Abstract
The incidence of pancreatic neuroendocrine tumors (panNETs) has increased worldwide in the last two decades. Given the indolent nature of these tumors, several patients are diagnosed with metastatic disease, which partially impairs the long-term efficacy of currently available treatments and reduces survival rates. The search for new therapeutic strategies for cancer patients has pushed towards the retrospective analysis of studies involving patients who concomitantly received other drugs together with standard anticancer agents. In this light, several retrospective analyses have shown that metformin use is associated with improved prognosis in patients with different tumor types treated with standard antitumor agents. Metformin, the cornerstone oral agent for the treatment of type 2 diabetes, plays a role in modulating glucose cell metabolism. Its potential ability to interfere with tumors may derive from the tight relationship between metabolic reprogramming in cancer cells and tumor progression. Indications for metformin use as an anticancer drug result from pre-clinical and clinical observations. In particular, metformin use in diabetic patients with advanced panNETs has been associated with better progression-free survival in patients treated with somatostatin analogues with or without metformin.
Keywords: cancer, everolimus, metformin, pancreatic neuroendocrine tumors, panNETs, review
Introduction
Pancreatic neuroendocrine tumors (panNETs) are a group of malignant neoplasms with heterogeneous biological traits and clinical behavior whose incidence has increased in the population worldwide.1–3 Unfortunately, because most panNETs have an indolent behavior and become symptomatic only at a late stage, the majority of patients are diagnosed with already metastatic disease. In particular, non-functional panNETs present in an asymptomatic and clinically silent course until the tumor has massively spread outside the pancreas, more frequently to the liver.4
Surgery is the only potentially definitive cure in the absence of metastatic spread. In the presence of metastatic disease, surgical resection of the primary tumor, coupled with the debulking of tumor metastases can still be feasible in some cases; however, these procedures are often palliative, while the majority of patients require additional medical and/or interventional therapies. Available medical and nuclear treatment options for unresectable panNETs include the use of peptide receptor radiotherapy, somatostatin analogs (which may relieve symptoms related to hormonal hypersecretion and produce antiproliferative effect), the mammalian target of rapamycin (mTOR) inhibitor everolimus, and the tyrosine kinase inhibitor sunitinib.5–10 Indeed, these treatments interfere with different biological mechanisms involved in tumorigenesis, also known as “hallmarks of cancer,”11 which have been revised and applied to the NET biology.12 According to this framework, somatostatin analogs interfere with proliferative signaling, mTOR inhibitor everolimus acts at different stages (angiogenesis, resisting to cell death, proliferative signaling), and tyrosine kinase inhibitor sunitinib interferes with angiogenesis.
The activity of cytotoxic chemotherapy is documented in metastatic pancreatic NETs but not in patients with carcinoid tumors, where clinical efficacy is also limited. In these latter patients, single agent or combinations of streptozocin, temozolomide, oxaliplatin or temozolomide, and capecitabine, were shown to be inactive and were also associated with substantial toxicity.13
Despite the availability of effective treatment options leading to a global improvement of patient-reported quality of life,9 most patients with advanced panNETs finally die as a consequence of disease progression.10 This underlines the urgency to identify new treatment strategies; in particular, identifying low-toxicity molecules that are able to synergize with already established anti-panNET treatments is an expanding research field.
In this clinical scenario, the antidiabetic compound metformin is gaining increasing attention as an anticancer agent. In fact, beside the well-known role in improving glucose homeostasis and in normalizing blood insulin levels in diabetic patients, suggestions of a direct or indirect antineoplastic activity of metformin are emerging from several epidemiological studies and meta-analyses.14,15 In this light, and considering the hallmarks of cancer,12 metformin can potentially act at different stages, that is, on cellular energetics, but also on proliferative signaling.
Here, we review available data regarding metformin’s potential anticancer activity in several solid tumors and in particular in patients with advanced panNETs, with a specific focus on ongoing clinical trials combining metformin with conventional anticancer drugs.
