Abstract
It is well known that radiation damage of the pharyngeal constrictor muscles, the glottic larynx, and the supraglottic larynx may lead to dysphagia, an unwanted effect of head and neck radiotherapy. The reduction of radiotherapy-induced dysphagia might be achieved by adaptive radiotherapy. Although the number of studies concerning adaptive radiotherapy of head and neck cancer is continuously increasing, there are only a few studies concerning changes in dysphagia-related structures during radiotherapy.
The goal of this review is to summarize the current knowledge about volumetric, dosimetric, and other changes of the pharyngeal constrictor muscles associated with head and neck radiotherapy. A literature search was performed in the MEDLINE database according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The conclusions of 8 studies that passed the criteria indicate a significant increase in the volume and the thickness of the pharyngeal constrictor muscles during radiotherapy. Moreover, the changes in magnetic resonance imaging signal intensity of the pharyngeal constrictor muscles correlate with the absorbed dose (typically higher than 50 Gy) and also with the grade of dysphagia. This systematic review presents 2 variables, which are suitable for estimation of radiotherapy-related pharyngeal constrictor muscles changes—magnetic resonance imaging signal intensity and the thickness. In the case of the thickness, there is no consensus in the level of the measurement—C2 vertebra, C3 vertebra, and the middle of the craniocaudal axis are used. It seems that reference to a position associated with a vertebral body could be more reproducible and beneficial for future research. Although late pharyngeal toxicity remains a challenge in head and neck cancer treatment, better knowledge of radiotherapy-related changes in the pharyngeal constrictor muscles contributes to adaptive radiotherapy development and thus improves the treatment results.
Keywords: radiotherapy, head and neck cancer, pharyngeal muscles, pharyngeal constrictors, dysphagia
Introduction
Radiation-induced dysphagia is one of the side effects of head and neck radiotherapy (RT) or chemo-RT, responsible for a change in the type of diet, prolongation of meal times,1,2 or even a need for tube feeding.3 These consequences have a strong negative impact on the patient’s quality of life. Dysphagia is possibly the most severe acute and late toxicity for patients with oropharyngeal cancer treated by chemo-RT.4 Consistently, Hunter et al 5 concluded that reduced quality of life after treatment correlates closely with the development of dysphagia. Moreover, Machtay et al 6 reported that 43% of patients in remission suffer from dysphagia grade.3-4 Although other toxicities such as xerostomia have been reduced significantly by developments in RT techniques in recent decades, dysphagia remains a challenge for radiation oncologists.
Radiotherapy is a primary modality in head and neck cancer treatment. New RT techniques such as intensity-modulated RT (IMRT), volumetric modulated arc therapy (VMAT), and image-guided RT (IGRT) for head and neck cancer have 2 main goals: the delivery of a curative dose to the tumor and the sparing of healthy tissues. Intensity-modulated RT has enabled dose escalation to the tumor by steep dose gradients between the target volume and healthy tissue. The use of VMAT has led to more homogeneous tumor coverage and more efficient normal tissue sparing.7 The utilization of these techniques has resulted in increased disease control and reduced xerostomia by sparing the parotid glands.8-10 The development of IGRT, especially daily cone-beam computed tomography (CBCT), has increased interfraction accuracy and shows geometrical and anatomical variations during the treatment.11 Adaptive RT (ART) as the next logical step in RT progress may achieve an additional reduction of dose to the organs at risk and may reduce the toxicity of the treatment and thus improve quality of life.12,13 The goal of ART is the modification of treatment in response to the tumor and the organs at risk using online or offline corrections of the treatment plans.14 However, the potential of ART remains much untapped. The tissue changes and biological responses need to be thoroughly investigated for the effective implementation of ART.15
It is evident that dysphagia can only be reduced after identifying all the structures which should be spared. Swallowing is a complex process, in which many anatomical structures participate: 30 pairs of muscles, 6 cranial nerves, and others.
Patients with RT-induced dysphagia have decreased pharyngeal peristalsis and poor synchronization between pharyngeal constrictor muscles (PCM) and other abnormalities.16 It seems that RT-induced damage of the PCM, the glottic larynx, and the supraglottic larynx contributes to the development of dysphagia.17-23 Various planning studies24-29 have confirmed a strong relationship between dose in swallowing structures mentioned above and dysphagia incidence. Nevertheless, according to Duprez et al 30 the most important structures associated with late swallowing disturbances are PCM because their mean dose is the most demonstrative predictor of dysphagia. Currently, a phase III randomized study31 of dysphagia optimized IMRT versus standard IMRT in head and neck cancer is launched, and the results will probably show us the importance of swallowing structures sparing.
Both the tumor and all organs at risk undergo volumetric changes during treatment. Volumetric, dosimetric, and other changes in the parotid glands have been documented in numerous studies.32 Most of them reported anatomic and dosimetric changes in the parotid glands associated with the incidence of xerostomia.11,33-36
Castelli et al 37 published a systematic review focused on ART for head and neck cancer in October 2018, including 29 studies, 11 of which reported benefits of ART, providing either a dosimetric or clinical result. However, none of these studies was engaged with the reduction of dysphagia. A few studies showing the changes in dysphagia-related structures during RT have been published. Thus, the goal of this review is to summarize them along with the current knowledge of volumetric, dosimetric, and other changes of PCM related to head and neck RT.
