Abstract
Endometrial cancer is the most common malignancies in developed countries. The present study aimed to identify the role of secretoglobin family 2A member 1 (SCGB2A1) expression in uteri corpus endometrial carcinoma (UCEC) from The Cancer Genome Atlas (TCGA) database, and determine the SCGB2A1-associated downstream signaling pathways. The clinicopathological characteristics and gene expression data were downloaded from TCGA database. The Kaplan-Meier method and Cox multivariate model were used for survival analysis. Logistic regression was used to analyze the association between the clinicopathological features and SCGB2A1 expression. For validation, data of SCGB2A1 mRNA expression and protein expression were obtained and then survival analysis was performed for 47 patients with endometrial cancer from the Fudan University Shanghai Cancer Center (FUSCC). In TCGA dataset, SCGB2A1 expression was significantly higher in tumor tissues (n=528) compared with normal tissues (n=23, P<0.001). The decrease in SCGB2A1 expression in UCEC was significantly associated with age at diagnosis, high tumor grade, residual tumor, positive peritoneal cytology, pelvic lymph node metastasis, para-aortic lymph node metastasis and advanced clinical stage with P<0.05. In the multivariate analysis, SCGB2A1 expression was identified as an independent prognostic factor. In the FUSCC validation set, low SCGB2A1 expression was also associated with worse survival compared with high expression in endometrial cancer (P<0.001). Gene Set Enrichment Analysis revealed that SCGB2A1 may be involved in tumor proliferation and cell cycle regulation. In conclusion, SCGB2A1 may have an important role in the prognosis of UCEC, and has value as a new target for novel therapeutic strategies.
Keywords: secretoglobin family 2A member 1, uteri corpus endometrial carcinoma, The Cancer Genome Atlas, biomarker, prognosis
Introduction
Endometrial cancer is the fourth most common cancer in women in the United States (1,2), and the most common gynecological malignancy in developed countries from Cancer Statistics in 2018 (3,4). Statistics obtained from the American Cancer Society suggest that there were 382,069 new cases and ~89,929 deaths associated with corpus uteri worldwide in 2018 (5,6). Endometrioid adenocarcinomas represent 80% of all endometrial cancers, and are viewed as estrogen-dependent endometrioid type I endometrial cancer and serous endometrial cancers are often referred to estrogen-independent carcinoma type II (2). The majority of newly diagnosed patients have a favorable prognosis with a 5-year survival rate of 81.8%, but patients with recurrent/metastatic disease have poor survival, with a 5-year survival rate of 55 and 42% after radiotherapy and chemotherapy, respectively (1,2). Clinical stage (7), histological subtype (8), grade (9), depth of invasive disease (10) and positive lymph node (11) have been reported as important prognostic factors. Elevated serum cancer antigen 125 level is observed during recurrence and tumor relapse (12). Surgery is the initial management for early-stage patients (2). However, due to the limits of effective biomarkers, developing novel treatment options, such as immunotherapy/targeted therapy for patients with recurrent/metastatic disease, remains as a challenge (13).
According to molecular features, The Cancer Genome Atlas (TCGA) divides endometrial cancer into four subgroups: POLE ultra-mutated, hypermutated/microsatellite unstable, copy number low/microsatellite stable and copy number high (serous-like) (1,14). Some studies have reported that 83% of endometrioid adenocarcinomas harbor PTEN mutations (13), which is the initial event occurring with co-mutations of PIK3CA and PIK3R1 (1). In serous endometrial cancers, TP53 mutations are the most common, with an incidence of 90% (13). Furthermore, F-box/WD repeat-containing protein 7, PIK3CA and serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A α isoform somatic mutations and G1/S-specific cyclin-E1 amplifications are observed in this type (1,15). Despite these findings for the diagnosis and differential diagnosis, the evaluation of novel biomarkers for targeted therapy/immunotherapy in endometrial cancer is needed to improve the treatment options.
Secretoglobin, family 2A, member 1 (SCGB2A1), which is also known as mammaglobin-B, is a member of the uteroglobin gene family and was first identified by Becker et al (16) in 1998. SCGB2A1 is highly expressed in human tears, breast and uterus tissue, and is involved in regulating androgen biosynthesis and the androgen receptor/steroid signaling pathway in prostate cancer (17). SCGB2A1 is the top differentially expressed gene in primary epithelial ovarian cancer with a 905-fold upregulation (18–20). In invasive and borderline ovarian carcinoma, presentation of SCGB2A1 expression was correlated to less aggressive behavior and a more favorable outcome (21). However, overexpression of SCGB2A2 is positively correlated with carcinogenesis, especially in advanced the Federation of Gynecology and Obsetrics stage for primary ovarian cancer, increased Silverberg tumor grade and the elevated mitotic index (22). Reference to ovarian cancer, SCGB2A1 maybe a novel prognostic biomarker for UCEC. Tassi et al (23) evaluated 70 patients to analyze SCGB2A1 gene expression by quantitative PCR and immunohistochemistry (IHC), and reported that SCGB2A1 is upregulated in endometrioid endometrial cancer, particularly in well- and moderately-differentiated tumors. However, the association between SCGB2A1 and endometrial cancer and its prognostic value for UCEC are not clear at present. Endometrial cancer cases are primarily estrogen-dependent endometroid types, which are known as type I, and these respond to estrogen/progesterone antagonists (10). SCGB2A1 may participate in estrogen/progesterone synthesis and the estrogen receptor/progesterone receptor signaling pathway, and maybe a potentially novel therapeutic target for endometrial cancer.
The present study aimed to determine the role of SCGB2A1 expression in UCEC using TCGA dataset, and to investigate the associated downstream signaling pathways. In addition, the study aimed to determine a novel candidate biomarker to inform targeted advanced therapeutics in endometrial cancer.
Materials and methods
Case identification and bioinformatics analysis
In total, data of 561 endometrial cancer cases were included in the present study, including 23 adjacent normal endometrium tissues and 552 tumor tissues. Clinicopathological characteristics and gene expression data were downloaded from TCGA official website for the UCEC project (https://portal.gdc.cancer.gov). After cases with incomplete gene expression and incomplete follow-up information were excluded, 528 tumor cases and 23 normal cases were finally identified. The staging system used in the present study was American Joint Committee on Cancer (AJCC) TNM (tumor-node-metastasis) staging system for endometrial cancer (24). For validation, surgical resected tissues were obtained from 47 patients with a median age of 52 years (age range, 42–69 years) who were diagnosed with endometrial cancer at Fudan University Shanghai Cancer Center (FUSCC) from January 2015 to June 2015. The inclusion criteria were as follows: Patients could accept surgical resection without distant disease and resected tumor tissues were available and sufficient for further research. Patients with incomplete follow-up information or unavailable tumor samples were excluded from the present study. All patients provided informed written consent.
