Abstract
Objectives
In 2014, the governor of New York announced the Ending the Epidemic (ETE) plan to reduce annual new HIV infections from 3000 to 750, achieve a first-ever decrease in HIV prevalence, and reduce AIDS progression by the end of 2020. The state health department undertook participatory simulation modeling to develop a baseline for comparing epidemic trends and feedback on ETE strategies.
Methods
A dynamic compartmental model projected the individual and combined effects of 3 ETE initiatives: enhanced linkage to and retention in HIV treatment, increased preexposure prophylaxis (PrEP) among men who have sex with men, and expanded housing assistance. Data inputs for model calibration and low-, medium-, and high-implementation scenarios (stakeholders’ rollout predictions, and lower and upper bounds) came from surveillance and program data through 2014, the literature, and expert judgment.
Results
Without ETE (baseline scenario), new HIV infections would decline but remain >750, and HIV prevalence would continue to increase by 2020. Concurrently implementing the 3 programs would lower annual new HIV infections by 16.0%, 28.1%, and 45.7% compared with baseline in the low-, medium-, and high-implementation scenarios, respectively. In all concurrent implementation scenarios, although annual new HIV infections would remain >750, there would be fewer new HIV infections than deaths, yielding the first-ever decrease in HIV prevalence. PrEP and enhanced linkage and retention would confer the largest population-level changes.
Conclusions
New York State will achieve 1 ETE benchmark under the most realistic (medium) implementation scenario. Findings facilitated framing of ETE goals and underscored the need to prioritize men who have sex with men and maintain ETE’s multipronged approach, including other programs not modeled here.
Keywords: health policy, HIV, computer simulation, preexposure prophylaxis
In 2014, New York State Governor Andrew M. Cuomo announced the Ending the Epidemic (ETE) plan to reduce the estimated number of annual new HIV infections from 3000 to 750, reduce AIDS progression by 50%, and achieve the first-ever decrease in HIV prevalence in New York State by the end of 2020.1 An ETE Metrics Committee comprising New York State and New York City health department staff members, researchers, medical and social service providers, consumers, and advocates developed population-level metrics with ambitious but achievable 2020 targets. Where comparable National HIV/AIDS Strategy2 metrics existed, the Committee chose more ambitious targets because New York State had higher baseline progress on each metric than other US states. With no uniform definition of “ending AIDS,” the Committee selected decreased HIV prevalence as its primary indicator. Achieving this goal would require a 50%-75% lower HIV incidence compared with pre-ETE levels, and the 75% target (corresponding to 750 new HIV infections annually) was adopted to ensure success.
The 3-point ETE plan encompassed (1) identifying undiagnosed persons and facilitating access to antiretroviral therapy (ARV) to achieve undetectable HIV viral loads, (2) retaining persons on ARV to maintain undetectable viral loads, and (3) facilitating access to preexposure prophylaxis (PrEP).1 These activities are important because undiagnosed persons have more unprotected sexual encounters than diagnosed persons,3 undetectable viral load is not transmittable sexually (U=U),4-6 and PrEP can reduce sexually transmitted HIV infections by >90%.7-9 The New York State Department of Health AIDS Institute led an appointed task force comprising academic, public health, and community stakeholders to develop the ETE Blueprint with evidence-based strategies to achieve goals.1 The Blueprint included expanded housing access as an important social determinant of health: compared with persons with stable housing, unstably housed persons have lower linkage to and retention in HIV care, lower adherence to ARV, and increased reported HIV risk behaviors.10,11
To improve ETE implementation, the AIDS Institute undertook a participatory modeling process for several purposes: (1) determine a baseline against which to compare epidemic trends as ETE was implemented; (2) identify AIDS Institute programs and New York State populations to prioritize; (3) estimate new HIV infections, a core ETE metric that is not directly measurable because persons only become cases in the surveillance registry after diagnosis; and (4) understand the effects of ETE programs. Other dynamic compartmental HIV models12 similar to our approach have examined related topics, including projecting progress toward the national Ending the Epidemic in America plan nationally and among certain cities13,14; the number of HIV infections averted and the cost effectiveness of HIV prevention interventions for persons who inject drugs15; the cost effectiveness of PrEP among men who have sex with men (MSM)16; requirements needed to achieve goals of the 2015 National HIV/AIDS Strategy; and how achieving the goals would improve racial/ethnic disparities, HIV epidemic outcomes, and economic outcomes.17,18 Other models examined policies similar to but not directly aligned with the ETE initiative, particularly the UNAIDS 90-90-90 and Fast-Track goals in low- and middle-income countries19-23 and Canada,24 as well as Getting to Zero in Illinois.25 Our study’s unique contributions were examining increasing access to housing, PrEP, and ARV, and our stakeholder-focused participatory modeling process.
Methods
System dynamics models, analogous to dynamic compartmental models, are suitable for analyzing complex systems.26,27 Participatory group modeling facilitates shared meanings among stakeholders, with model diagrams and output serving as tangible representations of policy problems, solutions, and consequences that can be used for discussion. Common meanings enable deep understandings, improved communication among stakeholders with specialized knowledge, and actionable insights for policy implementation.28 A dynamic compartmental model, whereby the rate of annual new HIV infections decreases as persons living with HIV are diagnosed and engaged in care and, thus, less likely to transmit infection, has a consistent framework with standard susceptible, infectious, and recovered models for infectious disease.29 (For HIV, there is no “recovered” compartment.)
Our model stakeholders comprised AIDS Institute staff members and executives representing diverse divisions and programs (surveillance, clinical care, linkage and retention programming, program evaluation, and PrEP) who met monthly from January 2016 through June 2017 to discuss model structure, core outcomes and specific programs for analysis, and findings. Additional meetings covered data inputs and calibration to HIV surveillance data, conceptual mapping of selected ETE programs, and policy parameters.
Model Structure
We adapted a previous dynamic model of New York State’s HIV testing law30,31 to reflect updates to the HIV surveillance system; distinguish intermittent and durable viral load suppression; separate all-cause mortality among diagnosed and undiagnosed persons, consistent with surveillance data; and stratify MSM and non-MSM populations.
