Abstract
Purpose
Some patients with novel coronavirus disease 2019 (COVID-2019) present with abdominal symptoms. Abdominal manifestations of COVID on imaging are not yet established. The goal of this study was to quantify the frequency of positive findings on abdominopelvic CT in COVID-positive patients, and to identify clinical factors associated with positive findings to assist with imaging triage.
Materials and methods
This retrospective study included adult COVID-positive patients with abdominopelvic CT performed within 14 days of their COVID PCR nasal swab assay from 3/1/2020 to 5/1/2020. Clinical CT reports were reviewed for the provided indication and any positive abdominopelvic findings. Demographic and laboratory data closest to the CT date were recorded. Multivariate logistic regression model with binary outcome of having no reported positive abdominopelvic findings was constructed.
Results
Of 141 COVID-positive patients having abdominopelvic CT (average age 64 years [± 16], 91 [64%] women), 80 (57%) had positive abdominopelvic findings. Abdominal pain was the most common indication, provided in 54% (43/80) and 74% (45/61) of patients with and without reported positive abdominopelvic findings, respectively (p = 0.015). 70% (98/141) of patients overall had reported findings in the lung bases. Findings either typical or intermediate for COVID were reported in 50% (40/80) and 64% (39/61) of patients with and without positive abdominopelvic findings, respectively (p = 0.099). Of 80 patients with positive abdominopelvic findings, 25 (31%) had an abnormality of gastrointestinal tract, and 14 (18%) had solid organ infarctions or vascular thromboses. In multivariate analysis, age (OR 0.85, p = 0.023), hemoglobin (OR 0.83, p = 0.029) and male gender (OR 2.58, p = 0.032) were independent predictors of positive abdominopelvic findings, adjusted for race and Charlson comorbidity index.
Conclusion
Abdominopelvic CT performed on COVID-positive patients yielded a positive finding in 57% of patients. Younger age, male gender, and lower hemoglobin were associated with higher odds of having reportable positive abdominopelvic CT findings.
Keywords: SARS-CoV-2, COVID-19, Pandemic, Coronavirus
Introduction
The novel coronavirus disease 2019 (COVID-2019) pandemic began in December 2019 in Wuhan, China and rapidly spread throughout the globe. There have been over 10 million confirmed cases globally and over 500,000 deaths with 2.5 million cases in USA at the time of this manuscript submission [1]. COVID-2019 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which enters host cells via angiotensin-converting enzyme 2 (ACE2) receptor, expressed in human airway epithelia as well as lung parenchyma, upper esophagus, ileum, colon, kidney, liver, and vascular endothelium [2, 3]. While patients most commonly present with symptoms of respiratory infection, including fever, cough, and shortness of breath [4], COVID-19 can cause multi-system disease with variable clinical presentations including abdominal pain, nausea and vomiting [2–5]. In fact, the first known patient in the USA of COVID-19 in Snohimish County, Washington reported a persistent dry cough, but also described a 2-day history of nausea, vomiting, and loose stool preceding admission [6]. Testing of both the stool and respiratory samples were ultimately positive for SARS-CoV-2 [6]. A cross-sectional multicenter study from Hubei, China reported that over 18% of COVID-positive patients presented with abdominal symptoms, and approximately 5% had isolated gastrointestinal symptoms, without respiratory complaints [3]. Imaging workup with abdominopelvic CT may reveal lung base imaging findings suggestive of COVID-19 pneumonia, without any significant findings below the diaphragm. In fact, there are convincing data that the radiologist may be the first to suggest COVID-19 diagnosis, based on the lung base appearance on abdominopelvic CT [7–9].
The mechanism of why some patients experience abdominal symptoms while others do not is poorly understood. A single recent preliminary report on abdominopelvic imaging findings in COVID-positive patients revealed a pattern of bowel wall thickening and fluid-filled colon in 31% of CTs, indicative/suggestive of diarrhea [10]. There are also reports on associated sequela of severe coagulopathy involving the pulmonary [11], cerebral [12], and renal circulations, with renal infarction identified on abdominal CT [13]. The rate of positive abdominopelvic CT findings in COVID-positive patients remains unknown. The goal of this study is to identify clinical factors associated with positive findings to assist with imaging triage.
Materials and methods
Study design
The institutional review board approved this retrospective study. The need for informed consent was waived. A search of the electronic medical records was performed to identify all CT abdomen and pelvis examinations, with or without full thoracic coverage performed on adult (> 18 years) patients from March 1, 2020 through May 1, 2020. Only exams performed on patients testing PCR nasal swab assay positive for SARS CoV-2 within 14 days of the CT exam were included in the search terms. Our academic medical center is located within a hot zone of the global pandemic in the USA. The selected time period corresponds to the rise, plateau and early fall of the springtime 2020 surge of COVID-19 infections in New York city.
