Jules D.E., Goldfarb D.S., Sevick M.A. Nutrient non-equivalence: does restricting high-potassium plant foods help to prevent hyperkalemia in hemodialysis patients? J Ren Nutr. 2016;26(5):282–287. doi: 10.1053/j.jrn.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keith N.M., Osterberg A.E., Burchell H.B. Some effects of potassium salts in man. Ann Intern Med. 1942;16(5):879–892. [Google Scholar]
- 50.Moorthi R.N., Armstrong C.L., Janda K., Ponsler-Sipes K., Asplin J.R., Moe S.M. The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am J Nephrol. 2014;40(6):582–591. doi: 10.1159/000371498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.St-Jules D.E., Woolf K., Pompeii M.L., Sevick M.A. Exploring problems in following the hemodialysis diet and their relation to energy and nutrient intakes: the BALANCEWISE study. J Ren Nutr. 2016;26(2):118–124. doi: 10.1053/j.jrn.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batlle D., Boobes K., Manjee K.G. The colon as the potassium target: entering the colonic age of hyperkalemia treatment? EBioMedicine. 2015;2(11):1562–1563. doi: 10.1016/j.ebiom.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agarwal R., Afzalpurkar R., Fordtran J.S. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology. 1994;107(2):548–571. doi: 10.1016/0016-5085(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 54.Mathialahan T., Maclennan K.A., Sandle L.N., Verbeke C., Sandle G.I. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol. 2005;206(1):46–51. doi: 10.1002/path.1750. [DOI] [PubMed] [Google Scholar]
- 55.Centers for Medicare & Medicaid Services Medicare and Medicaid programs; conditions for coverage for end-stage renal disease facilities; final rule. Fed Regist. 2008;73(73):20369–20484. [PubMed] [Google Scholar]
- 56.Oquendo L.G., Asencio J.M.M., de Las Nieves C.B. Contributing factors for therapeutic diet adherence in patients receiving haemodialysis treatment: an integrative review. J Clin Nurs. 2017;26(23-24):3893–3905. doi: 10.1111/jocn.13804. [DOI] [PubMed] [Google Scholar]
- 57.Gibson E.L., Held I., Khawnekar D., Rutherford P. Differences in knowledge, stress, sensation seeking, and locus of control linked to dietary adherence in hemodialysis patients. Front Psychol. 2016;7:1864. doi: 10.3389/fpsyg.2016.01864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lambert K., Mullan J., Mansfield K. An integrative review of the methodology and findings regarding dietary adherence in end stage kidney disease. BMC Nephrol. 2017;18(1):318. doi: 10.1186/s12882-017-0734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bataille S., Landrier J.F., Astier J. Haemodialysis patients with diabetes eat less than those without: a plea for a permissive diet. Nephrology (Carlton) 2017;22(9):712–719. doi: 10.1111/nep.12837. [DOI] [PubMed] [Google Scholar]
- 60.Epstein M., Reaven N.L., Funk S.E., McGaughey K.J., Oestreicher N., Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 suppl):S212–S220. [PubMed] [Google Scholar]
- 61.Jafar T.H., Schmid C.H., Landa M. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135(2):73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- 62.Karaboyas A., Xu H., Morgenstern H. DOPPS data suggest a possible survival benefit of renin angiotensin-aldosterone system inhibitors and other antihypertensive medications for hemodialysis patients. Kidney Int. 2018;94(3):589–598. doi: 10.1016/j.kint.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 63.Xie X., Liu Y., Perkovic V. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67(5):728–741. doi: 10.1053/j.ajkd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 64.Bandak G., Sang Y., Gasparini A. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) project. J Am Heart Assoc. 2017;6(7):e005428. doi: 10.1161/JAHA.116.005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jun M., Jardine M.J., Perkovic V. Hyperkalemia and renin-angiotensin aldosterone system inhibitor therapy in chronic kidney disease: a general practice-based, observational study. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0213192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.National Kidney Foundation Potassium and your CKD diet. https://www.kidney.org/atoz/content/potassium
- 67.American Heart Association The American Heart Association diet and lifestyle recommendations. https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/aha-diet-and-lifestyle-recommendations
- 68.Khoueiry G., Waked A., Goldman M. Dietary intake in hemodialysis patients does not reflect a heart healthy diet. J Ren Nutr. 2011;21(6):438–447. doi: 10.1053/j.jrn.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 69.Kalantar-Zadeh K., Kopple J.D., Deepak S., Block D., Block G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002;12(1):17–31. doi: 10.1053/jren.2002.29598. [DOI] [PubMed] [Google Scholar]
- 70.Kurniawan A.L., Hsu C.Y., Rau H.H., Lin L.Y., Chao J.C. Association of kidney function-related dietary pattern, weight status, and cardiovascular risk factors with severity of impaired kidney function in middle-aged and older adults with chronic kidney disease: a cross-sectional population study. Nutr J. 2019;18(1):27. doi: 10.1186/s12937-019-0452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Therrien M., Byham-Gray L., Denmark R., Beto J. Comparison of dietary intake among women on maintenance dialysis to a Women's Health Initiative cohort: results from the NKF-CRN Second National Research Question Collaborative Study. J Ren Nutr. 2014;24(2):72–80. doi: 10.1053/j.jrn.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Howard B.V., Van Horn L., Hsia J. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295(6):655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 73.Hassan K. Association of low potassium diet and folic acid deficiency in patients with CKD. Ther Clin Risk Manag. 