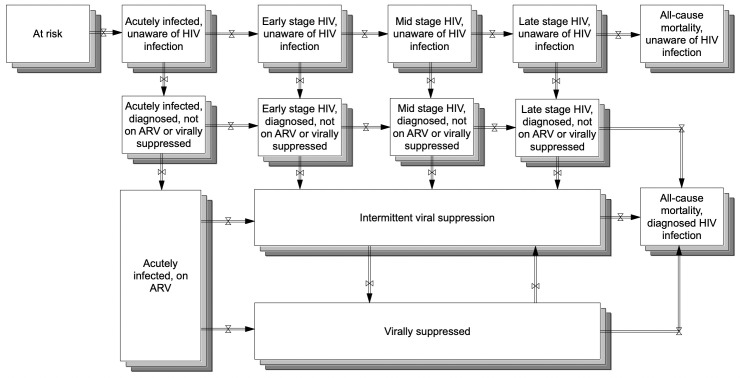
The stock-and-flow diagram (Figure 1) is analogous to compartments in dynamic compartmental models, with stocks (boxes) representing counts of persons at a point in time and flows (arrows) representing rates of persons moving across categories. In the following model description, italicized descriptions correspond to the variable names on the diagram. Persons who are at risk become infected through sexual or drug-related encounters with persons living with HIV. Stakeholders determined that modeling transmission mode was out of scope and not directly relevant to modeling short-term ETE effects. In addition, New York State–specific data were insufficient to support model inputs (eg, the New York State surveillance data identify transmission risk at the time of diagnosis without details on partnerships and subsequent changes in risk). Twenty-two categories of persons living with HIV are differentiated by the following characteristics: men who identify as gay or bisexual and other MSM or non-MSM, diagnosis status, intermittent vs durable viral suppression, and disease progression. MSM status is static: although MSM behavior may be fluid, the surveillance data list reported risk at time of diagnosis, and stakeholders desired consistency with surveillance data. We stratified the population in the model by MSM because this group was disproportionately affecting New York State’s current HIV epidemic.32
Figure 1.
Stock-and-flow diagram of New York State’s system of HIV testing and care for creating a participatory dynamic simulation model of the state’s Ending the Epidemic HIV Policy initiative.1 Boxes are stocks, representing the number of persons in a category at a particular time. Arrows (pipes with valves) are flows, representing movements between stocks over time. The population in each stock changes as persons move throughout the system. The stocks and flows are analogous to compartments and transition rates in dynamic compartmental models. After becoming infected (thereby moving from at risk to acutely infected, unaware of HIV infection), persons progress from left to right through the disease. They may also become diagnosed, subsequently linked to care and ARV, and virally suppressed (thereby moving down the diagram). Stocks have varying HIV transmission rates, with persons in the virally suppressed stock having zero transmissibility. Consequently, as persons move around the system and toward the stock of virally suppressed, the number of new HIV infections will decline. The model is disaggregated for the 2 subpopulations of MSM and non-MSM, represented by the overlapping shading. Abbreviations: ARV, antiretroviral therapy; MSM, men who have sex with men.
Persons who are unaware of HIV infection become aware of HIV infection, not on ARV upon HIV diagnosis. After linkage to care, persons are classified as having intermittent viral suppression upon their first viral load test <200 copies/mL. After 2 years of viral load tests <200 copies/mL, persons are considered continuously virally suppressed. They may transition to intermittent viral suppression because of treatment failure or low ARV adherence. Disease progression mirrors the Centers for Disease Control and Prevention’s (CDC’s) disease staging.33 Clinical experts recommended consolidating disease stages for intermittent viral suppression and virally suppressed because, although diagnosis stage is important for surveillance, undetectable viral load is clinically important.
Annual new HIV infections comprise infections among all persons living with HIV (diagnosed and undiagnosed). Categories have varying transmission rates because of factors that include high infectiousness during acute-stage disease with high viral load and no transmissions from undetectable viral load; frequency of contacts between persons with and without HIV; use of harm-reduction strategies such as condoms or sterile syringes; and the number of persons in each stock who might transmit infection. As previously noted, the model takes an aggregate perspective of “risk” within categories because of its boundary and available New York State data to support additional detail. MSM and non-MSM populations were assigned different transmission rates, described in detail hereinafter. Annual new HIV infections change when persons move between stocks; as persons are identified, on ARV, and virally suppressed, the number of HIV infections declines because more persons living with HIV are virally suppressed and can no longer transmit infections (U=U).6
The population in each stock in 2011 (the simulation model’s start date) and transition rates between stocks were from multiple data sources (Table 1). For calibration, we compared simulated findings with 2011-2014 historical data for estimated new HIV infections, all-cause mortality, prevalence, and new diagnoses by stage. These data were the most recent data available during model development and prepared by HIV surveillance staff members for the purposes of the model, and values are in a technical appendix (available from the authors upon request). We focused on short-term predictions because of recent changes in the surveillance data to account for persons who moved out of state and the HIV treatment and policy landscape.
Table 1.