Functional analysis
Gene Set Enrichment Analysis (GSEA) is a computational method that determines whether a defined set of genes exhibits differential expression, and the concordant differences between two biological states (25). There were 186 pathways for KEGG analysis and a total 10,192 biological processes for GO analysis, including 7,530 items for biological process (BP), 999 items for cellular component (CC), and 1,663 items for molecular functions (MF). In the present study, GSEA was performed to elucidate the overall survival difference between the high and low expression groups. Gene set permutations were performed for 1,000 times for each analysis. The most significant SCGB2A1-assocaited biological pathway (P<0.05) was selected for further analysis. The significant results of the GSEA were identified using the nominal P<0.05 and a false discovery rate <0.25. Gene Ontology (GO) (https://golang.org) analysis of these DEGs between UCEC and normal tissues was performed using blast2GO with a P≤0.05, and the pathway enrichment analysis was carried out against the Kyoto Encyclopedia of Genes and Genomes (KEGG) database with a Q-value of ≤1 (26). Genotype-Tissue Expression (GTEx) database is a powerful tool to unravel the complex patterns of genetic variation and gene regulation across diverse human tissue types (27). A total of 78 normal endometrium samples were collected from the GTEx database and combined with normal samples from the GTEx database and 23 normal tissues from TCGA database. Finally, differentially expressed genes (DEGs) in 101 normal endometrium tissues and 552 endometrial tumor tissues were analyzed.
RNA extraction and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from 47 endometrial cancer tissues using TRIzol® reagent (cat. no. 15596026; Thermo Fisher Scientific, Inc.). RNA was converted into cDNA using a PrimeScript™ RT Master Mix kit (cat. no. RR036A, Takara Biotechnology Co., Ltd.). Then, qPCR was performed using TB Green® Premix Ex Taq™ II (Tli RNaseH Plus kit, cat. no. RR820A, Takara Biotechnology Co., Ltd.). RT-qPCR analysis was performed according to the manufacturer's protocol: The procedure of RT reaction was 37°C for 15 min, followed by 85°C for 5 sec. The following thermocycling conditions were used for qPCR: 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec, with a final extension at 70°C for 10 sec. For measurement, the fluorescence level of TB Green (TB Green Premix Ex Taq II, Takara Bio, Inc.) was detected to measure the concentration of PCR production. The forward primer for SCGB2A1 mRNA was 5′-AAACTCCTGGAGGACATGGTT-3′. The reverse primer for SCGB2A1 mRNA was 5′-ACTGCTTGAATTTCCCCATAGC-3′. GAPDH mRNA was used as the internal reference. The forward primer for GAPDH mRNA was 5′-GGAGCGAGATCCCTCCAAAAT-3′. The reverse primer for GAPDH mRNA was 5′-GGCTGTTGTCATACTTCTCATGG-3′. Relative SCGB2A1 gene expression levels were calculated using the 2−ΔΔCq method (28).
External validation in multiple public databases and the FUSCC dataset
SCGB2A1 expression data were collected from other two databases to verify the role of SCGB2A1 in endometrial cancer. SCGB2A1 expression data in endometrial cancer tissues determined by immunohistochemistry (IHC) was obtained from The Human Protein Atlas (HPA) database (https://www.proteinatlas.org/). The expression of SCGB2A1 in cancer cell lines was obtained from Broad Institute Cancer Cell Line Encyclopedia (CCLE) database (https://portals.broadinstitute.org/). Furthermore, SCGB2A1 mRNA expression was analyzed using RT-qPCR and the 47 patients were divided into the high-expression and low expression group according to the median value (cycle threshold=24.385) of SCGB2A1 mRNA expression. Then, survival analysis was performed for the two groups in FUSCC dataset. SCGB2A1 protein expression was analyzed in these tumor tissues via IHC analysis, as previously described (29). Furthermore, to investigate aberrant expression of SCGB2A1 in UCEC, DNA methylation analysis was performed using the Mexpress tool (https://mexpress.be).
Statistical analysis
All statistical analyses were analyzed using R software version 3.6.1 software (https://www.r-project.org). Kaplan-Meier survival analysis was performed to compare the overall survival between the low-SCGB2A1 expression group and the high-SCGB2A1 expression group. Logistic regression was used to calculate the odds ratio (OR) of SCGB2A1 expression in groups with different clinical characteristics. The association between the clinicopathologic characteristics and SCGB2A1 expression was analyzed using a Wilcoxon rank sum test. The Cox repression univariate and multivariate model were used for the survival analysis according to overall survival. The cut-off value of continuous variables such as age at diagnosis (median age, 52 years) and SCGB2A1 expression data were determined according to the median value. P<0.05 was considered to indicate a statistically significant difference. P-value was adjusted using the Benjamini and Hochberg (BH) method to decrease sampling bias.
Results
SCGB2A1 expression between UCEC and normal tissues
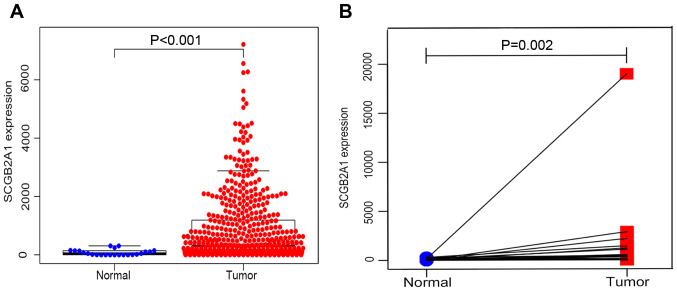
From the TCGA database, 23 normal samples and 528 tumor samples were obtained. As shown in Fig. 1A, SCGB2A1 gene expression was significantly higher in tumor tissues compared with normal tissues by unpaired t-test (P<0.001). In addition, paired analysis for the SCGB2A1 expression was performed in tumor tissues and normal tissues, demonstrating that SCGB2A1 expression was also elevated in tumor tissues by paired t-test (P=0.002, Fig. 1B). Both unpaired and paired t-test's confirmed that SCGB2A1 expression was elevated in UCEC compared with normal tissues.
Figure 1.
SCGB2A1 expression in UCEC compared with (A) normal tissues (n=23) from TCGA database. (B) Comparison of SCGB2A1 expression in 23 pairs of UCEC tissues and adjacent normal tissues. SCGB2A1, secretoglobin family 2A member 1; UCEC, uteri corpus endometrial carcinoma.
Clinicopathological characteristics of patients from the TCGA database and FUSCC dataset
As shown in Table I, 528 patients with UCEC with clinical and gene expression data were obtained from the TCGA database. The age at diagnosis ranged from 31 to 94 years old, with a median age of 64 years. The majority of patients were Caucasian, accounting for 72%. Furthermore, 89% of patients were post-menopausal vs. around 11% of patients were pre-menopausal. Moreover, about 61% of patients underwent open surgery, compared with 39% of patients who underwent minimally invasive surgery. As for the histological type, most of the tumors (76%, n=403) were endometrioid endometrial adenocarcinoma, 20% (n=104) were serous endometrial adenocarcinoma and 4% (n=21) were mixed serous and endometrioid. In TCGA cohort, approximately 19% of the tumors (n=98) were low grade and well-differentiated tumors, 22.73% (n=120) were moderately differentiated tumors and a large proportion of the tumors (58.71%, n=310) were high grade tumors with poor differentiation. Furthermore, 202 (44.30%) tumors had >50% depth of stromal invasion. The tumor status included 418 (85%) tumor-free and 73 (15%) with tumor. Moreover, 90.52% (n=363) reached R0 (no residual tumor), while 9.48% (n=38) had a positive margin (R1/R2). For the peritoneal washing, 343 (86.40%) were negative, while 13.60% (n=54) were positive. The majority of cases (83.88%, n=359) had a negative pelvic lymph node. The para-aortic lymph nodes were usually found to be negative in most cases (90.17%, n=321). In TCGA cohort, 332 patients (62.88%) were stage I, 51 patients (9.66%) were stage II, 119 patients (22.54%) were stage III and 26 patients (4.92%) were stage IV. The median survival time was 27.7 months (range, 0–228 months). The clinical characteristics of 47 patients with endometrial cancer from FUSCC were listed in Table SI.