2015;11:821–827. doi: 10.2147/TCRM.S83751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rebholz C.M., Coresh J., Grams M.E. Dietary acid load and incident chronic kidney disease: results from the ARIC study. Am J Nephrol. 2015;42(6):427–435. doi: 10.1159/000443746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Banerjee T., Crews D.C., Wesson D.E. High dietary acid load predicts ESRD among adults with CKD. J Am Soc Nephrol. 2015;26(7):1693–1700. doi: 10.1681/ASN.2014040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scialla J.J., Appel L.J., Astor B.C. Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int. 2012;82(1):106–112. doi: 10.1038/ki.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stenvinkel P., Heimburger O., Paultre F. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55(5):1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 78.Vaziri N.D., Wong J., Pahl M. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83(2):308–315. doi: 10.1038/ki.2012.345. [DOI] [PubMed] [Google Scholar]
- 79.Cases A., Cigarran-Guldris S., Mas S., Gonzalez-Parra E. Vegetable-based diets for chronic kidney disease? It is time to reconsider. Nutrients. 2019;11(6):1263. doi: 10.3390/nu11061263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mafra D., Lobo J.C., Barros A.F., Koppe L., Vaziri N.D., Fouque D. Role of altered intestinal microbiota in systemic inflammation and cardiovascular disease in chronic kidney disease. Future Microbiol. 2014;9(3):399–410. doi: 10.2217/fmb.13.165. [DOI] [PubMed] [Google Scholar]
- 81.Krishnamurthy V.M., Wei G., Baird B.C. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012;81(3):300–306. doi: 10.1038/ki.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mirmiran P., Yuzbashian E., Asghari G., Sarverzadeh S., Azizi F. Dietary fibre intake in relation to the risk of incident chronic kidney disease. Br J Nutr. 2018;119(5):479–485. doi: 10.1017/S0007114517003671. [DOI] [PubMed] [Google Scholar]
- 83.Feroze U., Noori N., Kovesdy C.P. Quality-of-life and mortality in hemodialysis patients: roles of race and nutritional status. Clin J Am Soc Nephrol. 2011;6(5):1100–1111. doi: 10.2215/CJN.07690910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rambod M., Bross R., Zitterkoph J. Association of malnutrition-inflammation score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. Am J Kidney Dis. 2009;53(2):298–309. doi: 10.1053/j.ajkd.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Palmer S.C., Hanson C.S., Craig J.C. Dietary and fluid restrictions in CKD: a thematic synthesis of patient views from qualitative studies. Am J Kidney Dis. 2015;65(4):559–573. doi: 10.1053/j.ajkd.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 86.Tafesse E, Jackson J, Moon R, Milligan GR, Kim J. Patient-reported quality of life in dialysis compared with non-dialysis CKD patients with hyperkalemia in the United States and European Union 5: results from the KDQOL. Presented at: American Society of Nephrology: Kidney Week, 2019; November 5-10, 2019; Washington, DC.
- 87.Tafesse E., Jackson J., Moon R., Milligan G.R., Kim J. Presented at: American Society of Nephrology: Kidney Week; 2019. Assessing the impact of hyperkalemia on the quality of life of dialysis patients compared with non-dialysis patients: results from a real-world study in the United States and European Union 5. November 5–10, 2019; Washington, DC. [Google Scholar]
- 88.Laureati P, Yang X, Trevisan M, et al. Initiation of sodium polystyrene sulfonate and the risk of gastrointestinal adverse events in advanced CKD: a nationwide study [published online ahead of print August 4, 2019]. Nephrol Dial Transplant: 10.1093/ndt/gfz150. [DOI] [PMC free article] [PubMed]
- 89.Ash S.R., Singh B., Lavin P.T., Stavros F., Rasmussen H.S. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int. 2015;88(2):404–411. doi: 10.1038/ki.2014.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Packham D.K., Rasmussen H.S., Lavin P.T. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372(3):222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 91.Kosiborod M., Rasmussen H.S., Lavin P. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312(21):2223–2233. doi: 10.1001/jama.2014.15688. [DOI] [PubMed] [Google Scholar]
- 92.Spinowitz B.S., Fishbane S., Pergola P.E. Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-month phase 3 study. Clin J Am Soc Nephrol. 2019;14(6):798–809. doi: 10.2215/CJN.12651018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pitt B., Anker S.D., Bushinsky D.A., Kitzman D.W., Zannad F., Huang I.Z. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32(7):820–828. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bakris G.L., Pitt B., Weir M.R. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314(2):151–161. doi: 10.1001/jama.2015.7446. [DOI] [PubMed] [Google Scholar]
- 95.Weir M.R., Bakris G.L., Bushinsky D.A. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 96.Fishbane S., Ford M., Fukagawa M. A phase 3b, randomized, double-blind, placebo-controlled study of sodium zirconium cyclosilicate for reducing the incidence of predialysis hyperkalemia. J Am Soc Nephrol. 2019;30(9):1723–1733. doi: 10.1681/ASN.2019050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kovesdy C.P., Rowan C.G., Conrad A. Real-world evaluation of patiromer for the treatment of hyperkalemia in hemodialysis patients. Kidney Int Rep. 2019;4(2):301–309. doi: 10.1016/j.ekir.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weir M.R., Bakris G.L., Gross C. Treatment with patiromer decreases aldosterone in patients with chronic kidney disease and hyperkalemia on renin-angiotensin system inhibitors. Kidney Int. 2016;90(3):696–704. doi: 10.1016/j.kint.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 99.Agarwal R, Rossignol P, Mayo MR, et al. Patiromer vs. placebo to enable spironolactone in patients with resistant hypertension and CKD according to baseline kidney function (AMBER trial). Presented at: American Society of Nephrology: Kidney Week, 2019; November 5-10, 2019; Washington, DC.