Summary of data sources used to develop a participatory dynamic simulation model of New York State’s Ending the Epidemic HIV policy initiativea and inputs for policy scenarios
| Model Component | Parameter | Scenario Values and Data Sources |
|---|---|---|
| Initial conditions and calibrationb | Calibration data series for number of new HIV infections | Incidence point estimate based on New York State HIV surveillance registry, using the CDC algorithm. (Data series covers 2011-2014.) |
| Number of new HIV diagnoses | New York State HIV surveillance registry. (Data series covers 2011-2014.) | |
| Number of persons living with diagnosed HIV infection, by stage and level of viral suppression | New York State HIV surveillance registry. (Analytic data set of viral load laboratory test values consists of a 2-year baseline period [2010-2011] and a 2-year follow-up period [2012-2013] to evaluate durable viral load suppression [all viral load tests ≤200 copies/mL during a 2-year period]. These initial conditions were based on the 2010-2011 baseline period.) | |
| Number of unaware persons living with HIV/AIDS, by stage | Published literature and model calibration. (See text for details.) | |
| Transition probabilities across disease stages for persons living with diagnosed HIV/AIDS | New York State HIV surveillance registry. (Analytic data set of viral load laboratory test values comprises a 2-year baseline period [2010-2011] and follow-up period [2012-2013] to evaluate durable viral load suppression [all viral load tests ≤200 copies/mL during a 2-year period]. A matrix of transition probabilities was derived from changes in durable viral load suppression between the baseline [2010-2011] and follow-up [2012-2013] period.) | |
| Transition probabilities across stages for unaware persons living with HIV/AIDS | Published literature, with an average time from HIV infection to death of 10 years, with approximately 2 months in acute stage, 24 months in late stage, and the remaining time divided into the early and mid stages.34,35 | |
| All-cause mortality, by stage and level of viral suppression | New York State HIV surveillance registry. (Data series covers 2011-2014.) | |
| HIV transmission rates, by stage and level of diagnosis, engagement in care, and viral suppression | Published literature and model calibration. (See text for details.) | |
| Enhanced linkage to and retention in HIV carec ,d | Increase in ARV initiation rate | Low implementation: 25% increase in ARV initiation rate Medium implementation: 50% increase in ARV initiation rate High implementation: 75% increase in ARV initiation rate |
| Increase in viral suppression rate | Low implementation: 85% increase in viral suppression rate Medium implementation: 110% increase in viral suppression rate High implementation: 135% increase in viral suppression rate |
|
| Implementation timeline | Start date: July 2014 2 years for full implementation Phased-in implementation using a smoothed first-order delay function |
|
| Uptake of PrEP among MSMd ,e | Coverage (current uptake in target population) | Low implementation: 25% uptake of PrEP Medium implementation: 50% uptake of PrEP High implementation: 75% uptake of PrEP |
| Adherence (portion of users who take medication as prescribed) | Low implementation: 50% adherence to PrEP Medium implementation: 85% adherence to PrEP High implementation: 100% adherence to PrEP |
|
| Clinical efficacy (effectiveness of medication if used as prescribed) | All scenarios: 90% clinical efficacy | |
| Implementation timeline | Start date: January 2014 4 years for full implementation Phased implementation using an S-shaped Bass diffusion curve |
|
| Expanded access to housingd ,f | Reduction in infectivity | Low implementation: 0% reduction in HIV infectivity Medium implementation: 5% reduction in HIV infectivity High implementation: 10% reduction in HIV infectivity |
| Increased rates of ARV initiation and viral suppression | Low implementation: 15% increase in rates of ARV initiation and viral suppression Medium implementation: 35% increase in rates of ARV initiation and viral suppression High implementation: 55% increase in rates of ARV initiation and viral suppression |
|
| Total enrollment and coverage | New York City scenario: 5480 persons Statewide scenario: 8540 persons |
|
| Implementation timeline | Start date: September 2016 2 years for full implementation Phased implementation using an S-shaped Bass diffusion curve |
Abbreviations: ARV, antiretroviral therapy; CDC, Centers for Disease Control and Prevention; MSM, men who have sex with men; PrEP, preexposure prophylaxis.
aSee the Blueprint for more details on the policy initiative.1
bAll model parameter values for the number of persons in each stock (ie, the categories depicted as box in Figure 1) at the start of the simulation (2011) and the flows (ie, transition rates depicted as arrows in Figure 1) are listed in the technical appendix (Appendices 3 and 5, available from the authors upon request). Each data series is stratified by MSM and non-MSM. Additional information about the data series is available from the authors upon request. At the time of model development, data were only available through 2014 because of a lag period for data to reach the HIV surveillance bureau, undergo quality assurance, and be prepared for analysis.
cIn the linkage to and retention-in-care program, increased rates of ARV initiation and viral suppression are operationalized as an increase in the transition rates from diagnosed, not on ARV or virally suppressed to intermittent viral suppression and from intermittent viral suppression to virally suppressed.
dAll sources used to derive program scenarios and diagrams of additional program-specific model structure are in Appendix 6, which is available from the authors upon request.
eIn the PrEP program, uninfected MSM at risk transition to a separate stock in which they are not susceptible to HIV infection. The medication confers imperfect protection, which is modeled by using separate variables for coverage (the percentage of MSM at risk on PrEP) and effectiveness (the product of adherence and clinical efficacy).
fIn the expanded access to housing scenario, housing recipients have a percentage reduction in their HIV transmissibility (likelihood of transmitting an infection in an encounter). In addition, they have increased rates of ARV initiation (operationalized as an increase in the transition rates from diagnosed, not on ARV or virally suppressed to intermittent viral suppression) and viral suppression (operationalized as an increase in the transition rates from intermittent viral suppression to virally suppressed). The total enrollment and coverage represent the number of persons with diagnosed HIV infection who will receive benefits, excluding persons anticipated to be in emergency housing or not using benefits. All diagnosed persons have an equal probability of housing eligibility, based on programmatic and survey data indicating that unstably housed persons with HIV in New York State can achieve viral suppression.
We estimated stock-specific HIV transmission rates by using methods from a previous New York State HIV testing law model.30,31 General published findings, such as higher transmission rates among persons who are unaware of their HIV infection compared with persons who are diagnosed with HIV infection and no sexual transmission from persons who are virally suppressed, estimated the relative contribution of HIV infections from persons in each category. We calculated stock-specific transmission rates from the relative contribution of HIV infections and the 2011 population in each stock (initial conditions).
The appendix (available from the authors upon request) contains all model views, including for ETE programs (Appendix 1), equations for replication (Appendix 2), values and data sources for the initial conditions and transition probabilities across stocks (Appendix 3), model validation tests including comparisons of historical and simulated data (Appendix 4), the derivation of transmission rates with references to support the values used (Appendix 5) and policy scenario parameters (Appendix 6), and definitions of each model outcome (Appendix 7).
