Graphical abstract
Abbreviations: DMSO, dimethyl sulfoxide; PBS, phosphate buffered saline; RBCs, red blood cells
Keywords: Toxicity, Rhodomyrtone, Rhodomyrtus tomentosa, Invertebrate, Vertebrate
Highlights
-
•
Rhodomyrtone, a bioactive acylphloroglucinol compound isolated from the leaves of Rhodomyrtus tomentosa, has been scientifically evidenced as a potential antibacterial agent.
-
•
Rhodomyrtone did not cause any signs of toxicity in invertebrate (Galleria mellonella) and vertebrate (zebrafish, murine) models.
-
•
Rhodomyrtone at 256 μg/mL did not cause any observable human erythrocyte haemolysis.
-
•
As the minimal inhibitory concentration of rhodomyrtone against most Gram-positive pathogens is 0.5-1 μg/mL, the results suggest that it should produce no toxic effects at concentrations used in human.
Abstract
Background
Rhodomyrtus tomentosa (Aiton) Hassk. has been traditionally used to relieve various diseases. Rhodomyrtone, a bioactive acylphloroglucinol compound isolated from the leaves of Rhodomyrtus tomentosa, has been scientifically evidenced as a potential antibacterial agent. This study aimed to assess safety of rhodomyrtone in both invertebrate and vertebrate models.
Material and Methods
Safety of rhodomyrtone was determined in an invertebrate model, Galleria mellonella as well as vertebrate models including zebrafish (Danio rerio) and murine. In addition, toxicity to human erythrocytes was also measured.
Results
Treatment of Galleria mellonella with rhodomyrtone at 100 mg/kg body weight up to four days showed no visible toxic effects (100 % survival). In zebrafish embryo model, at least 80 % survival of embryos was demonstrated when treated with rhodomyrtone at 0.5 μg/mL for three days. Prior to clinical trial, it is a prerequisite that rhodomyrtone has to be evaluated for its biocompatibility with human blood components. The results displayed that rhodomyrtone at 256 μg/mL did not cause any observable human erythrocyte haemolysis. Furthermore, preclinical assessment of rhodomyrtone formulation justified potential applications of rhodomyrtone in humans. Oral toxicity testing in a mouse model indicated the absence of systemic toxicity when the animals received up to 5000 mg/kg body weight of rhodomyrtone formulation for a period of fourteen days.
Conclusions
As the minimal inhibitory concentration of rhodomyrtone against most Gram-positive pathogens is 0.5−1 μg/mL, the results suggest that it should produce no toxic effects at concentrations used in human, thus support further development in pharmaceutical industries and public health applications.
1. Introduction
Increasing antimicrobial resistance and lack of novel antibiotic development are key challenges to global health. There is an urgent need to develop new agents for clinical practice. Natural products and their derivatives have been of crucial importance in identification and development of antibacterial agents [1]. Rhodomyrtus tomentosa (Aiton) Hassk. has been traditionally used in Southeast Asian countries to relieve various inflammatory symptoms such as diarrhoea, gynaecopathy, urinary tract infections, and wound infections [2]. Rhodomyrtone, a bioactive acylphloroglucinol compound isolated from Rhodomyrtus tomentosa leaves, has been proposed as a natural antibacterial agent for the treatment of Gram-positive bacterial infections [[3], [4], [5], [6], [7]]. Moreover, antioxidant [8], immunomodulatory [9,10], anti-proliferative [11], anti-acne [12,13], anti-metastatic [14], anti-inflammatory [9,15], anti-psoriatic [15], and anti-depressant effects [16] of rhodomyrtone have generated interest among researchers in the development and use of rhodomyrtone in public health applications.
Medicinal plant-based antimicrobials play a vital role in the development of effective therapeutics [17]. In order to reach safe applications in human, detailed studies on their toxicity issues have been reported, for example, Cassia fistula L. [18], Musa sp. [19], and Rubus fruticosus L. [20]. Similarly, safety assessment of rhodomyrtone is required for further development as human medicine. Up until now, very limited information is available on the toxicity of rhodomyrtone. Previous studies indicated that rhodomyrtone at concentrations higher than 200 μg/mL revealed very low cytotoxic effects on normal human fibroblasts [12]. Also, a brief report on the effects of rhodomyrtone on human erythrocytes has been documented [5]. In addition, rhodomyrtone formulation produced no skin irritation in rabbits indicating that the compound could be a novel candidate for clinical development [11]. However, there have been no in vivo toxicity tests of systemic application of rhodomyrtone.
