Abstract
Purpose
In a previous study, we identified the Asn450Tyr mutant myocilin gene (Myoc-N450Y) in the pedigree of families with juvenile open angle glaucoma (JOAG), but whether N450Y is a pathogenic mutation remained to be determined. The present study aimed at exploring the role of Myoc-N450Y in primary human trabecular meshwork (HTM) cells.
Methods
Primary HTM cells were infected with lentivirus with wild-type myocilin (Myoc-WT) or Myoc-N450Y. Primary HTM cells overexpressing Myoc-WT or Myoc-N450Y was treated with sodium 4-phenylbutyrate (4-PBA) or not. The secretion and intracellular distribution of Myoc were analyzed with western blotting and immunofluorescence. Expression of endoplasmic reticulum (ER) stress–related proteins was detected with quantitative real-time PCR (qRT-PCR) and western blotting. Cell viability, apoptosis, and expression of the related proteins were examined with Cell Counting Kit-8 (CCK-8), flow cytometry analysis, and western blotting, respectively.
Results
We found that non-secretion of Myoc-N450Y induced ER stress by colocalization with the ER marker calreticulin (CALR), and upregulating the expression of ER stress markers in primary HTM cells. Moreover, overexpression of Myoc-N450Y inhibited the viability and induced apoptosis of primary HTM cells, and inhibition of PI3K/AKT signaling was induced by ER stress. Reduction in ER stress with 4-PBA decreased the level of ER stress markers, promoted secretion, and prevented accumulation of myocilin in the Myoc-N450Y group. Apoptosis was rescued, and inhibition of PI3K/AKT signaling was reversed, after PBA treatment in primary HTM cells with Myoc-N450Y overexpression.
Conclusions
The study results suggest that Myoc-N450Y promotes apoptosis of primary HTM cells via the ER stress–induced apoptosis pathway, in which the PI3K/AKT signaling pathway plays a crucial role.
Introduction
Glaucoma is the leading cause of blindness, affecting 64 million people worldwide [1]. Primary open angle glaucoma (POAG) is the most common type of glaucoma [2]. Juvenile onset open angle glaucoma (JOAG) occurs at an early age and is more severe [3]. The elevated intraocular pressure (IOP) caused by aqueous humor outflow resistance is the major risk factor, and the main treatment strategy for glaucoma is to reduce intraocular pressure with drugs, lasers, or surgery [4]. The trabecular meshwork (TM) is responsible for the dynamic balance between aqueous humor generation and outflow to maintain normal IOP [5,6]. Therefore, investigating the biologic function and properties of the TM may improve our understanding of the pathogenesis of glaucoma.
The glucocorticoid-induced reactive protein gene (myocilin), located at 1q21–24, has three exons encoding a secreted protein of 504 amino acids. The biologic function of myocilin in glaucoma is controversial [7-9], although mutations of this gene have been suggested to be involved in the pathogenesis of POAG [10,11]. Several studies have indicated a gain of function mechanism for mutant myocilin causing POAG due to the lack of glaucoma phenotype in myocilin-heterozygous and myocilin-null mice [8,12]. Most glaucoma-causing mutant forms of myocilin are secretion incompetent and aggregate at the endoplasmic reticulum (ER) of human TM (HTM) cells, leading to HTM cell apoptosis [13-15], which is associated with the pathogenesis of glaucoma. We identified the Asn450Tyr mutant myocilin gene (Myoc-N450Y) in the pedigree of JOAG [16], and found that overexpression of Myoc-N450Y upregulates the fibrosis-related genes in a previous study [17], suggesting its role in glaucoma, but the mechanism requires further study.
ER is involved in the synthesis and processing of secreted proteins and membrane proteins. Disruption of ER homeostasis may induce ER stress, and persistent ER stress causes damage to cells [18]. Accumulating evidence has linked the glaucoma-causing mutant myocilin with ER stress. Non-secretion of mutant myocilin protein is retained in the ER of TM cells, induces ER stress, and contributes to TM cell dysfunction, resulting in increased IOP [10,13-15,19]. However, some POAG-causing mutant myocilin proteins are also secreted, including R126W, T377M, and A427T [20]. Therefore, alternative mechanisms may exist in mutant myocilin-caused glaucoma. Thus far, knowledge of Myoc-N450Y in glaucoma is unclear. The present study aimed to investigate the association of Myoc-N450Y with ER stress in primary HTM cells and clarify the related mechanism. Recently, studies have shown that the PI3K/AKT signaling pathway is involved in ER stress–initiated apoptosis [21,22]. However, the relationship between PI3K/AKT signaling and ER stress in primary HTM cells is unknown. We proposed for the first time that Myoc-N450Y-induced ER stress–mediated apoptosis in primary HTM cells is associated with inhibition of the PI3K/AKT signaling pathway.
Methods
Cell culture
Primary human trabecular meshwork cells were kindly provided by Professor Wei Zhu (Qingdao University, Qingdao, China) [23]. This study was approved by the Eye Bank Association of America and the Institutional Review Boards of Beijing Tongren Hospital. Nineteen short tandem repeat (STR) loci plus the gender-determining locus, amelogenin, were amplified using STR Multi-amplification Kit (Basic Cognitive Technology, Beijing, China). The cell strain sample was processed using the ABI 3730xl DNA Analyzer. Data were analyzed using GeneMapper3.2 software (Applied Biosystems, Suzhou, China). Appropriate positive and negative controls were run and confirmed for each sample submitted. The STR analyses are presented in Appendix 1. The cells were cultured in MEM Alpha basic medium (Gibco, Grand Island, NY) supplemented with 20% fetal bovine serum (FBS) and 1% penicillin/streptomycin solution (Gibco). The cells were maintained at 37 °C with 5% CO2.