Policy Scenarios
We selected 3 ETE programs for modeling: enhanced linkage to and retention in care, PrEP for MSM, and expanded housing assistance. Each program comprises multiple new or expanded activities under ETE. Studies have examined single interventions, such as the effectiveness23 and costs36 of multisite retention-in-care interventions and the efficacy of PrEP among MSM.37 ETE entails implementation of numerous interventions within programs, and it was neither feasible nor useful to stakeholders to model the incremental effects of each ETE activity. After enumerating activities within each program, we reviewed published literature and internal and external data sources to develop low-, medium-, and high-implementation scenarios. The medium-implementation scenario represents stakeholders’ rollout predictions based on early program data and their decades of experience implementing AIDS Institute programming. The low- and high-implementation scenarios represent lower and upper bounds of anticipated rollout.
For each program, we summarize hereinafter the major activities, model structure, and scenario parameters (Table 1). Additional details, including internal and external data, literature, and logic guiding input values, are available in an appendix (available from the authors upon request).
Linkage and retention interventions engage diagnosed persons in HIV care to achieve undetectable viral load and prevent transmission of new infections. Examples of ETE activities to enhance ARV initiation include requiring diagnosing providers to schedule HIV medical appointments within 30 days of diagnosis; funding medical providers, community-based organizations, and Medicaid managed care plans to engage persons who are no longer in care or not virally suppressed; and making available 7-day ARV packs in New York City sexual health clinics. ETE activities to leverage surveillance data to identify persons no longer in care include (1) expanded partner services, whereby disease intervention specialists contact persons with diagnosed HIV who are potentially out of care, and (2) a Medicaid managed care data match program, whereby Medicaid plans receive information on members potentially out of care and/or not virally suppressed. These activities were operationalized as a percentage increase over the baseline transition rates from the diagnosed, not on ARV or virally suppressed stock to the intermittent viral suppression stock (linkage to care); and as a percentage increase in the transition rate from intermittent viral suppression to virally suppressed (re-linkage). We based the medium-implementation scenario (50% increase in the transition rate to intermittent viral suppression and 110% increase in the transition rate to virally suppressed) on (1) internal program data from the linkage-to-care activities (for increased linkage to care) and (2) an average of published findings from the expanded partner services pilot38 and unpublished early findings (New York State Department of Health AIDS Institute, 2017) from the Medicaid data match made available to our modeling team, weighted by the proportion of persons with intermittent viral suppression who would be reached by expanded partner services and/or the Medicaid data match (for increased re-linkage to care). Stakeholders defined the high- and low-implementation scenario values (low: 25% increase in ARV initiation and 85% increase in viral suppression; high: 75% increase in ARV initiation and 135% increase in viral suppression) based on their experience implementing similar programs.
Examples of ETE-related PrEP activities include social marketing; HIV service provider education; promotion in community health centers, hospitals, sexual health clinics, and syringe services programs; adherence counseling; advertising payment options; and coverage of copays. We focused on PrEP among MSM because they were prioritized by early ETE PrEP activities. To model this program, additional structure moves MSM at risk into another MSM on PrEP stock outside the at risk population. Under 100% PrEP coverage, all HIV-negative MSM would be in MSM on PrEP. A separate input implements imperfect clinical efficacy and adherence, which we combine into a more general “effectiveness” parameter (the product of efficacy and adherence). Our “effectiveness” parameter multiplied efficacy and adherence, consistent with other models.39,40 The medium-implementation scenario assumes 50% PrEP coverage by December 2017, based on extrapolation of 2012-2015 New York State PrEP initiation data from the manufacturer of the ARV, Truvada (low: 25%; high: 75%). All scenarios assumed 90% clinical efficacy among persons using PrEP as prescribed.37,41 The medium-implementation scenario assumed 85% adherence, consistent with multi-city demonstration projects among MSM and transgender women7 and a multi-city clinical trial among MSM.42 Adherence in the low- and high-implementation scenarios was 50% and 100%, respectively.
Although New York State implemented several new or expanded programs to expand housing access, we focused on the New York City HIV/AIDS Service Administration (HASA) “HASA for All” rental assistance. Pre-ETE, HASA provided rental subsidies, emergency housing, and other social services to low-income persons who met HIV medical eligibility of having clinically defined AIDS. Under ETE, medical eligibility was removed and HASA benefits were extended to all low-income New York City residents living with HIV.43 We selected this activity because it received considerable attention, enrollment data were accessible, and stakeholders believed it was the most important ETE-related housing program. The model (1) reduces transmissibility among all HIV-infected persons by 5% (excluding those who are unaware of their HIV status; low and high implementation: 0% and 10% reduction, respectively) and (2) increases transition rates from diagnosed, not on ARV or virally suppressed to intermittent viral suppression (linkage to HIV care) by 35% (low and high implementation: 15% and 55%, respectively). Parameter estimates came from our comprehensive literature review; literature, relevant empirical findings, and logic used to synthesize results into parameters are in Appendix 6 (available from the authors upon request). A housing program stock constrained the number of persons experiencing these improvements; persons outside the program retained the same infectivity and transition rates as baseline. The main scenario assumed benefits accrued to New York City residents, with 5480 diagnosed persons gaining eligibility based on HASA experts’ projections. (Calculations are in Appendix 6, which is available from the authors upon request.) Statewide policy scenarios considered expanding housing assistance beyond New York City.
We modeled programs alone and concurrently. In the concurrent implementation scenarios, we enabled the model structure for each program; for example, MSM at risk moved into a different stock of receiving protection from PrEP (thereby reducing the number of persons who might acquire HIV), and transmissibility among diagnosed persons (within the housing program enrollment constraint) decreased after the housing policy was implemented.