Rodent models have been proposed as the gold standard for toxicity assessment, however, there are limitations of high costs, inconsistent responses, and ethical issues [21,22]. Recently, alternative lower hierarchy animal models such as zebrafish (Danio rerio) [[23], [24], [25]] and insect larvae (Galleria mellonella) [[26], [27], [28]] have been employed as they offer various benefits such as reduced ethical concerns, high throughput, and in some cases, easier genetic manipulation, compared with traditional rodent models. In addition, both zebrafish [29] and larvae [30,31] have innate immune system similar as in mammals or jawed vertebrates. Therefore, assessment of rhodomyrtone toxicity in invertebrate model may additionally facilitate the identification of organ-specific or systemic toxicity in mammals.
In order to characterize and further justify the potential use of rhodomyrtone in pharmaceutical industries and public health applications, this study aimed to assess the safety of rhodomyrtone in an invertebrate model, Galleria mellonella, and vertebrate models including zebrafish (Danio rerio) and mice. Additional experiments were also carried out on human erythrocytes.
2. Material and methods
2.1. Rhodomyrtone purification
Rhodomyrtone was isolated from Rhodomyrtus tomentosa leaves by our research group [3] and the purity of rhodomyrtone was confirmed by nuclear magnetic resonance and mass spectrometry [32]. Stock solutions of rhodomyrtone were prepared by dissolving 50 mg of the compound in 1 mL of 100 % DMSO and stored at −20 °C until further used.
2.2. Preparation of rhodomyrtone formulation
Rhodomyrtone formulation was previously described by Chorachoo et al. [11]. The composition of the formulation was carbopol ultrez 21 0.2 g, DC RM 2051 2 g, fumed silica 0.5 g, glycerin 20 g, mineral oil 26 g, propylene glycol 30 g, rhodomyrtone 0.01−0.9 g, and distilled water q.s. to 100 g. The raw materials used in the formulation base were accurately weighed and the mixture was stirred until congealed at room temperature.
2.3. Galleria mellonella survival assay
Galleria mellonella larvae were obtained from UK Waxworms Ltd (Sheffield, UK) and stored at room temperature in darkness with a nonrestricted diet. Larvae weighing within the range of 250–350 mg were selected for each experiment and were used within one week of receipt. Briefly, each group of fifteen randomly-selected larvae was injected with 10 μL of rhodomyrtone (50 and 100 mg/kg). PBS-injected and unmanipulated control groups were included with each experiment. Larvae were incubated at 37 °C and the survival rate of larvae was measured at one day interval over four days incubation. Experiments were performed in duplicate using larvae from different batches [33].
2.4. Zebrafish embryo toxicity assay
Zebrafish embryo toxicity was carried out following the method from Morash et al. [34]. Zebrafish embryos at 24 h post fertilization were used to determine the toxic effect of rhodomyrtone in 96-well plates. The embryos were manually dechorionated, placed directly into E3 medium (15 mm NaCl, 0.5 mm KCl, 1 mm CaCl2, 1 mm MgSO4, 0.05 mm Na2HPO4, 0.7 mm NaHCO3), with or without rhodomyrtone. Rhodomyrtone was tested on 40 embryos at 0.125, 0.5, 2, and 8 μg/mL for five days. Twenty microliters of the compound was added in 180 μL of E3 medium supplemented with 0.01 % methylene blue. The plates were incubated at 28 °C and monitored for survival at regular intervals using stereomicroscope. The scoring of living versus dead embryos was assayed by the presence of a heartbeat and circulating blood. Control embryo with 1 % DMSO was incubated under the same conditions. For rhodomyrtone injection into zebrafish, dechorionated embryos were anesthetized with 0.4 % tricaine prior to injection. Two nanoliters of rhodomyrtone (0.5, 5, 10, 20, and 40 μg/mL) were microinjected into the yolk circulation valley of the embryos. Injected embryos were returned to E3 medium and monitored for survival as described previously.
2.5. Erythrocyte haemolysis assay
Haemolytic activity was determined following a modified method of Lin and Haynes, [35]. Briefly, 5 mL of blood sample was added to 10 mL of PBS, and then red blood cells (RBCs) were isolated from serum by centrifugation at 10,000 rpm for 10 min.. The RBCs were further washed five times with 10 mL of PBS solution. The purified blood was diluted in 50 mL of PBS. RBCs, incubated with 0.5 % Triton-X and PBS, were used as positive and negative controls, respectively. Cells were incubated with 16, 32, 64, 128, 256, and 512 μg/mL rhodomyrtone at room temperature for 0.5, 1, 2, and 3 h. Finally, the mixtures were centrifuged at 10,000 rpm for 3 min. and 100 μL of supernatant of all samples was transferred to a 96-well plate. The absorbance values of the supernatants at 570 nm were determined using a microplate reader. The percent haemolysis of RBCs was calculated according to the equation: percent haemolysis = [(sample absorbance - negative control absorbance)/(positive control absorbance - negative control absorbance)] × 100.