Plasmid construction and lentivirus packaging
The wild-type myocilin (Myoc-WT) and Myoc-N450Y sequences were synthesized and cloned into lentivirus vector pLVX-Puro. Then the sequences were verified with a DNA sequencer. Empty vector, pLVX-Myoc-WT vector, or pLVX-Myoc-N450Y vector was cotransfected with the packaging plasmids PMD2G and PXPAX2 into human embryonic kidney 293 cells (HEK 293T). Three days after transfection, the virus supernatants were filtered with 0.45-μm filters (BD, Franklin Lakes, NJ) and used for the infection of primary HTM cells.
Infection of HTM cells
The day before infection, HTM cells were seeded into a six-well plate at a density of 2.0 × 105 per well. The next day, HTM cells reaching 70–80% confluence were infected with Empty, Myoc-WT, or Myoc-N450Y lentivirus at a multiplicity of infection (MOI) of 30, and Polybrene (Sigma, Darmstadt, Germany) was added to the cells at a concentration of 5 μg/ml. After 20 h of infection, the culture medium was replaced with fresh medium.
Quantitative real-time PCR (qRT-PCR) analysis
The cells were harvested using TRIzol reagent (Invitrogen, Carlsbad, CA), and the RNA was extracted according to the manufacturer’s instructions. Five hundred nanograms of total RNA was reversely transcribed with HiScriptII Q RT SuperMix for qPCR (Vazyme, Nanjing, China). cDNA quantification was analyzed with ChamQTM Universal SYBR qPCR Master Mix (Vazyme) on a Bio-Rad machine (Hercules, CA). PCR reaction was run at 95 °C for 30 s, followed by 40 cycles at 95 °C for 10 s, at 60 °C for 30 s and at 72 °C for 30 s. The primer sequences were listed in Table 1.
Table 1. Primers.
| Gene |
Primer (5‘-3’) |
| myocilin |
F: GCGTGGAAGAGGCGATAGTG |
| |
R: CTGCTGCCAGCGTGTAAGTG |
| Grp 78 |
F: GGAGGTGGGCAAACAAAGAC |
| |
R: CCAGCAATAGTTCCAGCGTCT |
| Grp 94 |
F: AAGAAGAAGCAGCCAAAGAAGAG |
| |
R: ATATCATTCATAAGTTCCCAGTCCC |
| PDI |
F: GGCCAAGTCAAGCCCTATCT |
| |
R: CCATCTCCTTGCAGTGGGT |
| XBP1s |
F: AGACTACGTGCACCTCTGCAG |
| |
R: TCTGGGTAGACCTCTGGGAGC |
| Chop |
F: AATCTTCACCACTCTTGACCCTG |
| |
R: AATGACCACTCTGTTTCCGTTTC |
| GAPDH |
F: TGACTTCAACAGCGACACCCA |
| R: CACCCTGTTGCTGTAGCCAAA |
Western blotting analysis
Proteins were extracted using radioimmunoprecipitation assay (RIPA) buffer (Thermo, Waltham, MA). Equal amounts of the total proteins were separated with sodium dodecyl sulfate-PAGE (SDS–PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Thermo). After blocking with 5% nonfat milk (Thermo) for 2 h, the membranes were incubated with primary antibodies at 4 °C overnight and then with secondary antibodies at room temperature for 2 h. The membranes were washed with 0.1% Tris-buffered saline with Tween (TBST) and subjected to chemiluminescence analysis using the enhanced chemiluminescence (ECL) kit (CWBIO, Beijing, China). The antibodies against myocilin (sc-515500) and GAPDH (sc-25778) were purchased from Santa Cruz Biotechnology (Dallas, TX). Antibodies against Grp78 (3177), Grp94 (2104), PDI (2446), calnexin (2679), XBP1s (27,901), CHOP (2895), eIF2α (5324), phosphorylated eIF2α (3398), cleaved caspase 3(9664), cleaved caspase 9 (7237), and IRE1 (3194) were purchased from Cell Signaling Technology (Boston, MA). Phosphorylated IRE1 antibody was purchased from Abcam (ab124945, Cambridge, MA). All secondary antibodies, anti-mouse immunoglobulin (IgG; 7076) and anti-rabbit IgG (7074), were purchased from Cell Signaling Technology.
Immunofluorescence
Primary HTM cells were plated in a confocal dish at a density of 8.0 × 104 per dish, infected with lentivirus carrying empty vector, Myoc-WT, or Myoc-N450Y at an MOI of 30, and cultured for 72 h. Then, the supernatant was discarded, and the cells were washed with PBS (1X; 155 mM NaCl, 3 mM Na2HPO4, 1 mM KH2PO4, pH 7.4). The cells were fixed with 4% paraformaldehyde (CWBIO) for 20 min, permeabilized with 0.3% TritonX-100 (CWBIO), and blocked with 5% bovine serum albumin (BSA) for 1 h. Primary antibody myocilin (sc-515500, Santa Cruz Biotechnology) and calreticulin (12,238, CST) were diluted in 0.5% BSA at a concentration of 1:100. Then, the cells were incubated with primary antibodies at 4 °C overnight, followed by a 1-h incubation with Alexa Fluor 488 (A21206; Thermo) and Alexa Fluor 546 (A10036; Thermo) secondary antibodies. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Peterborough, UK). Images were captured with a Zeiss and Leica imaging system (Carl Zeiss; Hertfordshire, UK).