Materials and Methods
Search Strategy
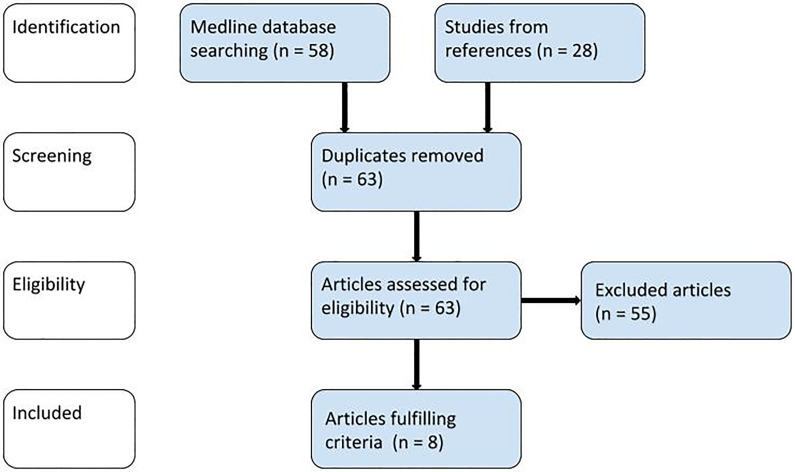
The search of the articles included in this review (Figure 1) was performed in the MEDLINE database according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.38 The following keywords were used: ((pharyngeal constrictor muscles) OR (pharyngeal constrictors)) AND (radiotherapy) AND (head and neck). The search was completed in January 2019. As the review focused only on this topic has not yet been published, the search duration was not restricted; 58 results were found in the MEDLINE database, and 28 records were identified in references (of which 23 were duplicates). In total, 63 articles were reviewed.
Figure 1.
PRISMA flow diagram of the literature search. PRISMA indicates Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Selection Criteria and Data Synthesis
The articles had to be in accordance with the following criteria in order to be included in this review: (1) describing anatomical, dosimetric, or other changes of PCM related to RT, (2) to be written in English, and (3) to be available in full-text form. To avoid biasing the outcome of this review, no other criteria such as “only statistically significant findings” were added. Two reviewers reviewed the search results independently and agreed on the following data extraction.
In all, 47 articles were excluded because they were dealing with another topic: contouring (6), imaging (3), treatment planning (7), and toxicity of treatment (31). Furthermore, 2 clinical investigations reported anatomical changes but unfortunately did not mention PCM (also excluded). Another 4 studies were written in a language other than English, and 2 articles not relating to RT were not included. In total, 55 reviewed records did not meet the criteria for inclusion. The following studies were found appropriate for this review: 4 articles report anatomical changes (2 of them report dosimetric changes as well), 3 articles deal with magnetic resonance imaging (MRI) signal changes, and 1 study is focused on computed tomography (CT) perfusion changes (Table 1). The risk of bias was assessed according to the Cochrane Handbook for Systematic reviews39 (Table 1). Although the majority of the articles did not achieve high scoring, they were all included in our study due to the critically low number of publications focused on the given topic so far.
Table 1.
Studies Included in This Review and Risk Assessment of Individual Studies According To Higgins and Green.39
| Risk of bias in individual studies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of patients | Site | Prescribed dose | Modality | PCM’s parameters | Study method | SG | AC | BP | IO | SO | OS |
| Ricchetti et al 40 | 26 | Oropharynx | 70 Gy | kV CT weekly | Volume, mean dose | Prospective pilot study, consecutive patients | + | ? | ? | – | ? | ? |
| Kumarasiri et al 41 | 23 | Oropharynx | 60-70 Gy | CBCT daily | Volume, thickness, mean dose | Retrospective analysis | ? | ? | ? | + | ? | ? |
| Duffy et al 44 | 5 | More sites | 60-70 Gy | CBCT weekly | Volume, mean dose, V50 | Retrospective analysis | – | ? | ? | + | ? | ? |
| Eisbruch et al 42 | 29 | Base of tongue, larynx, tonsil, hypopharynx | 70 Gy | Endoscopy (3 months after), kV CT (pre, 3 months after) | Thickness | Trial phase I, consecutive patients | + | ? | ? | – | ? | ? |
| Popovtzer et al 43 | 12 | Tonsil, base of tongue, unknown, nasopharynx, hypopharynx | 70 Gy | MRI (before and 3 months after) | Signal intensity, mean dose, thickness | Prospective pilot study | ? | ? | ? | – | ? | ? |
| Meheissen et al 45 | 46 | Oropharynx | 70 Gy | MRI (before, mid, after) | Signal intensity | Randomized trial phase II/III | + | – | – | + | ? | + |
| Messer et al 46 | 72 | Nasopharynx | 70 Gy | MRI (before, early after, follow up) | Signal intensity, mean dose | Retrospective analysis | – | ? | ? | + | ? | ? |
| Minh Tam Truong et al 47 | 15 | Nasopharynx, oropharynx, hypopharynx, other | 70 Gy | CT (second, fourth, sixth week and 6 weeks after) | Blood flow, blood volume, mean transit time, capillary permeability | Prospective single arm study | ? | ? | ? | – | ? | ? |
Abbreviations: AC, allocation concealment; BP, blinding of participants, personnel, and outcome; CBCT, cone-beam computed tomography; CT, computed tomography; kV CT, kilovolt computed tomography; IO, incomplete outcome data; MRI, magnetic resonance imaging; OS, other sources of bias; PCM, pharyngeal constrictor muscles; SG, sequence generation; SO, selective outcome reporting; V50, volume of the PCM receiving more than 50 Gy.