2.6. Acute oral toxicity study in mouse model
In accordance to Organisation for Economic Co-operation and Development (OECD) guidelines no. 425 [36], animal experiments were approved by the Ethics Committee for Animal Experiments of Thailand Institute of Scientific and Technological Research (No. TS-59001). Thirty mice from Institute of Cancer Research (ICR) were employed and acclimatized to the laboratory environment for one week, then the mice were divided into three groups with ten mice per group. Animals were fasted for 8 h prior to dosing but had access to water. A single dose of each formulation was administered via oral gavages according to body weight (approximately 0.5 mL/animal). Groups were treated as follows: group I; normal control mice treated with distilled water, group II; treated with a single dose of 2000 mg/kg body weight of rhodomyrtone formulation, and group III; treated with a single dose of 5000 mg/kg body weight of rhodomyrtone formulation. The animals were closely observed for the first 30 min., then for 1 and 3 h. Food was withheld after 3−4 h of dosing. Each group was noticed closely for any toxic effects within the first 4 h and then at regular intervals for a total period of fourteen days. Body weights of animals were taken on day 1, 7, and 14. At the end of the study, surviving animals were sacrificed and both internal organs including kidney and liver were removed for histopathological evaluation.
3. Results and discussion
3.1. Toxicity of rhodomyrtone in Galleria mellonella
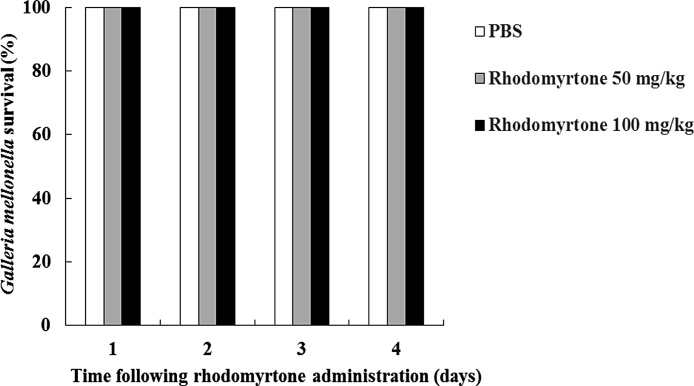
Caterpillars of the Greater Wax moth, Galleria mellonella represents a useful preliminary model for assessing in vivo efficacy of new antibacterial agents before proceeding to mammalian studies. The invertebrate model Galleria mellonella is simple to use, inexpensive, and no ethical approval is required [26]. Use of a Galleria mellonella model to determine compound toxicity revealed a strong positive correlation with data obtained from mammalian models, for example, mice [28] and rats [27,28]. In this study, Galleria mellonella larvae were used to study acute systemic toxicity of rhodomyrtone. The larvae were injected with 50 and 100 mg/kg of the compound and monitored for a four-day period. As shown in Fig. 1, the compound at both concentrations did not exert any toxic effects in the larvae up to four days post-treatment (100 % survival). A number of works confirmed strong antibacterial potency of rhodomyrtone against a wide range of Gram-positive bacteria with low minimal inhibitory concentration values (0.5−1 μg/mL), comparable to vancomycin [3,5].
Fig. 1.
Survival rate of Galleria mellonella larvae following feeding with different concentrations of rhodomyrtone (50 and 100 mg/kg) at one day interval over four days of incubation. Experiments were performed in duplicate using larvae from different batches.