Phenylbutyrate (PBA) treatment
Dimethyl sulfoxide (DMSO; Thermo) or PBA (1 mM; Thermo) was added to the culture medium of primary HTM cells with Myoc-WT or Myoc-N450Y overexpression for 48 h. The culture medium containing DMSO or PBA was replaced daily.
Cell viability detection
HTM cells expressing empty vector, Myoc-WT, or Myoc-N450Y were seeded in 96-well plates at a density of 1,000 cells/well. Then, 1, 2, 3, and 4 days later, 10 μl of CCK-8 solution was added to each well. The plates were maintained at 37 °C for 2 h. Spectrometric absorbance at 450 nm was detected on the microplate reader (Thermo).
Apoptosis assay
An Annexin V-fluorescein isothiocyanate/propidium iodine (FITC/PI) apoptosis detection kit (CWBIO) was used to measure the apoptosis of primary HTM cells according to the manufacturer’s protocol. Primary HTM cells expressing empty vector, Myoc-WT, or Myoc-N450Y were washed with PBS (Gibco) and trypsinized with 0.05% trypsin (Thermo). Then, the cells were harvested and resuspended in 250 μl of binding buffer to reach a concentration of 1.0 × 106/ml. Five microliters of annexin V-FITC and 10 μl of propidium iodine (PI) was added to 100 μl of cell suspension. The mixture was incubated at room temperature for 15 min, and then subjected to flow cytometry examination.
Statistical analysis
All the statistical data are shown as the mean ± standard error of the mean (SEM) of at least the independent repeats. Statistical analysis was performed with GraphPad Prism software. The Student t test was used to analyze the difference between two groups. One-way analysis of variance was used when there were more than two groups. A p value of less than 0.05 was considered statistically significant.
Results
Characterization and overexpression of Myoc-WT and Myoc-N450Y in primary HTM cells
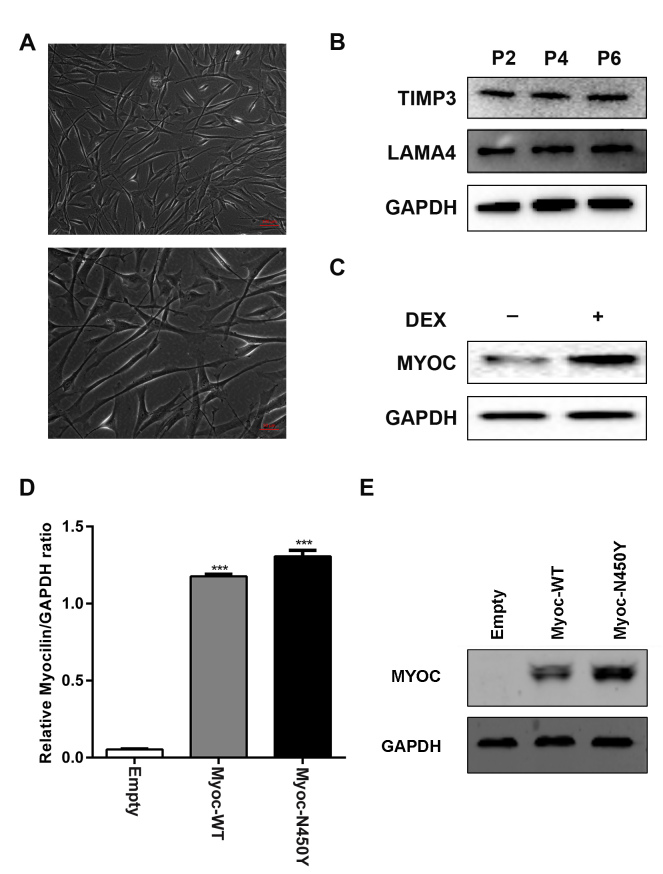
First, we characterized HTM cells as previously [24,25]. The morphology of the cells is shown in Figure 1A. The expression of TIMP3 and LAMA4 in the HTM cells was detected with western blotting (Figure 1B). Glucocorticoid (dexamethasone) treatment induced the expression of myocilin in HTM cells (Figure 1C). To investigate the function of Myoc-N450Y in primary HTM cells, lentiviruses with Myoc-WT and Myoc-N450Y were successfully constructed, and we generated primary HTM cells overexpressing Myoc-WT and Myoc-N450Y. The empty group was designed to quantify the endogenous expression of Myoc and compared with the Myoc-WT group to confirm Myoc-WT has no effect on HTM cells. The qRT-PCR and western blotting results showed that Myoc-WT and Myoc-N450Y were efficiently overexpressed (Figure 1D,E).
Figure 1.
Characterization and overexpression of Myoc-WT and Myoc-N450Y in primary HTM cells. A: Phase contrast microscopic images of human trabecular meshwork (HTM) cells. B: Expression of TIMP3 and LAMA4 in HTM cells at passage 2 (P2), passage 4 (P4), and passage 6 (P6). C: Myocilin expression in HTM cells with or without dexamethasone treatment. D: mRNA levels of Myoc-WT and Myoc-N450Y were elevated in cultured primary HTM cells. ***p<0.001. E: Protein levels of Myoc-WT and Myoc-N450Y were elevated in cultured primary HTM cells. Data were quantified from three independent experiments (n = 3).