CT technique
CT examinations were performed with 64-detector row CT scanners (LightSpeed VCT [GE Healthcare, Milwaukee, WI] LightSpeed Xtra [GE Healthcare, Milwaukee, WI]), or IQon [Philips Healthcare, Best, Netherlands]. Axial CT Images were acquired with the following parameters: 120 kVp; mAs 200–650 adjusted according to patient size; section thickness of 0.625 mm, table speed of 13.75 mm per rotation, pitch of 1.375. All images were reconstructed with a section thickness of 2.5–5 mm. Multiplanar reconstructed images were created in sagittal and coronal planes, with reconstructed section thickness of 3 mm. For contrast-enhanced studies, 90–120 mL of iopamidol (300 mg iodine per milliliter [Isovue 300] or 370 mg iodine per milliliter [Isovue 300]; Bracco Diagnostics, Monroe Township, NJ) was injected at a rate of 2–4 mL/s. Bolus-tracking software was used for image timing, depending on the protocol.
Data analysis
Clinical CT reports were reviewed by one of the authors (AF). The following information was extracted from the reports: coverage (chest, abdomen, and pelvis versus abdomen and pelvis only), use of intravenous contrast, patient location (emergency department, inpatient or outpatients), clinical indication and symptoms as provided by the ordering clinician, and the presence of any positive CT findings within the abdomen or pelvis. Positive findings were defined as those that potentially could cause the indicated symptoms. Our criteria were very inclusive in our attempt capture all positive results on CT. In cases where prior studies were referenced and the finding was reported as stable, the finding was considered to be non-positive, however, findings potentially accounting for abdominal symptoms on prior CT report from the same admission were considered as positive. CT findings on chest CT or in the partially imaged lung bases of the CT abdomen/pelvis were coded as typical of COVID, intermediate probability of COVID, low probability of COVID or normal, based on RSNA reporting guidelines [14]. Electronic medical records were queried to extract patient demographic characteristics and laboratory findings closest to the date of the CT. Extracted laboratory data included complete metabolic profile (CMP), complete blood count (CBC), and coagulation profile. Modified Charlson comorbidity index, a validated tool for comorbidity adjustment [15, 16], was calculated based on the data from the outpatient, inpatient, and emergency department encounters within 180 days prior to the CT date. Documented inhouse death and the date were recorded.
Statistical analysis
Statistical analyses were performed using STATA version 13.1 (StataCorp LP, College Station TX). Statistical significance was set at p < 0.05. Continuous variables were summarized as mean and standard deviations or median and interquartile range (IQR), depending on normality of distribution. Bivariate associations for continuous variables were compared using t test or Kruskal–Wallis rank test, for variables with normal and non-normal distribution, respectively. Bivariate associations of the categorical variables were compared using χ2 test or Fisher’s exact test, as appropriate.
The study population was separated into two groups: those with no reported positive abdominopelvic findings and those with at least one reported positive abdominopelvic finding. Multivariate logistic regression model with binary outcome of having reported positive abdominopelvic findings was constructed. The initial model included all covariates with the p value < 0.10 on bivariate associations. The exit criteria were: p value of the covariate of > 0.05 and < 10% change in the coefficient(s) of other covariates in the model. Due to collinearity between hemoglobin and hematocrit, only hemoglobin was included in the model.
Results
The initial search identified 198 patients who tested positive for SARS-CoV-2 and underwent abdominopelvic CT during the study period. 57 patients were excluded because the time period between CT and a positive COVID PCR nasal swab assay was greater than 14 days. The final study population included 141 patients (average age 64.2 years [± 15.5], 91 [64.5%] women), of which 80 (57%) had reported positive abdominopelvic findings. Of 141 patients, 111 (78.7%) were inpatients at the time of CT, 27 (19.2%) were imaged in the emergency department, and 3 (2.1%) were imaged as outpatients. 97.9% (138/141) of CTs were performed with intravenous contrast. Patient demographic characteristics and laboratory findings are summarized in Table 1. Statistically significant differences between the two groups were seen in the alkaline phosphatase, albumin, total bilirubin, direct bilirubin, hematocrit, prothrombin time and international normalized ratio (Table 1).
Table 1.