3.2. Toxicity of rhodomyrtone on zebrafish embryos
Zebrafish is a prominent vertebrate model for research in genetics, development, regeneration, and toxicology. In addition, zebrafish has become a popular and powerful model over other vertebrate species because of its small size, easy husbandry, and prolific breeding [23,25]. In this study, zebrafish embryos provide a rapid approach to determine cytotoxicity of rhodomyrtone. Early development of zebrafish embryos corresponds to the most sensitive phase to external stimuli such as toxicants, chemicals, and mechanical stress [37]. As shown in Table 1, embryos kept in E3 medium with rhodomyrtone at 0.125 and 0.5 μg/mL for five days showed no toxicity (100 % survival). However, in higher concentrations of rhodomyrtone (2 and 8 μg/mL), it was noted that E3 medium became highly turbid which directly affected the overall activity of the zebrafish embryos after certain period. It is well-documented that water turbidity can affect fish behaviour [38]. In addition, it has been clearly demonstrated that zebrafish kept in water of higher turbidity displayed lower activity level, lower aggression, and higher shoaling tendency [39]. Therefore, in the next series of experiments, we directly injected rhodomyrtone (0.5, 5, 10, 20, and 40 μg/mL) and 1 % DMSO as a control, into the yolk cells. One day post-fertilization, 82.5–90 % survival embryos in rhodomyrtone treatment group were observed (Table 2). With 1 % DMSO in the control group, 77.5 % were detected. Other works have reported similar percentage of spontaneous early mortality [40,41]. Up to 70 % is acceptable for fertilization rate (OECD guidelines no. 236 [42]). Rhodomyrtone at 0.5−20 μg/mL resulted in 75–90 % survival of embryos throughout the treatment period (4 days) (Table 2). However, similar survival rate in rhodomyrtone and 1% DMSO were noted on day 4 which may due to external stimuli [37].
Table 1.
Survival rate of zebrafish embryos (n = 40) after treatment with rhodomyrtone.
| Treatment |
Zebrafish embryos survival (%) |
|||||
|---|---|---|---|---|---|---|
| 1 h | 1 Day | 2 Day | 3 Day | 4 Day | 5 Day | |
| Rhodomyrtone (μg/mL) | ||||||
| 0.125 | 100 | 100 | 100 | 100 | 100 | 100 |
| 0.5 | 100 | 100 | 100 | 100 | 100 | 100 |
| 2 | 100 | NA | NA | NA | NA | NA |
| 8 | 100 | NA | NA | NA | NA | NA |
| 1% DMSO | 100 | 100 | 100 | 100 | 100 | 100 |
1% DMSO was used as a negative control.
NA: not applicable.
Table 2.
Survival rate of zebrafish embryos (n = 40) after rhodomyrtone injection.
| Treatment |
Zebrafish embryos survival (%) |
|||
|---|---|---|---|---|
| 1 Day | 2 Day | 3 Day | 4 Day | |
| Rhodomyrtone (μg/mL) | ||||
| 0.5 | 87.5 | 82.5 | 80 | 75 |
| 5 | 90 | 82.5 | 77.5 | 72.5 |
| 10 | 90 | 80 | 77.5 | 72.5 |
| 20 | 87.5 | 75 | 75 | 75 |
| 40 | 82.5 | 77.5 | 75 | 65 |
| 1% DMSO | 77.5 | 70 | 67.5 | 67.5 |
3.3. Haemolytic property of rhodomyrtone on human erythrocytes