Aggregation of Myoc-N450Y induces ER stress and activates UPR
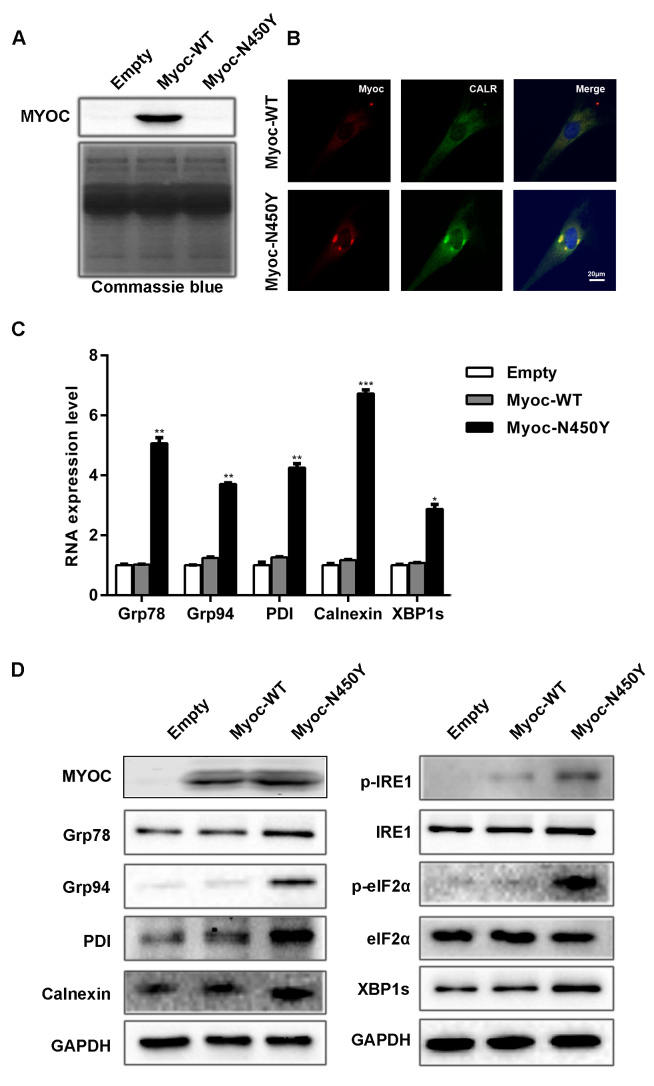
Previous studies have shown that mutant myocilin is secretion incompetent and accumulates inside the cell, thus inducing ER stress [15,19,26]. Then, UPR is activated to alleviate ER stress by sensing ER stress via IRE1, PERK, and ATF6 [27]. We investigated whether Myoc-N450Y induces ER stress and activates UPR in primary HTM cells. Western blotting results showed that Myoc-N450Y was non-secreted (Figure 2A). Based on the immunofluorescence assay, Myoc-N450Y colocalized with ER marker CALR as shown in Figure 2B. Increased mRNA levels of Grp78, Grp94, PDI, calnexin, and XBP1s were observed in primary HTM cells expressing Myoc-N450Y compared with the Myoc-WT group (Figure 2C). There was no statistically significant difference between the Myoc-WT group and the empty vector group. Similarly, western blotting results showed that the protein levels of Grp78, Grp94, PDI, calnexin p-IRE1, p-eIF2α, and XBP1s were upregulated in the Myoc-N450Y group (Figure 2D). Collectively, these findings indicate that Myoc-N450Y aggregates at the ER, induces ER stress, and activates UPR.
Figure 2.
Aggregation of Myoc-N450Y induces ER stress and activates UPR. A: No myocilin secretion in the Myoc-N450Y group. B: Myoc-N450Y accumulates in the endoplasmic reticulum (ER) of primary human trabecular meshwork (HTM) cells. Colocalization of Myoc (Red) with CALR (green) was examined with immunofluorescence. C: Increased mRNA levels of ER stress markers in the Myoc-N450Y group. *p<0.01, **p<0.05, ***p<0.001. D: Increased protein level of ER stress markers in the Myoc-N450Y group. Data were quantified from three independent experiments (n = 3).
