Abstract
Rationale & Objective
Surrogate outcomes for end-stage kidney disease often assume linear changes, which may not reflect true estimated glomerular filtration rate (eGFR) trajectories. This study’s objective was to characterize nonlinear eGFR trajectories in nephrotic syndrome.
Study Design
Observational cohort study.
Setting & Participants
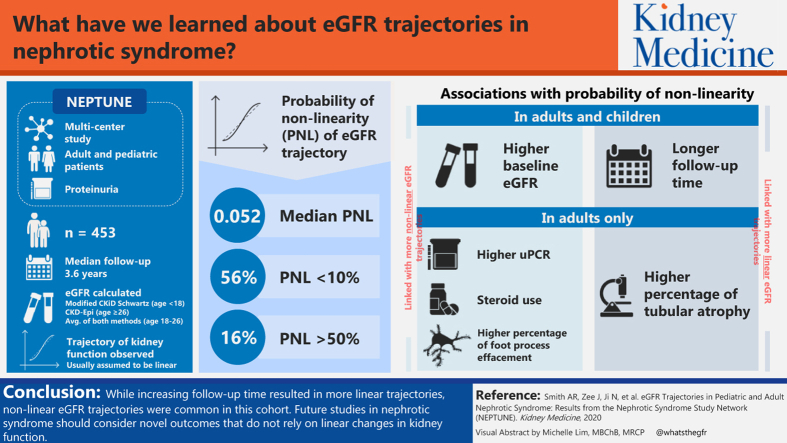
Nephrotic Syndrome Study Network (NEPTUNE) is a multicenter study of adult and pediatric patients with proteinuria enrolled at clinically indicated kidney biopsy or initial presentation of disease (pediatric only).
Predictors
Patient demographic, clinical, and pathology variables at study enrollment and follow-up time.
Outcome
eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (patients ≥ 18 years old) or modified Chronic Kidney Disease in Children Study–Schwartz (patients < 18 years) formulas. The probability of nonlinearity (PNL) was calculated for individual eGFR trajectories.
Analytical Approach
Associations between predictors and PNL were assessed using multivariable linear regression.
Results
453 patients with ≥3 eGFR measurements and 1 or more year of follow-up were included (median follow-up, 3.6 years). Median PNL was 0.052; 56% and 16% had PNL < 10% and >50%, respectively. In both adults and pediatric patients, higher baseline eGFR was associated with higher PNL, whereas longer follow-up time was associated with lower PNL. Higher urine protein-creatinine ratio and steroid use were also associated with higher PNL in adults. Higher percentages of tubular atrophy and foot-process effacement were associated with lower and higher PNLs, respectively, in adults.
Limitations
Relatively short follow-up time, inability to assess acute kidney injury events, and variable eGFR measurement frequency across patients.
Conclusions
Although increasing follow-up time resulted in more linear trajectories, nonlinear eGFR trajectories were common in this cohort. Future studies in nephrotic syndrome should consider novel outcomes that do not rely on linearity assumptions.
Index Words: Glomerular disease, non-linear eGFR, surrogate outcomes, ESKD
Graphical abstract
Plain-Language Summary.
Kidney function decline is often assumed to occur at a constant rate; however, this may not always be the case in patients with glomerular disease. Using data from the Nephrotic Syndrome Study Network (NEPTUNE), we explored participants’ estimated glomerular filtration rate (eGFR) trajectories and found that about half exhibited some nonlinear changes over time. Adult participants with higher baseline eGFR and urine protein-creatinine ratio values and steroid use at baseline were more likely to have nonlinear disease progression, as were those with less tubular atrophy and more foot-process effacement. Longer follow-up time was associated with more linear trajectories; however, shorter-term studies that assess kidney disease progression in this population should consider end points that do not rely on constant kidney function decline.
Several chronic kidney disease (CKD) populations contain subgroups of patients who experience nonlinear estimated glomerular filtration rate (eGFR) trajectories, with wide variability in reported prevalences of nonlinearity, including 5% in the hospital-based NephroTest cohort, 10% of Veterans Affairs Healthcare System patients with CKD stage 4, 15% to 20% in patients with CKD from 6 clinical trials, and 66% of patients in the African American Study of Kidney Disease and Hypertension (AASK).1, 2, 3, 4 However, none of these studies assessed nonlinear eGFR trajectories among patients with glomerular disease, so the prevalence in this population remains unknown.
Studies of rare glomerular disease are often challenged by small sample sizes and low rates of clinical end point occurrence, making novel treatment discovery especially challenging and the use of surrogate eGFR-based outcomes especially appealing. However, disease activity, as well as the medications used to treat glomerular disease, can cause abrupt changes in eGFRs. Given these and other unique characteristics of patients with glomerular disease, our objective was to characterize the prevalence of nonlinear eGFR trajectories in the Nephrotic Syndrome Study Network (NEPTUNE), an incident glomerular disease cohort. In addition, we sought to identify demographic, clinical, and pathology predictors of nonlinearity.
Methods
Study Design and Patient Population
NEPTUNE is an ongoing multicenter prospective observational cohort study of adult and pediatric patients with proteinuria at screening with protein excretion > 0.5 g/d (>1.5 g/d in the second study phase).5 Patients were enrolled at the time of a clinically indicated first kidney biopsy or initial presentation of disease for pediatric patients without biopsy. Patients were included in the study if their biopsy-confirmed diagnosis or clinical diagnosis without biopsy was minimal change disease, focal segmental glomerulosclerosis (FSGS), or membranous nephropathy. Although not one of the primary glomerular diseases of interest in NEPTUNE, patients with other diagnoses (primarily immunoglobulin A nephropathy or lupus) who had been retained for follow-up in NEPTUNE as a relevant comparison cohort were also included in this study.
All patients enrolled in the NEPTUNE study provided informed consent. This study received institutional review board approval under IRBMED #HUM00026609.
Probability of Nonlinearity in eGFR Outcome
The current study included patients with at least 3 eGFR measurements in 3 separate months and at least 1 year of total follow-up. eGFR was calculated using the CKD Epidemiology Collaboration (CKD-EPI) formula for patients older than 26 years and the modified Chronic Kidney Disease in Children Study (CKiD)-Schwartz formula for patients younger than 18 years, with the average of both formulas used for patients aged between 18 and 26 years.6 All available serum creatinine measurements were used, which includes measurements taken between visits and recorded in the patient medical record, in addition to those measured during NEPTUNE study visits. Measurements in the same month were averaged, and patients were censored if they progressed to end-stage kidney disease (ESKD).