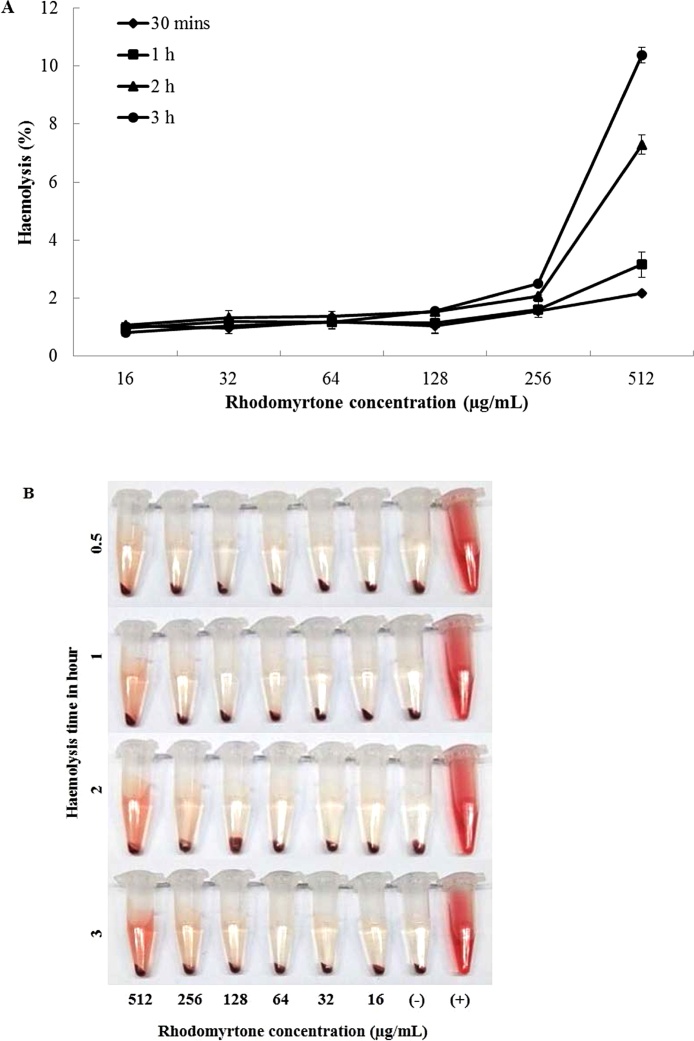
Haemolytic property is a major factor limiting the clinical use of antimicrobial compounds. The use of human erythrocytes as a test to evaluate cytotoxicity of new antimicrobial agents is commonly employed. The erythrocyte model is fast, reproducible, and inexpensive and thus contributes to decreasing, refining, and replacing studies conducted with animals [35,43]. A previous study demonstrated the safety of rhodomyrtone at 64 μg/mL in human erythrocytes after 30 min. [5]. In this study, extended studies on human erythrocytes were set up. Human erythrocyte haemolysis assay after exposure to rhodomyrtone at 16, 32, 64, 128, 256, and 512 μg/mL for 0.5, 1, 2, and 3 h was performed and is shown in Fig. 2. Compared to a positive control of 0.5 % Triton X-100 where 100 % haemolysis was observed, rhodomyrtone did not cause any observable haemoglobin release even at 256 μg/mL. In addition, after 3 h incubation, rhodomyrtone caused less than 11 % haemolysis even at the highest concentration tested. Different degrees of cytotoxicity are classified as non-toxic (0–9 %), slightly toxic (10–49 %), toxic (50–89 %), and highly toxic (90–100 %) [32].
Fig. 2.
Haemolytic property of rhodomyrtone on human erythrocytes. The percent haemolytic of red blood cells (RBS) of various concentrations of rhodomyrtone (A) and images of RBCs treated with rhodomyrtone (B). The positive and negative controls used in this study were 0.5 % Triton-X and PBS, respectively. Values are expressed as mean ± SEM.
3.4. Mortality and histological findings after oral treatment of rhodomyrtone formulation in a mouse model
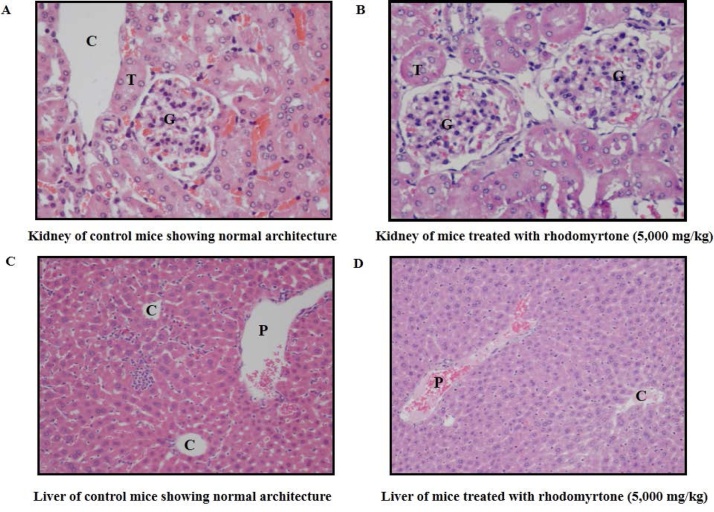
Mouse models have been used as predictors of human responses as they have genetic and physiological similarities between both species [44,45]. Mice offer a number of benefits, for instance, small, inexpensive to maintain, and easy to ship. In addition, they have short generation times and produce large numbers of offspring [44]. Safety assessment of rhodomyrtone formulation obtained from the mouse model provides further evidence that development of rhodomyrtone for possible use in humans is warranted. In this study, oral administration of rhodomyrtone formulation at single doses of 2000 and 5000 mg/kg for fourteen days indicated no significant abnormal change in behavioral properties of mice and no mortality. Moreover, the body weight of tested animals of both control and treated groups increased gradually throughout the study period as presented in Table 3. The difference in body weight between the control and the tested groups were not statistically significant. Regarding the histopathological evaluation, all internal organs including kidney and liver did not show any gross pathological changes (Fig. 3). The results clearly demonstrated that rhodomyrtone formulation was safe up to 5000 mg/kg in mice.