Table I.
Clinical characteristics of 528 patients with uteri corpus endometrial carcinoma from the Cancer Genome Atlas database.
| Clinicopathological characteristics | Number (%) |
|---|---|
| Age at diagnosisa, years | 64 (31–90) |
| Ethnicity | |
| Caucasian | 361 (72.34) |
| African American | 105 (21.04) |
| Other | 33 (6.61) |
| Menopause status | |
| Pre | 51 (10.56) |
| Post | 432 (89.44) |
| Surgical approach | |
| Minimally invasive | 199 (39.25) |
| Open | 308 (60.75) |
| Histological type | |
| Serous endometrial adenocarcinoma | 104 (19.70) |
| Endometrioid endometrial adenocarcinoma | 403 (76.33) |
| Mixed serous and endometrioid | 21 (3.98) |
| Grade | |
| Low G1 | 98 (18.56) |
| Moderate G2 | 120 (22.73) |
| High G3 | 310 (58.71) |
| Tumor invasion depth, % | |
| <50 | 254 (55.70) |
| ≥50% | 202 (44.30) |
| Tumor status | |
| Tumor free | 418 (85.13) |
| With tumor | 73 (14.87) |
| Residual tumor | |
| Without, R0 | 363 (90.52) |
| With, R1/R2 | 38 (9.48) |
| Peritoneal washing | |
| Negative | 343 (86.40) |
| Positive | 54 (13.60) |
| Pelvic lymph node status | |
| Negative | 359 (83.88) |
| Positive | 69 (16.12) |
| Para-aortic lymph node status | |
| Negative | 321 (90.17) |
| Positive | 35 (9.83) |
| Stage | |
| I | 332 (62.88) |
| II | 51 (9.66) |
| III | 119 (22.54) |
| IV | 26 (4.92) |
Presented as median (range).
Association between SCGB2A1 expression and clinicopathological variables
A total of 528 samples identified from TCGA database were analyzed for the association with SCGB2A1 expression and clinicopathological characteristics. As shown in Table II, the decrease in SCGB2A1 expression was significantly associated with the age at diagnosis (>64 years old vs. <64 years old, P<0.001), grade (high vs. low/moderate, P<0.001), tumor status (with tumor vs. tumor free, P<0.001), residual tumor (R1/R2 vs. R0, P=0.046), peritoneal cytology (positive vs. negative, P=0.004), pelvic lymph node (positive vs. negative, P<0.001), para-aortic lymph node (positive vs. negative, P=0.024) and clinical stage (II vs. I, P<0.001). Elevated SCGB2A1 expression was significantly associated with the histological type (endometrioid vs. serous, P<0.001). Therefore, it was hypothesized that patients with UCEC with a decreased SCGB2A1 expression were more likely to possess high grade, lymph node metastasis and advanced clinical stage.
Table II.
Association between SCGB2A1 expression and clinicopathological variables.
| Characteristics (group A vs. group B) | OR of SCGB2A1 expression (95% CI) | Median (P25,P75) of SCGB2A1 expression (group A) | Median (P25,P75) of S CGB2A1 expression (group B) | P-value |
|---|---|---|---|---|
| Age at diagnosis, >64 vs. <64 years | 0.41 (0.29-0.58) | 561 (122,1696) | 207 (40,799) | <0.001 |
| Ethnicity, African American vs. Caucasian | 0.87 (0.64-1.16) | 174 (36,1334) | 388 (77,1289) | 0.344 |
| Menopause status, post vs. pre | 0.89 (0.54-1.44) | 296 (55,1088) | 926 (263,2069) | 0.622 |
| Surgical approach, open vs. minimally invasive | 0.98 (0.68-1.40) | 334 (62,1169) | 374 (61,1355) | 0.899 |
| Histological type, endometrioid vs. serous | 4.00 (2.60-6.33) | 555 (142,1710) | 48 (13,141) | <0.001 |
| Grade, high vs. low/moderate | 0.11 (0.07-0.17) | 113 (26,420) | 1,166 (415,2264) | <0.001 |
| Tumor invasion depth, ≥50 vs. <50% | 0.73 (0.50-1.05) | 297 (34,1186) | 491 (100,1517) | 0.090 |
| Tumor status, with vs. tumor free | 0.28 (0.15-0.48) | 81 (16,380) | 448 (102,1466) | <0.001 |
| Residual tumor, R1/R2 vs. R0 | 0.49 (0.24-0.97) | 82 (15,549) | 350 (68,1355) | 0.046 |
| Peritoneal washing, positive vs. negative | 0.41 (0.22-0.75) | 99 (26,544) | 388 (68,1335) | 0.004 |
| Pelvic lymph node metastasis, yes vs. no | 0.27 (0.15-0.47) | 89 (11,349) | 426 (82,1467) | <0.001 |
| Para-aortic lymph node metastasis, yes vs. no | 0.42 (0.19-0.87) | 141 (8,411) | 362 (66,1205) | 0.024 |
| Stage, II vs. I | 0.59 (0.49-0.71) | 373 (78,782) | 484 (104,1654) | <0.001 |
OR, odds ratio; CI, confidence interval; SCGB2A1, secretoglobin family 2A member1
Univariate analysis and multivariate analysis for UCEC
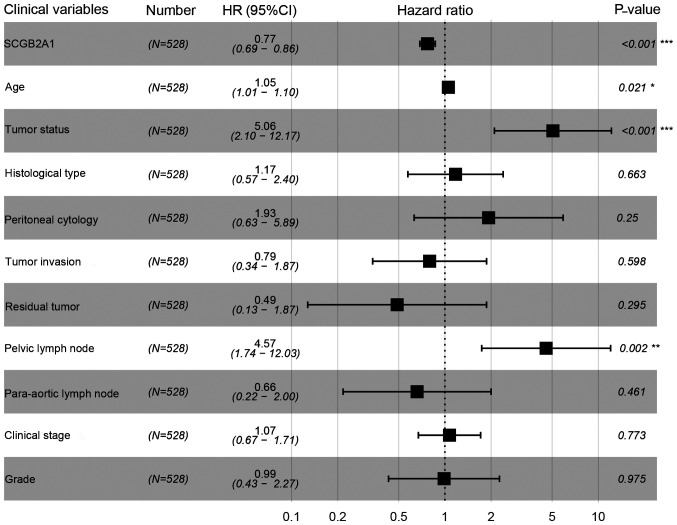
In Fig. 2, Kaplan-Meier survival analysis was performed for patients with UCEC with low and high SCGB2A1 gene expression. The results suggested that low SCGB2A1 expression was associated with worse survival compared with high expression (P<0.001). In Table III, it was illustrated that the decrease in SCGB2A1 expression (P<0.001), age at diagnosis >64 years old (P=0.007), histological type (P=0.033), high grade (P<0.001), deep tumor invasion (P<0.001), with tumor (P<0.001), residual tumor (P<0.001), positive peritoneal cytology (P<0.001), positive pelvic/para-aortic lymph node (P<0.001) and clinical stage (P<0.001) predicted poorer prognosis in the univariate analysis. In the multivariate analysis, SCGB2A1 expression was significantly associated with the prognosis (high vs. low, HR=0.77, P<0.001). SCGB2A1 expression (P<0.001), age at diagnosis (P=0.021), tumor status (P<0.001) and positive lymph node (P=0.002) plotted in the forest map (Fig. 3) were independent prognostic factors.