We used a Bayesian penalized spline regression model developed by Crainiceanu et al7 and modified by Li et al1 for eGFR trajectories. The model was fit to all available eGFR measurements for each patient individually as a function of follow-up time with knots at midpoints between observation times. The algorithm produced 3,000 smoothed eGFR trajectories that could have produced the patient’s observed eGFR measurements. Based on the approach described by Li et al1 for application to eGFR trajectories in AASK and subsequently used by others,4 a probability of nonlinearity (PNL) was estimated by the percentage of trajectories of 3,000 that showed designated deviations from linearity (see Li et al1 for full technical details). The algorithm produces numerous possible eGFR trajectories; therefore, PNL captures the uncertainty in the true underlying trajectory’s shape. With this method, even patients with limited numbers of observed eGFR measurements can be included in the analysis, albeit with greater variability in their possible trajectories.
To describe changes in trajectory as individuals accrued follow-up time, the PNL for each patient was also calculated sequentially based on eGFR measurements in the first year of follow-up, in the first 2 years of follow-up, etc, up to using the first 5 or more years of follow-up. Each interval included only patients with follow-up through the end of the interval and a minimum of 3 measurements during the interval.
Independent Variables
Patient demographics, clinical variables, morphologic characteristics, and follow-up time were used to predict PNL. Demographic factors included age, sex, race, and ethnicity. Baseline clinical characteristics included disease diagnosis (minimal change disease, membranous nephropathy, FSGS, or other), eGFR (parameterized as a linear spline with a knot at eGFR of 120 mL/min/1.73 m2, which may suggest hyperfiltration), urine protein-creatinine ratio (UPCR), and use of steroids, renin-angiotensin-aldosterone system inhibitors (RAASis), calcineurin inhibitors, and diuretics. Having a high eGFR and medication use during follow-up were also compared with PNL in descriptive analyses. Morphologic characteristics from biopsy assessment through the NEPTUNE Digital Pathology Scoring System8 included interstitial fibrosis (IF), tubular atrophy (TA), global sclerosis, and foot-process effacement.
Statistical Analysis
Independent variables were summarized using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. The distribution of PNL was summarized within patient subgroups using median and interquartile range (IQR) and by frequencies and percentages of patients with <10%, 10% to 50%, and >50% PNL. PNL by years of follow-up (ie, first 1, 2, 3, 4, or 5+ years) was compared within subgroups. To assess whether the frequency of measurements was driving linearity, PNL by years of follow-up was also stratified by number of eGFR measurements available. Further descriptive analyses compared PNL by medication use and having high eGFR during follow-up.
Associations between PNL and baseline characteristics were estimated using multivariable linear regression models separately for pediatric and adult patients based on complete-case analyses. PNL was log-transformed for regression models due to its right-skewed distribution. Models excluding and including pathology variables were fitted because these variables were available among only a subset of patients. We hypothesized that the association between baseline eGFR and PNL may differ at low or high eGFRs; therefore, a linear spline was tested. Estimated regression coefficients were exponentiated and are presented as percentage difference in PNL per unit increase in continuous predictors or percentage difference between groups for categorical predictors.
Estimation of PNL was conducted in R, version 3.5.1 (R Core Team), and all other analyses were conducted in SAS, version 9.4 (SAS Institute Inc).
Results
Sample Characteristics
Of 1,033 enrolled NEPTUNE patients, 312 were deemed ineligible for the NEPTUNE study after enrollment and an additional 268 were excluded from the current study due to having less than 1 year of follow-up (n = 145), having fewer than 3 eGFR measurements (n = 6), or missing disease diagnosis (n = 106) or baseline eGFR (n = 11; Fig S1). A total of n = 453 NEPTUNE patients with a median of 11 (range, 3-32) eGFR measurements over a median follow-up of 3.6 years were included in the current study. More than half had FSGS or minimal change disease (n = 148 and n = 139, respectively) and n = 80 had membranous nephropathy (Table 1). Nearly two-thirds of patients were at least 18 years old at baseline, 60% were male, 56% were white, and 26% were black. Mean eGFR at baseline in the sample was 84.8 mL/min/1.73 m2 and median UPCR at baseline was 2.2 mg/mg. About one-third of patients were taking steroids or diuretics at baseline, almost half (47%) were using RAASis, and 9% were using a calcineurin inhibitor.