Summary of demographic and laboratory data on 141 patients
| Variable* | CT with no positive findings (n = 61) | CT w positive findings (n = 80) | p value |
|---|---|---|---|
| Age (years) | 66.0 (14.2) | 62.9 (16.4) | 0.245 |
| Gender (n, % female) | 44 (72.1) | 47 (58.8) | 0.1 |
| Race (n, %) | 0.46 | ||
| Hispanic | 21 (34.4) | 35 (43.8) | |
| African American | 21 (34.4) | 28 (35.0) | |
| White | 5 (8.2) | 7 (8.8) | |
| Asian | 3 (4.9) | 2 (2.5) | |
| Other/mixed | 5 (8.2) | 6 (7.5) | |
| Unknown | 6 (9.8) | 2 (2.5) | |
| Charlson score | 1 (0 to 4) | 2 (0 to 4.5) | 0.092 |
| Death (n, %) | 5 (8.2) | 10 (12.5) | 0.412 |
| Time period between CT and death (days) | 7 (5 to 12) | 6.5 (5 to 12) | 0.902 |
| Time period between CT and COVID test (days) | 0 (0 to 0) | 0 (− 1 to 1) | 0.841 |
| CT location (n, %) | 0.582 | ||
| Emergency department | 13 (21.3) | 14 (17.5) | |
| Inpatient | 46 (75.4) | 65 (81.2) | |
| Outpatient | 2 (3.3) | 1 (1.2) | |
| No intravenous contrast administered | 3 (4.9) | 0 (0) | 0.079 |
| Thorax coverage included | 12 (19.2) | 15 (18.8) | 0.89 |
| CT findings typical or intermediate for COVID | 39 (63.9) | 40 (50.0) | 0.099 |
| Time period between CT and CMP (days)a | 0 (0 to 0) | 0 (0 to 0) | 0.698 |
| Complete metabolic profile | |||
| Sodiuma (mEq/L) | 137.9 (6.3) | 138.2 (8.0) | 0.768 |
| Potassiumb (mEq/L) | 4.3 (0.6) | 4.2 (0.6) | 0.511 |
| Chloridea (mmol/L) | 98.8 (6.5) | 99.9 (8.3) | 0.422 |
| Carbon dioxidea (mmol/L) | 22.9 (3.4) | 22.5 (4.1) | 0.534 |
| Anion gapa (mEq/L) | 16.1 (3.2) | 15.9 (4.6) | 0.746 |
| Blood urea nitrogenb (mg/dL) | 16 (11 to 22) | 17 (11 to 24) | 0.454 |
| Creatininea (mg/dL) | 0.9 (0.7 to 1.2) | 0.9 (0.7 to 1.2) | 0.988 |
| GFRa (mL/min) | 69 (47 to 91) | 71 (54 to 98) | 0.645 |
| Glucosec (mg/dL) | 127.0 (100 to 171) | 131.0 (96 to 184) | 0.826 |
| Magnesiumd (mEq/L) | 2.0 (0.3) | 1.9 (0.3) | 0.331 |
| Alkaline phosphatasee (IU/L) | 75.5 (62 to 92) | 102.5 (76 to 140) | < 0.001 |
| Aspartate aminotransferasef (IU/L) | 28 (23 to 43) | 34 (23 to 52) | 0.165 |
| Alanine aminotransferasef (IU/L) | 21.5 (15 to 32.5) | 26 (17 to 39) | 0.185 |
| Albumine (g/dL) | 4.0 (0.5) | 3.6 (0.8) | 0.014 |
| Total bilirubing (mg/dL) | 0.4 (0.3 to 0.6) | 0.6 (0.3 to 0.8) | 0.036 |
| Direct bilirubing (mg/dL) | 0.15 (0.1 to 0.2) | 0.2 (0.1 to 0.3) | 0.05 |
| Lactate dehydrogenaseh (U/L) | 354 (231 to 463) | 304 (244 to 361) | 0.43 |
| Total proteini (g/dL) | 7.2 (0.7) | 7.1 (1.1) | 0.46 |
| Amylasej (U/L) | 80 (46 to 100) | 66 (56 to 84) | 0.63 |
| Lipasek (U/L) | 32 (19 to 69) | 42 (22 to 60) | 0.63 |
| Time period between CT and CBC (days)a | 0 (0 to 0) | 0 (0 to 0) | 0.988 |
| Complete blood count | |||
| White blood cell counta (mln cells/mL) | 4.5 (4.0 to 5.2) | 4.3 (3.3 to 5.1) | 0.123 |
| Hemoglobina (g/dL) | 12.7 (2.1) | 11.5 (2.9) | 0.007 |
| Hematocrita (%) | 39.9 (6.1) | 36.4 (8.4) | 0.005 |
| Platelet counta (mln cells/mL) | 219 (156 to 283) | 212 (146.5 to 292.5) | 0.578 |
| Neutrophil (%)a | 74 (66 to 81) | 74 (62 to 82) | 0.818 |
| Lymphocyte (%)a | 14 (11 to 25) | 15.5 (9 to 23.5) | 0.676 |
| Monocyte (%)a | 8 (6 to 10) | 8 (5 to 10) | 0.538 |
| Eosinophil (%)a | 0 (0 to 1) | 0 (0 to 1) | 0.167 |
| Basophil (%)a | 0 (0 to 0) | 0 (0 to 0) | 0.635 |
| Time period between CT and coagulation profile (days)l | 0 (0 to 0) | 0 (0 to 0) | 0.727 |
| Prothrombin timef (s) | 14.0 (13.5 to 14.8) | 14.7 (13.9 to 16.2) | 0.002 |
| Partial thromboplastin timem (s) | 36.8 (27.5 to 36.8) | 31.7 (28.9 to 38.5) | 0.424 |
| International normalized ratiof | 1.1 (1.1 to 1.1) | 1.1 (1.1 to 1.3) | 0.013 |
*Reported as mean and standard deviation, unless noted otherwise
aIn 139 patients with nonmissing data
bIn 129 patients with nonmissing data
cIn 138 patients with nonmissing data
dIn 81 patients with nonmissing data
eIn 126 patients with nonmissing data
fIn 124 patients with nonmissing data
gIn 125 patients with nonmissing data
hIn 59 patients with nonmissing data
iIn 90 patients with nonmissing data
jIn 19 patients with nonmissing data
kIn 63 patients with nonmissing data
lIn 134 patients with nonmissing data
mIn 113 patients with nonmissing data
Symptoms listed in the indications for the included CTs are summarized in Table 2. The most commonly listed symptom was abdominal pain, reported in 45 (73.8%) of 61 patients with no reported positive abdominopelvic findings, and in 43 (53.8%) of 80 patients with reported positive abdominopelvic findings (p = 0.015). There were no statistically significant differences between the groups based on any other listed indications (Table 2).