Table 3.
Effects of rhodomyrtone formulation on body weight and mortality of mice in acute toxicity study.
| Groups | Body Weight (g) |
Mortality | ||
|---|---|---|---|---|
| 1 Day | 7 Day | 14 Day | ||
| Rhodomyrtone formulation | ||||
| 2000 mg/kg | 31.7 ± 0.74 | 34.1 ± 0.85 | 35.6 ± 0.77 | Not found |
| 5000 mg/kg | 31.2 ± 0.65 | 32.9 ± 0.72 | 34.7 ± 0.60 | Not found |
| Vehicle control | 31.6 ± 0.68 | 34.2 ± 0.81 | 36 ± 0.60 | Not found |
Values were presented as mean ± SEM. (n = 10).
Fig. 3.
Effects of rhodomyrtone formulation (5000 mg/kg) on mouse organ histomorphologies (kidney: A and B; liver: C and D) in acute oral toxicity study. (C: central vein; G: glomerulus; T: tubule; P: portal area).
4. Conclusions
The findings revealed that rhodomyrtone did not cause any signs of toxicity in both invertebrate and vertebrate models. It is evident that the compound did not produce any interaction with red blood cells. This study provides valuable information to support the development of rhodomyrtone in pharmaceutical industries and public health applications.
Consent for publication
Not applicable.
Authors' contributions
TS, JC, SL, and SS designed and performed experiments, analyzed data, prepared figures and tables, and wrote the first draft of a manuscript. SPV and PJC supervised throughout the process and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Availability of data and material
The data and materials are included within the article.
Funding
This research was funded by the Thailand Research Fund Senior Research Scholar (Grant number RTA6180006).
CRediT authorship contribution statement
Thanyaluck Siriyong: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing. Julalak Chorachoo Ontong: Conceptualization, Methodology, Investigation, Writing - original draft. Sukanlaya Leejae: Investigation. Sakol Suwalak: Investigation. Peter John Coote: Supervision. Supayang Piyawan Voravuthikunchai: Supervision.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
Not applicable.
References
- 1.Moloney M.G. Natural products as a source for novel antibiotics. Trends Pharmacol. Sci. 2016;37:689–701. doi: 10.1016/j.tips.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Hamid H.A.B.D., Mutazah S.S.Z.R., Yusoff M.M. Rhodomyrtus tomentosa: a phytochemical and pharmacological review. Asian J. Pharm. Clin. Res. 2017;10:10–16. [Google Scholar]
- 3.Limsuwan S., Trip E.N., Kouwen T.R., Piersma S., Hiranrat A., Mahabusarakam W., Voravuthikunchai S.P., van Dijl J.M., Kayser O. Rhodomyrtone: a new candidate as natural antibacterial drug from Rhodomyrtus tomentosa. Phytomedicine. 2009;16:645–651. doi: 10.1016/j.phymed.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Sianglum W., Srimanote P., Taylor P.W., Rosado H., Voravuthikunchai S.P. Transcriptome analysis of responses to rhodomyrtone in methicillin-resistant Staphylococcus aureus. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leejae S., Taylor P.W., Voravuthikunchai S.P. Antibacterial mechanisms of rhodomyrtone against important hospital-acquired antibiotic-resistant pathogenic bacteria. J. Med. Microbiol. 2013;62:78–85. doi: 10.1099/jmm.0.049205-0. [DOI] [PubMed] [Google Scholar]
- 6.Mitsuwan W., Olaya-Abril A., Calderón-Santiago M., Jiménez-Munguía I., González-Reyes J.A., Priego-Capote F., Voravuthikunchai S.P., Rodríguez-Ortega M.J. Integrated proteomic and metabolomic analysis reveals that rhodomyrtone reduces the capsule in Streptococcus pneumoniae. Sci. Rep. 2017;7:2715. doi: 10.1038/s41598-017-02996-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saeloh D., Wenzel M., Rungrotmongkol T., Hamoen L.W., Tipmanee V., Voravuthikunchai S.P. Effects of rhodomyrtone on Gram-positive bacterial tubulin homologue FtsZ. Peer J. 2017;5:e2962. doi: 10.7717/peerj.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leejae S., Hasap L., Voravuthikunchai S.P. Inhibition of staphyloxanthin biosynthesis in Staphylococcus aureus by rhodomyrtone, a novel antibiotic candidate. J. Med. Microbiol. 2013;62:421–428. doi: 10.1099/jmm.0.047316-0. [DOI] [PubMed] [Google Scholar]