Table 1.
Sample Baseline Characteristics by Disease Cohort
| Overall (n = 453) | FSGS (n = 148) | MCD (n = 139) | MN (n = 80) | Other (n = 86) | |
|---|---|---|---|---|---|
| Age, y | 32.9 (22.5) | 33.8 (21.8) | 20.1 (19.3) | 51.6 (14.1) | 34.8 (21.8) |
| <18 | 170 (37.5%) | 56 (37.8%) | 89 (64.0%) | 1 (1.3%) | 24 (27.9%) |
| Sex | |||||
| Female | 183 (40.4%) | 51 (34.5%) | 68 (48.9%) | 31 (38.8%) | 33 (38.4%) |
| Male | 270 (59.6%) | 97 (65.5%) | 71 (51.1%) | 49 (61.3%) | 53 (61.6%) |
| Race | |||||
| White | 253 (56.3%) | 74 (50.3%) | 74 (53.2%) | 60 (75.0%) | 45 (54.2%) |
| Black | 116 (25.8%) | 55 (37.4%) | 34 (24.5%) | 11 (13.8%) | 16 (19.3%) |
| Other | 80 (17.8%) | 18 (12.2%) | 31 (22.3%) | 9 (11.3%) | 22 (26.5%) |
| Hispanic | 90 (19.9%) | 32 (21.6%) | 26 (18.7%) | 14 (17.5%) | 18 (20.9%) |
| Baseline eGFR, mL/min/1.73 m2 | 84.8 (40.8) | 73.8 (32.7) | 106.1 (50.6) | 81.0 (28.0) | 73.0 (31.8) |
| eGFR ≤ 60 | 128 (28.3%) | 62 (41.9%) | 15 (10.8%) | 18 (22.5%) | 33 (38.4%) |
| eGFR of 60-120 | 269 (59.4%) | 74 (50.0%) | 93 (66.9%) | 55 (68.8%) | 47 (54.7%) |
| eGFR > 120 | 56 (12.4%) | 12 (8.1%) | 31 (22.3%) | 7 (8.8%) | 6 (7.0%) |
| Baseline UPCR, mg/mg | 2.2 [0.7-4.5] | 2.4 [0.9-4.4] | 0.9 [0.1-4.0] | 4.1 [2.7-7.6] | 1.4 [0.6-2.9] |
| UPCR 0-0.3 | 73 (16.6%) | 12 (8.3%) | 48 (36.6%) | 0 (0.0%) | 13 (15.1%) |
| UPCR 0.3-1.0 | 72 (16.3%) | 27 (18.8%) | 22 (16.8%) | 8 (10.0%) | 15 (17.4%) |
| UPCR 1.0-3.0 | 121 (27.4%) | 45 (31.3%) | 22 (16.8%) | 15 (18.8%) | 39 (45.3%) |
| UPCR > 3.0 | 175 (39.7%) | 60 (41.7%) | 39 (29.8%) | 57 (71.3%) | 19 (22.1%) |
| Baseline medication use (multiple possible) | |||||
| Steroids | 161 (35.5%) | 39 (26.4%) | 87 (62.6%) | 9 (11.3%) | 26 (30.2%) |
| RAASi | 212 (46.8%) | 75 (50.7%) | 37 (26.6%) | 50 (62.5%) | 50 (58.1%) |
| CNI | 41 (9.1%) | 11 (7.4%) | 21 (15.1%) | 4 (5.0%) | 5 (5.8%) |
| Diuretics | 151 (33.3%) | 42 (28.4%) | 41 (29.5%) | 45 (56.3%) | 23 (26.7%) |
| Days from biopsy to baseline | 19.0 [8.0-31.5] | 16.0 [8.0-27.0] | 17.0 [7.0-35.0] | 17.0 [10.5-27.0] | 22.0 [14.0-32.0] |
| Follow-up time, mo | 3.6 [2.1-4.6] | 4.1 [2.5-4.6] | 3.6 [2.1-4.5] | 4.0 [2.3-4.6] | 2.5 [1.8-3.6] |
| Pathology | |||||
| % Interstitial fibrosis | 5.0 [1.0-20.0] | 16.0 [3.0-27.0] | 1.0 [0.0-4.0] | 7.0 [4.0-12.0] | 21.0 [9.5-35.0] |
| % Tubular atrophy | 5.0 [0.0-16.0] | 13.0 [3.0-25.0] | 0.0 [0.0-2.0] | 6.0 [3.0-11.0] | 21.0 [8.0-34.5] |
| % Global sclerosis | 3.3 [0.0-17.9] | 9.0 [0.0-32.7] | 0.0 [0.0-3.6] | — | 4.9 [0.0-33.3] |
| Foot-process effacement | |||||
| 0%-10% | 16 (6.2%) | 4 (3.5%) | 12 (12.8%) | 0 (0%) | 0 (0%) |
| 11%-25% | 29 (11.2%) | 13 (11.3%) | 13 (13.8%) | 1 (2.3%) | 2 (28.6%) |
| 26%-50% | 29 (11.2%) | 18 (15.7%) | 10 (10.6%) | 0 (0%) | 1 (14.3%) |
| 51%-75% | 43 (16.5%) | 23 (20.0%) | 11 (11.7%) | 7 (15.9%) | 2 (28.6%) |
| 76%-100% | 143 (55.0%) | 57 (49.6%) | 48 (51.1%) | 36 (81.8%) | 2 (28.6%) |
Note: Values for categorical variables are given as percentages; values for continuous variables are given as mean (standard deviation) or median [interquartile range]. Missingness: race, <1%; ethnicity, <1%; baseline UPCR, <5%; interstitial fibrosis, tubular atrophy, 30.3%; % global sclerosis, 53.9%; foot-process effacement, 38.9%.
Abbreviations: CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MN, membranous nephropathy; RAASi, renin-angiotensin-aldosterone system inhibitor; UPCR, urinary protein-creatinine ratio.
Distribution of PNL
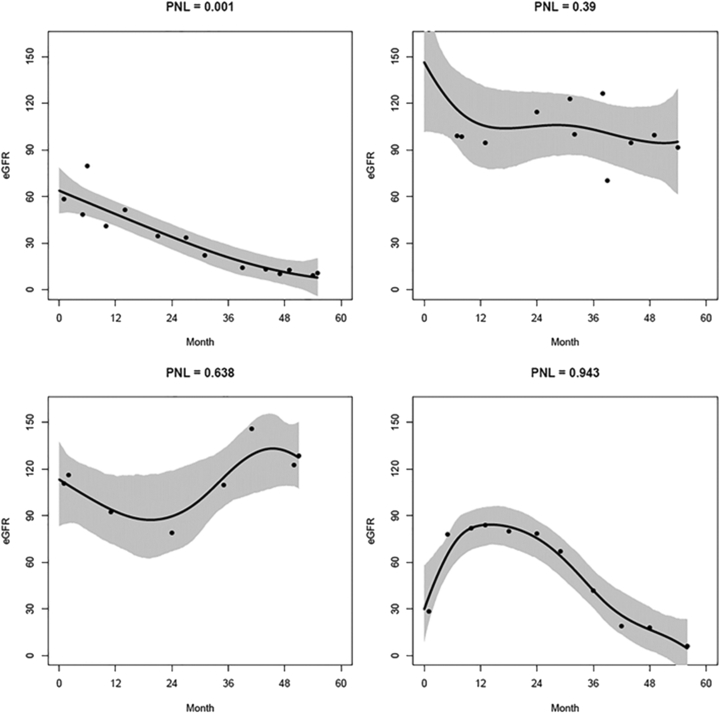
Figure 1 shows several examples of smoothed eGFR trajectories for low and high PNLs. The sample median PNL was 0.052 (IQR, 0.0023-0.292), with 253 (56%) having PNL < 10% and 71 (16%) having PNL > 50%. Adult patients and those with lower baseline eGFRs had lower PNLs, indicating more linear eGFR trajectories among these subgroups (Table 2). Hispanic patients and those taking steroids or RAASis at baseline had higher PNLs, indicating more nonlinear eGFR trajectories among these subgroups.
Figure 1.
Smoothed estimated glomerular filtration rate (eGFR) trajectories for 4 example patients. The black line represents the mean estimated eGFR trajectory based on 3,000 smoothed trajectories (with 95% pointwise credible intervals represented by the gray shaded area) from a Bayesian penalized spline regression model that could have produced the patient’s observed eGFR measurements (dots). Abbreviation: PNL, probability of nonlinearity.
Table 2.