Table 2.
Summary of symptoms, signs, and lab abnormalities listed in indication
| Symptom | CT with no positive findings (n = 61) | CT w positive findings (n = 80) | p value |
|---|---|---|---|
| Shortness of breath | 2 (3.3) | 5 (6.3) | 0.421 |
| Abdominal pain (any location) | 45 (73.8) | 43 (53.8) | 0.015 |
| Abdominal pain (location not specified) | 28 (45.9) | 32 (40.0) | 0.483 |
| Right upper quadrant pain | 4 (6.6) | 1 (1.2) | 0.166 |
| Left upper quadrant pain | 2 (3.3) | 2 (2.5) | > 0.999 |
| Right lower quadrant pain | 5 (8.2) | 4 (5.0) | 0.501 |
| Left lower quadrant pain | 7 (11.5) | 2 (2.5) | 0.04 |
| Epigastric pain | 4 (6.6) | 4 (5.0) | 0.727 |
| Flank pain | 1 (1.6) | 1 (1.2) | > 0.999 |
| Vomiting | 9 (14.8) | 12 (15.0) | > 0.999 |
| Fever | 12 (19.7) | 12 (15.0) | 0.503 |
| Abnormal liver function tests | 1 (1.6) | 1 (1.2) | > 0.999 |
| Sepsis | 1 (1.6) | 7 (8.8) | 0.138 |
| Fatigue | 1 (1.6) | 1 (1.2) | > 0.999 |
| Diarrhea | 4 (6.6) | 1 (1.2) | 0.166 |
| Melena | 2 (3.3) | 1 (1.2) | 0.578 |
| Constipation | 2 (3.3) | 1 (1.2) | 0.578 |
| Nausea | 10 (16.4) | 5 (6.2) | 0.06 |
| Cancer staging | 3 (4.9) | 8 (10.0) | 0.35 |
| Imaging prior to G-tube placement | 1 (1.6) | 3 (4.9) | 0.316 |
| Drop in hematocrit | 2 (3.3) | 4 (5.0) | 0.698 |
| Abdominal distension | 2 (3.3) | 5 (6.2) | 0.699 |
| Tachycardia | 3 (4.9) | 3 (3.8) | > 0.999 |
| Evaluate for pulmonary embolism | 2 (3.3) | 1 (1.2) | 0.578 |
| Chest pain | 0 (0) | 2 (2.5) | 0.506 |
| Elevated white blood cell count | 1 (1.6) | 3 (3.8) | 0.633 |
| Cough | 1 (1.6) | 0 (0) | 0.433 |
| Hematuria | 0 (0) | 3 (3.8) | 0.258 |
| Hypotension | 0 (0) | 1 (1.2) | > 0.999 |
| Other | 0 (0) | 2 (2.5) | 0.506 |
An abnormality in the imaged portion of the thorax was reported in 98 (69.5%) of 141 patients. The findings either typical or intermediate for COVID were reported in 39/61 (63.9%) patients with no positive abdominopelvic findings and in 40/80 (50.0%) patients with positive abdominopelvic findings (p = 0.099) (Fig. 1).
Fig. 1.
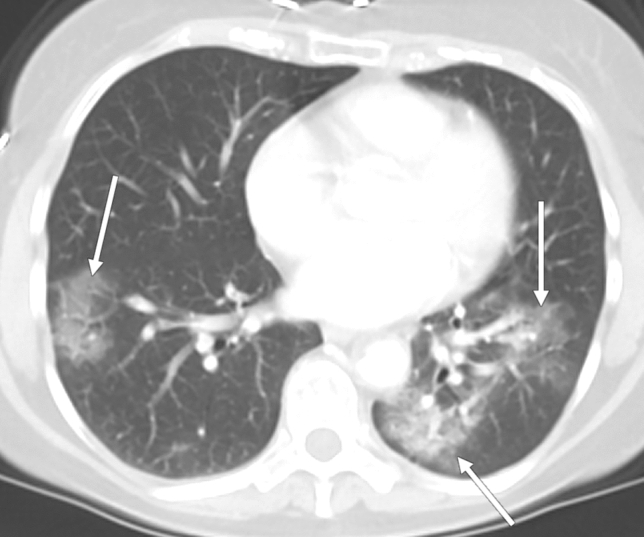
CT in a 70-year-old female with clinical indication of epigastric abdominal pain. Axial image through the lung bases demonstrates multiple bilateral peripheral ground-glass opacities in rounded configuration (arrows), reported as typical features of COVID pneumonia. No positive abdominopelvic findings were reported
Table 3 summarizes the reported positive abdominopelvic findings in the 80 patients with at least one reported positive finding (Figs. 2, 3). The most commonly reported findings were related to gastrointestinal tract (25/80, 31.2%), with mural thickening of a portion of the GI tract being the most common (Fig. 4), reported in 12 (15.0%) of 80 patients.
Table 3.