Figure 2.
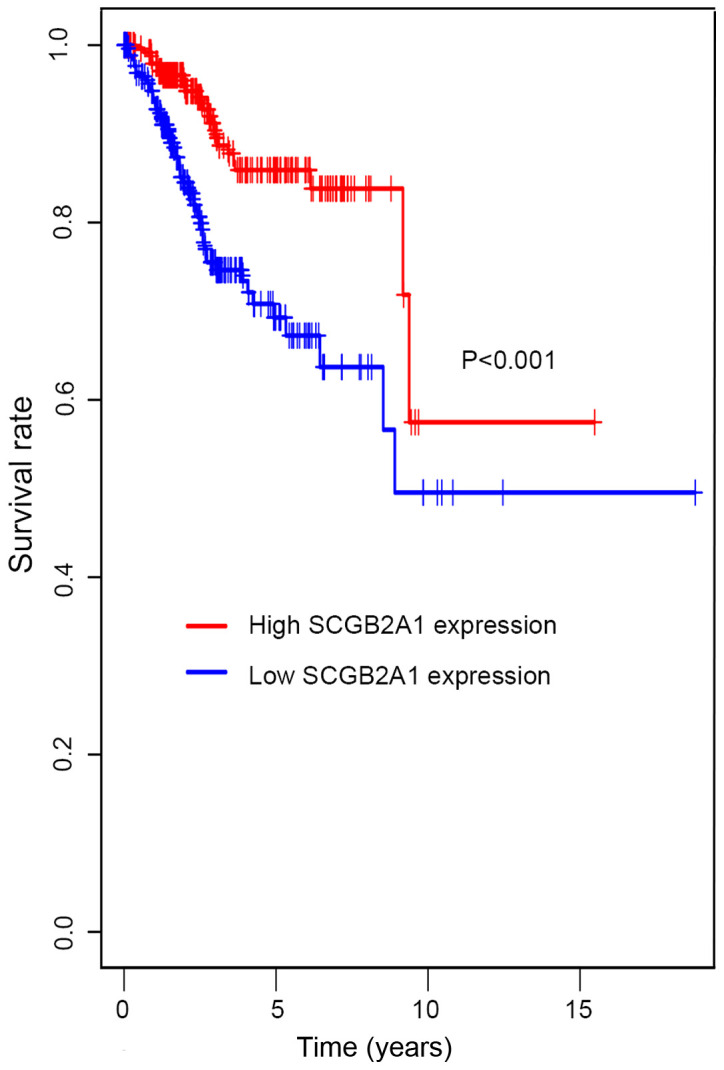
Kaplan-Meier survival analysis for high and low SCGB2A1 expression groups. All patients were divided into high and low expression groups according to > and < median value of SCGB2A1 expression, respectively. SCGB2A1, secretoglobin family 2A member 1.
Table III.
Univariate analysis and multivariate analysis for uteri corpus endometrial carcinoma according to overall survival.
| A, Univariate analysis | ||
|---|---|---|
| Clinical characteristics | HR (95% CI) | P-value |
| SCGB2A1 expression | 0.75 (0.71-0.80) | <0.001 |
| Age at diagnosis | 1.03 (1.00-1.05) | 0.007 |
| Race | 0.95 (0.66-1.36) | 0.769 |
| Menopause status | 0.68 (0.37-1.26) | 0.221 |
| Surgical approach | 0.85 (0.52-1.37) | 0.499 |
| Histological type | 0.60 (0.37-0.96) | 0.033 |
| Grade | 3.49 (2.12-5.75) | <0.001 |
| Tumor invasion depth | 2.43 (1.56-3.87) | <0.001 |
| Tumor status | 6.85 (4.57-10.26) | <0.001 |
| Residual tumor | 2.65 (1.54-4.55) | <0.001 |
| Peritoneal washing | 3.05 (1.85-5.04) | <0.001 |
| Pelvic lymph node metastasis | 4.50 (2.87-7.05) | <0.001 |
| Para-aortic lymph node metastasis | 3.70 (2.11-6.49) | <0.001 |
| Stage | 1.80 (1.51-2.15) | <0.001 |
| B, Multivariate analysis | ||
| Clinical characteristics | HR (95% CI) | P-value |
| SCGB2A1 expression | 0.77 (0.69-0.86) | <0.001 |
| Age at diagnosis | 1.05 (1.01-1.10) | 0.021 |
| Tumor status | 5.06 (2.10-12.17) | <0.001 |
| Pelvic lymph node | 4.57 (1.73-12.03) | 0.002 |
HR, hazard ratio; CI, confidence interval; SCGB2A1, secretoglobin family 2A member 1.
Figure 3.
Forest map of multivariate analysis for SCGB2A1 expression levels as well as clinical variables in UCEC prognosis. A cox regression model was conducted to identify independent factors for UCEC. SCGB2A1, secretoglobin family 2A member 1; UCEC, uteri corpus endometrial carcinoma; HR, hazard ratio.
GSEA for the SCGB2A1-associated pathways
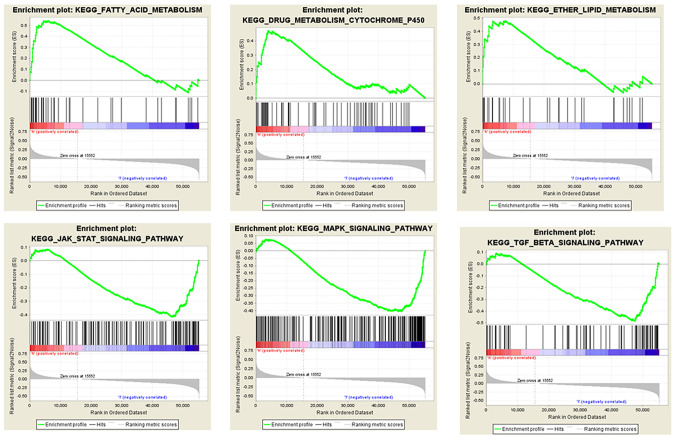
GSEA was conducted for the low and high SCGB2A1 expression groups in order to identify the SCGB2A1-related pathways. It was found that the high SCGB2A1 expression was enriched in ‘fatty acid metabolism’, ‘lipid metabolism’ and ‘cytochrome P450 drug metabolism’. In the low SCGB2A1 expression group, there were 31 predicted pathways with significance. Furthermore, it was found that low SCGB2A1 expression was enriched in the ‘transforming growth factor (TGF)-β signaling pathway’, ‘Janus kinase (JAK)-STAT pathway’ and ‘mitogen-activated protein kinase (MAPK) signaling pathway’ (Fig. 4).
Figure 4.
Multi-GSEA for SCGB2A1-associated signaling pathways. All patients were divided into high and low expression groups and GSEA enrichment analysis was performed. Each column represents SCGB2A1 expression, including high and low expression. The peak value of green curve represents the enrichment score, and high scores indicated that the pathway was mostly enriched. SCGB2A1, secretoglobin family 2A member 1; KEGG, Kyoto Encyclopedia of Genes and Genomes; GSEA, Gene Set Enrichment Analysis.