Distribution of PNL by Baseline Subgroups and Follow-up Time
| Overall PNL | PNL < 10% | PNL 10%-50% | PNL > 50% | |
|---|---|---|---|---|
| Age, y | ||||
| <18 | 0.24 [0.05-0.56] | 54 (31.8%) | 67 (39.4%) | 49 (28.8%) |
| ≥18 | 0.01 [0.00-0.12] | 199 (70.3%) | 62 (21.9%) | 22 (7.8%) |
| Sex | ||||
| Female | 0.05 [0.00-0.27] | 105 (57.4%) | 55 (30.1%) | 23 (12.6%) |
| Male | 0.05 [0.00-0.30] | 148 (54.8%) | 74 (27.4%) | 48 (17.8%) |
| Race | ||||
| White | 0.03 [0.00-0.21] | 155 (61.3%) | 67 (26.5%) | 31 (12.3%) |
| Black | 0.09 [0.00-0.35] | 62 (53.4%) | 35 (30.2%) | 19 (16.4%) |
| Other | 0.12 [0.00-0.49] | 36 (45.0%) | 25 (31.3%) | 19 (23.8%) |
| Ethnicity | ||||
| Hispanic | 0.11 [0.01-0.43] | 42 (46.7%) | 30 (33.3%) | 18 (20.0%) |
| Non-Hispanic | 0.04 [0.00-0.27] | 210 (58.5%) | 98 (27.3%) | 51 (14.2%) |
| Diagnosis | ||||
| FSGS | 0.03 [0.00-0.14] | 50 (62.5%) | 23 (28.8%) | 7 (8.8%) |
| MCD | 0.17 [0.04-0.47] | 52 (37.4%) | 54 (38.8%) | 33 (23.7%) |
| MN | 0.01 [0.00-0.26] | 52 (60.5%) | 24 (27.9%) | 10 (11.6%) |
| Other | 0.01 [0.00-0.18] | 99 (66.9%) | 28 (18.9%) | 21 (14.2%) |
| Baseline eGFR, mL/min/1.73 m2 | ||||
| ≤60 | 0.00 [0.00-0.10] | 96 (75.0%) | 24 (18.8%) | 8 (6.3%) |
| 60-120 | 0.07 [0.01-0.30] | 145 (53.9%) | 78 (29.0%) | 46 (17.1%) |
| >120 | 0.37 [0.13-0.56] | 12 (21.4%) | 27 (48.2%) | 17 (30.4%) |
| Baseline UPCR, mg/mg | ||||
| 0-0.3 | 0.08 [0.01-0.23] | 41 (56.2%) | 18 (24.7%) | 14 (19.2%) |
| 0.3-1.0 | 0.05 [0.00-0.26] | 44 (61.1%) | 18 (25.0%) | 10 (13.9%) |
| 1.0-3.0 | 0.01 [0.00-0.14] | 83 (68.6%) | 30 (24.8%) | 8 (6.6%) |
| >3.0 | 0.11 [0.01-0.39] | 84 (48.0%) | 56 (32.0%) | 35 (20.0%) |
| Baseline medication use | ||||
| Steroids | 0.17 [0.03-0.49] | 64 (39.8%) | 57 (35.4%) | 40 (24.8%) |
| RAASi | 0.02 [0.00-0.19] | 130 (61.3%) | 60 (28.3%) | 22 (10.4%) |
| CNI | 0.27 [0.05-0.60] | 12 (29.3%) | 12 (29.3%) | 17 (41.5%) |
| Diuretics | 0.03 [0.00-0.24] | 88 (58.3%) | 42 (27.8%) | 21 (13.9%) |
| Follow-up duration | ||||
| 1 y | 0.28 [0.09-0.58] | 24 (25.0%) | 42 (43.8%) | 30 (31.3%) |
| 2 y | 0.11 [0.01-0.45] | 45 (46.4%) | 32 (33.0%) | 20 (20.6%) |
| 3 y | 0.04 [0.00-0.16] | 47 (62.7%) | 20 (26.7%) | 8 (10.7%) |
| 4 y | 0.01 [0.00-0.12] | 123 (72.4%) | 35 (20.6%) | 12 (7.1%) |
| 5 y | 0.00 [0.00-0.04] | 14 (93.3%) | 0 (0%) | 1 (6.7%) |
Note: Values for categorical variables are given as percentages; values for continuous variables are given as median [interquartile range].
Abbreviations: CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MN, membranous nephropathy; PNL, probability of nonlinearity; RAASi, renin-angiotensin-aldosterone system inhibitor; UPCR, urinary protein-creatinine ratio.
Predictors of PNL
Multivariable models without pathology variables were fitted for 278 adults and 149 pediatric patients. In adults, FSGS and other disease cohorts and longer follow-up time were associated with lower PNLs, whereas higher UPCRs and steroid use at baseline were associated with higher PNLs (Table 3, full sample). Higher baseline eGFR was associated with higher PNL, but the magnitude of this effect was substantially higher for patients with baseline eGFRs > 120 mL/min/1.73 m2 (+102%; 95% confidence interval [CI], +37% to +198%; P < 0.01 for eGFRs > 120 vs +6%; 95% CI, +1% to +13%; P = 0.03 for eGFRs ≤ 120 mL/min/1.73 m2; see Fig S2a for graphical illustration). For pediatric patients, longer follow-up time was associated with lower PNLs, whereas higher baseline eGFRs were associated with higher PNLs, but only for those with baseline eGFRs ≤ 120 mL/min/1.73 m2 (Table 4, full sample; Fig S2b).
Table 3.
Baseline Clinical Predictors of PNL of eGFR Trajectories Based on Multivariable Linear Regression Models of log(PNL) Among Adult Patients
| Predictor | Full Sample (n = 278) |
Pathology Subsample (n = 149) |
||||
|---|---|---|---|---|---|---|
| % Difference in PNL | 95% CI | P | % Difference in PNL | 95% CI | P | |
| Age, per 10 y | +1% | −8% to +11% | 0.85 | −12% | −23% to −0% | 0.04 |
| Male sex (ref: female sex) | +29% | −3% to +71% | 0.07 | +57% | +10% to +123% | 0.01 |
| Black race (ref: all other races) | +14% | −19% to +63% | 0.45 | +28% | −18% to +99% | 0.27 |
| Hispanic (ref: non-Hispanic) | −2% | −33% to +41% | 0.90 | +14% | −26% to +77% | 0.55 |
| Diagnosis (ref: MCD) | ||||||
| MN | −24% | −51% to +18% | 0.22 | −25% | −56% to +25% | 0.26 |
| FSGS | −50% | −67% to −22% | <0.01 | −37% | −61% to +3% | 0.06 |
| Other | −60% | −75% to −37% | <0.01 | −50% | −85% to +68% | 0.26 |
| Follow-up time, per y | −45% | −50% to −39% | <0.01 | −41% | −49% to −32% | <0.01 |
| Baseline eGFR, per 10 mL/min/1.7 3 m2 for eGFR ≤ 120 | +6% | +1% to +13% | 0.03 | −6% | −14% to +2% | 0.14 |
| Baseline eGFR, per 10 mL/min/1.73 m2 for eGFR > 120 | +102% | +37% to +198% | <0.01 | +145% | +71% to +251% | <0.01 |
| Baseline UPCR, per mg/mg | +5% | +1% to +8% | <0.01 | +3% | −1% to +8% | 0.16 |
| Baseline steroid use | +50% | +6% to +113% | 0.02 | +27% | −20% to +102% | 0.30 |
| Baseline CNI use | −23% | −66% to +74% | 0.53 | +71% | −38% to +370% | 0.30 |
| Baseline RAASi use | +3% | −22% to +36% | 0.85 | +10% | −23% to +56% | 0.60 |
| Baseline diuretic use | +7% | −20% to +42% | 0.66 | −14% | −41% to +25% | 0.44 |
| Tubular atrophy, per 10% | — | — | — | −20% | −30% to −9% | <0.01 |
| Foot-process effacement, per unit increase in ordinal scalea | — | — | — | +21% | +3% to +41% | 0.02 |
Note: Coefficients (beta) were transformed to 100×[exp(beta) − 1] to represent percentage change in PNL. Negative percent differences in PNL indicate more linear eGFR trajectories for a 1-unit increase in continuous variables or difference between groups for categorical variables, whereas positive percent differences in PNL indicate more nonlinear eGFR trajectories. R2 for full sample model was 0.42 and for pathology subsample model was 0.49.