Myoc-N450Y regulates cell viability and apoptosis in primary HTM cells
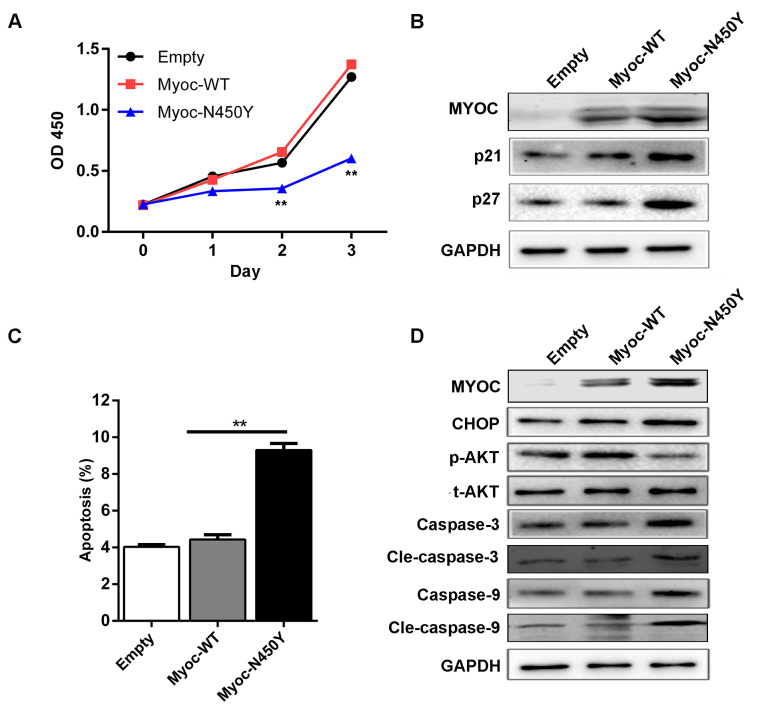
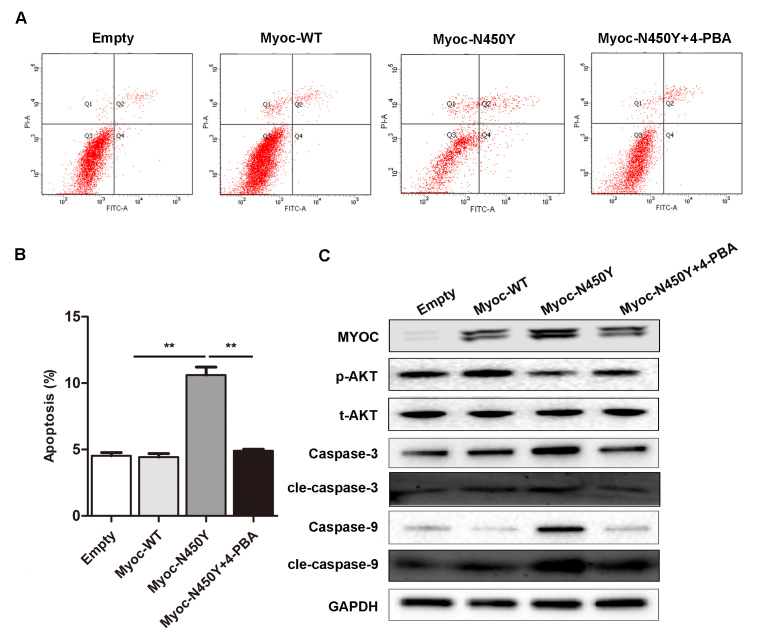
If UPR is insufficient to alleviate ER stress, chronic ER stress and ER dysfunction may impair HTM cells. We examined the viability and apoptosis of HTM cells expressing Myoc-WT and Myoc-N450Y. The CCK-8 results showed that the viability was decreased in the Myoc-N450Y group compared with the Myoc-WT group, and there was no statistically significant difference between the Myoc-WT and empty vector groups (Figure 3A). The protein levels of P21 and P27, the inhibitors of cell cycle progression, were markedly increased in the Myoc-N450Y group (Figure 3B). Apoptosis was determined with flow cytometry analysis. As illustrated in Figure 3C, the apoptosis rate in the Myoc-N450Y group was increased compared with that of the Myoc-WT group. Consistent with the flow cytometry analysis results, the western blotting results indicated that ectopic expression of Myoc-N450Y upregulated the ER-initiated apoptosis-related protein CHOP, and caspase cascade–associated proteins caspase 3, cleaved caspase 3 (cle-caspase 3), caspase 9, and cleaved caspase-9 (cle-caspase 9; Figure 3D). Many studies have indicated that PI3K/AKT plays a crucial role in the process of ER stress–induced apoptosis [21,22]. Therefore, we measured the activity of the PI3K/AKT signaling pathway. Western blotting showed that PI3K/AKT signaling was inhibited in the primary HTM cells with Myoc-N450Y overexpression (Figure 3D). The results indicated that Myoc-N450Y promoted ER stress–induced apoptosis in primary HTM cells by regulating PI3K/AKT signaling.
Figure 3.
Myoc-N450Y regulates cell viability and apoptosis in primary HTM cells. A: Cell viability was detected with Cell Counting Kit-8 (CCK-8). **p<0.05. B: The protein level of P21 and P27 in primary human trabecular meshwork (HTM) cells with Myoc-WT or Myoc-N450Y overexpression. C: Myoc-WT or Myoc-N450Y overexpression was subjected to flow cytometry analysis of apoptosis. **p<0.05. D: Western blotting was used to detect the expression of apoptosis-related proteins. Data were quantified from three independent experiments (n = 3).