Summary of reported positive reported findings in 80 patients
| Finding | n (%) |
|---|---|
| Liver findings (any) | 5 (6.2) |
| Hepatomegaly | 4 (5.0) |
| Periportal edema | 1 (1.2) |
| Biliary dilatation | 8 (10.0) |
| Gallbladder findings (any) | 12 (15.0) |
| Distension | 5 (6.2) |
| Mural edema | 2 (2.5) |
| Possible/definite acute cholecystitis | 5 (5.0) |
| Pancreatic ductal dilatation | 3 (3.8) |
| Spleen findings (any) | 6 (8.8) |
| Splenomegaly | 2 (2.5) |
| Splenic infarct(s) | 4 (5.0) |
| Renal findings (any) | 16 (20.0) |
| Renal infarcts | 4 (5.0) |
| Nonspecific stranding | 8 (10.0) |
| Possible/definite pyelonephritis | 4 (5.0) |
| Gastrointestinal tract findings (any) | 25 (31.2) |
| Acute appendicitis | 2 (2.5) |
| Retained colonic stool | 5 (6.2) |
| SBO | 1 (1.2) |
| SB ileus | 1 (1.2) |
| Mural thickening (any) | 12 (15.0) |
| Mural thickening (colon) | 5 (6.2) |
| Mural thickening (small bowel) | 2 (2.5) |
| Mural thickening (esophagus) | 3 (3.8) |
| Mural thickening (stomach) | 2 (2.5) |
| Acute colonic diverticulitis | 2 (2.5) |
| Other | 2 (2.5) |
| Urinary bladder findings (any) | 18 (22.5) |
| Mural thickeninga | 12 (15.0) |
| Cystitis | 4 (5.0) |
| Intraluminal air | 1 (1.2) |
| Possible/definite blood | 1 (1.2) |
| Pelvic organs findings (any) | 4 (5.0) |
| Possible adnexal torsion | 2 (2.5) |
| Hydrosalpinx | 2 (2.5) |
| Vascular findings (any) | 6 (7.5) |
| Deep vein thrombosis | 3 (3.8) |
| Arterial occlusion | 3 (3.8) |
| Peritoneum findings (any) | 19 (23.8) |
| Ascites | 15 (18.8) |
| Free airb | 4 (5.0) |
| Soft tissues findings (any) | 11 (13.8) |
| Edema/anasarca | 5 (6.2) |
| Phlegmon/abscess | 3 (3.8) |
| Decubitus ulcer | 2 (2.5) |
| Hematoma | 1 (1.2) |
| Retroperitoneal lymphadenopathy | 4 (5.0) |
| Infiltration of retroperitoneal fat | 3 (3.8) |
| Skeletal findings (any) | 5 (6.2) |
| Osteomyelitis | 3 (3.8) |
| Fracture(s) | 2 (2.5) |
aExcluding cases with definite cystitis
bOne of the 4 cases was associated with acute diverticulitis, and one was in the setting of a recent laparotomy
Fig. 2.
CT in a 21-year-old female with clinical indication of abdominal pain and diarrhea. Axial images through the upper abdomen (a) and pelvis (b) demonstrate liquid stool in the colon (long arrow, a) and rectum (long arrow, b). There is marked mural edema in the gallbladder (short arrow, a). Axial image through the lung bases c demonstrates several nonrounded peripheral ground-glass opacities (arrows) at the right lung base, reported as intermediate confidence features for COVID pneumonia
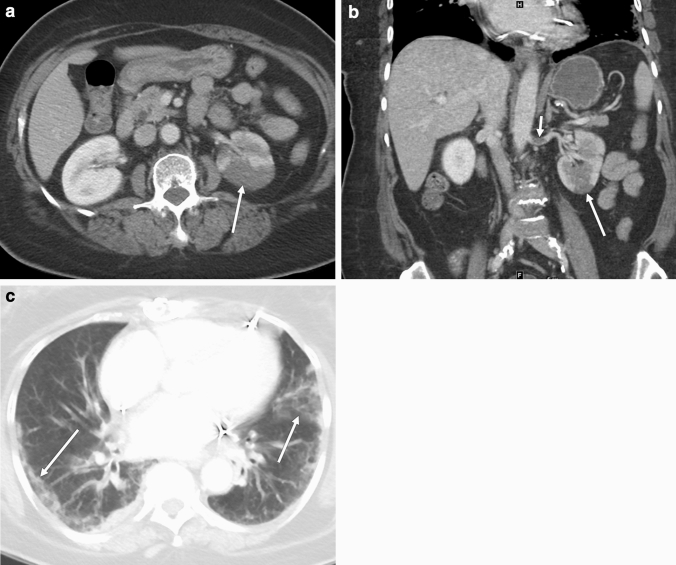
Fig. 3.
CT in a 69-year-old female with clinical indication of abdominal pain. Axial (a) and coronal (b) images through the abdomen demonstrate wedge-shaped areas of decreased enhancement in the left kidney (long arrows), consistent with renal infarcts. A filling defect in the left renal artery (short arrow, b) is consistent with an arterial thrombus. Axial image through the lung bases c demonstrates multiple peripheral ground-glass opacities (arrows) at the lung bases bilaterally, reported as typical features for COVID pneumonia
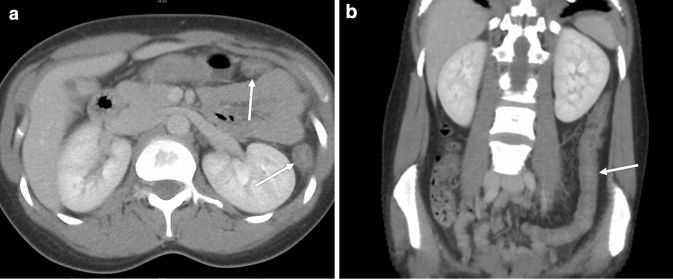
Fig. 4.