Results
Anticipated Epidemic Outcomes Without ETE
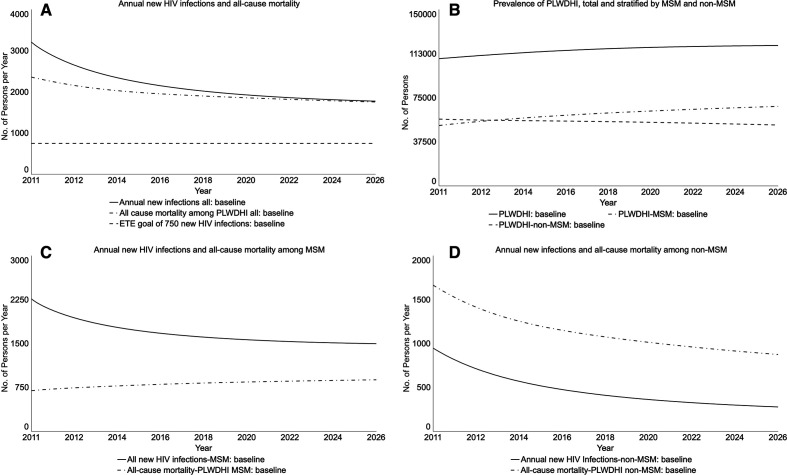
Without ETE (baseline), the number of annual new HIV infections and all-cause mortality would decline by 18.0% and 8.7%, respectively, from December 2014 to December 2020 (Figure 2A). However, the number of annual new HIV infections would not meet the 750 goal by December 2020 or 5 years after ETE (December 2025) without additional intervention. The prevalence of HIV infection would increase slightly because the infection rate would exceed the death rate until their curves approach each other by the period end (Figure 2B).
Figure 2.
Projected HIV population outcomes for New York State in the baseline of no policy initiative in place, from a participatory dynamic simulation model. New York State’s Ending the Epidemic (ETE) policy initiative is the state’s action plan to end the HIV epidemic, with targets of reducing the estimated annual number of new HIV infections from 3000 to 750, reducing AIDS progression by 50%, and achieving a first-ever decrease in HIV prevalence by the end of 2020.1 Graphs run from January 2011 (simulation start) to January 2026; Table 2 reports percentage changes from baseline in December 2020 (the end of the ETE 2020 milestone) and December 2025 (5 years later). Abbreviations: MSM, men who have sex with men; PLWDHI, persons living with diagnosed HIV infection.
Trends for MSM and non-MSM baseline projections, which were based on historical patterns and other model inputs such as their varying infectivity, differed: HIV prevalence would increase among MSM but decline among non-MSM (Figure 2B). Although the number of annual new HIV infections would decline among both populations (Figure 2C and D), the projected number of new HIV infections would be higher than the number of deaths among MSM. Throughout the period, most HIV infections would be among MSM.
Anticipated Impact of ETE Programs
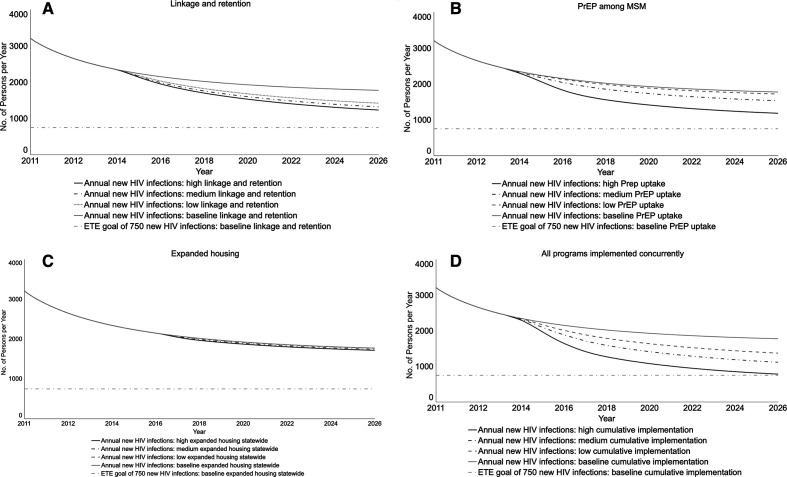
Compared with baseline, if the ETE linkage and retention program were implemented alone, the annual number of new HIV infections in the low-, medium-, and high-implementation scenarios would decrease by 13.8%, 18.1%, and 21.9%, respectively, by December 2020 (Table 2; Figure 3A). This ETE program would have the largest effect on all-cause mortality. In all scenarios, its implementation could meet one ETE Blueprint goal of fewer new HIV infections than deaths. These curves would intersect by about 2019 in the high-implementation scenario and in 2020 in the medium- and low-implementation scenarios.
Table 2.
Projected incremental 5-year differences in HIV population outcomes in New York State under 3 Ending the Epidemic programs compared with baseline projections of no initiative, from a participatory dynamic simulation modela
| Variable | % Difference From Baseline in December 2020 | % Difference From Baseline in December 2025 | ||||
|---|---|---|---|---|---|---|
| Program and Outcome | Low | Medium | High | Low | Medium | High |
| Linkage to and retention in care | ||||||
| No. of annual new HIV infections | −13.8 | −18.1 | −21.9 | −19.9 | −25.6 | −30.5 |
| Annual all-cause mortality | −13.5 | −16.5 | −16.5 | −12.2 | −15.6 | −18.4 |
| Prevalence of PLWDHI | 0.4 | 0.6 | 0.8 | 0.4 | 0.6 | 0.8 |
| No. of annual new HIV diagnoses | −5.4 | −7.3 | −9.0 | −13.4 | −17.5 | −21.1 |
| PrEP (full population)b | ||||||
| No. of annual new HIV infections | −2.3 | −10.6 | −27.6 | −3.1 | −13.7 | −33.4 |
| Annual all-cause mortality | −0.1 | −0.3 | −0.9 | −0.2 | −1.0 | −2.8 |
| Prevalence of PLWDHI | 0 | −0.3 | −0.8 | −0.2 | −0.9 | −2.4 |
| No. of annual new HIV diagnoses | −1.1 | −5.4 | −14.7 | −2.3 | −10.3 | −26.0 |
| Housing (statewide) | ||||||
| No. of annual new HIV infections | −0.4 | −1.6 | −2.8 | −0.3 | −1.7 | −3.2 |
| Annual all-cause mortality | −0.3 | −0.6 | −0.9 | −0.1 | −0.3 | −0.5 |
| Prevalence of PLWDHI | 0.1 | 0.1 | 0.1 | 0 | 0 | −0.1 |
| No. of annual new HIV diagnoses | −0.2 | −0.7 | −1.2 | −0.3 | −1.4 | −2.5 |
| All programs concurrently | ||||||
| No. of annual new HIV infections | −16.0 | −28.1 | −45.7 | −22.7 | −37.1 | −55.8 |
| Annual all-cause mortality | −10.3 | −14.1 | −17.5 | −12.5 | −16.6 | −20.6 |
| Prevalence of PLWDHI | 0.4 | 0.3 | 0.1 | 0.3 | −0.3 | −1.4 |
| No. of annual new HIV diagnoses | −6.5 | −12.6 | −22.5 | −15.5 | −26.7 | −42.4 |
Abbreviations: ARV, antiretroviral therapy; MSM, men who have sex with men; PLWDHI, persons living with diagnosed HIV infection; PrEP, preexposure prophylaxis.