PBA treatment promotes secretion of Myoc-N450Y
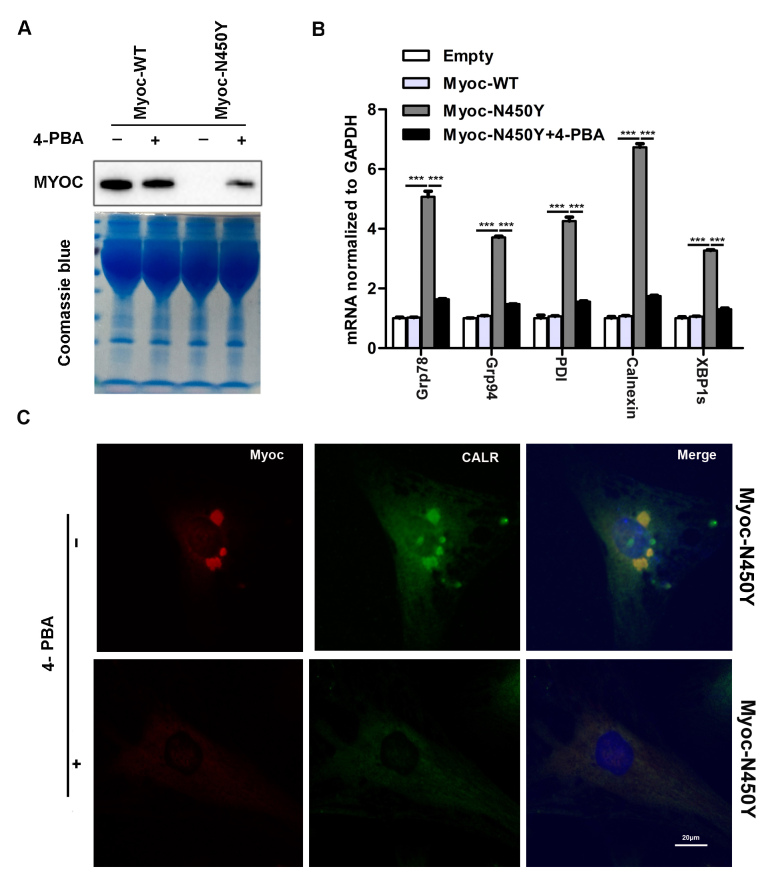
4-PBA, a chemical chaperone, has been reported to rescue ER stress [28]. Primary HTM cells expressing Myoc-WT and Myoc-N450Y were treated with PBA for 48 h, and DMSO was used as the control treatment. PBA treatment promoted the secretion of Myoc-N450Y in the cell medium (Figure 4A) and reduced the expression of ER stress markers Grp78, Grp94, PDI, calnexin, Chop, and XBP1s in the Myoc-N450Y group (Figure 4B). In contrast, the expression of ER stress markers was statistically significantly upregulated in the Myoc-N450Y group without PBA treatment (Figure 4B). Consistent with the increased secretion of Myoc-N450Y, PBA treatment reduced the accumulation of Myoc-N450Y in primary HTM cells (Figure 4C). Taken together, relieving ER stress promotes secretion of Myoc-N450Y.
Figure 4.
Effect of PBA on MYOC secretion and localization. A: Secretion of myocilin was determined with western blotting. B: Endoplasmic reticulum (ER) stress–associated molecules were evaluated with quantitative real-time PCR (qRT-PCR). ***p<0.001. C: Colocalization of Myoc-N450Y with ER marker CALR was detected with immunofluorescence. Data were quantified from three independent experiments (n = 3).
Relieving ER stress rescues apoptosis of primary HTM cells
To further confirm the axis of Myoc-N450Y accumulation-ER stress-PI3K/AKT-apoptosis, we analyzed the apoptosis and activity of PI3K/AKT signaling in primary HTM cells after PBA treatment. As expected, PBA treatment reversed the apoptosis and expression of caspase 3, cle-caspase 3, caspase 9, and cle-caspase 9, and rescued the activity of PI3K/AKT signaling in primary HTM cells with Myoc-N450Y overexpression compared with the Myoc-WT group (Figure 5A–C). The Myoc-N450Y group without PBA treatment exhibited opposite results. The results indicated that Myoc-N450Y aggregates at the ER and induces ER stress–mediated apoptosis via inhibition of the PI3K/AKT signaling pathway.
Figure 5.
Relieving of ER stress rescues apoptosis of primary HTM cells. A: Primary human trabecular meshwork (HTM) cells with Myoc-WT or Myoc-N450Y overexpression were subjected to flow cytometry analysis of apoptosis and quantification of apoptosis assay (B). **p<0.05. C: The activity of the PI3K/AKT signaling pathway and expression of apoptosis-related proteins were detected with western blotting. Data were quantified from three independent experiments (n = 3).
Discussion
The myocilin gene mutation is the earliest discovery of a disease-causing mutation in POAG [29]. Until now, it has been reported that nearly 300 different myocilin mutations have been identified, among which approximately 100 mutations are pathogenic [30]. However, the mechanism by which mutant myocilin may cause glaucoma remains elusive. Previously, we identified the myocilin Asn450Tyr mutation in the pedigree of JOAG, and affected members carrying Myoc-N450Y experienced more severe symptoms at an earlier age [16]. Thus, understanding the pathogenic mechanisms is particularly meaningful for developing early diagnosis strategies and intervention targets. In this study, we showed for the first time that Myoc-N450Y induces ER stress–mediated apoptosis, which is involved in the inhibition of PI3K/AKT signaling pathway.
Studies have shown that aggregation of mutant myocilin, including G364V, I477N, and Y437H, in HTM cells may cause cell death [13,15,31]. However, the related mechanism is unclear. Here, we found that Myoc-N450Y accumulates intracellularly in primary HTM cells. Additionally, Myoc-N450Y aggregates at the ER of primary HTM cells, evidenced by colocalization with ER marker CALR. The results also showed that Myoc-N450Y upregulates ER stress signaling. Upon ER stress, Grp 78 is generally induced as an ER molecular chaperone helping to relieve ER stress by chaperoning the misfolded and unfolded proteins [32]. Similarly, the results demonstrated that Myoc-N450Y upregulates the expression of Grp78 transcriptionally and translationally.