Glucose metabolism, insulin receptor (IR)-IGF-1-receptor/PI3K/AKT/mTORC1 pathway, and cancer progression: the hidden link
The close relationship between metabolic reprogramming in cancer cells and tumor progression is a matter of debate since the discovery of the Warburg effect in 1924.16 Indeed, the notion that the tumor fulfills its energy requirements preferentially through glucose catabolism via the glycolytic pathway instead of oxidative phosphorylation even when the oxygen supply is prominent – a phenomenon known as aerobic glycolysis or the Warburg effect – indicates that higher glucose availability to cancer cells, for instance as a result of higher plasma glucose levels, could result in accelerated tumor cell growth, proliferation, and metastatic spread.17 Moreover, hyperglycemia is frequently accompanied by hyperinsulinemia, which could further promote tumor growth through direct and indirect effects. As a direct effect, insulin stimulates glucose uptake and consumption by the cells, thus stimulating their proliferation. As an indirect effect, insulin displays mitogenic actions promoting cell division, migration, and inhibiting apoptosis through the activation of the insulin receptor (IR)-IGF-1-receptor/phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mTORC1 pathway. Moreover, hyperinsulinemia downregulates the expression of IGF-1-binding proteins, which, in turn, enhance the bioavailability of IGF-1 and promote its binding to IGF1R.18 Finally, insulin can directly activate the PI3K/AKT/mTORC1 pathway by binding to IR.19
Deregulation of PI3K/AKT/mTOR signaling pathway is commonly involved in panNET tumorigenesis.20 mTOR inhibitors used in clinical practice are derived from rapamycin, an anticancer agent also used as an immunosuppressor after organ transplantation. In particular, everolimus – a mTORC1 inhibitor – has been approved for the treatment of patients with advanced well/moderately differentiated panNETs. While everolimus inhibits mTORC1, it does not affect mTORC2 activation. Although everolimus significantly prolongs the progression-free survival (PFS), a number of patients with panNETs did not benefit from the drug due to early or late progression.5
Consistent with the importance of glucose metabolism and blood insulin levels in tumor formation and progression, patients with type 2 diabetes mellitus (T2DM), who have higher blood glucose and insulin levels, are at higher risk of developing cancer as demonstrated by epidemiological studies.21,22 T2DM and cancer are strongly associated to each other; for instance, diabetes has been observed as a comorbidity in up to 75% of patients with exocrine pancreatic cancer23 and an increased risk of developing cancer has been found in diabetic patients.24
Metformin: new applications for an old drug
Metformin is the cornerstone oral agent for the treatment of T2DM patients. Metformin has been used in the treatment of diabetes for more than 50 years in Europe and from 1994 in the USA,25 with a reported 14.4 million patients receiving prescription for metformin or metformin-containing combinations in 2017.26,27 Its widespread use not only results from its indisputable clinical efficacy, but also from its safety profile and scarce adverse effects, including rare cases of lactic acidosis and the absence of risk of causing hypoglycemia. In fact, although in the USA the use of metformin was limited to patients suffering from kidney impairment, in 2016 the US Food and Drug Administration has expanded the criteria for using metformin to patients with mild and moderate impaired kidney function.28,29
Metformin can be easily manufactured in a generic form by a large number of drug makers all over the world with a very cheap cost of production.
When administered orally, metformin is adsorbed by the small intestine and delivered to the tissues via the blood.30 Consistent accumulation of the drug is reached in the liver, which is assumed to be the first site of action where it reduces the glucose synthesis via gluconeogenesis.31,32 Another possible effect is the improvement of insulin action through the modulation of hepatic AMPK33 – one of the energy sensors of the cell.
As previously mentioned, there are indications in support of metformin in reducing the risk of severe complications from T2DM. For instance, metformin use is associated with lower risks of myocardial infarction34 and chronic heart failure35 in diabetic patients. Despite the positive outcomes on diabetic patients, the beneficial effect of metformin on non-diabetic patients is controversial.36,37
Moreover, retrospective studies highlight a potential role of metformin in modulating different tumor-related events, including cancer incidence, recurrence, and survival, mostly in diabetic patients. The association between metformin use and better cancer outcomes emerged from retrospective analyses and has promptly supported the initiation of a series of prospective studies aiming to investigate the efficacy of the antidiabetic medication in the prevention and treatment of different types of malignancies. To date (18 November 2019), a search with the entries “metformin” and “cancer” in the clinical trial database (ClinicalTrials.gov) retrieved 32 results for active, non-recruiting studies and 83 studies that are currently recruiting patients. Considering the completed studies (n = 131) and the ones with unknown status (n = 34), there are a total of 280 records, highlighting the effort to shed light on metformin role as an anticancer drug.
Metformin as anticancer agent: preclinical observations
Metformin acts as an inhibitor of the NADH:ubiquinone oxidoreductase complex in the oxidative phosphorylation pathway.25 The inhibition of the mitochondrial respiratory chain results in the decrease of the energy status of the cells (increased AMP:ATP and ADP:ATP ratios). The lower energy status activates the AMPK energy sensor that switches off cellular processes consuming ATP (i.e. cell division), just like the mTOR pathways aforementioned.33 Other biochemical pathways targeted by metformin are hepatic gluconeogenesis31,32 and lipid metabolism, with an increment in fatty acid oxidation and lipogenesis inhibition, likely due to AMPK action.25
The rationale behind the effect of metformin on cancer development is based either on a possible modulation of systemic glucose metabolism or on a direct antitumor activity.
Regarding systemic metabolic effects, metformin reduces blood glucose concentration in diabetic patients by sensitizing peripheral tissues (i.e. skeletal muscle, liver, adipose tissue) to the effects of insulin, thus promoting glucose uptake in normal cells and consequently reducing blood insulin levels. These systemic metabolic effects could participate in metformin antitumor activity by reducing glucose availability to cancer cells and also by reducing insulin-mediated activation of the insulin receptor (IR)/PI3K/AKT/mTORC1 pathway, which plays an important role in stimulating panNET cell growth, proliferation, and survival.