Results
Anatomical Changes of PCM During RT
Ricchetti et al 40 reported a statistically significant increase in the volume of PCM in 91.6% of analyzed patients treated by chemo-RT (CBCT weekly). The mean volume growth (± standard deviation) of PCM was 0.7 ± 0.9 cm3 (4.8% ± 6.3%) during the first week. The highest volume increase was measured in the seventh week as 2.5 ± 2.9 cm3 (16.9% ± 18.9%).
Kumarasiri et al 41 analyzed the volume and thickness of PCM at the center of the C3 vertebral body during treatment (CBCT daily, measured on every fifth CBCT). The authors measured the mean volume increase in PCM as 11.9 ± 7.6 cm3 (54% ± 33%) over the treatment course. The thickness increased by 2.9 ± 1.9 mm (63 ± 39%) as well (Table 2).
Table 2.
The Thickness of Pharyngeal Constrictor Muscles.
| PCM thickness, mm | |||||
|---|---|---|---|---|---|
| Author | Slice of measurement | Group of patients | Pre-RT | Post-RT | 3 Months post-RT |
| Kumarasiri et al 41 | Center of the C3 | All patients | 4.3 ± 0.7 | 6.9 ± 1.6 | |
| Eisbruch et al 42 | Center of the C2 | Gemcitabine (50-150 mg/m2) | 2.5 (range, 1-5) | 7 (range, 5-11) | |
| Gemcitabine (10 mg/m2) | No difference between the pre-RT and post-RT | ||||
| Popovtzer et al 43 | Middle of the craniocaudal axis of PCM | All patients | 2.9 ± 0.9 | 5.4 ± 1.5 | |
| PCM mean dose < 50 Gy | 3.3 ± 1.0 | 5.3 ± 1.7 | |||
| PCM mean dose >50 Gy | 2.7 ± 0.8 | 5.7 ± 1.4 | |||
Abbreviations: PCM, pharyngeal constrictor muscles; RT, radiotherapy.
Eisbruch et al 42 evaluated PCM changes using several techniques, including endoscopy and CT. All patients were scanned by CT pre-RT and 3 months post-RT. Thickness of PCM was measured on both CT scans. The thickness of the PCM was measured at the center of the C2 vertebral body. The cohort of patients was divided into 2 groups according to gemcitabine dose levels. The group at the higher dose (50-150 mg/m2) possessed a median pre-RT constrictor thickness of 2.5 mm and a median post-RT thickness of 7 mm (Table 2). The group at the lower gemcitabine dose (10 mg/m2) showed no statistically significant difference in PCM thickness between pre-RT and post-RT CT scans.
Popovtzer et al 43 measured the thickness of PCM in the middle of their craniocaudal axis. The PCM thickness significantly increased from 2.9 ± 0.9 mm pre-RT to 5.4 ± 1.5 mm, 3 months post-RT. The increase in thickness was more pronounced in muscles receiving >50 Gy (Table 2).
Doses Received by PCM and Associated Functional Changes
Ricchetti et al 40 reported the mean dose to PCM as 61.7 ± 4.3 Gy due to the lack of sparing at the planning (Table 3). At a median follow-up of 13.0 months, 5 from 26 patients were still percutaneous endoscopic gastrostomy-tube dependent. The authors stated that 23% of patients reported dysphagia and a weight loss >10% and estimated that the changes were probably caused by inflammation or edema.
Table 3.
Mean Doses of Pharyngeal Constrictor Muscles Reviewed in This Study.
| Author | PCM mean dose, Gy | Note |
|---|---|---|
| Ricchetti et al 40 | 61.7 ± 4.3 | |
| Kumarasiri et al 41 | 62.3 | Cumulative after recalculation—63.2 Gy |
| Duffy et al 44 | 37.12 | Cumulative after recalculation—37.83 Gy |
| Popovtzer et al 43 | 52 ± 18 | Superior PCM 59 ± 13 Gy, middle PCM 56 ± 15 Gy, inferior PCM 41 ± 22 Gy |
| Messer et al 46 | 62.4 ± 8.7 | Superior PCM |
| Meheissen et al 45 | 65 | Deducted from the chart |
Abbreviation: PCM, pharyngeal constrictor muscles.
Kumarasiri et al 41 calculated the dose of the day for all fractions to estimate the delivered dose to PCM. The mean cumulative dose to the PCM was 63.2 ± 4.7 Gy, which was 0.9 ± 0. Gy (1.4% ± 1.3%) more than planned (Table 3). A strong correlation between the PCM changes mentioned above and the mean dose to PCM was found. Unexpectedly, mid-course adaptive replanning showed only a small decrease in mean dose to PCM, and the authors assumed it to be not large enough to influence clinical outcomes. Regrettably, no relationship between toxicity and dose or changes of PCM was published in this article. It is unclear if more frequent replanning would provide better results.
Eisbruch et al 42 also described the results of endoscopies 3 months post-RT. Strictures involving the inferior PCM at the postcricoid level were identified in 7 of 22 cases. In 3 of these 7 cases, the stricture volume received 70 Gy. The lowest dose to most PCM causing stricture was 50 Gy.