To alleviate ER stress, the cytoprotective UPR is activated, which is illustrated by phosphorylation of the ER sensor, p-IRE1, and upregulation of downstream signaling proteins, including p-eIF2α, Grp78, Grp 94, PDI, and calnexin. We did not observe any difference in most ER stress– and UPR-related proteins between the Myoc-WT and empty groups, consistent with the study by Gulab S. Zode’s group [15]. We speculate a possible explanation: ER stress is a dynamic process generally induced by various stressful conditions. In the present study, misfolded mutant MYOC was continuously synthesized and aggregated. The accumulated mutant proteins exceeded the capability of UPR. Thus, the ER stress was intensive and continuous, although we did not observe any changes in ER stress in the Myoc-WT group. A large quantity of normal protein expression may add to the misfolding rate, but the rate varies in different cellular conditions. ER stress also exists in normal physical conditions, but we cannot detect small changes. In the case of Myoc-WT, we speculate only a few Myoc-WT cells were misfolded and then corrected immediately. Even if amounts of Myoc-WT cause ER stress, it is not a continuous process. Thus, detecting a small change in ER stress is difficult. If UPR fails to restore normal function of ER, persistent ER stress may lead to dysfunction and death of primary HTM cells [33]. Then, we detected the viability and apoptosis of primary HTM cells with Myoc-WT or Myoc-N450Y overexpression. The viability of primary HTM cells was statistically significantly decreased, and the apoptosis rate was increased in the Myoc-N450Y group. ER stress induces apoptosis mainly through two pathways. One pathway is related to a proapoptosis factor, CHOP, via suppression of prosurvival proteins and activation of proapoptosis proteins [34-36]. ER stress can also mediate apoptosis by translocating caspase 12 from the ER to the cytosol which directly cleaves procaspase 9, finally activating the subsequent caspase cascade [37,38]. In this study, we showed that CHOP, as well as caspase cascade apoptosis-related proteins caspase 3, cle-caspase 3, caspase 9, and cle-caspase 9, was increased upon ER stress induced by the accumulation of Myoc-N450Y in the ER of primary HTM cells. 4-PBA, a chemical chaperone, is reported to alleviate ER stress in glaucoma and other diseases [15,39,40]. We also found that ER stress was inhibited, and apoptosis was rescued in primary HTM cells overexpressing Myoc-N450Y after PBA treatment.
Recent studies have indicated that PI3K/AKT plays a vital role in the inhibition of ER stress–induced apoptosis [21,22]. Li et al. showed that after spinal cord injury, PI3K/AKT signaling is inhibited by ER stress–induced apoptosis [21]. Deng et al. also indicated that FABP4 silencing exerts protective effects against hypoxia reoxygenation injury through inhibiting ER stress–induced cell apoptosis via activation of the PI3K/Akt pathway [22]. Similarly, the present results proposed that inhibition of PI3K/AKT signaling involved in ER stress–induced apoptosis and alleviation of ER stress rescue the activity of PI3K/AKT signaling in primary HTM cells. This study is the first to correlate the PI3K/AKT signaling pathway with ER stress in primary HTM cells. However, the specific mechanism modulating PI3K/AKT signaling is unclear. The present study demonstrated that Myoc-N450Y promotes primary HTM cell apoptosis via the ER stress–induced apoptosis pathway, in which the PI3K/AKT signaling pathway plays a crucial role.
Acknowledgments
This work was supported by Beijing Natural Science Foundation (7204243), National Natural Science Foundation of China (81730027), and Beijing Hospitals Authority Youth Programme (QML20180208).
Appendix 1. STR analysis.
To access the data, click or select the words “Appendix 1.”
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta D, Chen PP. Glaucoma. Am Fam Physician. 2016;93:668–74. [PubMed] [Google Scholar]
- 3.Kaur A, Vanita V, Singh J. Screening of CYP1B1 Arg368His as predominant mutation in North Indian primary open angle glaucoma and juvenile onset glaucoma patients. Mol Biol Res Commun. 2018;7:181–6. doi: 10.22099/mbrc.2018.30630.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lusthaus J, Goldberg I. Current management of glaucoma. Med J Aust. 2019;210:180–7. doi: 10.5694/mja2.50020. [DOI] [PubMed] [Google Scholar]
- 5.Rohen JW, Witmer R. Electrn microscopic studies on the trabecular meshwork in glaucoma simplex. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1972;183:251–66. doi: 10.1007/BF00496153. [DOI] [PubMed] [Google Scholar]
- 6.Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24:612–37. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Fautsch MP, Bahler CK, Jewison DJ, Johnson DH. Recombinant TIGR/MYOC increases outflow resistance in the human anterior segment. Invest Ophthalmol Vis Sci. 2000;41:4163–8. [PubMed] [Google Scholar]
- 8.Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, Smith RS, Tomarev SI, John SW, Johnson RL. Targeted Disruption of the Myocilin Gene (Myoc) Suggests that Human Glaucoma-Causing Mutations Are Gain of Function. Mol Cell Biol. 2001;21:7707–13. doi: 10.1128/MCB.21.22.7707-7713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould DB, Miceli-Libby L, Savinova OV, Torrado M, Tomarev SI, Smith RS, John SW. Genetically increasing Myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma. Mol Cell Biol. 2004;24:9019–25. doi: 10.1128/MCB.24.20.9019-9025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain A, Zode G, Kasetti RB, Ran FA, Yan W, Sharma TP, Bugge K, Searby CC, Fingert JH, Zhang F, Clark AF, Sheffield VC. CRISPR-Cas9-based treatment of myocilin-associated glaucoma. Proc Natl Acad Sci USA. 2017;114:11199–204. doi: 10.1073/pnas.1706193114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia LY, Gong B, Pang CP, Huang Y, Lam DS, Wang N, Yam GH. Correction of the disease phenotype of myocilin-causing glaucoma by a natural osmolyte. Invest Ophthalmol Vis Sci. 2009;50:3743–9. doi: 10.1167/iovs.08-3151. [DOI] [PubMed] [Google Scholar]