Abbreviations: CI, confidence interval; CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MN, membranous nephropathy; PNL, probability of nonlinearity; RAASi, renin-angiotensin-aldosterone system inhibitor; ref, reference; UPCR, urinary protein-creatinine ratio.
Foot-process effacement was recorded on an ordinal scale with 0 corresponding to 0% to 10% of the glomerular capillary surface area affected by effacement, 1 = 11% to 25%, 2 = 26% to 50%, 3 = 51% to 75%, and 4 = 76% to 100%.
Table 4.
Baseline Clinical Predictors of PNL of eGFR Trajectories Based on Multivariable Linear Regression Models of log(PNL) Among Pediatric Patients
| Predictor | Full Sample (n = 155) |
Pathology Subsample (n = 100) |
||||
|---|---|---|---|---|---|---|
| % Difference in PNL | 95% CI | P | % Difference in PNL | 95% CI | P | |
| Age, per 10 y | −23% | −51% to +20% | 0.25 | −29% | −61% to +31% | 0.27 |
| Male sex (ref: female sex) | +22% | −16% to +75% | 0.29 | +10% | −34% to +84% | 0.71 |
| Black race (ref: all other races) | +7% | −29% to +60% | 0.75 | +11% | −35% to +90% | 0.70 |
| Hispanic (ref: non-Hispanic) | +43% | −8% to +122% | 0.11 | +18% | −33% to +108% | 0.57 |
| Diagnosis (ref: MCD) | ||||||
| FSGS | −27% | −53% to +13% | 0.16 | −31% | −61% to +23% | 0.21 |
| Other | −25% | −57% to +30% | 0.30 | +14% | −73% to +383% | 0.86 |
| Follow-up time, per y | −32% | −42% to −21% | <0.01 | −31% | −45% to −13% | <0.01 |
| Baseline eGFR, per 10 mL/min/1.73 m2 for eGFR ≤ 120 | +18% | +7% to +31% | <0.01 | +12% | −4% to +30% | 0.15 |
| Baseline eGFR, per 10 mL/min/1.73 m2 for eGFR>120 | +2% | −4% to +8% | 0.51 | +1% | −9% to +13% | 0.80 |
| Baseline UPCR, per mg/mg | +2% | −1% to +4% | 0.15 | +2% | −1% to +6% | 0.24 |
| Baseline steroid use | −5% | −37% to +43% | 0.80 | −34% | −62% to +14% | 0.14 |
| Baseline CNI use | +33% | −16% to +111% | 0.23 | +20% | −32% to +114% | 0.52 |
| Baseline RAASi use | +12% | −26% to +68% | 0.59 | +37% | −23% to +142% | 0.28 |
| Baseline diuretic use | −15% | −49% to +45% | 0.56 | −18% | −60% to +70% | 0.59 |
| Interstitial fibrosis, per 10% | — | — | — | −8% | −28% to +18% | 0.52 |
| Foot-process effacement, per unit increase in ordinal scalea | — | — | — | +6% | −12% to +28% | 0.52 |
Note: Coefficients (beta) were transformed to 100×[exp(beta) − 1] to represent percentage change in PNL. Negative percent differences in PNL indicate more linear eGFR trajectories for a 1-unit increase in continuous variables or difference between groups for categorical variables, whereas positive percent differences in PNL indicate more nonlinear eGFR trajectories. R2 for full sample model was 0.34 and for pathology subsample model was 0.30.
Abbreviations: CI, confidence interval; CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; PNL, probability of nonlinearity; RAASi, renin-angiotensin-aldosterone system inhibitor; UPCR, urinary protein-creatinine ratio.
Foot-process effacement was recorded on an ordinal scale with 0 corresponding to 0% to 10% of the glomerular capillary surface area affected by effacement, 1 = 11% to 25%, 2 = 26% to 50%, 3 = 51% to 75%, and 4 = 76% to 100%.
IF, TA, and global sclerosis were highly correlated (Pearson correlations between 0.70 and 0.99), so these features were tested separately along with the clinical predictors and foot-process effacement in the subset of patients with pathology data (n = 149 adults, n = 100 pediatric patients). Although all 3 glomerular/tubulointerstitial features yielded similar R2 values (R2 adult/pediatric = 0.495/0.270 for TA model vs 0.485/0.302 for IF model and 0.490/0.265 for global sclerosis model), the TA model was chosen for the final adult model and the IF model was chosen for the final pediatric model because they demonstrated the best model fit.
Adjusted for TA and foot-process effacement, older age and longer follow-up were associated with lower PNL, whereas male sex and higher eGFR at baseline for patients with baseline eGFRs > 120 mL/min/1.73 m2 were associated with higher PNL in adults (Table 3, pathology subsample). TA was associated with lower PNL (−20% per 10% increase in TA; 95% CI, −30% to −9%), whereas foot-process effacement was associated with higher PNL (+21% per unit increase in ordinal scale; 95% CI, +3%-+41%). In pediatric patients, longer follow-up time was associated with lower PNL, and neither IF nor foot-process effacement were associated with PNL (Table 4, pathology subsample).