Duffy et al 44 recalculated the original plan on weekly performed CBCTs. The PCM mean dose and the volume of PCM receiving more than 50 Gy (V50) were compared against the original plan. They described a statistically significant decrease in V50, unfortunately, without quantification. The greatest decrease in V50 of PCM was described in the oropharyngeal case. The reference value of V50 was 9.30 cm3, while the recalculated V50 was 7.70 ± 0.51 cm3. Different results were obtained in the case of bilateral neck disease, where the mean dose to PCM increased. The reference mean dose was 33.50 Gy, while the recalculated mean dose was 36.50 ± 0.41 Gy. The authors linked this change to nonpredictable differences in laryngeal position and neck flexion. Any toxicity information is missing in this article.
Popovtzer et al 43 reported doses to the superior, middle, and inferior PCM as 59 ± 13 Gy, 56 ± 15 Gy, and 41 ± 22 Gy, respectively (Table 3). All the PCM received 52 ± 18 Gy in total. From the cohort of 12 patients, at 3 months post-RT, 2 patients whose PCM received mean doses >60 Gy were gastric tube-dependent, 2 other patients required liquid food, and 8 patients had no or mild dysphagia.
Meheissen et al 45 evaluated doses in PCM only in a chart available in Supplement data. It seems that PCM received doses from 45 to 72 Gy with the mean dose of approximately 65 Gy (Table 3). The authors provided detailed information about dysphagia distribution pre-RT “and post-RT (median follow-up was 7.8 months): 54% patients grade 0, 39% grade 1, 7% grade 2, 0% grade >2 at pre-RT; 17% grade 0, 44% grade 1, 27% grade 2, and 12% grade 3 at post-RT.
Messer et al 46 described the mean dose to the superior PCM as 62.4 ± 8.7 Gy (Table 3) without any reference to dysphagia or another side effect.
Non-negligible anatomic changes in PCM lead to the question: How does the delivered dose differ from the planned one? Several authors reported mean planned doses received by PCM (Table 3). Ricchetti et al 40, Kumarasiri et al 41, and Messer et al 46 measured doses around 62 Gy, while Popovtzer et al 43 measured 52 Gy and Duffy et al 44 only 37 Gy. Besides, 2 studies dealt with recalculation of the PCM cumulative dose based on daily or weekly performed CBCT.41,44 Both of them reported only a slight discrepancy between planned and delivered doses.
Magnetic Resonance Imaging Signal and CT Perfusion Parameter Changes
Popovtzer et al 43 measured and compared the signal intensities of the PCM in pre-RT and 3 months post-RT MRI scans. The signal in T1W scans decreased significantly in each of the 3 PCM (superior, middle, and inferior) receiving a dose higher than 50 Gy. No signal changes were observed in PCM with doses lower than 50 Gy. A significant increase in the T2W signal was described in PCM irradiated throughout the dose range (Table 4). The increase correlated linearly with dose.
Table 4.
MRI Signal Intensity Changes.
| T1W signal intensity | ||||||
|---|---|---|---|---|---|---|
| Author | Group of patients | Pre-RT | Mid-RT | Post-RT | 3 Months post-RT | Late post-RT |
| Popovtzer et al 43 | All patients | 0.87 ± 0.15 | 0.80 ± 0.19 | |||
| PCM mean dose < 50 Gy | 0.85 ± 0.12 | 0.86 ±0.16 | ||||
| PCM mean dose >50 Gy | 0.88 ± 0.16 | 0.77 ± 0.20 | ||||
| Messer et al 46 | All patients | 1.5 ± 0.4 | 1.4 ± 0.4 | |||
| PCM mean dose < 62.25 Gy | 1.3 ± 0.4 | 1.6 ± 0.4 | ||||
| PCM mean dose > 62.25 Gy | 1.6 ± 0.4 | 1.3 ± 0.4 | ||||
| T2W signal intensity | ||||||
| Popovtzer et al 43 | All patients | 0.62 ± 0.5 | 1.14 ± 0.9 | |||
| PCM mean dose < 50 Gy | 0.42 ± 0.07 | 0.60 ± 0.18 | ||||
| PCM mean dose > 50 Gy | 0.71 ± 0.57 | 1.38 ± 1.00 | ||||
| Meheissen et al 45 | All patients | 0.6 | 0.8 | 0.9 | ||
| Messer et al 46 | All patients | 0.48 ± 0.1 | 0.73 ± 0.2 | 0.52 ± 0.2 | ||
| PCM mean dose < 62.25 Gy | 0.48 ± 0.2 | 0.71 ± 0.2 | ||||
| PCM mean dose > 62.25 Gy | 0.48 ± 0.1 | 0.74 ± 0.2 | ||||
| T1W + contrast signal intensity | ||||||
| Meheissen et al 45 | All patients | 0.9 | 1.2 | 1.4 | ||
Abbreviations: PCM, pharyngeal constrictor muscles; RT, radiotherapy.
Meheissen et al 45 collected data from 3 MRIs at pre-RT, mid-RT, and post-RT. The MRI signal intensities in the superior and middle constrictors were evaluated in the same way as did Popovtzer et al.43 Percentage signal changes of each muscle were calculated for pre-RT, mid-RT, and post-RT scans. A significant increase in signal intensity in T1W with contrast was discovered for the superior and middle PCM (Table 4). The T2W signal increase was significant only for the middle PCM. The received dose correlated weakly, albeit still significantly with both T1W with contrast and T2W signal changes for PCM. The percentage signal intensity change was significantly higher in the group of patients with radiation-induced dysphagia than in other patients. The strength of the association of MRI signal changes was calculated not only for the received dose but also for dysphagia grade. Patients with dysphagia grade >2 showed significantly higher changes in T2 signal intensity relative to patients with no or mild dysphagia.