CT in a 30-year-old female with clinical indication of abdominal pain. Axial (a) and coronal (b) CT images through the abdomen demonstrate mural thickening in the left colon (arrows). No abnormalities were reported in the lung bases
Table 4 summarizes the results of the multivariate analysis. Hemoglobin, age and gender were independent predictors of having positive abdominopelvic findings, adjusted for race and Charlson index. The odds of having positive abdominopelvic findings decreased 17% per each unit of hemoglobin increase (p = 0.029), decreased 15% per each 5-year age increment (p = 0.023), and were 2.6 times higher in men compared to women (p = 0.032).
Table 4.
Multivariate analysis with positive abdominopelvic findings as a dichotomous outcome
| Variable | Odds ratioa | 95% CI | p value |
|---|---|---|---|
| Age (per 5-year increment) | 0.85 | 0.74–0.98 | 0.023 |
| Sex (female as a reference) | 2.58 | 1.09–6.14 | 0.032 |
| Hemoglobin (per 1 unit increment) | 0.83 | 0.70–0.98 | 0.029 |
aAdjusted for race and Charlson score
Discussion
In this study, 57% of abdominopelvic CT exams performed on symptomatic COVID-positive patients had positive CT findings in the abdomen or pelvis. In patients with reported positive abdominopelvic findings, an abnormality of the gastrointestinal tract was the most common (31%), of which the finding of mural thickening was the most frequent. Abdominal pain was overall the most common clinical indication for abdominopelvic CT. Lower hemoglobin, younger age and male gender were independent predictors of having positive abdominopelvic CT findings. In addition, this study found that 64% of patients without abdominopelvic CT findings had findings suggestive of COVID-19 pneumonia at the lung bases.
To our knowledge, this is the largest study evaluating findings on abdominopelvic CT in patients with COVID-19. Nearly all (97%) patients were imaged in the emergency or inpatient hospital setting. Since only slightly more than half (57%) of acutely ill COVID-positive patients in our study were found to have positive abdominopelvic findings on CT, this result suggests that abdominal symptoms may be present in COVID-19 patients without correlative CT imaging findings. Of patients with no positive abdominopelvic findings, 64% demonstrated either intermediate or typical COVID-19 lung findings at the lung bases. The implications of this are important for evaluation and workup of symptomatic patients with abdominal pain, especially during a period of high prevalence of infection, but also when COVID-19 is not suspected. Prior studies have shown that findings on abdominal CT may be the first indication of COVID-19 on the basis of partly imaged lung base parenchymal findings [7–9]. Our report further supports this concept noting that lung parenchymal findings may indeed be the only indication of COVID-19 infection on abdominopelvic CT in patients presenting with abdominal symptoms.
A recent meta-analysis by Sultan et al. reported a prevalence of abdominal pain in COVID-positive patients of 3.6% overall, and up to 5.3% in some populations, including the USA [5]. In our cohort, abdominal pain was the most common clinical indication for abdominopelvic CT (62%). This is similar to the study by Bhayana et al., in which abdominal pain was also the most common symptom in COVID-19 inpatients undergoing abdominopelvic CT, reported in 33% [10]. Gastrointestinal tract abnormalities were the most common CT findings in our study. This is concordant with the results reported by Bhayana et al., who described predominately bowel abnormalities, including ischemia, in the 42 evaluated CT scans [10]. The authors found that 29% of CTs showed bowel wall thickening involving colon or small bowel. Findings of ischemia with pneumatosis or portal venous gas and bowel perforation were also described [10]. Other CT findings included fluid-filled colon in 43% of patients, suggestive of diarrhea [10]. The frequency of GI tract mural thickening in our study was found to be slightly lower, at 15%. The differences may at least in part be due to the fact that the current study evaluated clinical reports rather than re-review of CT images. It is possible that the mural thickening may be relatively under-reported in the clinical setting as compared with the experimental setting. Similarly, the finding of a fluid-filled colon seen in diarrhea was not reported in our cohort, possibly due to under-reporting of this CT finding in our clinical practice. In our cohort, there were no reported cases of mesenteric or bowel ischemia on CT, but there were 2 cases of free air, without an exact reportable cause.