PNL Over Follow-up Time
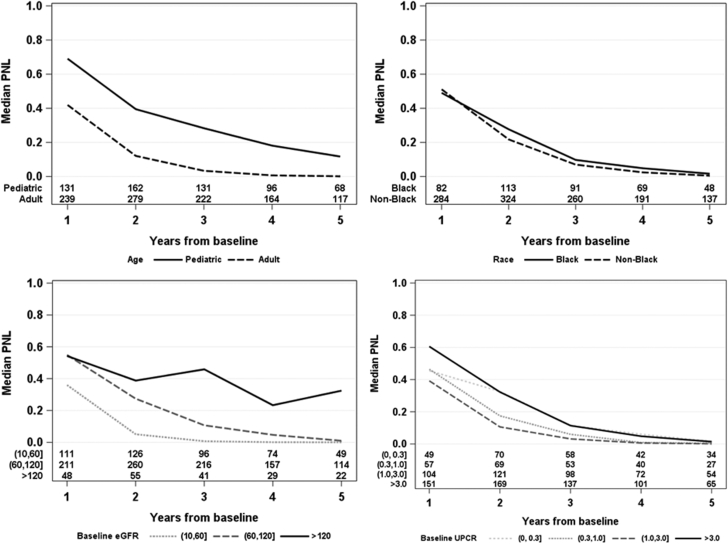
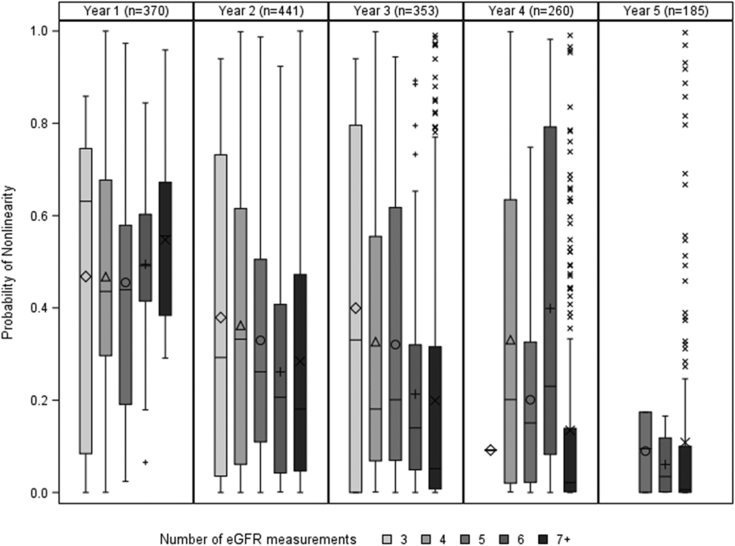
As follow-up time increased, PNL decreased on average. This trend of increased linear trajectory over time was also apparent among patient subgroups by age, black versus nonblack race, eGFR, and UPCR (Fig 2). The differences in PNL between pediatric and adult subgroups and between black and nonblack patients were sustained throughout different follow-up times. However, although patients with baseline eGFRs ≤ 60 mL/min/1.73 m2 had much lower PNLs than those with baseline eGFRs > 60 mL/min/1.73 m2 in the first year of follow-up, patients with baseline eGFRs ≤ 60 and those with 60 < eGFR ≤ 120 mL/min/1.73 m2 had similarly low PNLs when including 5 or more years of follow-up. Furthermore, PNLs differed by baseline UPCR in the first year of follow-up but were low across all levels of baseline UPCR when including 5 or more years of follow-up. Although PNL was lower for patients with longer follow-up overall (Fig S3), the distributions of PNL within a given amount of follow-up was largely overlapping for varying numbers of eGFR measurements, indicating that lower PNL was not systematically driven by having more eGFR measurements available (Fig 3).
Figure 2.
Median probability of nonlinearity (PNL) by year from baseline and (top left) age, (top right) race, (bottom left) baseline estimated glomerular filtration rate (eGFR), and (bottom right) baseline urinary protein-creatinine ratio (UPCR). Note that patients contribute to median PNL estimates for multiple years from baseline up to their maximum observed follow-up time.
Figure 3.
Distribution of probability of nonlinearity (PNL) grouped by years from baseline for varying numbers of estimated glomerular filtration rate (eGFR) measurements across follow-up time. Note that patients contribute to median PNL estimates for multiple years from baseline up to their maximum observed follow-up time. Each box represents the interquartile range (IQR; 25th-75th percentile) of the PNL distribution, with horizontal line at the median and symbol within the box representing the mean. Whiskers represent the range of observations within 1.5×IQR from the box and symbols represent outlier observations.
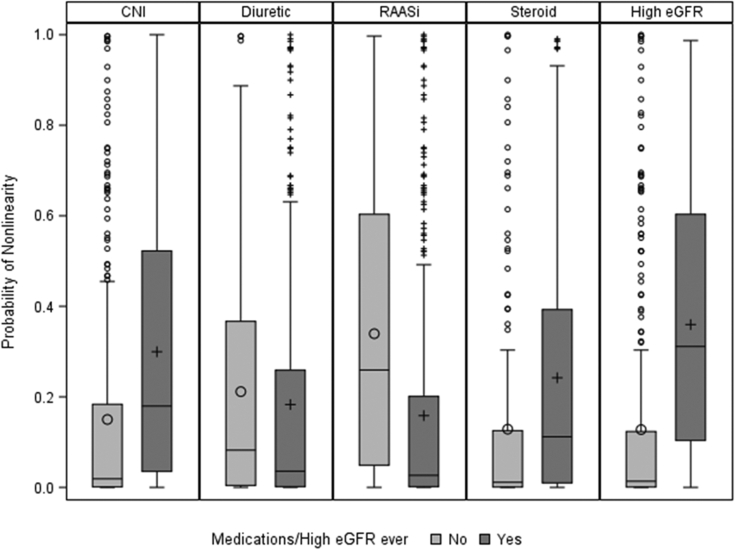
Patients who had high eGFRs (ie, eGFRs > 120 mL/min/1.73 m2) during follow-up or those who used calcineurin inhibitors or steroids at least once during follow-up had higher PNLs (Fig 4) despite similar median follow-up times. Conversely, patients who had ever used diuretics or RAASis had similar or lower PNLs than those who had never used diuretics or RAASis during follow-up. Follow-up time was similar for patients who ever versus never used diuretics, but patients who ever used RAASis had a longer median follow-up time compared with those who never used RAASis (43 vs 31 months).
Figure 4.
Distribution of probability of nonlinearity (PNL) by medication use or incidence of high estimated glomerular filtration rate (eGFR; >120 mL/min/1.73 m2) at least once between screening and end of follow-up. Each box represents the interquartile range (IQR; 25th-75th percentile) of the PNL distribution, with horizontal line at the median and symbol within the box representing the mean. Whiskers represent the range of observations within 1.5×IQR from the box and symbols represent outlier observations. Abbreviations: CNI, calcineurin inhibitor; RAASi, renin-angiotensin-aldosterone system inhibitor.
Rates of progression to ESKD or 40% decline in eGFR across categories of PNL (<10%, 10%-50%, and >50%) showed somewhat higher event rates for PNLs > 50% compared with PNLs < 10% or 10% to 50% (13.7 per 1,000 person-months vs 6.6-9.2 per 1,000 person-months; Table S1). These rates were similar when assessing PNLs within the first year, first 2 years, etc. Linear eGFR slope per month was also steeper overall for patients with PNLs > 50% (−0.53 per month vs −0.24 to −0.43 per month).
Discussion
A nontrivial minority of patients with glomerular disease in our study exhibited nonlinearity in their eGFR trajectories, with 17% having PNLs > 50%. This result is similar to the prevalence of nonlinearity observed in nondiabetic patients with CKD from clinical trials.4 Pediatric patients were more likely to have nonlinear eGFR trajectories, as were adults with minimal change disease, higher UPCRs and eGFRs at baseline, and more foot-process effacement, whereas adults with more TA had more linear eGFR trajectories. Relationships between PNL and age, sex, and eGFR are consistent with other studies that used similar methodology to calculate PNL.1,4
As a whole, patients with characteristics consistent with increased disease activity and current immunosuppressive treatment were more likely to have nonlinear trajectories. This could have several underlying mechanisms, such as fluid shifts, medication changes, and measurement error in eGFR, as well as transient morphologic changes such as foot-process effacement. For example, because use of RAASi has a hemodynamic effect and was hypothesized to be associated with higher PNLs, it may be that our finding of lower PNLs with RAASi use is due to confounding by indication, that is, clinicians are more likely to use this medication when patients have achieved a more stable trajectory. Conversely, those with evidence of chronic scarring, lower disease activity, and low eGFRs were more likely to have linear trajectories. However, due to the observational nature of the study and potential confounding by indication, we are unable to assign causality to any factors associated with PNL, particularly related to medication use.