Differentially expressed genes in UCEC and normal tissues
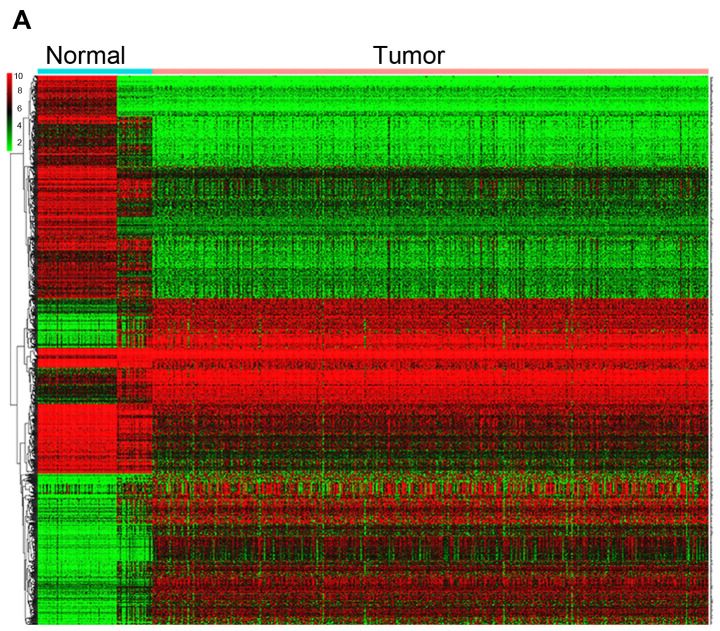
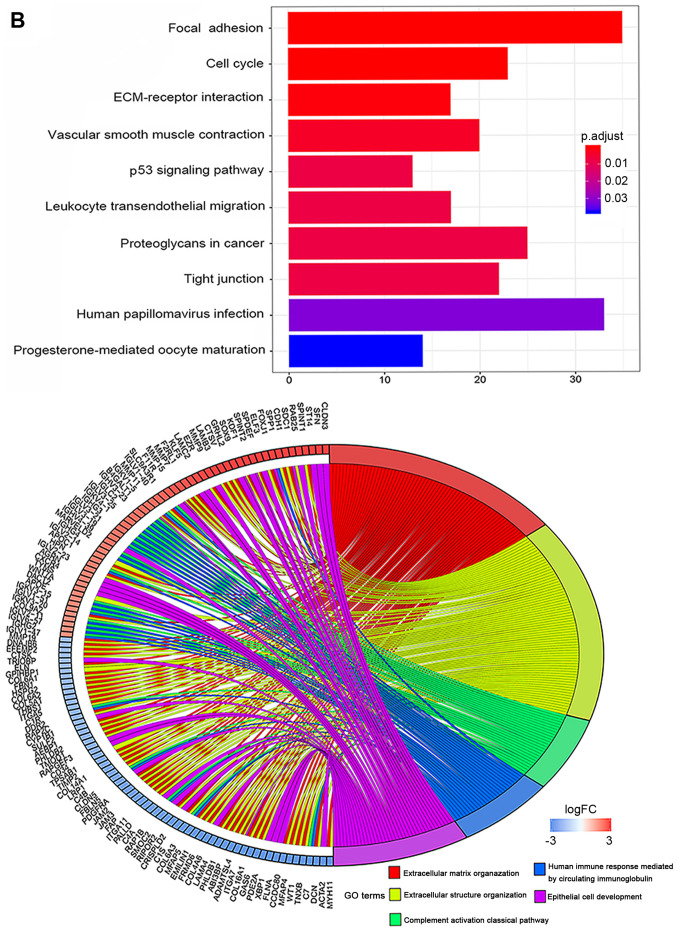
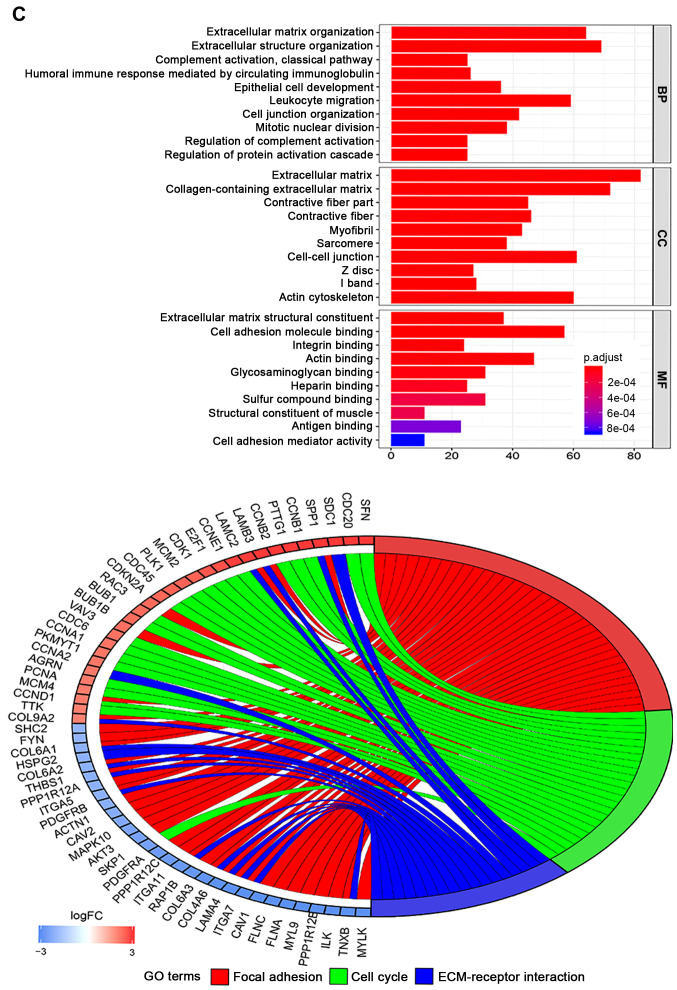
In total, 78 normal endometrial tissues with gene expression data were identified from the Genotype-Tissue Expression (GTEx) database. Then, the expression data of normal and tumor tissues from the TCGA and GTEx databases were merged. Finally, 101 normal tissues and 552 tumor samples were obtained for further analysis. These results revealed that 1,192 genes were differentially expressed in tumors and normal tissues, both with |Log fold-change |≥2 and P<0.05 (Table SII). Heatmaps were established for the differential genes, as shown in Fig. 5A. The GO and KEGG analysis were also conducted with the DEGs (Fig. 5B and C). DEGs were enriched in focal adhesion, cell cycle, ECM-receptor interaction, both P<0.05 in KEGG analysis. DEGs were mostly enriched in extracellular matrix/structure organization for biological process (BP), extracellular matrix/collagen-containing extracellular matrix for cell component (CC) and extracellular matrix structural constituent/cell adhesion molecule binding for molecular function (MF), with P<0.05 in GO analysis.
Figure 5.
DEGs between UCEC and normal tissues. (A) Heatmap for 1,192 DEGs in endometrial cancer and normal tissues. Each column represents one sample and each row represents one gene. The red color and the blue color represent tumor and normal tissues. The gradual color ranging from green to red represent the extent of down to upregulation, respectively. DEGs between UCEC and normal tissues. (B) KEGG bar plot and circle plot for DEGs. The upper graph represents the significant biological process that DEGs were enriched in. The bottom diagram shows the DEGs and their associated biological processes. DEGs between UCEC and normal tissues. (C) GO bar plot and circle plot for DEGs. The upper graph presents the enrichment of DEGs in the three parts of GO terms as BP, CC and MF. The bottom diagram shows the DEGs and mostly significant BPs. DEG, differentially expressed gene; KEGG, Kyoto Encyclopedia of Genes and Genomes; GO, Gene Ontology; BP, biological process; CC, cellular component; MF, molecular function; FC, fold-change.