Messer et al 46 characterized MRI signal changes of the superior PCM. T1W and T2W MRI scans were obtained pre-RT, early post-RT, and late post-RT after treatment. The median time to post-RT and late post-RT was 4 months and 41 months, respectively. Data analysis showed the T1W signal decrease in the superior PCM, probably caused by late scarring and fibrosis (Table 3). Patients whose superior PCM received more than 62.25 Gy were associated with a significant fall of the T1W signal. Post-RT T2W scans revealed a signal increase associated with acute edema developed around the superior PCM. The signal at late post-RT on T2W scans had returned to the level at pre-RT. The dose–response relationship of the MRI signal change was obtained.
Not only MRI signal but also CT parameters can contribute to a better understanding of PCM alteration during RT and related dysphagia. Truong et al 47 measured changes in CT perfusion parameters. The spot dose in the area of PCM perfusion measurement was 70 Gy. Patients underwent CT perfusion imaging 4 times in total (second, fourth, and sixth week during treatment, and 6 weeks after). The authors reported the development of dysphagia grade 3 in 7 of 15 patients and grade 0 to 2 in 8 patients during and after chemo-RT (grade 4 was not observed). In 7 patients, grades 1, 2, or 3 (2, 2, and 3 patients, respectively) were present as a baseline before treatment. The mean value of blood flow (BF), blood volume (BV), and capillary permeability (CP) measured in PCM increased over time in all patients included in the study. The values of BF and BV increased substantially during the first 2 weeks of treatment in patients associated with dysphagia development. The CP decreased during chemo-RT relative to baseline in patients with dysphagia grade 3, whereas CP increased in patients with a lower grade. No trend was identified in mean transit time in relation to dysphagia.
Discussion
In this review, we summarized the current knowledge about volumetric, dosimetric, and other changes in PCM related to head and neck RT. In general, there are significant PCM volume and thickness alterations caused by inflammation and edema during RT.40 -43 Moreover, other factors such as concurrent chemotherapy can contribute to PCM volume change, as demonstrated by Eisbruch et al.42 Furthermore, MRI signal changes are also commonly observed, and they correlate with dose, typically higher than 50 Gy.43, 46 Importantly, it is not only the dose–response relationship that is associated with MRI changes but also the grade of dysphagia.45 Cumulative dose recalculation based on daily or weekly performed CBCT showed a slight discrepancy between the planned and delivered dose.41,44
It is known that skeletal muscles are relatively resistant to RT. However, Eisbruch et al 42 stated that evidence of fibrosis of the submucosa and muscle layers of the esophagus and pharynx were shown in patients having RT-induced dysphagia.48 Pharyngeal constrictor muscles are located very close to the submucosa and are affected by submucosal inflammation by the increase in proinflammatory cytokines.49 The presence of the cytokines causes secondary edema and fibrosis of underlying muscles such as PCM.49 These findings could explain volumetric and other changes in PCM summarized in this review.
Although the number of studies concerning ART of head and neck cancer is continuously increasing, only a few studies have been published so far dealing with PCM changes and we tried to summarize them in this review. Each one is focused on a different aspect of volume increase after RT. On the other hand, the data regarding PCM changes are not contradictory, and the conclusions of individual studies support each other.
The greatest limitation of the studies included in this review is the low number of patients involved in the studies. Firstly due to low statistical significance and secondly due to increased heterogeneity of the cohort as most of the articles describe various treated sites with the different prescribed dose. Thus, the relation of PCM changes to the tumor location, and the dose is not obvious. For instance, Duffy et al 44 described a significant decrease in V50 Gy but without quantification and, unfortunately, only on 5 patients with different tumor location. Another drawback of the currently available studies is the low methodological rigor stemming from incomplete information about patient selection, blinding, and outcome data, or other sources of bias (Table 1).
Three of the 9 studies included in this review evaluated thickness changes of PCM during RT. Unfortunately, each team selected a different PCM level for its measurement: C2 vertebra, C3 vertebra, and the middle of the craniocaudal axis. It makes the mutual comparison of the results more demanding. It seems that reference to a position associated with a vertebral body could be more reproducible and less subjective than referring to the middle of the craniocaudal axis. That said, it would be beneficial to find a consensus for future research.
It is well known that MRI provides superior tissue contrast resolution and can detect soft tissue disease. Magnetic resonance imaging parameter that could assess post-RT dysphagia even before clinically apparent development would allow radiation oncologists to adjust the treatment and possibly reduce the risk of toxicity. Such a parameter could be useful, especially when the number of ART dedicated machines combining an MRI scanner and linear accelerator is currently increasing. Although both T1 and T2 signal intensity in PCM correlate with dose and grade of dysphagia,43,45,46 further research with a broader set of patients and better methodology is needed.
Recently, the clinical value of ART has been studied intensively. Although it is evident that substantial anatomic variations such as weight loss, tumor shrinkage, edema, or inflammation are a natural part of head and neck RT, it is still challenging to decide which patients should undergo adaptive replanning and how often. It has been shown that only 20% to 30% of head and neck patients should benefit from ART.33,50 Weekly replanning or only 1 early replanning session seems to be suitable ART methods for sparing the parotid glands for locally advanced head and neck patients.51-53 The advantage of ART for submandibular glands, oral cavity, and dysphagia-related structures has not yet been sufficiently explored.