Cell autonomous, direct antitumor effects of metformin have been investigated in vitro. Data regarding the behavior of panNET cell lines upon metformin treatment are limited due to the complication of handling these cells in culture. One study reported that metformin reduced BON-1 cell viability in a dose-dependent manner acting on AMPK and inhibiting mTORC1/S6K/S6 pathway, with only a slight inhibition of insulin receptor protein expression. Interestingly, the study reported a dual effect of metformin on the ERK signaling pathway, depending on the cell line: a potent induction of ERK phosphorylation in BON-1 cells but an inhibition of ERK phosphorylation in midgut GOT-1 cells. Despite the different levels of ERK activation, the outcome was a reduced cell viability in both cell lines, with the highest reduction obtained in GOT-1 cells. This difference highlights the complexity of signaling pathways stimulated by metformin. It is speculated that the dual effect on ERK activation may depend on a compensatory mechanism of the tumor cells in response to inhibition of mTOR signaling by metformin. In BON-1 cells, there is a compensatory activation of ERK in response to mTOR inhibition, whereas the compensation is not efficient in GOT-1 cells where metformin induces a more potent antiproliferative effect.38 Interestingly, metformin influenced cell distribution in cell cycle, reducing the percentage of cells in the S phase with concomitant increase of the cells in G0/G1 phase.38 The direct role of metformin on panNET cell behavior was confirmed by another study where BON-1 and QGP-1 panNET cells showed reduced survival and diminished migration ability upon metformin exposure, with the involvement of the ERK/AKT pathway and no effect on apoptosis. Unlike the former study, here BON-1 cells showed a clear ERK inhibition upon metformin treatment. The opposite result obtained on ERK phosphorylation in BON-1 cells in response to metformin could be explained by the different setting of the experiments, in particular incubation time (48 h in the study by Vlotides et al.38 and 8 min in the study by Herrera-Martinez et al.39). Moreover, in BON-1 cells, metformin strongly reduced the expression of insulin receptor gene.39 These studies support the concept that metformin could act on ERK/AKT and mTOR signaling pathways that in the end reduce cell proliferation. A possible involvement of insulin receptor gene is also speculated with metformin, which can downregulate the expression of the upstream receptor of the mTOR pathway.38,39 Another metformin target is glycogen synthase kinase (GSK) 3, which was inactivated upon metformin treatment. GSK 3 is also involved in cell proliferation and has been associated with cancer.40,41 Another intriguing hypothesis considers the systemic role of metformin in the regulation of the tumor microenvironment with effects on cancer associated fibroblasts, immune cells (macrophages and T cells), and endothelial cells, which synergize in the determination of cancer cell fate.42
One downside of in vitro investigations is that the drug concentration used to appreciate cell responses is by far higher than the one found in the plasma according to pharmacokinetics assessments (1–20 mM versus 18 µM).30 It is therefore risky to speculate that the effect displayed in vitro would be simply replicated in vivo. In addition, a compound administered in vivo is subjected to a variety of cross-functional interactions and to systemic metabolism, which must be considered in the evaluation of the outcome in patients.
Metformin as an anticancer agent: clinical data
Non-neuroendocrine tumors
Most clinical data supporting the association of metformin with positive cancer outcome derive from retrospective studies. These studies have been undertaken with the purpose of identifying adjuvant medications that could improve clinical outcomes when combined with standard antineoplastic treatments. Since metformin is a glucose lowering medication, most of the studies investigated cancer outcome in diabetic patients. Overall, these analyses showed a positive association between metformin use and longer patient survival. Nevertheless, conflicting findings are reported when considering individual tumor types.
The first evidence of an association between metformin use and cancer risk came from a case–control study published in 2005, in which diabetic patients taking metformin were shown to have overall reduced risk of developing malignant tumors [odds ratio: 0.86, 95% confidence interval (CI): 0.73–1.02].43 Moreover, case–control and cohort studies reported a 10–40% reduction of cancer incidence for different site-specific tumors (breast, colon, liver, pancreas, prostate, endometrium, lung) in patients taking metformin.44 Disappointingly, meta-analyses of randomized control trials failed to confirm an association between metformin use and cancer risk. However, these studies were designed to monitor different outcomes that included, but that were not limited to cancer development, and were not powered to assess a difference in cancer incidence between metformin users and non-users. Moreover, these studies had a short observation time and did not properly monitor patients for cancer development during the follow-up.45 Therefore, the role of metformin use in preventing tumor incidence remains to be fully addressed.
Regarding the potential impact of metformin on the outcomes of patients with already established neoplasms, a meta-analysis including 24,178 patients with four different types of tumors (colorectal, prostate, breast, urothelial) reported better clinical outcomes [recurrence-free survival, overall survival (OS), cancer-specific survival] in patients taking metformin, notwithstanding a series of considerations. First, the impact of metformin use in patients with colorectal cancer reached significance only in non-western patients, while only a trend towards better outcomes was observed in western patients. Second, in patients with prostate cancer, metformin use was associated with better clinical outcomes in patients receiving radiotherapy but not in those undergoing prostatectomies. Finally, only a non-significant association between metformin use and improved clinical outcomes was observed in breast cancer patients, while no clear associations were found in urothelial cancer patients.14 Despite the large sample of patients included in this meta-analysis, clear conclusions cannot be drawn from this study as the settings of the studies were highly variable, not only for the type and progress grade of cancer, but also for the definition of metformin use (before surgery, at surgery, before diagnosis, at diagnosis, as cumulative exposure) and the follow-up period.