Validation of the role of SCGB2A1 in endometrial cancer
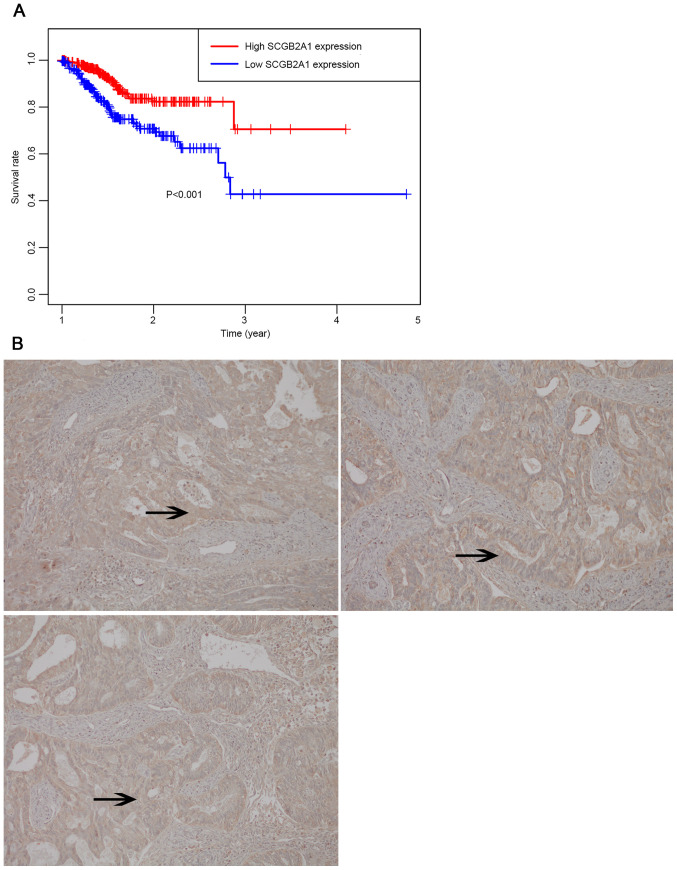
To identify the role of SCGB2A1 in endometrial cancer, SCGB2A1 expression data in endometrial cancer cell lines and tissues was obtained from the CCLE database and HPA database. It was demonstrated that SCGB2A1 expression was higher in endometrial cancer cell lines compared with other cancer cell lines (in Fig. S1). The results demonstrated that SCGB2A1 was mostly overexpressed in prostate cancer cell lines, endometrial cancer cell lines and small cell lung cancer cell lines. The expression of SCGB2A1 was stronger in patient tissues with endometrial cancer (in Fig. S2). In the FUSCC validation set, it was reported that the low-expression group had a less favorable overall survival compared with the high-expression group (P<0.001, Fig. 6A). Moreover, immunohistochemical analysis of SCGB2A1 protein in FUSCC tumor tissues was used to verify SCGB2A1 protein expression in endometrial cancer. It was demonstrated that SCGB2A1 was more highly expressed in patients with endometrial cancer compared with normal tissues (Fig. 6B), which was consistent with findings from the HPA database.
Figure 6.
External validation of the role of SCGB2A1 in UCEC. (A) Survival analysis of the low expression and the high expression group in the FUSCC cohort. In total, 47 patients were divided into the high and low expression groups depending on > or < the median mRNA SCGB2A1 expression value, respectively. (B) SCGB2A1 expression detected using immunohistochemistry in endometrial cancer tissues from the FUSCC cohort. The expression of SCGB2A1 in endometrial cancer was strong positive in brown color (magnification, ×20). The arrows highlight areas with high SCGB2A1 expression. SCGB2A1, secretoglobin family 2A member 1; UCEC, uteri corpus endometrial carcinoma; Fudan University Shanghai Cancer Center, FUSCC.
To investigate the mechanisms underlying aberrant SCGB2A1 gene expression in endometrial cancer, DNA methylation analysis was performed. It was demonstrated that five methylation sites of the SCGB2A1 CpG island (cg05277881, cg16986846, cg06334737, cg23206745 and cg14265033) were significantly correlated with SCGB2A1 gene expression regulation, but with a weak association in UCEC (P<0.001, Fig. S3).
Discussion
Endometrial cancer has been reported to have favorable prognosis (10). However, over the past few years, the morality rate has rapidly increased (5) due to its advanced stage and high risk factors (13). Furthermore, local treatment such as surgery or radiotherapy is not sufficient for advanced diseases. Hence, novel biomarkers for novel therapeutic strategies are needed (13). SCGB2A1, a member of the secretoglobin family of proteins (17), has been considered as a candidate diagnostic biomarker for detecting metastasis in breast cancer. In a study conducted by Mercatali et al (29), SCGB2A1 was detected in the peripheral blood in 7% of patients with breast cancer, while this was not detected in healthy donors. Furthermore, this was positivity correlated with advanced pathological stage (P=0.013) and node metastasis. Limited reports have revealed the role of SCGB2A1 in gynecological cancer types (19,30,31). It has been reported that SCGB2A1 is significantly elevated in epithelial ovarian cancer tissue compared with normal ovarian tissues (32). Elevated SCGB2A1 expression has been reported to present with less aggressive behavior with a decreased risk of recurrence and disease progression, and be correlated with a favorable outcome in ovarian cancer (21). Furthermore, Dieters-Castator et al (19) proposed that SCGB2A1 has value as an endometrioid carcinoma-specific diagnostic biomarker, as well as 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase β-1 and UPF0577 protein KIAA1324, which were also significantly associated with favorable prognosis of patients with endometrioid carcinoma. In addition, Aihara et al (30) reported that the MGB2 gene is a novel biomarker for lymph node micrometastasis in patients with abdominal cancer types. However, research investigating the association between SCGB2A1 and endometrial cancer is limited.
Previous research has reported that the SCGB2A1 gene, which is mediated by the Sp family transcription factors, is essential for androgen synthesis in prostate cancer (17). Androgen is a precursor to the synthesis of estrogen and progesterone, and SCGB2A1 is a crucial regulatory factor for androgen/steroid hormone synthesis, which is associated with the biological process in endometrial cancer (17). Therefore, it was hypothesized that SCGB2A1 may function in regulating female hormone biosynthesis, and that it may be a novel target in UCEC. Tassi et al (23) detected the expression levels of the SCGB2A1 gene in UCEC, and reported that SCGB2A1 expression is higher in endometrial cancer tissue when compared with normal tissues, and that higher SCGB2A1 expression is correlated with good differentiation in tumor tissues. The present study demonstrated that SCGB2A1 expression was higher in endometrial cancer compared with normal tissues (P<0.001). To investigate the mechanisms underlying aberrant SCGB2A1 gene expression in UCEC, methylation analysis was performed. The data revealed that SCGB2A1 CpG island methylation was significantly correlated with SCGB2A1 gene expression regulation. CpG island hypermethylation generally decreases gene expression; however, the results of the present study failed to demonstrate that SCGB2A1 expression was hypermethylated or low-methylated in endometrial cancer. Epigenetic alterations, such as DNA methylation, have been associated with cancer development, and CpG islands methylation is a key contributor to carcinogenesis by silencing tumor-suppressor genes and activating oncogenes in endometrial cancer (31). Based on the aforementioned results, it was hypothesized that SCGB2A1 DNA methylation maybe one of mechanisms underpinning abnormal SCGB2A1 gene expression in UCEC. Further experiments on DNA methylation, histone modification and chromatin remodeling should be performed to investigate the potential mechanism of abnormal SCGB2A1 expression in epigenetic perspective.