Various factors influence tissue behavior during treatment, and many parameters can be monitored. Many radiation oncologists focus their efforts on searching for suitable ones, which could make the replanning strategy decision easier.
Conclusion
In summary, this systematic review presents 2 variables, which are suitable for estimation of RT-related PCM changes—PCM thickness measured at the C3 or C2 level and MRI signal intensity. The current literature indicates a significant increase in PCM volume and thickness during RT. In addition, PCM signal intensity changes in MRI scans correlate with the absorbed dose (typically higher than 50 Gy) and also with the grade of dysphagia. Although we assume that PCM changes and functional abnormalities are related to absorbed dose, a generally accepted relationship that has been described in detail is still missing. Obviously, further research in this field is needed. For example, it is still not clear which dosimetric parameter is the most appropriate for the estimation of the development of side effects such as dysphagia, aspiration, or nonoral feeding.
On the other hand, this review contributed to this field of research by a conclusion that PCM thickness and MRI signal intensity could be useful parameters for the estimation of the dysphagia development during or after head and neck RT.
In any case, late pharyngeal toxicity remains a challenge in ART. However, as collaborative research and funding in the field increase, advances that improve the treatment of head and neck cancer are expected to be rapid in the next decade.
Acknowledgments
The authors would like to thank to Dr Ian McColl for proof-reading the manuscript. The authors are grateful to Miroslav Hodek, PhD; Petr Paluska, PhD; and Petra Sykorova for assistance with the manuscript.
Abbreviations
- ART
adaptive radiotherapy
- BF
blood flow
- BV
blood volume
- CBCT
cone-beam computed tomography
- CP
capillary permeability
- CT
computed tomography
- IGRT
image-guided RT
- IMRT
intensity-modulated radiotherapy
- MRI
magnetic resonance imaging
- PCM
pharyngeal constrictor muscles
- RT
radiotherapy
- VMAT
volumetric modulated arc therapy
- V50
volume of PCM receiving more than 50 Gy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Long-term organization development plan no. 1011, Ministry of Defence of the Czech Republic, and Ministry of Health MHCZ-DRO (UHHK, 00179906).
ORCID iD: Jakub Grepl  https://orcid.org/0000-0002-8262-5703
https://orcid.org/0000-0002-8262-5703
References
- 1. Rademaker AW, Vonesh EF, Logemann JA, et al. Eating ability in head and neck cancer patients after treatment with chemoradiation: a 12-month follow-up study accounting for dropout. Head Neck. 2003;25(12):1034–1041. doi:10.1002/hed.10317 [DOI] [PubMed] [Google Scholar]
- 2. Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24(17):2636–2643. doi:10.1200/JCO.2006.06.0079 [DOI] [PubMed] [Google Scholar]
- 3. Morton RP, Crowder VL, Mawdsley R, Ong E, Izzard M. Elective gastrostomy, nutritional status and quality of life in advanced head and neck cancer patients receiving chemoradiotherapy. ANZ J Surg. 2009;79(10):713–718. doi:10.1111/j.1445-2197.2009.05056.x [DOI] [PubMed] [Google Scholar]
- 4. Stenson KM, MacCracken E, List M, et al. Swallowing function in patients with head and neck cancer prior to treatment. Arch Otolaryngol Head Neck Surg. 2000;126(3):371 doi:10.1001/archotol.126.3.371 [DOI] [PubMed] [Google Scholar]
- 5. Hunter KU, Schipper M, Feng FY, et al. Toxicities affecting quality of life after chemo-IMRT of oropharyngeal cancer: prospective study of patient-reported, observer-rated, and objective outcomes. Int J Radiat Oncol Biol Phys. 2013;85(4):935–940. doi:10.1016/j.ijrobp.2012.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26(21):3582–3589. doi:10.1200/JCO.2007.14.8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verbakel WFAR, Cuijpers JP, Hoffmans D, Bieker M, Slotman BJ, Senan S. Volumetric intensity-modulated arc therapy vs. conventional IMRT in head-and-neck cancer: a comparative planning and dosimetric study. Int J Radiat Oncol Biol Phys. 2009;74(1):252–259. doi:10.1016/j.ijrobp.2008.12.033 [DOI] [PubMed] [Google Scholar]
- 8. Dijkema T, Raaijmakers CPJ, Ten Haken RK, et al. Parotid gland function after radiotherapy: the combined Michigan and Utrecht experience. Int J Radiat Oncol Biol Phys. 2010;78(2):449–453. doi:10.1016/j.ijrobp.2009.07.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deasy JO, Moiseenko V, Marks L, Chao KSC, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S58–S63. doi:10.1016/j.ijrobp.2009.06.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee AWM, Ng WT, Chan LLK, et al. Evolution of treatment for nasopharyngeal cancer--success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110(3):377–384. doi:10.1016/j.radonc.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 11. Barker JL, Garden AS, Ang KK, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;59(4):960–970. doi:10.1016/j.ijrobp.2003.12.024 [DOI] [PubMed] [Google Scholar]