Another meta-analysis of nine studies (five retrospective and four cohort studies) including a total of 9265 patients with pancreatic cancer and type 2 diabetes from different countries (USA, Korea, Japan, UK) found that patients taking metformin had significantly better OS when compared with those not taking metformin. Again, when population subgroups were considered separately, the significant association was reported for Asian patients and not for western patients.15
Regarding pancreatic exocrine tumors, five prospective studies evaluating metformin effect have been completed on the 18 November 2019 (search performed with the terms “pancreatic cancer” and “metformin” on the ClinicalTrials.gov database). Results of these trials seem to exclude a clinically meaningful impact of combining metformin with standard antitumor therapies in improving patient outcomes.46–48 No clinical benefit was observed also when metformin dosage was increased during the course of the study. Moreover, more adverse events were reported in metformin-taking patients.46 One study enrolling 60 patients was ended for futility at the preplanned interim analysis.47 Despite the small sample size, inadequate plasma metformin assessment, and unavailability of many tumor tissue samples, this study pointed out a good tolerability of metformin when administered in combination with chemotherapy. In one other case, the treatment resulted in being poorly tolerated by the patients and the study was not continued further.48 Two further studies have been completed during 2019, but results have not been published yet; these two studies may be helpful in enriching the knowledge about metformin role (NCT01666730 and NCT02005419).
A summary of the most recent analyses on metformin association with cancer incidence and patient survival is reported in Table 1.
Table 1.
Table summarizing the outcomes of published meta-analyses analyzing the association of metformin use with cancer incidence and outcome.
| Year & Ref | Cancer site | Cancer incidence, relative risk (95% CI) | Cancer outcome (95% CI) |
|---|---|---|---|
| 201949 | Lung | 0.89 (0.83−0.96) | NR |
| 201850 | Lung | NR | OS HR = 0.77 (0.68–0.86) |
| 201851 | Pancreatic adenocarcinoma | NR | OS HR = 0.88 (0.80−0.97) |
| 201852 | Bladder | No association | RFS HR = 0.55 (0.35−0.88) |
| 201853 | Endometrium | No association | OS HR = 0.61 (0.48−0.77) |
| 201754 | Endometrium | 0.87 (0.80−0.95) | HR = 0.63 (0.45−0.87) |
| 201755 | Endometrium | NR | OS HR = 0.82 (0.70−0.95) |
| 201756 | Colon-rectum | NR | OS HR 0.75 (0.65−0.87) |
| 201757 | Pancreatic adenocarcinoma | NR | OS HR = 0.86 (0.76−0.97) |
| 201758 | Kidney | NR | OS HR = 0.643 (0.520−0.795) |
| 201659 | Liver | 0.49 (0.25−0.97) | NR |
| 201660 | Colon | 0.75 (0.65−0.87) | NR |
| 201661 | Lung | NR | OS HR = 0.78 (0.64−0.93) |
| 201614 | Colon−rectum | NR | OS HR = 0.69 (0.58−0.83) |
| Prostate | NR | OS HR 0.82 (0.73−0.93) | |
| 201562 | Breast | No association | Decreased mortality, RR = 0.652 (0.488−0.873) |
| 201463 | Pancreas | 0.63 (0.46−0.86) | NR |
CI, confidence interval; HR, hazard ratio; NR, not reported; OS, overall survival; RFS, recurrence-free survival; RR, reduced risk.
In all the mentioned studies, the general association of improved survival with metformin often masks a series of discrepancy when considering subgroup populations due to the heterogeneity of the studies. In order to further assess these general retrospective observations, more controlled studies on well-defined populations together with homogeneous type of metformin administration need to be designed.