In present findings, SCGB2A1 was highly expressed in tumors, and the decrease in SCGB2A1 expression was correlated with higher grade (P<0.001), advanced stage (P<0.001) and worse overall survival (P<0.001). Huang et al (33) established a five gene biomarker model (consisting of ASRGL1, RHEX, SCGB2A1, SOX17 and STX18) to predict the lymph node metastasis in early-stage endometrial cancer. The group reported that low SCGB2A1 expression is associated with the lymph node metastasis. Meanwhile, in the present findings, SCGB2A1 expression was significantly associated with histological grade, clinical stage and overall survival for patients with UCEC. It was revealed that SCGB2A1 expression was an independent prognostic factor for endometrial cancer (high vs. low, HR=0.77, P<0.001) and it was hypothesized that high SCGB2A1 expression was a protective factor in UCEC.
To investigate the involvement of SCGB2A1 in endometrial cancer, functional analysis was conducted to determine the associated signaling pathways. GSEA enrichment analysis was performed, and it was revealed that the high SCGB2A1 expression was correlated with lipid metabolism. Furthermore, it was reported that SCGB2A1 may participate in cell proliferation by regulating the MAPK and JAK-STAT signaling pathways, which may have an effect on tumor growth and progression. Similar to the study conducted by Bignotti et al (32), it was revealed that lipophilin B has a significant correlation with mammaglobin B in ovarian carcinoma. Consistent with GSEA that suggested that SCGB2A1 may participate in lipid metabolism process, lipophilin B, as a homolog to SCGB2A1, was confirmed to be involved in lipid metabolism. Furthermore, Bellone et al (18) reported that SCGB2A1 may be a novel target of immunotherapy in patients with recurrent disease resistant to chemotherapy in ovarian cancer. The group illustrated that CD8+ cytotoxic T lymphocytes populations could decrease SCGB2A1 expression and were able to consistently induce the lysis of autologous primary (chemo-naive) and metastatic/recurrent (chemo-resistant) target tumor cells expressing SCGB2A1. Previous studies have revealed that SCGB2A1 might have a potential as a new biomarker for predicting metastasis (27), and a new target for novel therapy in ovarian cancer (28). However, the role of SCGB2A1 in endometrial cancer has not been elucidated at present. Based on these present results, it was hypothesized that SCGB2A1 may be involved in the estrogen-progestogen signaling pathway and subsequently associated with tumor proliferation and progression in UCEC.
In general, the present study is the first to illustrate the prognostic value of SCGB2A1 for UCEC, to the best of our knowledge, and analyzed the association between clinicopathological factors and SCGB2A1 expression obtained from a large-scale public databases. In the present study, it was demonstrated that low SCGB2A1 expression is indeed correlated with clinical characteristics, such as advanced clinical stage, higher tumor grade, histological type and lymph node metastasis. Furthermore, SCGB2A1 was significantly associated with overall survival, and is an independent prognostic factor. Then, it was reported that SCGB2A1 is associated with tumor growth and progression through the TGF-β, JAK and MAPK signaling pathways, but this needs further research. In the present study, the potential molecular mechanism of SCGB2A1 participating in tumorigenesis and tumor progression of endometrial cancer was not fully elucidated. In vitro (SCGB2A1-silenced/overexpressed cancer cell lines) and in vivo (SCGB2A1 knockdown) xenograft mouse models should be established to provide more evidence.
In conclusion, SCGB2A1 has potential as a new target for predicting the prognosis in UCEC, and the associated downstream pathways of TGF-β, JAK and MAPK signaling may be helpful for further research. The present study highlights the potential of SCGB2A1 as a novel prognostic biomarker for advanced therapy target in UCEC. However, further experimental research is needed to verify the prognostic value of SCGB2A1 in UCEC.
Supplementary Material
Acknowledgements
The authors of the present study would like to thank Professor Xueke Zhou from the Department of Pathology, Fudan University Shanghai Cancer Center (Shanghai, China) and Professor Bin Chang from the Department of Pathology, Fudan University Shanghai Cancer Center (Shanghai, China) for their support in providing immunohistochemistry analyses.
Glossary
Abbreviations
- SCGB2A1
secretoglobin family 2A member 1
- UCEC
uteri corpus endometrial carcinoma
- TCGA
The Cancer Genome Atlas
- GSEA
Gene Set Enrichment Analysis
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- DEGs
differentially expressed genes
Funding
The present study was supported by the Natural Science Foundation of Shanghai (grant no. 17ZR1406000). The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors' contributions
XC and HZ conceived the study. HZ and HL designed the study, analyzed the data and drafted the initial manuscript. XZ helped revised the manuscript critically for important intellectual content and interpreted the data with constructive suggestion. TL and LC helped collect the data and performed statistical analysis. All authors read and approved the final manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the The Cancer Genome Atlas (https://cancergenome.nih.gov/), the Broad Institute Cancer Cell Line Encyclopedia (https://portals.broadinstitute.org/) and The Human Protein Atlas (https://www.proteinatlas.org/) repositories.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center (approval no. 050432-4-1212B). All patients provided informed written consent.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interest.