- 9.Srisuwan S., Tongtawe P., Srimanote P., Voravuthikunchai S.P. Rhodomyrtone modulates innate immune responses of THP-1 monocytes to assist in clearing methicillin-resistant Staphylococcus aureus. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Na-Phatthalung P., Teles M., Voravuthikunchai S.P., Tort L., Fierro-Castro C. Immunomodulatory effects of Rhodomyrtus tomentosa leaf extract and its derivative compound, rhodomyrtone, on head kidney macrophages of rainbow trout (Oncorhynchus mykiss) Fish Physiol. Biochem. 2018;44:543–555. doi: 10.1007/s10695-017-0452-2. [DOI] [PubMed] [Google Scholar]
- 11.Chorachoo J., Saeloh D., Srichana T., Amnuaikit T., Musthafa K.S., Sretrirutchai S., Voravuthikunchai S.P. Rhodomyrtone as a potential anti-proliferative and apoptosis inducing agent in HaCaT keratinocyte cells. Eur. J. Pharmacol. 2016;772:144–151. doi: 10.1016/j.ejphar.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Saising J., Voravuthikunchai S.P. Anti Propionibacterium acnes activity of rhodomyrtone, an effective compound from Rhodomyrtus tomentosa (Aiton) Hassk. leaves. Anaerobe. 2012;18:400–404. doi: 10.1016/j.anaerobe.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Wunnoo S., Saising J., Voravuthikunchai S.P. Rhodomyrtone inhibits lipase production, biofilm formation, and disorganizes established biofilm in Propionibacterium acnes. Anaerobe. 2017;43:61–68. doi: 10.1016/j.anaerobe.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Tayeh M., Nilwarangoon S., Mahabusarakum W., Watanapokasin R. Anti-metastatic effect of rhodomyrtone from Rhodomyrtus tomentosa on human skin cancer cells. Int. J. Oncol. 2017;50:1035–1043. doi: 10.3892/ijo.2017.3845. [DOI] [PubMed] [Google Scholar]
- 15.Chorachoo J., Lambert S., Furnholm T., Roberts L., Reingold L., Auepemkiate S., Voravuthikunchai S.P., Johnston A. The small molecule rhodomyrtone suppresses TNF-α and IL-17A-induced keratinocyte inflammatory responses: a potential new therapeutic for psoriasis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chai H., Liu B., Zhan H., Li X., He Z., Ye J., Guo Q., Chen J., Zhang J., Li S. Antidepressant effects of rhodomyrtone in mice with chronic unpredictable mild stress-induced depression. Int. J. Neuropsychopharmacol. 2019;22:157–164. doi: 10.1093/ijnp/pyy091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta P.D., Birdi T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017;8:266–275. doi: 10.1016/j.jaim.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaerunisaa A.Y., Susilawati Y., Muhaimin M., Milanda T., Hendriani R., Subarnas A. Antibacterial activity and subchronic toxicity of Cassia fistula L. barks in rats. Toxicol. Rep. 2020;7:649–657. doi: 10.1016/j.toxrep.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Mqbali L.R.A., Hossain M.A. Cytotoxic and antimicrobial potential of different varieties of ripe banana used traditionally to treat ulcers. Toxicol. Rep. 2019;6:1086–1090. doi: 10.1016/j.toxrep.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weli A.M., Al-Saadi H.S., Al-Fudhaili R.S., Hossain A., Putit Z.B., Jasim M.K. Cytotoxic and antimicrobial potential of different leaves extracts of R. fruticosus used traditionally to treat diabetes. Toxicol. Rep. 2020;7:183–187. doi: 10.1016/j.toxrep.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merlot C. Computational toxicology-a tool for early safety evaluation. Drug Discov. Today. 2010;15:16–22. doi: 10.1016/j.drudis.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Sun H., Xia M., Austin C.P., Huang R. Paradigm shift in toxicity testing and modeling. AAPS J. 2012;14:473–480. doi: 10.1208/s12248-012-9358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill A.J., Teraoka H., Heideman W., Peterson R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- 24.Mathias J.R., Saxena M.T., Mumm J.S. Advances in zebrafish chemical screening technologies. Future Med. Chem. 2012;4:1811–1822. doi: 10.4155/fmc.12.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyers J.R. Zebrafish: development of a vertebrate model organism. Curr. Protoc. Essent. Lab. Tech. 2018:e19. [Google Scholar]
- 26.Desbois A.P., Coote P.J. Utility of greater wax moth larva (Galleria mellonella) for evaluating the toxicity and efficacy of new antimicrobial agents. Adv. Appl. Microbiol. 2012;78:25–53. doi: 10.1016/B978-0-12-394805-2.00002-6. [DOI] [PubMed] [Google Scholar]