aNew York State’s Ending the Epidemic (ETE) policy initiative is the state’s action plan to end the HIV epidemic, with targets of reducing estimated annual new HIV infections from 3000 to 750, reducing AIDS progression by 50%, and achieving a first-ever decrease in HIV prevalence by the end of 2020.1 Numbers represent differential changes in outcomes comparing the ETE scenarios with the no-ETE scenario (baseline projection), for each ETE program examined separately and in combination. Low, medium, and high correspond to the 3 levels of implementation. For each outcome measure, the projected value for the ETE scenario is compared with the projected baseline value in the scenario without ETE in place.
bThe PrEP program modeled here targets only MSM. The values presented in this table are for the full New York population, including MSM and non-MSM.
Figure 3.
Projected impact of 3 programs in New York State’s Ending the Epidemic (ETE) policy initiative on the number of annual new HIV infections, from a participatory dynamic simulation model. New York State’s ETE initiative is the state’s action plan to end the HIV epidemic, with targets of reducing the estimated number of annual new HIV infections from 3000 to 750, reducing AIDS progression by 50%, and achieving a first-ever decrease in HIV prevalence by the end of 2020.1 The 3 programs modeled here are linkage to and retention in care, preexposure prophylaxis for men who have sex with men (MSM), and expanded housing. Each panel displays findings for the baseline projection and 3 implementation scenarios for the outcome of annual new HIV infections. The black line displays the policy goal of 750 new HIV infections. The housing policy uses the most optimistic scenario of statewide implementation. Graphs run from January 2011 (simulation start) to January 2026; Table 2 reports percentage changes from baseline in December 2020 (the end of the ETE 2020 milestone) and December 2025 (5 years later). The lines for the housing policy appear to overlap because the values for different scenarios are similar to the baseline. Abbreviation: PrEP, preexposure prophylaxis.
Compared with baseline, if the PrEP for MSM program were implemented alone, the annual number of new HIV infections in the low-, medium-, and high-implementation scenarios would decrease by 2.3%, 10.6%, and 27.6%, respectively, by December 2020 (Table 2; Figure 3B). The high- and medium-implementation scenarios would meet the ETE goal of fewer new HIV infections than deaths, with curves crossing by about 2016 and 2017, respectively. The curves would cross in 2022 in the low-implementation scenario. Findings on PrEP for MSM demonstrated an interactive effect of levels of both coverage and adherence, with high levels of both parameters required to achieve substantial reductions in the number of annual new HIV infections (data not shown).
When expanded housing was implemented alone, the projected number of annual new HIV infections was similar to baseline (Table 2; Figure 3C). Even in the scenario of high statewide implementation, the curves for new HIV infections and all-cause mortality would not intersect by 2020 (data not shown). The New York City scenario, with fewer persons receiving the housing benefit than in the statewide scenario, was nearly identical to the statewide scenario.
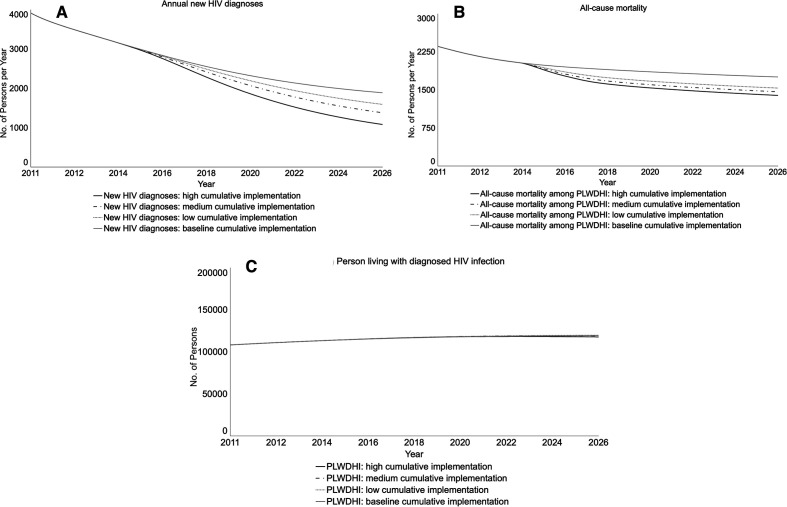
In the concurrent implementation of the 3 ETE programs, the policy goal of <750 annual new HIV infections would be approached by December 2025 (projection: 785) (data not shown). Compared with baseline, the low-, medium-, and high-implementation scenarios would yield 16.0%, 28.1%, and 45.7% declines, respectively, in annual new HIV infections by December 2020 (Table 2; Figure 3D). The rate of annual new HIV diagnoses—persons whose HIV infections are detected and thereafter included in the HIV surveillance registry as “cases”—would decline compared with baseline, reflecting fewer new HIV infections (Figure 4A). The numbers of annual new HIV infections and diagnoses would differ because the system includes persons who are currently infected but are unaware and will become cases upon diagnosis. All-cause mortality would decline (Figure 4B) because of the enhanced linkage and retention program; persons who are virally suppressed live longer than persons who are not virally suppressed. However, prevalence would be similar to baseline because persons in care live longer, thereby remaining in the system (Figure 4C). All scenarios with concurrent implementation of the 3 programs would meet the ETE benchmark of fewer HIV infections than deaths (data not shown).