Pancreatic neuroendocrine tumors
Clinical data on the impact of metformin use on the prognosis of patients with advanced panNETs is limited overall. A small retrospective analysis conducted in 31 patients with advanced grade (G) 1–2 panNETs who were treated between 2009 and 2012 at Fondazione Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Nazionale dei Tumori, Milan, Italy (European Neuroendocrine Tumor Society [ENETS] Center of excellence and a referral Institute for the treatment of panNETs in Northern Italy) revealed for the first time a significant association between metformin use and longer PFS in patients with advanced panNETs.20 In this study, all patients were treated with everolimus at a daily dosage of 10 mg in combination with octreotide LAR 30 mg i.m. every 28 day until disease progression or unbearable toxicity. Out of 31 patients, 12 were diabetic treated with either insulin or metformin, and 19 were normoglycemic. Of note, median PFS was 29 months in diabetic patients taking metformin (95% CI: 17.21–38.46) compared with 11 months in normoglycemic patients (95% CI: 8.41–16.96), with a statistically significant PFS difference (p = 0.018).20
To confirm and expand these findings, we recently conducted a larger, retrospective multicentric analysis involving 445 patients with advanced panNETs, who were treated at 24 medical centers in Italy from 1999 to 2015.64 The primary aim of the study was to investigate the potential association between T2DM and PFS in advanced panNET patients and, as a secondary objective, to assess the association between metformin treatment and clinical outcomes (PFS and OS) among diabetic patients. Patients enrolled in this study received everolimus with or without somatostatin analogues (SAs), and were divided according to the onset of diabetes, before or during the anticancer treatment. This study confirmed and expanded preliminary observations from our previous smaller study. In particular, patient PFS was significantly longer in diabetic patients than in non-diabetic ones (median: 32.0 versus 15.1 months), with diabetic patients receiving metformin showing remarkably better PFS when compared with diabetics receiving other glucose-lowering treatments (median: 44.2 versus 20.8 months), Moreover, there was no significant association between metformin dosage and patient PFS. Overall, the beneficial effect of metformin on PFS was independent of its dosage, type of concomitant antitumor therapy, and, even more relevant, of patient glycemic status.64 Further evidence of a possible anticancer effect of metformin emerged from a landmark analysis of the same study,64 which demonstrated longer PFS in patients who were on metformin treatment before or within 3 months from everolimus or SAs initiation when compared with both patients who did not take metformin and patients who started metformin more than 3 months after treatment initiation. One limitation of the study was the impossibility to investigate a potential association between metformin dosages and clinical outcomes; this was mainly due to the fact that metformin dosages were not reliably assessed and recorded in patients during the course of diabetes before they started the anticancer regimen.
Despite the availability of promising data from retrospective analyses, prospective studies are now needed to assess whether metformin can really improve clinical outcomes in patients with advanced panNETs when used in combination with standard anti-panNET treatments. Moreover, the potential impact of metformin on clinical outcomes should be tested not only on diabetic patients, but also in patients with normoglycemic status.
To date (18 November 2019), there is only one prospective study that is evaluating the impact of adding metformin to everolimus and octreotide in patients with advanced panNETs irrespective of their diabetic status. This single-arm, open-label MetNet1 trial (NCT02294006) was designed to evaluate the efficacy of the experimental treatment, as defined as median PFS at 12 months of treatment, and likely will add new information on the efficacy of metformin use in this setting.65
Expert opinion
Despite promising retrospective data and encouraging results of preclinical experiments, crucial aspects regarding the role of metformin in the treatment of panNET patients still need to be elucidated.
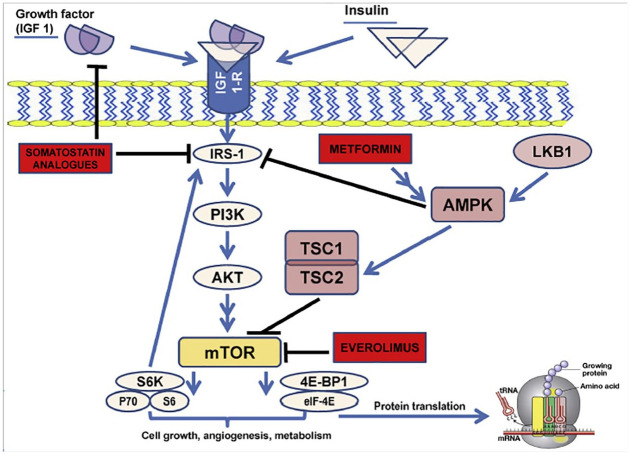
First of all, the molecular mechanism of metformin antitumor activity needs to be unequivocally addressed; in particular, it is essential to clarify if it is more the result of direct antitumor effects of this compound, or the consequence of metformin-induced modifications of systemic metabolism (i.e. blood glucose and insulin concentration). A direct action of metformin on tumor cells may disclose a universal potentiality of this compound whose effect would be beneficial for cancer patients regardless of their diabetic status. Conversely, should metformin effect be related exclusively to its ability to regulate systemic metabolism, and in particular to reduce blood glucose and insulin concentration, diabetic patients would be more likely to benefit from this agent due to more frequently dysregulated metabolic pathways at baseline. However, this latter hypothesis seems to be less likely based on our recent findings showing that metformin use is associated with better patient PFS independently of concomitant insulin use, as well as in patients treated with either SAs or everolimus, which display opposite effects on blood insulin levels.64 Based on these data, a direct, cell-autonomous antitumor activity of metformin against panNET cells could be more reasonable. In particular, AMPK-mediated inhibition of the mTOR pathway by metformin could act synergistically with everolimus and/or SAs, which also result in the inhibition of this signaling pathway that is crucially implicated in panNET cell growth and proliferation (Figure 1).20,64
Figure 1.
Schematic representation of IGF-1/IGF-1-receptor/PI3K/AKT/mTOR axis and the inhibitory effects exercised by everolimus, SAs, and metformin at different levels of the transduction pathway. (Reproduced with permission from20).