- 12.Wiggs JL, Vollrath D. Molecular and clinical evaluation of a patient hemizygous for TIGR/MYOC. Arch Ophthalmol. 2001;119:1674–8. doi: 10.1001/archopht.119.11.1674. [DOI] [PubMed] [Google Scholar]
- 13.Joe MK, Sohn S, Hur W, Moon Y, Choi YR, Kee C. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;312:592–600. doi: 10.1016/j.bbrc.2003.10.162. [DOI] [PubMed] [Google Scholar]
- 14.Yam GH, Gaplovska-Kysela K, Zuber C, Roth J. Aggregated myocilin induces russell bodies and causes apoptosis: implications for the pathogenesis of myocilin-caused primary open-angle glaucoma. Am J Pathol. 2007;170:100–9. doi: 10.2353/ajpath.2007.060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2011;121:3542–53. doi: 10.1172/JCI58183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao X, Yang C, Tong Y, Zhang X, Xu L, Li Y. Identification a novel MYOC gene mutation in a Chinese family with juvenile-onset open angle glaucoma. Mol Vis. 2010;16:1728–35. [PMC free article] [PubMed] [Google Scholar]
- 17.Yan XJ, Wu S, Liu Q, Zhang JX. Myocilin Asn 450 Tyr promotes the expression of extracellular matrix protein in human primary trabecular meshwork cells and the significance. Ophthalmology in China. 2019;28:374–80. [Google Scholar]
- 18.Kim C, Kim B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients. 2018;10:1021. doi: 10.3390/nu10081021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Vollrath D. Reversal of mutant myocilin non-secretion and cell killing: implications for glaucoma. Hum Mol Genet. 2004;13:1193–204. doi: 10.1093/hmg/ddh128. [DOI] [PubMed] [Google Scholar]
- 20.Gobeil S, Letartre L, Raymond V. Functional analysis of the glaucoma-causing TIGR/myocilin protein: integrity of N-terminal coiled-coil regions and olfactomedin homology domain is essential for extracellular adhesion and secretion. Exp Eye Res. 2006;82:1017–29. doi: 10.1016/j.exer.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Zhang X, Qi X, Zhu X, Cheng L. Icariin Inhibits Endoplasmic Reticulum Stress-induced Neuronal Apoptosis after Spinal Cord Injury through Modulating the PI3K/AKT Signaling Pathway. Int J Biol Sci. 2019;15:277–86. doi: 10.7150/ijbs.30348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng T, Wang Y, Wang C, Yan H. FABP4 silencing ameliorates hypoxia reoxygenation injury through the attenuation of endoplasmic reticulum stress-mediated apoptosis by activating PI3K/Akt pathway. Life Sci. 2019;224:149–56. doi: 10.1016/j.lfs.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 23.Yu H, Miao Y, Chen W, Qi X, Yang X, Pan X, Wang KW, Zhu W. Expressional and functional involvement of gap junctions in aqueous humor outflow into the ocular trabecular meshwork of the anterior chamber. Mol Vis. 2019;25:255–65. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu W, Gramlich OW, Laboissonniere L, Jain A, Sheffield VC, Trimarchi JM, Tucker BA, Kuehn MH. Transplantation of iPSC-derived TM cells rescues glaucoma phenotypes in vivo. Proc Natl Acad Sci USA. 2016;113:E3492–500. doi: 10.1073/pnas.1604153113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamer WD, Clark AF. The many faces of the trabecular meshwork cell. Exp Eye Res. 2017;158:112–23. doi: 10.1016/j.exer.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Sheffield VC. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001;10:117–25. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko M, Imaizumi K, Saito A, Kanemoto S, Asada R, Matsuhisa K, Ohtake Y. ER Stress and Disease: Toward Prevention and Treatment. Biol Pharm Bull. 2017;40:1337–43. doi: 10.1248/bpb.b17-00342. [DOI] [PubMed] [Google Scholar]
- 28.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–70. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 30.Hewitt AW, Mackey DA, Craig JE. Myocilin allele-specific glaucoma phenotype database. Hum Mutat. 2008;29:207–11. doi: 10.1002/humu.20634. [DOI] [PubMed] [Google Scholar]
- 31.Carbone MA, Ayroles JF, Yamamoto A, Morozova TV, West SA, Magwire MM, Mackay TF, Anholt RR. Overexpression of myocilin in the Drosophila eye activates the unfolded protein response: implications for glaucoma. PLoS One. 2009;4:e4216. doi: 10.1371/journal.pone.0004216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 33.Haze K, Okada T, Yoshida H, Yanagi H, Yura T, Negishi M, Mori K. Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem J. 2001;355:19–28. doi: 10.1042/0264-6021:3550019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang XZ, Kuroda M, Sok J, Batchvarova N, Kimmel R, Chung P, Zinszner H, Ron D. Identification of novel stress-induced genes downstream of chop. EMBO J. 1998;17:3619–30. doi: 10.1093/emboj/17.13.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 37.Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186–94. doi: 10.1196/annals.1299.032. [DOI] [PubMed] [Google Scholar]
- 38.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–70. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng M, Sang W, Chen S, Chen R, Zhang H, Xue F, Li Z, Liu Y, Gong Y, Zhang H, Kong X. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol Lett. 2017;271:26–37. doi: 10.1016/j.toxlet.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Reddy SS, Shruthi K, Joy D, Reddy GB. 4-PBA prevents diabetic muscle atrophy in rats by modulating ER stress response and ubiquitin-proteasome system. Chem Biol Interact. 2019;306:70–7. doi: 10.1016/j.cbi.2019.04.009. [DOI] [PubMed] [Google Scholar]