In our cohort, CT findings relating to the gallbladder and biliary system were found in 25% of patients, including gallbladder distension, mural edema (Fig. 2), and findings reported as possible or definite acute cholecystitis; 10% of patients had biliary ductal dilation. Right upper quadrant ultrasound results previously reported on 37 COVID-positive patients showed similar distribution of pathology, with approximately 60% demonstrating gallbladder sludge, 3% demonstrating wall thickening and 3% showing pericholecystic fluid [10]. We acknowledge that some of these positive findings may be related to chronic disease, and more critically, may be unrelated to COVID-19. There is emerging evidence of hepatobiliary injury in COVID-19 patients [5, 10] Almost 40% of COVID-positive patients have abnormal liver function tests (LFT) on admission, and LFT abnormalities are associated with higher fevers, higher levels of C-reactive protein and longer hospital stay [17].LFT elevation indicates the damage to hepatocytes, possibly resulting from a more pronounced inflammatory response, as evidenced by higher fevers and higher levels of inflammatory markers [17]. Additionally, a not-yet-established virus-induced cytopathic effect or a direct ACE-2-mediated viral infection of hepatocytes may be present [5, 17] Since gallbladder wall edema is a common finding in acute hepatitis, and is an independent predictor of a more severe clinical course [18], it is possible that gallbladder wall edema seen in some of our patients is a reflection of hepatocellular damage, either directly by SARS-CoV-2, or by a reactive inflammatory response. Further studies are necessary to determine the implications of the findings of biliary and gallbladder pathology, and the relationship to COVID-19 positivity is uncertain.
In our study, the frequency of abdominal pain as the exam clinical indication was higher in patients with no reported positive abdominopelvic findings. A meta-analysis by Cheung et al. reported that the prevalence of GI symptoms is higher in patients with a more severe COVID course [19]. In our cohort, younger age and male gender were independent predictors of having reported positive abdominopelvic findings on CT. It is known that older age and male gender are associated with higher morbidity and mortality in the COVID-positive population [20, 21]. However, the relationship between GI symptoms, age and gender in the COVID-positive population is not yet clear. One retrospective study from Asia comparing COVID-positive patients presenting primarily with GI symptoms and those with respiratory symptoms failed to demonstrate either age or gender differences between the groups [22]. Conversely, a prospective case–control US-based study demonstrated that male sex was independently associated with a positive COVID test in patients presenting with GI symptoms [23]. Further and larger studies are needed to explore the relationship between age, gender, abdominopelvic CT findings and presenting GI symptoms.
While the exact pathophysiology of SARS-CoV-2 and its effect on the human organism is not yet fully understood, data are emerging that hematopoiesis and coagulation pathways may be adversely affected [4, 24]. In our study, 18% of patients with positive abdominopelvic findings had findings related to a hypercoagulable state, such as solid organ infarctions and vascular thromboses (Fig. 3). Additionally, our results demonstrate that lower hemoglobin levels were independently associated with increased odds of having positive abdominopelvic findings on CT. Several reports from Asia demonstrated that hemoglobin is lower in patients with a more severe clinical course of COVID-19 [25, 26]. It is possible that lower hemoglobin levels identify a subset of patients with more severe manifestations of COVID-19, which translate into identifiable imaging findings, e.g., solid organ infarctions, arterial thromboses, etc.
At our institution, imaging of COVID-positive patients on a fixed modality (e.g., CT or MRI) in the radiology department requires attending-to-attending approval prior to exam performance. This workflow was established to reduce in-hospital virus transmissions to patients and staff, and to ensure that imaging is performed only when the outcome would affect patient management. Radiology attendings are intimately involved in the triage of these patients and are actively engaged in their care. The goal of this workflow is not to deter imaging but rather to partner with referring physicians and direct appropriate imaging care. This approach of reserving COVID-19 imaging for those in which there will be an impact on patient management is endorsed by the RSNA COVID-19 Task Force [27]. The results of this study may be helpful in risk-stratification of which patients would most benefit from abdominopelvic CT imaging. It is possible that for older women with higher hemoglobin levels with COVID-19 and abdominal pain, CT is less likely to reveal an identifiable cause for the pain. This postulate invites further prospective investigation.
This study is limited by its retrospective design which may introduce selection bias. Our demographics reflect a mostly Hispanic and African American study population (74%), and the findings of this study may not be generalizable to different patient populations. Another limitation is that the analysis is based on the data extracted from clinical reports, rather than reassessment of imaging under the experimental conditions. However, the strength of this approach is that it reflects the real-life clinical interpretation of the imaging studies, and is more reflective of what can be expected in the clinical setting. Our criteria for positive findings were very inclusive to reduce false negative results; this may have incurred a higher false positive rate. Importantly, it is unclear whether all diagnoses considered positive were COVID-19 related; some findings may be the result of stress response related to hospitalization or may be due to pre-existing condition unrelated to COVID-19. Lack of a COVID-negative control group in the current study is limits drawing conclusions about which of the reported findings are directly related to COVID, and which are prevalent in the population at the baseline. However, incorporation of Charlson comorbidity index in the multivariate model allowed for an internal control for many common comorbidities, such myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, liver disease, diabetes, renal disease, malignancy, and human immunodeficiency virus. Further multi-institutional study with a contemporary COVID-negative control group can help establish whether the factors associated with having a positive abdominopelvic CT are the same in COVID-positive and COVID-negative patients, and to establish which of the reported abdominopelvic findings are a result of COVID infection. Lastly, we only reviewed the clinical symptomatology as mentioned in a report’s indication, and we were unable to assess the impact of the CT results on the clinical management. As we know, at the time of clinical interpretation of these exams, the existing published data on Abdominopelvic CT in COVID-9 was quite limited and significant under-reporting due to lack of awareness of typical findings is possible.