Another aspect that needs to be discussed regards an inherent limitation of retrospective studies, which typically do not consider the starting point of metformin use, thus limiting the possibility to assess a duration–effect relationship. This is an important point, because the global time of patient exposure to metformin may play a crucial role in modifying the biology of the tumor, as well as in determining patient probability to respond to specific treatments. Other sources of potential biases in retrospective studies consist in the treatment recall bias, time-on-treatment bias, differences in study design, and type of interventions across different studies.66
For these reasons, further prospective studies are necessary to understand whether metformin could have a stronger impact when administered before diagnosis, during tumor progression, or if it could be effective also in the case of established tumors.20
Recent studies suggest that metformin antitumor activity needs to be evaluated in the light of other systemic metabolic pathways, such as systemic and tumor lipid metabolism. In fact, we found that everolimus-treated advanced panNET patients who displayed higher baseline blood cholesterol levels or increased blood triglycerides during the first 3 months of therapy had significantly lower PFS when compared with patients with normal lipid levels.67 Moreover, higher intratumor expression of the acetyl-CoA carboxylase 1 (ACC1) enzyme, which catalyzes the limiting-step reaction in the fatty acid de novo biosynthesis pathway, was associated with significantly lower patient PFS. Since metformin affects lipid metabolism at both systemic – that is, by reducing blood triglycerides and cholesterol – and tumor level – that is, by indirectly inhibiting ACC1 – this study suggests that metformin could also work by reducing blood or intratumor lipid levels, thus inhibiting another important source of energy units and biomass in panNET cells.
Acknowledgments
Editorial assistance was provided by Barbara Bartolini, PhD, and Aashni Shah (Polistudium srl, Milan, Italy). This assistance was supported by internal funds. We also thank the Associazione Italiana per la Ricerca sul Cancro (AIRC) for supporting our research.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC) (Vernieri C., MFAG-2019 n. 22977).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Sara Pusceddu  https://orcid.org/0000-0002-5096-660X
https://orcid.org/0000-0002-5096-660X
Contributor Information
Sara Pusceddu, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Via Venezian 1, Milan, 20133, Italy.
Claudio Vernieri, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Milan, Italy The FIRC Institute of Molecular Oncology (IFOM), Milan, Italy.
Natalie Prinzi, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Milan, Italy.
Martina Torchio, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Milan, Italy.
Jorgelina Coppa, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Milan, Italy.
Maria Antista, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Milan, Italy.
Monica Niger, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Milan, Italy.
Massimo Milione, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Milan, Italy.
Luca Giacomelli, Polistudium SRL (Milan, Italy) and Department of Surgical Sciences and Integrated Diagnostics, University of Genoa, Genoa, Italy.
Francesca Corti, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Milan, Italy.
Michele Prisciandaro, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Milan, Italy.
Michela Monteleone, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Milan, Italy.
Elena Colombo, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Milan, Italy.
Maria Di Bartolomeo, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Milan, Italy.
Filppo de Braud, Fondazione IRCCS Istituto Nazionale dei Tumori, ENETS Center of Excellence, Milan, Italy University of Milan, Milan, Italy.
References
- 1. Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015; 121: 589–597. [DOI] [PubMed] [Google Scholar]
- 2. Fraenkel M, Kim M, Faggiano A, et al. Incidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literature. Endocr Relat Cancer 2014; 21: R153–R163. [DOI] [PubMed] [Google Scholar]
- 3. Asa SL, Mete O. Endocrine pathology: past, present and future. Pathology 2018; 50: 111–118. [DOI] [PubMed] [Google Scholar]
- 4. Orditura M, Petrillo A, Ventriglia J, et al. Pancreatic neuroendocrine tumors: nosography, management and treatment. Int J Surg 2016; 28(Suppl. 1): S156–S162. [DOI] [PubMed] [Google Scholar]
- 5. Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011; 364: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011; 364: 501–513. [DOI] [PubMed] [Google Scholar]
- 7. Caplin ME, Pavel M, Ruszniewski P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014; 371: 1556–1557. [DOI] [PubMed] [Google Scholar]
- 8. Dash A, Chakraborty S, Pillai MR, et al. Peptide receptor radionuclide therapy: an overview. Cancer Biother Radiopharm 2015; 30: 47–71. [DOI] [PubMed] [Google Scholar]
- 9. Vinik A, Bottomley A, Korytowsky B, et al. Patient-reported outcomes and quality of life with Sunitinib versus Placebo for pancreatic neuroendocrine tumors: results from an international phase III trial. Target Oncol 2016; 11: 815–824. [DOI] [PubMed] [Google Scholar]