- 12. Nishi T, Nishimura Y, Shibata T, Tamura M, Nishigaito N, Okumura M. Volume and dosimetric changes and initial clinical experience of a two-step adaptive intensity modulated radiation therapy (IMRT) scheme for head and neck cancer. Radiother Oncol. 2013;106(1):85–89. doi:10.1016/j.radonc.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 13. Yan D, Liang J. Expected treatment dose construction and adaptive inverse planning optimization: implementation for offline head and neck cancer adaptive radiotherapy. Med Phys. 2013;40(2):021719 doi:10.1118/1.4788659 [DOI] [PubMed] [Google Scholar]
- 14. Schwartz DL. Current progress in adaptive radiation therapy for head and neck cancer. Curr Oncol Rep. 2012;14(2):139–147. doi:10.1007/s11912-012-0221-4 [DOI] [PubMed] [Google Scholar]
- 15. Grégoire V, Langendijk JA, Nuyts S. Advances in radiotherapy for head and neck cancer. J Clin Oncol. 2015;33(29):3277–3284. doi:10.1200/JCO.2015.61.2994 [DOI] [PubMed] [Google Scholar]
- 16. Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53(1):23–28. doi:10.1016/s0360-3016(02)02712-8 [DOI] [PubMed] [Google Scholar]
- 17. Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85(1):64–73. doi:10.1016/j.radonc.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 18. Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93(3):539–544. doi:10.1016/j.radonc.2009.09.017 [DOI] [PubMed] [Google Scholar]
- 19. Mortensen HR, Jensen K, Aksglæde K, Behrens M, Grau C. Late dysphagia after IMRT for head and neck cancer and correlation with dose-volume parameters. Radiother Oncol. 2013;107(3):288–294. doi:10.1016/j.radonc.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 20. Deantonio L, Masini L, Brambilla M, Pia F, Krengli M. Dysphagia after definitive radiotherapy for head and neck cancer. Correlation of dose-volume parameters of the pharyngeal constrictor muscles. Strahlenther Onkol. 2013;189(3):230–236. doi:10.1007/s00066-012-0288-8 [DOI] [PubMed] [Google Scholar]
- 21. Dirix P, Abbeel S, Vanstraelen B, Hermans R, Nuyts S. Dysphagia after chemoradiotherapy for head-and-neck squamous cell carcinoma: dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2009;75(2):385–392. doi:10.1016/j.ijrobp.2008.11.041 [DOI] [PubMed] [Google Scholar]
- 22. Caudell JJ, Schaner PE, Desmond RA, Meredith RF, Spencer SA, Bonner JA. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76(2):403–409. doi:10.1016/j.ijrobp.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 23. MD Anderson Head and Neck Cancer Symptom Working Group. Beyond mean pharyngeal constrictor dose for beam path toxicity in non-target swallowing muscles: dose-volume correlates of chronic radiation-associated dysphagia (RAD) after oropharyngeal intensity modulated radiotherapy. Radiother Oncol. 2016;118(2):304–314. doi:10.1016/j.radonc.2016.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caglar HB, Tishler RB, Othus M, et al. Dose to Larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(4):1110–1118. doi:10.1016/j.ijrobp.2008.02.048 [DOI] [PubMed] [Google Scholar]
- 25. Li B, Li D, Lau DH, et al. Clinical-dosimetric analysis of measures of dysphagia including gastrostomy-tube dependence among head and neck cancer patients treated definitively by intensity-modulated radiotherapy with concurrent chemotherapy. Radiat Oncol. 2009;4(1):52 doi:10.1186/1748-717X-4-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peponi E, Glanzmann C, Willi B, Huber G, Studer G. Dysphagia in head and neck cancer patients following intensity modulated radiotherapy (IMRT). Radiat Oncol. 2011;6(1):1 doi:10.1186/1748-717X-6-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dornfeld K, Simmons JR, Karnell L, et al. Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet- and speech-related quality of life. Int J Radiat Oncol Biol Phys. 2007;68(3):750–757. doi:10.1016/j.ijrobp.2007.01.047 [DOI] [PubMed] [Google Scholar]
- 28. Mazzola R, Ricchetti F, Fiorentino A, et al. Dose–volume-related dysphagia after constrictor muscles definition in head and neck cancer intensity-modulated radiation treatment. BJR. 2014;87(1044):20140543 doi:10.1259/bjr.20140543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68(5):1289–1298. doi:10.1016/j.ijrobp.2007.02.049 [DOI] [PubMed] [Google Scholar]
- 30. Duprez F, Madani I, De Potter B, Boterberg T, De Neve W. Systematic review of dose--volume correlates for structures related to late swallowing disturbances after radiotherapy for head and neck cancer. Dysphagia. 2013;28(3):337–349. doi:10.1007/s00455-013-9452-2 [DOI] [PubMed] [Google Scholar]
- 31. Petkar I, Rooney K, Roe JWG, et al. DARS: a phase III randomised multicentre study of dysphagia- optimised intensity- modulated radiotherapy (Do-IMRT) versus standard intensity- modulated radiotherapy (S-IMRT) in head and neck cancer. BMC Cancer. 2016;16(1):770 doi:10.1186/s12885-016-2813-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brouwer CL, Steenbakkers RJHM, Langendijk JA, Sijtsema NM. Identifying patients who may benefit from adaptive radiotherapy: does the literature on anatomic and dosimetric changes in head and neck organs at risk during radiotherapy provide information to help? Radiother Oncol. 2015;115(3):285–294. doi:10.1016/j.radonc.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 33. Lee C, Langen KM, Lu W, et al. Assessment of parotid gland dose changes during head and neck cancer radiotherapy using daily megavoltage computed tomography and deformable image registration. Int J Radiat Oncol Biol Phys. 2008;71(5):1563–1571. doi:10.1016/j.ijrobp.2008.04.013 [DOI] [PubMed] [Google Scholar]