We also found that eGFR trajectories were more linear after observing patients for longer follow-up times. Although a previous study emphasized that more follow-up could be associated with more nonlinearity due to increased opportunity to detect nonlinearity,4 there are several differences in study characteristics. First, our sample was composed of newly diagnosed pediatric patients and patients with nephrotic syndrome enrolled at the time of kidney biopsy, whose disease activity and medication changes shortly after study enrollment may have been more variable compared with later times in follow-up or compared with more stable prevalent patients. This is a relevant population for determining optimal surrogate outcomes for clinical research studies because identifying predictive biomarkers at the time of disease onset or biopsy is critical to informing therapeutic choices.
Second, our study of nephrotic patients may have different disease progression trajectories compared with general patients with CKD due to the interaction between high-grade proteinuria and kidney function.
Third, the frequency of high eGFR events, which we found to be associated with nonlinearity, may be more common at the beginning of follow-up in incident nephrotic patients.
Lastly, because nephrotic syndrome is a relapsing and remitting disease and our results showed decreasing UPCRs over time on average, the impact of follow-up time on PNL differs from more common kidney diseases such as diabetic nephropathy.
Although we demonstrated that the likelihood of nonlinear eGFR is low 4 or 5 years after disease onset, clinical trials are not always limited to incident patients and rarely will include more than 2 years of follow-up. Although this was an observational study demonstrating patterns of nonlinearity under usual clinical care with medication cessation and initiation that would not occur in a clinical trial, our preliminary assessment of eGFR slope by PNL categories for varying follow-up periods demonstrated that the timing of assessment of nonlinearity may affect the calculation of traditional surrogate outcomes for ESKD. Therefore, shorter-term studies examining changes in eGFR will likely be affected by nonlinear eGFR trajectories. This is particularly true if target populations include patients with characteristics more likely to present with these trajectories. Furthermore, our preliminary finding of higher rates of progression to ESKD or 40% decline in eGFR among those with more nonlinear eGFR trajectories calls into question the suitability of percentage eGFR decline outcomes in the presence of nonlinearity. Future research is thus underway to determine optimal methods for accounting for nonlinearity in surrogate CKD progression outcomes.
Our study has several strengths, including the application of a novel method for quantification of nonlinearity of eGFR trajectories to patients with glomerular disease.1 Another commonly used method, latent class mixed models, fits linear mixed models to the observed eGFR measurements, which can be noisy due to mismeasurement of serum creatinine, estimation bias, and short-term biological variation. The smoothing method applied in our report addresses these issues by resampling, which allows for estimation of the uncertainty regarding the true trajectory given the variation in the observed measures.1 Our study also included a large proportion of pediatric patients, among whom research in eGFR trajectories has been sparse. Finally, we considered several data domains from the NEPTUNE study to assess predictors of nonlinearity, including not only demographics and clinical characteristics but also pathology data.
However, our study is not without limitations. Follow-up time was relatively short, with a median follow-up of 3.6 (IQR, 2.1-4.6; range, 1.1-6.2) years. Despite this, we were able to detect nonlinear trajectories in this cohort that would likely be representative of a population of newly diagnosed patients undergoing usual clinical care. That said, the prevalence of nonlinear trajectories should be confirmed in other glomerular disease cohorts. The 1-year minimum follow-up requirement prevented us from assessing PNL in patients with rapid early declines to ESKD. In addition, the study cohort combined patients with multiple types of glomerulonephritis whose outcomes are varied, and some subgroup comparisons may be underpowered. Therefore, relationships between PNL and clinical characteristics and outcomes need to be verified in larger more homogeneous cohorts. Another limitation was our inability to assess the effect of acute kidney injury events on PNL because this information was not collected directly and serum creatinine measurements were not frequent enough to detect these events based on KDIGO (Kidney Disease: Improving Global Outcomes) guidelines. It is hypothesized that acute kidney injury events may increase PNL, and consideration of whether these events should be considered part of the true eGFR trajectory deserves further consideration.
This study included both protocol-driven and clinically driven eGFR measurements, and patients with more clinically driven measurements may be experiencing periods of more active disease. However, we did not observe clear associations between PNL and number of eGFR measurements in our study. Furthermore, in patients with longer follow-up, we expect that any effect of eGFR frequency was dampened by longer periods of stable disease, as demonstrated by lower PNLs with longer follow-up. Finally, the inclusion of baseline eGFR as a covariate in PNL models may have resulted in some bias due to measurement error because baseline eGFR was also included in the calculation of PNL. However, removing it from the models would likely result in larger biases due to confounding. We included baseline eGFR in the calculation of PNL because for patients with protocol-driven eGFR measurements only, the change from baseline to the second measurement provides important information about nonlinearity.
Nonlinear eGFR trajectories occur in a subset of patients with glomerular disease and should be accounted for when estimating disease progression outcomes and rates of decline. In studies of patients with glomerular disease with shorter follow-up times, nonlinear trajectories will be more prevalent regardless of the number of creatinine measurements. In addition, medication use and high eGFRs, which are relatively common, increase PNLs substantially. Future work that can further elucidate these different mechanisms and shapes of nonlinearity will be useful, for example, to understand the timing of nonlinearity. Furthermore, there is a need for strategies and novel statistical methods that account for these events. Recent methods that calculate time to percentage eGFR decline by fitting linear regression models to eGFR trajectories attempt to address the potential error in eGFR; however, they still cannot accommodate nonlinear trajectories.9 When follow-up is sufficiently long, linear assumptions for calculating eGFR slope or time to percentage decline may be appropriate for most patients. However, it will not be applicable to all, and flexible forms for time (eg, polynomial terms in regression models) should be considered when eGFR is used as an outcome in clinical research.
Article Information
Authors’ Full Names and Academic Degrees
Abigail R. Smith, PhD, Jarcy Zee, PhD, Nan Ji, MS, Jonathan P. Troost, PhD, Brenda W. Gillespie, PhD, Viji Nair, MS, Qian Liu, MPH, Keisha L. Gibson, MD, Matthias Kretzler, MD, and Laura H. Mariani, MD.
Authors’ Contributions
Research idea and study design: JZ, ARS, LHM, BWG; data acquisition: MK, KLG, LHM; data analysis/interpretation: JZ, ARS, MK, QL, KLG, NJ, LHM, JT, BWG, VN; statistical analysis: JZ, ARS, NJ, QL; supervision or mentorship: JZ, ARS, MK, KLG, LHM, BWG. ARS and JZ contributed equally as primary authors. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The NEPTUNE Consortium, U54-DK-083912, is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network, supported through a collaboration between the Office of Rare Diseases Research, National Center for Advancing Translational Sciences (NCATS) and the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK). Additional funding and/or programmatic support for this project has also been provided by the University of Michigan, NephCure Kidney International, and the Halpin Foundation. This study was also supported in part by the NCATS for the Michigan Institute for Clinical and Health Research (UL1TR002240).