- 27.Maguire R., Duggan O., Kavanagh K. Evaluation of Galleria mellonella larvae as an in vivo model for assessing the relative toxicity of food preservative agents. Cell Biol. Toxicol. 2016;32:209–216. doi: 10.1007/s10565-016-9329-x. [DOI] [PubMed] [Google Scholar]
- 28.Allegra E., Titball R.W., Carter J., Champion O.L. Galleria mellonella larvae allow the discrimination of toxic and non-toxic chemicals. Chemosphere. 2018;198:469–472. doi: 10.1016/j.chemosphere.2018.01.175. [DOI] [PubMed] [Google Scholar]
- 29.Meijer A.H., Spaink H.P. Host-pathogen interactions made transparent with the zebrafish model. Curr. Drug Targets. 2011;12:1000–1017. doi: 10.2174/138945011795677809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cytrynska M., Zdybicka-Barabas A., Jakubowicz T. The involvement of protein kinase A in the immune response of Galleria mellonella larvae to bacteria. Acta Biochim. Pol. 2007;54:167–174. [PubMed] [Google Scholar]
- 31.Browne N., Heelan M., Kavanagh K. An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence. 2013;4:597–603. doi: 10.4161/viru.25906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salni D., Sargent M.V., Skelton B.W., Soediro I., Sutisna M., White A.H., Yulinah E. Rhodomyrtone, an antibiotic from Rhodomyrtus tomentosa. Aust. J. Chem. 2002;55:229–232. [Google Scholar]
- 33.Desbois A.P., Coote P.J. Wax moth larva (Galleria mellonella): an in vivo model for assessing the efficacy of antistaphylococcal agents. J. Antimicrob. Chemother. 2011;66:1785–1790. doi: 10.1093/jac/dkr198. [DOI] [PubMed] [Google Scholar]
- 34.Morash M.G., Douglas S.E., Robotham A., Ridley C.M., Gallant J.W., Soanes K.H. The zebrafish embryo as a tool for screening and characterizing pleurocidin host-defense peptides as anti-cancer agents. Dis. Model. Mech. 2011;4:622–633. doi: 10.1242/dmm.007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin Y.S., Haynes C.L. Impacts of mesoporous silica nanoparticle size, pore ordering, and pore integrity on hemolytic activity. J. Am. Chem. Soc. 2010;132:4834–4842. doi: 10.1021/ja910846q. [DOI] [PubMed] [Google Scholar]
- 36.Organisation for Economic Co-operation and Development . 2001. Guideline for Testing of Chemicals, Guideance, no. 425. [Google Scholar]
- 37.Mandrell D., Truong L., Jephson C. Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. J. Lab. Autom. 2012;17:66–74. doi: 10.1177/2211068211432197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meager J.J., Batty R.S. Effects of turbidity on the spontaneous and prey-searching activity of juvenile Atlantic cod (Gadus morhua) Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2007;362:2123–2130. doi: 10.1098/rstb.2007.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wojtas K., Pokorny P., Mucha A. Effect of water turbidity on zebrafish behaviour. Front. Mar. Sci. 2015 [Google Scholar]
- 40.Fraysse B., Mons R., Garric J. Development of a zebrafish 4-day embryo-larval bioassay to assess toxicity of chemicals. Ecotoxicol. Environ. Saf. 2006;63:253–267. doi: 10.1016/j.ecoenv.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Ali S., van Mil H.G., Richardson M.K. Large-scale assessment of the zebrafish embryo as a possible predictive model in toxicity testing. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Organisation for Economic Co-operation and Development . 2013. Guideline for Testing of Chemicals, Guideance, no. 236. [Google Scholar]
- 43.Pagano M., Faggio C. The use of erythrocyte fragility to assess xenobiotic cytotoxicity. Cell Biochem. Funct. 2015;33:351–355. doi: 10.1002/cbf.3135. [DOI] [PubMed] [Google Scholar]
- 44.Uhl E.W., Warner N.J. Mouse models as predictors of human responses: evolutionary medicine. Curr. Pathobiol. Rep. 2015;3:219–223. doi: 10.1007/s40139-015-0086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perlman R.L. Mouse models of human disease: an evolutionary perspective. Evol. Med. Public Health. 2016;1:170–176. doi: 10.1093/emph/eow014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials are included within the article.