- 34. Han C, Chen Y-J, Liu A, Schultheiss TE, Wong JYC. Actual dose variation of parotid glands and spinal cord for nasopharyngeal cancer patients during radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(4):1256–1262. doi:10.1016/j.ijrobp.2007.10.067 [DOI] [PubMed] [Google Scholar]
- 35. Robar JL, Day A, Clancey J, et al. Spatial and dosimetric variability of organs at risk in head-and-neck intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68(4):1121–1130. doi:10.1016/j.ijrobp.2007.01.030 [DOI] [PubMed] [Google Scholar]
- 36. Hansen EK, Bucci MK, Quivey JM, Weinberg V, Xia P. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;64(2):355–362. doi:10.1016/j.ijrobp.2005.07.957 [DOI] [PubMed] [Google Scholar]
- 37. Castelli J, Simon A, Lafond C, et al. Adaptive radiotherapy for head and neck cancer. Acta Oncol. 2018;57(10):1284–1292. doi:10.1080/0284186X.2018.1505053 [DOI] [PubMed] [Google Scholar]
- 38. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097 doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Higgins JPT, Green S, Cochrane Collaboration, eds. Cochrane Handbook for Systematic Reviews of Interventions. Wiley-Blackwell; 2008. [Google Scholar]
- 40. Ricchetti F, Wu B, McNutt T, et al. Volumetric change of selected organs at risk during IMRT for oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2011;80(1):161–168. doi:10.1016/j.ijrobp.2010.01.071 [DOI] [PubMed] [Google Scholar]
- 41. Kumarasiri A, Liu C, Kamal M, et al. Changes in pharyngeal constrictor volumes during head and neck radiation therapy: Implications for dose delivery. J Cancer Res Ther. 2017;13(2):218–223. doi:10.4103/0973-1482.183176 [DOI] [PubMed] [Google Scholar]
- 42. Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT?. Int J Radiat Oncol Biol Phys. 2004;60(5):1425–1439. doi:10.1016/j.ijrobp.2004.05.050 [DOI] [PubMed] [Google Scholar]
- 43. Popovtzer A, Cao Y, Feng FY, Eisbruch A. Anatomical changes in the pharyngeal constrictors after chemo-irradiation of head and neck cancer and their dose-effect relationships: MRI-based study. Radiother Oncol. 2009;93(3):510–515. doi:10.1016/j.radonc.2009.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Duffy O, Forde E, Leech M. The dilemma of parotid gland and pharyngeal constrictor muscles preservation-Is daily online image guidance required? A dosimetric analysis. Med Dosim. 2017;42(1):24–30. doi:10.1016/j.meddos.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 45. Meheissen M, Kamal M, Hernandez M, et al. A prospective longitudinal assessment of MRI signal intensity kinetics of non-target muscles in patients with advanced stage oropharyngeal cancer in relationship to radiotherapy dose and post-treatment radiation-associated dysphagia: preliminary findings from a randomized trial. Radiother Oncol. 2019;130:46–55. doi:10.1016/j.radonc.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Messer JA, Mohamed ASR, Hutcheson KA, et al. Magnetic resonance imaging of swallowing-related structures in nasopharyngeal carcinoma patients receiving IMRT: Longitudinal dose-response characterization of quantitative signal kinetics. Radiother Oncol. 2016;118(2):315–322. doi:10.1016/j.radonc.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Truong MT, Lee R, Saito N, et al. Correlating computed tomography perfusion changes in the pharyngeal constrictor muscles during head-and-neck radiotherapy to dysphagia outcome. Int J Radiat Oncol Biol Phys. 2012;82(2):e119–127. doi:10.1016/j.ijrobp.2011.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fajardo LF, Berthrong M, Anderson RE. Radiation Pathology. Oxford University Press; 2001. [Google Scholar]
- 49. Sonis ST, Peterson RL, Edwards LJ, et al. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncology. 2000;36(4):373–381. doi:10.1016/S1368-8375(00)00012-9 [DOI] [PubMed] [Google Scholar]
- 50. Castadot P, Geets X, Lee JA, Grégoire V. Adaptive functional image-guided IMRT in pharyngo-laryngeal squamous cell carcinoma: is the gain in dose distribution worth the effort?. Radiother Oncol. 2011;101(3):343–350. doi:10.1016/j.radonc.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 51. Hunter KU, Fernandes LL, Vineberg KA, et al. Parotid glands dose-effect relationships based on their actually delivered doses: implications for adaptive replanning in radiation therapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2013;87(4):676–682. doi:10.1016/j.ijrobp.2013.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang P, Simon A, Rigaud B, et al. Optimal adaptive IMRT strategy to spare the parotid glands in oropharyngeal cancer. Radiother Oncol. 2016;120(1):41–47. doi:10.1016/j.radonc.2016.05.028 [DOI] [PubMed] [Google Scholar]
- 53. Castelli J, Simon A, Rigaud B, et al. A nomogram to predict parotid gland overdose in head and neck IMRT. Radiat Oncol. 2016;11:79 doi:10.1186/s13014-016-0650-6 [DOI] [PMC free article] [PubMed] [Google Scholar]