Financial Disclosure
Dr Mariani received consulting fees or paid advisory boards from Reata Pharmaceuticals in the last 2 years and has a grant from NIH-NIDDK. Dr Gibson received consulting fees or paid advisory boards from Reata Inc, along with travel support., and receives grant support from Aurinia Inc and Glaxo Smith Kline Inc. Dr Kretzler receives grant support from NIH, University of Michigan, Nephcure Kidney International, Lilly, Astra-Zeneca, Gilead, NovoNordisc, Merck, Goldfinch, Elpidera, and Boehringer-Ingelheim. Dr Troost has equity ownership/stock options in General Electric and Procter & Gamble and receives current grant support from Pfizer Inc. The remaining authors declare that they have no relevant financial interests.
Acknowledgements
Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial support for this report.
Peer Review
Received August 14, 2019. Evaluated by 3 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form March 16, 2020.
Members of NEPTUNE. NEPTUNE Enrolling Centers
Case Western Reserve University, Cleveland, OH: J. Sedor,a K. Dell,b M. Schachere,c J. Negreyc; Children’s Hospital, Los Angeles, CA: K. Lemley,a S. Tangc; Children’s Mercy Hospital, Kansas City, MO: T. Srivastava,a A. Garrettc; Cohen Children’s Hospital, New Hyde Park, NY: C. Sethna,a R. Odusayanac; Columbia University, New York, NY: G. Appel,a M. Toledoc; Duke University, Durham, NC: L. Barisonia; Emory University, Atlanta, GA: L. Greenbaum,a C. Wang,b B. Leec; Harbor-University of California Los Angeles Medical Center: S. Adler,a C. Nast,a,d J. LaPagec; John H. Stroger Jr. Hospital of Cook County, Chicago, IL: A. Athavalea; Johns Hopkins Medicine, Baltimore, MD: A. Neu,a S. Boyntonc; Mayo Clinic, Rochester, MN: F. Fervenza,a M. Hogan,b J. Lieske,a V. Chernitskiyc; Montefiore Medical Center, Bronx, NY: F. Kaskel,a N. Kumar,a P. Flynnc; NIDDK Intramural, Bethesda, MD: J. Kopp,a J. Blakec; New York University Medical Center, New York, NY: H. Trachtman,a O. Zhdanova,b F. Modersitzki,c S. Ventoc; Stanford University, Stanford, CA: R. Lafayette,a K. Mehtac; Temple University, Philadelphia, PA: C. Gadegbeku,a D. Johnstone,b S. Quinn-Boylec; University Health Network Toronto: D. Cattran,a M. Hladunewich,b H. Reich,b P. Ling,c M. Romanoc; University of Miami, Miami, FL: A. Fornoni,a C. Bidotc; University of Michigan, Ann Arbor, MI: M. Kretzler,a D. Gipson,a A. Williams,c J. LaVignec; University of North Carolina, Chapel Hill, NC: V. Derebail,a K. Gibson,a E. Cole,c S. Grubbsc; University of Pennsylvania, Philadelphia, PA: L. Holzman,a K. Meyers,b K. Kallem,c J. Lallic; University of Texas Southwestern, Dallas, TX: K. Sambandam,a Z. Wang,c F. Chambersc; University of Washington, Seattle, WA: A. Jefferson,a S. Hingorani,b K. Tuttle,b,e N. Johnson,c S. Dismuke,c A. Cooperc,e; Wake Forest University, Winston-Salem, NC: B. Freedman,a J.J. Lin,b S. Grayc; Data Analysis and Coordinating Center: M. Kretzler, L. Barisoni, C. Gadegbeku, B. Gillespie, D. Gipson, L. Holzman, L. Mariani, M. Sampson, P. Song, J. Troost, J. Zee, E. Herreshoff, S. Li, C. Lienczewski, T. Mainieri, M. Wladkowski, A. Williams, D. Zinsser; NIDDK Program Office: C. Roy, S. Mendley; The NCATS Program Office: T. Urv, P.J. Brooks. aPrincipal Investigator; bCo-investigator;; cStudy Coordinator; dCedars-Sinai Medical Center, Los Angeles, CA; eProvidence Medical Research Center, Spokane, WA.
Footnotes
Complete author and article information provided before references.
Figure S1: Enrollment flow chart for the study.
Figure S2: Relationship between baseline eGFR and PNL modeled as a linear spline in (A) adults and (B) pediatric patients.
Figure S3: Median PNL by years from baseline grouped by total follow-up time.
Table S1: Rates of progression to ESKD or 40% decline in eGFR across categories of PNL.
Supplementary Material
Figure S1-S3, Table S1
References
- 1.Li L., Astor B.C., Lewis J. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis. 2012;59(4):504–512. doi: 10.1053/j.ajkd.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie Y., Bowe B., Xian H. Estimated GFR trajectories of people entering CKD stage 4 and subsequent kidney disease outcomes and mortality. Am J Kidney Dis. 2016;68(2):219–228. doi: 10.1053/j.ajkd.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 3.Boucquemont J., Loubere L., Metzger M. Identifying subgroups of renal function trajectories. Nephrol Dial Transplant. 2017;32(suppl 2):ii185–ii193. doi: 10.1093/ndt/gfw380. [DOI] [PubMed] [Google Scholar]
- 4.Weldegiorgis M., de Zeeuw D., Li L. Longitudinal estimated GFR trajectories in patients with and without type 2 diabetes and nephropathy. Am J Kidney Dis. 2018;71(1):91–101. doi: 10.1053/j.ajkd.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Gadegbeku C.A., Gipson D.S., Holzman L.B. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 2013;83(4):749–756. doi: 10.1038/ki.2012.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng D.K., Schwartz G.J., Schneider M.F. Combination of pediatric and adult formulas yield valid glomerular filtration rate estimates in young adults with a history of pediatric chronic kidney disease. Kidney Int. 2018;94(1):170–177. doi: 10.1016/j.kint.2018.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crainiceanu C.M., Ruppert D., Wand M.P. Bayesian analysis for penalized spline regression using WinBUGS. J Stat Softw. 2005;14(14):1–24. [Google Scholar]
- 8.Barisoni L., Nast C.C., Jennette J.C. Digital pathology evaluation in the multicenter Nephrotic Syndrome Study Network (NEPTUNE) Clin J Am Soc Nephrol. 2013;8(8):1449–1459. doi: 10.2215/CJN.08370812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zee J., Mansfield S., Mariani L.H. Using all longitudinal data to define time to specified percentages of estimated GFR decline: a simulation study. Am J Kidney Dis. 2019;73(1):82–89. doi: 10.1053/j.ajkd.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1-S3, Table S1