References
- 1.Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer. 2019;19:510–521. doi: 10.1038/s41568-019-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, Sessa C, ESMO Guidelines Working Group Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi33–vi38. doi: 10.1093/annonc/mdt353. [DOI] [PubMed] [Google Scholar]
- 3.Amant F, Mirza MR, Koskas M, Creutzberg CL. Cancer of the corpus uteri. Int J Gynaecol Obstet. 2018;143(Suppl 2):S37–S50. doi: 10.1002/ijgo.12612. [DOI] [PubMed] [Google Scholar]
- 4.Saso S, Chatterjee J, Georgiou E, Ditri AM, Smith JR, Ghaem-Maghami S. Endometrial cancer. BMJ. 2011;343:d3954. doi: 10.1136/bmj.d3954. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 6.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 7.Tangjitgamol S, Anderson BO, See HT, Lertbutsayanukul C, Sirisabya N, Manchana T, Ilancheran A, Lee KM, Lim SE, Chia YN, et al. Management of endometrial cancer in Asia: Consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1119–1127. doi: 10.1016/S1470-2045(09)70290-6. [DOI] [PubMed] [Google Scholar]
- 8.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka K, Kobayashi Y, Sugiyama J, Yamazaki T, Dozono K, Watanabe M, Shibuya H, Nishigaya Y, Momomura M, Matsumoto H, et al. Histologic grade and peritoneal cytology as prognostic factors in type 1 endometrial cancer. Int J Clin Oncol. 2017;22:533–540. doi: 10.1007/s10147-016-1079-5. [DOI] [PubMed] [Google Scholar]
- 10.Sorosky JI. Endometrial cancer. Obstet Gynecol. 2012;120:383–397. doi: 10.1097/AOG.0b013e3182605bf1. [DOI] [PubMed] [Google Scholar]
- 11.Odagiri T, Watari H, Kato T, Mitamura T, Hosaka M, Sudo S, Takeda M, Kobayashi N, Dong P, Todo Y, et al. Distribution of lymph node metastasis sites in endometrial cancer undergoing systematic pelvic and para-aortic lymphadenectomy: A proposal of optimal lymphadenectomy for future clinical trials. Ann Surg Oncol. 2014;21:2755–2761. doi: 10.1245/s10434-014-3663-0. [DOI] [PubMed] [Google Scholar]
- 12.Reijnen C, IntHout J, Massuger LFAG, Strobbe F, Küsters-Vandevelde HVN, Haldorsen IS, Snijders MPLM, Pijnenborg JMA. Diagnostic accuracy of clinical biomarkers for preoperative prediction of lymph node metastasis in endometrial carcinoma: A systematic review and meta-analysis. Oncologist. 2019;24:e880–e890. doi: 10.1634/theoncologist.2019-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arend RC, Jones BA, Martinez A, Goodfellow P. Endometrial cancer: Molecular markers and management of advanced stage disease. Gynecol Oncol. 2018;150:569–580. doi: 10.1016/j.ygyno.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Le Gallo M, Bell DW. The emerging genomic landscape of endometrial cancer. Clin Chem. 2014;60:98–110. doi: 10.1373/clinchem.2013.205740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murali R, Delair DF, Bean SM, Abu-Rustum NR, Soslow RA. Evolving roles of histologic evaluation and molecular/genomic profiling in the management of endometrial cancer. J Natl Compr Canc Netw. 2018;16:201–209. doi: 10.6004/jnccn.2017.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker RM, Darrow C, Zimonjic DB, Popescu NC, Watson MA, Fleming TP. Identification of mammaglobin B, a novel member of the uteroglobin gene family. Genomics. 1998;54:70–78. doi: 10.1006/geno.1998.5539. [DOI] [PubMed] [Google Scholar]
- 17.Xiao F, Mirwald A, Papaioannou M, Baniahmad A, Klug J. Secretoglobin 2A1 is under selective androgen control mediated by a peculiar binding site for Sp family transcription factors. Mol Endocrinol. 2005;19:2964–2978. doi: 10.1210/me.2004-0408. [DOI] [PubMed] [Google Scholar]
- 18.Bellone S, Tassi R, Betti M, English D, Cocco E, Gasparrini S, Bortolomai I, Black JD, Todeschini P, Romani C, et al. Mammaglobin B (SCGB2A1) is a novel tumour antigen highly differentially expressed in all major histological types of ovarian cancer: Implications for ovarian cancer immunotherapy. Br J Cancer. 2013;109:462–471. doi: 10.1038/bjc.2013.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieters-Castator DZ, Rambau PF, Kelemen LE, Siegers GM, Lajoie GA, Postovit LM, Köbel M. Proteomics-derived biomarker panel improves diagnostic precision to classify endometrioid and high-grade serous ovarian carcinoma. Clin Cancer Res. 2019;25:4309–4319. doi: 10.1158/1078-0432.CCR-18-3818. [DOI] [PubMed] [Google Scholar]
- 20.Tassi RA, Bignotti E, Rossi E, Falchetti M, Donzelli C, Calza S, Ravaggi A, Bandiera E, Pecorelli S, Santin AD. Overexpression of mammaglobin B in epithelial ovarian carcinomas. Gynecol Oncol. 2007;105:578–585. doi: 10.1016/j.ygyno.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 21.Tassi RA, Calza S, Ravaggi A, Bignotti E, Odicino FE, Tognon G, Donzelli C, Falchetti M, Rossi E, Todeschini P, et al. Mammaglobin B is an independent prognostic marker in epithelial ovarian cancer and its expression is associated with reduced risk of disease recurrence. BMC Cancer. 2009;9:253. doi: 10.1186/1471-2407-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer K, von Brünneck AC, Hornung D, Denkert C, Ufer C, Schiebel H, Kuhn H, Borchert A. Differential expression of secretoglobins in normal ovary and in ovarian carcinoma-overexpression of mammaglobin-1 is linked to tumor progression. Arch Biochem Biophys. 2014;547:27–36. doi: 10.1016/j.abb.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Tassi RA, Bignotti E, Falchetti M, Calza S, Ravaggi A, Rossi E, Martinelli F, Bandiera E, Pecorelli S, Santin AD. Mammaglobin B expression in human endometrial cancer. Int J Gynecol Cancer. 2008;18:1090–1096. doi: 10.1111/j.1525-1438.2007.01137.x. [DOI] [PubMed] [Google Scholar]
- 24.McCluggage WG. Pathologic staging of endometrial carcinomas: Selected areas of difficulty. Adv Anat Pathol. 2018;25:71–84. doi: 10.1097/PAP.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 25.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang BL, Li X, Liu P, Ma L, Wu W, Zhang X, Li Z, Huang B. Transcriptomic analysis of Eruca vesicaria subs. sativa lines with contrasting tolerance to polyethylene glycol-simulated drought stress. BMC Plant Biol. 2019;19:419. doi: 10.1186/s12870-019-1997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Hua J, Shi S, Xu J, Wei M, Zhang Y, Liu J, Zhang B, Yu X. Expression patterns and prognostic value of DNA damage repair proteins in resected pancreatic neuroendocrine neoplasms. Ann Surg. 2020 Mar 20; doi: 10.1097/SLA.0000000000003884. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 29.Mercatali L, Valenti V, Calistri D, Calpona S, Rosti G, Folli S, Gaudio M, Frassineti GL, Amadori D, Flamini E. RT-PCR determination of maspin and mammaglobin B in peripheral blood of healthy donors and breast cancer patients. Ann Oncol. 2006;17:424–428. doi: 10.1093/annonc/mdj109. [DOI] [PubMed] [Google Scholar]
- 30.Aihara T, Fujiwara Y, Miyake Y, Okami J, Okada Y, Iwao K, Sugita Y, Tomita N, Sakon M, Shiozaki H, Monden M. Mammaglobin B gene as a novel marker for lymph node micrometastasis in patients with abdominal cancers. Cancer Lett. 2000;150:79–84. doi: 10.1016/S0304-3835(99)00378-X. [DOI] [PubMed] [Google Scholar]
- 31.Bartosch C, Lopes JM, Jeronimo C. Epigenetics in endometrial carcinogenesis-part 1: DNA methylation. Epigenomics. 2017;9:737–755. doi: 10.2217/epi-2016-0166. [DOI] [PubMed] [Google Scholar]
- 32.Bignotti E, Tassi RA, Calza S, Ravaggi A, Rossi E, Donzelli C, Todeschini P, Romani C, Bandiera E, Zanotti L, et al. Secretoglobin expression in ovarian carcinoma: Lipophilin B gene upregulation as an independent marker of better prognosis. J Transl Med. 2013;11:162. doi: 10.1186/1479-5876-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang CY, Liao KW, Chou CH, Shrestha S, Yang CD, Chiew MY, Huang HT, Hong HC, Huang SH, Chang TH, Huang HD. Pilot study to establish a novel five-gene biomarker panel for predicting lymph node metastasis in patients with early stage endometrial cancer. Front Oncol. 2019;9:1508. doi: 10.3389/fonc.2019.01508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the The Cancer Genome Atlas (https://cancergenome.nih.gov/), the Broad Institute Cancer Cell Line Encyclopedia (https://portals.broadinstitute.org/) and The Human Protein Atlas (https://www.proteinatlas.org/) repositories.