- 10. Rinke A, Wittenberg M, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): results of long-term survival. Neuroendocrinology 2017; 104: 26–32. [DOI] [PubMed] [Google Scholar]
- 11. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 12. Walenkamp A, Crespo G, Fierro Maya F, et al. Hallmarks of gastrointestinal neuroendocrine tumours: implications for treatment. Endocr Relat Cancer 2014; 21: R445–R460. [DOI] [PubMed] [Google Scholar]
- 13. Pavel M, de Herder WW. ENETS consensus guidelines for the standard of care in neuroendocrine tumors. Neuroendocrinology 2017; 105: 193–195. [DOI] [PubMed] [Google Scholar]
- 14. Coyle C, Cafferty FH, Vale C, et al. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol 2016; 27: 2184–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou PT, Li B, Liu FR, et al. Metformin is associated with survival benefit in pancreatic cancer patients with diabetes: a systematic review and meta-analysis. Oncotarget 2017; 8: 25242–25250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Potter M, Newport E, Morten KJ. The Warburg effect: 80 years on. Biochem Soc Trans 2016; 44: 1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr 2001; 131: 3109S–3120S. [DOI] [PubMed] [Google Scholar]
- 19. Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov 2014; 13: 140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pusceddu S, Buzzoni R, Vernieri C, et al. Metformin with everolimus and octreotide in pancreatic neuroendocrine tumor patients with diabetes. Future Oncol 2016; 12: 1251–1260. [DOI] [PubMed] [Google Scholar]
- 21. Wojciechowska J, Krajewski W, Bolanowski M, et al. Diabetes and cancer: a review of current knowledge. Exp Clin Endocrinol Diabetes 2016; 124: 263–275. [DOI] [PubMed] [Google Scholar]
- 22. Andersen DK, Korc M, Petersen GM, et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes 2017; 66: 1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gong J, Robbins LA, Lugea A, et al. Diabetes, pancreatic cancer, and metformin therapy. Front Physiol 2014; 5: 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nicolucci A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol 2010; 47: 87–95. [DOI] [PubMed] [Google Scholar]
- 25. Foretz M, Guigas B, Bertrand L, et al. Metformin: from mechanisms of action to therapies. Cell Metab 2014; 20: 953–966. [DOI] [PubMed] [Google Scholar]
- 26. IMS Health. Total patient tracker. Extracted September 2015. 2014, https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/closing-the-healthcare-gap.pdf?la=en&hash=D8C2C80539B75803510BB4BC6916DD4B.
- 27. U.S. Food & Drug Administration. FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function, https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-warnings-regarding-use-diabetes-medicine-metformin-certain (2017).
- 28. Rachmani R, Slavachevski I, Levi Z, et al. Metformin in patients with type 2 diabetes mellitus: reconsideration of traditional contraindications. Eur J Intern Med 2002; 13: 428. [DOI] [PubMed] [Google Scholar]
- 29. Ekström N, Schiöler L, Svensson AM, et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open 2012; 2: pii: e001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tucker GT, Casey C, Phillips PJ, et al. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol 1981; 12: 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnson AB, Webster JM, Sum CF, et al. The impact of metformin therapy on hepatic glucose production and skeletal muscle glycogen synthase activity in overweight type II diabetic patients. Metabolism 1993; 42: 1217–1222. [DOI] [PubMed] [Google Scholar]
- 32. Perriello G, Misericordia P, Volpi E, et al. Acute antihyperglycemic mechanisms of metformin in NIDDM. Evidence for suppression of lipid oxidation and hepatic glucose production. Diabetes 1994; 43: 920–928. [DOI] [PubMed] [Google Scholar]
- 33. Fullerton MD, Galic S, Marcinko K, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med 2013; 19: 1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 35. Eurich DT, Weir DL, Majumdar SR, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail 2013; 6: 395–402. [DOI] [PubMed] [Google Scholar]
- 36. Preiss D, Lloyd SM, Ford I, et al. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. Lancet Diabetes Endocrinol 2014; 2: 116–124. [DOI] [PubMed] [Google Scholar]
- 37. Lexis CP, van der Horst IC, Lipsic E, et al. Effect of metformin on left ventricular function after acute myocardial infarction in patients without diabetes: the GIPS-III randomized clinical trial. JAMA 2014; 311: 1526–1535. [DOI] [PubMed] [Google Scholar]
- 38. Vlotides G, Tanyeri A, Spampatti M, et al. Anticancer effects of metformin on neuroendocrine tumor cells in vitro. Hormones (Athens) 2014; 13: 498–508. [DOI] [PubMed] [Google Scholar]
- 39. Herrera-Martínez AD, Pedraza-Arevalo S, L-López F, et al. Type 2 diabetes in neuroendocrine tumors: are biguanides and statins part of the solution? J Clin Endocrinol Metab 2019; 104: 57–73. [DOI] [PubMed] [Google Scholar]
- 40. Mancinelli R, Carpino G, Petrungaro S, et al. Multifaceted roles of GSK-3 in cancer and autophagy-related diseases. Oxid Med Cell Longev 2017; 2017: 4629495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aristizabal Prada ET, Weis C, Orth M, et al. GSK3α/β: a novel therapeutic target for neuroendocrine tumors. Neuroendocrinology 2018; 106: 335–351. [DOI] [PubMed] [Google Scholar]
- 42. Kurelac I, Umesh Ganesh N, Iorio M, et al. The multifaceted effects of metformin on tumor microenvironment. Semin Cell Dev Biol 2020. 98:90–97. [DOI] [PubMed] [Google Scholar]