Figure 4.
Projected impact of 3 programs in New York State’s Ending the Epidemic (ETE) initiative on other HIV epidemic outcomes, from a participatory dynamic simulation model. New York State’s ETE initiative is the state’s action plan to end the HIV epidemic, with targets of reducing the estimated number of annual new HIV infections from 3000 to 750, reducing AIDS progression by 50%, and achieving a first-ever decrease in HIV prevalence by the end of 2020.1 The 3 programs modeled here are linkage to and retention in care, preexposure prophylaxis for men who have sex with men (MSM), and expanded housing. Each panel displays findings for the baseline projection and 3 implementation scenarios for the 3 ETE programs implemented concurrently. The housing policy uses the most optimistic scenario of statewide implementation. Graphs run from January 2011 (simulation start) to January 2026; Table 2 reports percentage changes from baseline in December 2020 (the end of the ETE 2020 milestone) and December 2025 (5 years subsequent). Abbreviation: PLWDHI, persons living with diagnosed HIV infection.
Discussion
Modeling provided several critical insights about ETE goals. First, although the goal of <750 new HIV infections by the end of 2020 is unlikely, New York State can likely surpass its ETE goal of fewer new HIV infections than deaths and, thereby, a first-ever decline in HIV prevalence. Our projections of continued high HIV prevalence after the inflection point support the need to maintain investments in prevention, diagnosis, and linkage to and retention in care to prevent a rebound in new HIV infections. Second, reducing the number of new HIV infections among MSM is necessary to meet ETE goals. Third, enhanced linkage to and retention in care and PrEP will likely have the largest system influence. Increasing durable viral suppression reduces the number of new HIV infections because persons with undetectable viral loads cannot transmit infections sexually. Fourth, although PrEP can protect against HIV infection, it requires high levels of both coverage and adherence.
Our findings are consistent with the limited existing literature. Despite numerous studies on achieving UNAIDS 90-90-90 and Fast-Track goals in low- and middle-income countries19-23 and Canada,24 their conclusions are less directly relevant to the United States and New York State, whose baseline HIV epidemic outcomes were ahead of the national average pre-ETE. The Canadian study projected its targets to be on schedule23 but did not directly model what it means to end HIV as an epidemic. Compared with US studies of local epidemics, an agent-based model of young black MSM in Illinois examined the effect of initiating and adhering to PrEP under its Getting to Zero initiative.25 Consistent with our findings, the highest PrEP implementation scenario would not achieve Getting to Zero policy goals without other concurrent programs. Also consistent with our findings, another study that projected HIV incidence in 6 cities estimated continued declines in New York City, representing a substantially higher improvement than in other cities that were modeled, and concluded that targeted combination strategies are needed to meet national Ending the Epidemic in America goals.13 Evaluations of early outcomes of Getting to Zero in San Francisco44,45 and Miami46 show promising results, but their ability to fully assess the end of HIV as an epidemic was limited to existing data with time lags and process-oriented measures, supporting the value of our formative modeling approach to guide policy implementation.
Our finding about the ETE housing program was unexpected. Literature documents the important role of housing to individuals,10 and national core indicators of HIV care include housing.47 However, we projected a limited effect at the population level. We conclude that expanded housing should be maintained to address social determinants of health, but other ETE programs are more influential than expanding housing on ETE goals. We have 2 likely explanations for our unexpected findings for the housing program. First, this program affects relatively few persons: 4.9% and 7.9% of persons with diagnosed HIV infection would gain coverage in the New York City and statewide scenarios, respectively. This explanation is consistent with findings from the New York State Medical Monitoring Program survey that, during 2009-2014, 27% of respondents reported needing shelter or housing, 22% received shelter or housing assistance, and 5% reported an unmet housing need.48 Second, many unstably housed persons in New York State have already achieved viral load suppression: survey data from the New York City metropolitan area documented lower self-reported viral load suppression among homeless and unstably housed persons than among persons who were stably housed, but most were virally suppressed at last interview (personal communication, Angela Aidala, Columbia, University, March 2017). Subject matter experts, who were consulted throughout the model development process, believe that New York State’s extensive HIV care system and wraparound services have improved viral load suppression among unstably housed persons.
Participatory modeling facilitated idea exchanges and common understandings of New York State’s HIV testing and care system. Collaborations among stakeholders with specialized knowledge (eg, health department staff members, community partners, clinicians) can enhance innovation, but specialization can also hinder implementation.49 Solving complex tasks requires that stakeholders develop common terminologies, meanings, and frameworks to describe phenomena and interdependencies.50 In our participatory modeling process, conceptual framework discussions propelled stakeholders to harmonize definitions of viral load suppression with various viral load suppression measures used for annual point-in-time estimates prepared for CDC,51 expanded partner services,38 Medicaid managed care data match programs, and our model. Other conflicting viewpoints reconciled through structured conversations include discrepant classifications of disease stages and risk groups in clinical practice vs surveillance systems, and individual vs population-level perspectives. Challenges to the participatory process included staff members’ time commitment, funding for academic partners to conduct modeling, and openness to alternative views; however, these challenges were mitigated by stakeholders’ motivations to meet ETE goals, strong executive support for the modeling project and evidence-informed decision making, and the modeling team’s existing practitioner/academic partnership, which improved trust.
The participatory process facilitated reflective discussions about communicating to stakeholders the modeling findings on the goal of 750 new HIV infections. Although the 750 benchmark is widely cited, the 3 programs analyzed were not projected to achieve the 2020 benchmark. It is important to note that New York State’s ETE efforts comprise more than these 3 initiatives, and our findings do not necessarily mean that New York State will fail to achieve its goal of <750 new HIV infections by the end of 2020. In addition, since the ETE’s targets were set, CDC changed its HIV incidence calculation methodology and incidence is 30% higher under the new formula; this, combined with New York State’s decision to select a “greater-than-necessary” incidence target, suggests that HIV prevalence will decline at a rate of >750 new HIV infections annually. The intersection of new HIV infections and mortality, another definition in the ETE Blueprint, is an important measure of success to communicate because this accomplishment marks the first-ever decline in HIV prevalence.