In conclusion, the frequency of positive imaging findings reported on abdominopelvic CT in symptomatic COVID-positive patients was 57%, indicating that nearly half of CT exams demonstrated no positive finding. Younger age, male gender, and lower hemoglobin levels were independent predictors of having positive findings on abdominopelvic CT. Additional studies are required to further evaluate these preliminary results.
Acknowledgments
Disclosures
V Chernyak: Bayer, consultant. J. Yee: Research Grants: Philips, GE Healthcare, Echopixel. S. Goldberg-Stein, A. Fink, V Paroder, Mariya Kobi: No disclosures.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.COVID-19 Dashboard by the Center for Systems Science and Engineering at John Hopkins University https://www.coronavirusjhuedu/maphtml. [DOI] [PMC free article] [PubMed]
- 2.Wadman M, Couzin-Frankel J, Kaiser J, Matacic C. A rampage through the body. Science. 2020;368(6489):356–360. doi: 10.1126/science.368.6489.356. [DOI] [PubMed] [Google Scholar]
- 3.Luo S, Zhang X, Xu H. Don’t Overlook Digestive Symptoms in Patients With 2019 Novel Coronavirus Disease (COVID-19). Clin Gastroenterol Hepatol. 2020. [DOI] [PMC free article] [PubMed]
- 4.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, et al. AGA Institute Rapid Review of the GI and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed]
- 6.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dane B, Brusca-Augello G, Kim D, Katz DS. Unexpected Findings of Coronavirus Disease (COVID-19) at the Lung Bases on Abdominopelvic CT. AJR Am J Roentgenol. 2020:1-4. [DOI] [PubMed]
- 8.Hossain R, Lazarus MS, Roudenko A, Dako F, Mehta V, Alis J, et al. CT Scans Obtained for Nonpulmonary Indications: Associated Respiratory Findings of COVID-19. Radiology. 2020:201743. [DOI] [PMC free article] [PubMed]
- 9.Siegel A, Chang PJ, Jarou ZJ, Paushter DM, Harmath CB, Arevalo JB, et al. Lung Base Findings of Coronavirus Disease (COVID-19) on Abdominal CT in Patients With Predominant Gastrointestinal Symptoms. AJR Am J Roentgenol. 2020:1-3. [DOI] [PubMed]
- 10.Bhayana R, Som A, Li MD, Carey DE, Anderson MA, Blake MA, et al. Abdominal Imaging Findings in COVID-19: Preliminary Observations. Radiology. 2020:201908. [DOI] [PMC free article] [PubMed]
- 11.Poyiadi N, Cormier P, Patel PY, Hadied MO, Bhargava P, Khanna K, et al. Acute Pulmonary Embolism and COVID-19. Radiology. 2020:201955. [DOI] [PMC free article] [PubMed]
- 12.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020. [DOI] [PMC free article] [PubMed]
- 13.Lushina N, Kuo JS, Shaikh HA. Pulmonary, Cerebral, and Renal Thromboembolic Disease Associated with COVID-19 Infection. Radiology. 2020:201623. [DOI] [PMC free article] [PubMed]
- 14.Prokop M, van Everdingen W, van Rees Vellinga T, Quarles van Ufford J, Stoger L, Beenen L, et al. CO-RADS - A categorical CT assessment scheme for patients with suspected COVID-19: definition and evaluation. Radiology. 2020:201473. [DOI] [PMC free article] [PubMed]
- 15.Austin SR, Wong YN, Uzzo RG, Beck JR, Egleston BL. Why Summary Comorbidity Measures Such As the Charlson Comorbidity Index and Elixhauser Score Work. Medical care. 2015;53(9):e65–e72. doi: 10.1097/MLR.0b013e318297429c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. American journal of epidemiology. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 17.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2020;18(7):1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SJ, Kim JD, Seo YS, Park BJ, Kim MJ, Um SH, et al. Computed tomography findings for predicting severe acute hepatitis with prolonged cholestasis. World journal of gastroenterology: WJG. 2013;19(16):2543–2549. doi: 10.3748/wjg.v19.i16.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung KS, Hung IF, Chan PP, Lung KC, Tso E, Liu R, et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples from the Hong Kong Cohort and Systematic Review and Meta-analysis. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed]
- 20.Organization WH, Organization WH. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). Geneva; 2020.
- 21.Kakodkar P, Kaka N, Baig MN. A Comprehensive Literature Review on the Clinical Presentation, and Management of the Pandemic Coronavirus Disease 2019 (COVID-19) Cureus. 2020;12(4):e7560. doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen A, Agarwal A, Ravindran N, To C, Zhang T, Thuluvath PJ. Are Gastrointestinal Symptoms Specific for COVID-19 Infection? A Prospective Case-Control Study from the United States. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed]
- 24.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun S, Cai X, Wang H, He G, Lin Y, Lu B, et al. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou. China. Clin Chim Acta. 2020;507:174–180. doi: 10.1016/j.cca.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y, Sun LJ, Xu M, Pan J, Zhang YT, Fang XL, et al. Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou. China. J Zhejiang Univ Sci B. 2020;21(5):378–387. doi: 10.1631/jzus.B2000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.RSNA COVID-19 Task Force rsnaorg/COVID-19.