- 43. Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005; 330: 1304–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, et al. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia 2017; 60: 1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stevens RJ, Ali R, Bankhead CR, et al. Cancer outcomes and all-cause mortality in adults allocated to metformin: systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia 2012; 55: 2593–2603. [DOI] [PubMed] [Google Scholar]
- 46. Kordes S, Pollak MN, Zwinderman AH, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 2015; 16: 839–847. [DOI] [PubMed] [Google Scholar]
- 47. Reni M, Dugnani E, Cereda S, et al. (Ir)relevance of metformin treatment in patients with metastatic pancreatic cancer: an open-label, randomized phase II trial. Clin Cancer Res 2016; 22: 1076–1085. [DOI] [PubMed] [Google Scholar]
- 48. Braghiroli MI, de Celis Ferrari AC, Pfiffer TE, et al. Phase II trial of metformin and paclitaxel for patients with gemcitabine-refractory advanced adenocarcinoma of the pancreas. Ecancermedicalscience 2015; 9: 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yao L, Liu M, Huang Y, et al. Metformin use and lung cancer risk in diabetic patients: a systematic review and meta-analysis. Dis Markers 2019; 2019: 6230162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xin WX, Fang L, Fang QL, et al. Effect of hypoglycemic agents on survival outcomes of lung cancer patients with diabetes mellitus: a meta-analysis. Medicine (Baltimore) 2018; 97: e0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wan G, Sun X, Li F, et al. Survival benefit of metformin adjuvant treatment for pancreatic cancer patients: a systematic review and meta-analysis. Cell Physiol Biochem 2018; 49: 837–847. [DOI] [PubMed] [Google Scholar]
- 52. Hu J, Chen JB, Cui Y, et al. Association of metformin intake with bladder cancer risk and oncologic outcomes in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Medicine (Baltimore) 2018; 97: e11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chu D, Wu J, Wang K, et al. Effect of metformin use on the risk and prognosis of endometrial cancer: a systematic review and meta-analysis. BMC Cancer 2018; 18: 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tang YL, Zhu LY, Li Y, et al. Metformin use is associated with reduced incidence and improved survival of endometrial cancer: a meta-analysis. Biomed Res Int 2017; 2017: 5905384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meireles CG, Pereira SA, Valadares LP, et al. Effects of metformin on endometrial cancer: systematic review and meta-analysis. Gynecol Oncol 2017; 147: 167–180. [DOI] [PubMed] [Google Scholar]
- 56. Meng F, Song L, Wang W. Metformin improves overall survival of colorectal cancer patients with diabetes: a meta-analysis. J Diabetes Res 2017; 2017: 5063239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li X, Li T, Liu Z, et al. The effect of metformin on survival of patients with pancreatic cancer: a meta-analysis. Sci Rep 2017; 7: 5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li Y, Hu L, Xia Q, et al. The impact of metformin use on survival in kidney cancer patients with diabetes: a meta-analysis. Int Urol Nephrol 2017; 49: 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhou YY, Zhu GQ, Liu T, et al. Systematic review with network meta-analysis: antidiabetic medication and risk of hepatocellular carcinoma. Sci Rep 2016; 6: 33743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rokkas T, Portincasa P. Colon neoplasia in patients with type 2 diabetes on metformin: a meta-analysis. Eur J Intern Med 2016; 33: 60–66. [DOI] [PubMed] [Google Scholar]
- 61. Wan G, Yu X, Chen P, et al. Metformin therapy associated with survival benefit in lung cancer patients with diabetes. Oncotarget 2016; 7: 35437–35445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yang T, Yang Y, Liu S. Association between metformin therapy and breast cancer incidence and mortality: evidence from a meta-analysis. J Breast Cancer 2015; 18: 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang Z, Lai ST, Xie L, et al. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 2014; 106: 19–26. [DOI] [PubMed] [Google Scholar]
- 64. Pusceddu S, Vernieri C, Di Maio M, et al. Metformin use is associated with longer progression-free survival of patients with diabetes and pancreatic neuroendocrine tumors receiving everolimus and/or somatostatin analogues. Gastroenterology 2018; 155: 479–489e477. [DOI] [PubMed] [Google Scholar]
- 65. Pusceddu S, de Braud F, Concas L, et al. Rationale and protocol of the MetNET-1 trial, a prospective, single center, phase II study to evaluate the activity and safety of everolimus in combination with octreotide LAR and metformin in patients with advanced pancreatic neuroendocrine tumors. Tumori 2014; 100: e286–e289. [DOI] [PubMed] [Google Scholar]
- 66. Farmer RE, Ford D, Forbes HJ, et al. Metformin and cancer in type 2 diabetes: a systematic review and comprehensive bias evaluation. Int J Epidemiol 2017; 46: 728–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vernieri C, Pusceddu S, Fucà G, et al. Impact of systemic and tumor lipid metabolism on everolimus efficacy in advanced pancreatic neuroendocrine tumors (pNETs). Int J Cancer 2019; 144: 1704–1712. [DOI] [PubMed] [Google Scholar]