Finally, the participatory modeling process identified future research priorities. Discussions to reconcile competing viral load suppression definitions and triangulate viral load suppression findings from internal data created interest in developing best practices for linking programmatic data for HIV programming, monitoring, and evaluation. Subject matter and data experts’ descriptions of geographic differences in the epidemic created interest in further modeling tailored regional strategies.
To enhance ETE implementation, we communicated findings to AIDS Institute executive leadership and staff members, local health departments, community partners, advocacy groups, and other key ETE stakeholders. After results were presented internally, a new “Achieving ETE Goals” meeting series among division directors and executive staff members was initiated to leverage the modeling results to promote data-driven decision making on HIV programming. For example, additional review of modeling results revealed that by 2020, 60% of new HIV infections would be generated by undiagnosed persons. This realization increased attention on the scope and efficacy of HIV testing programs and a reexamination of existing contracts and subsequent requests for applications. As New York State develops its “2020 and Beyond” initiative, additional external research partners will be invited to establish metrics and goals.
Limitations
Our model had several limitations. First, dynamic compartmental models such as ours and agent-based models have strengths and weaknesses, and the model’s purpose should guide the approach.52 We made simplifying assumptions about homogenous behaviors within stocks, whereas agent-based models can capture complexities that we do not include, such as varying risk behaviors over time; regional differences in population density, community prevalence, and sexual partner networks; and persons receiving various combinations of interventions. However, agent-based models have higher computational requirements, an important consideration for our time-sensitive formative evaluation. Additional simplifying assumptions were omitting transmission modes and an age-structured population. We made these decisions to improve comprehension among broader ETE stakeholders who lack modeling expertise and because these additional details were insufficiently supported with New York State–specific data, which are important for ensuring credibility among ETE stakeholders.
A second limitation was generalizability. Our model was uniquely tailored to the New York State context, which enhanced its utility for decision making, but our tailored approach also limited the generalizability of some findings. For example, our finding that MSM are an important population is generalizable because this population has the highest rates of HIV diagnosis nationally53; however, other jurisdictions with limited syringe services programs may find advocacy to establish these programs to be an important priority for their local ETE plans. Our finding that housing would have limited population-level benefits may be unique to New York State because this benefit was previously available to many (although not all) vulnerable populations and because New York State has robust social support services.
Finally, our work was completed in June 2017, and important program and policy changes have since occurred that may have altered our findings. For example, in 2018, the AIDS Institute implemented a rapid initiation of HIV treatment programs whereby every new HIV diagnosis outside New York City is monitored in real time to ensure HIV treatment and viral suppression are achieved within 30 and 90 days, respectively.
Conclusions
As policy initiatives to end HIV as an epidemic are implemented nationally, systems modeling can synthesize diverse data for real-time actionable insights. Participatory group model-building can enable leadership and staff members to develop common understandings of their policy systems, exchange ideas and specialized knowledge, create collective ownership of actionable findings, and enhance evidence-informed policy implementation.
Acknowledgments
The authors acknowledge the support of the AIDS Institute’s modeling steering committee for providing regular input on the model, including Bridget Anderson, Ira Feldman, John Fuller, Charles Gonzales, Jayleen Gunn, Franklin Laufer, John Leung, Alison Muse, Deepa Rajulu, Mona Scully, Ling Wang, and Janet Wikoff. Additional subject matter experts who provided invaluable insights into programs were Angela Aidala, Joe Losowski, Cindy Ravida, Ginny Shubert, and Daniel Tietz (housing); and Lillian Lee and Wendy Levey (linkage and retention). Ling Wang and Janet Wikoff additionally provided some of the data series to set the initial conditions and calibrate the model. Mitchel Abolafia, Thomas Gais, Luis Luna-Reyes, and Patricia Strach provided helpful comments on an early draft of this article.
This work was commissioned, designed, and executed under the former director of the New York State Department of Health AIDS Institute.
Footnotes
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: At the time of analysis, Daniel Gordon, John Helmeset, Travis O’Donnell, Carol-Ann Swain, and James Tesoriero were employed by the New York State Department of Health AIDS Institute, the agency that is implementing the ETE initiative.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a contract from the New York State Department of Health from December 1, 2015, through May 31, 2017.
ORCID iDs
Erika G. Martin https://orcid.org/0000-0003-2607-8933
James M. Tesoriero https://orcid.org/0000-0002-7100-0791
References
- 1. New York State Department of Health 2015 blueprint for achieving the goal set forth by Governor Cuomo to end the epidemic in New York State by the end of 2020. Accessed March 17, 2019 https://www.health.ny.gov/diseases/aids/ending_the_epidemic/docs/blueprint.pdf
- 2. The White House National HIV/AIDS Strategy. Accessed April 27, 2020 https://www.hiv.gov/federal-response/national-hiv-aids-strategy/strategy-history
- 3. Marks G., Crepaz N., Senterfitt JW., Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39(4):446-453. 10.1097/01.qai.0000151079.33935.79 [DOI] [PubMed] [Google Scholar]
- 4. Cohen MS., Chen YQ., McCauley M. et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493-505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodger AJ., Cambiano V., Bruun T. et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171-181. 10.1001/jama.2016.5148 [DOI] [PubMed] [Google Scholar]
- 6. U=U taking off in 2017. Lancet HIV. 2017;4(11):e475. 10.1016/S2352-3018(17)30183-2 [DOI] [PubMed] [Google Scholar]
- 7. Grant RM., Anderson PL., McMahan V. et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820-829. 10.1016/S1473-3099(14)70847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu AY., Cohen SE., Vittinghoff E. et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med. 2016;176(1):75-84. 10.1001/jamainternmed.2015.4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCormack S., Dunn DT., Desai M. et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53-60. 10.1016/S0140-6736(15)00056-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aidala AA., Wilson MG., Shubert V. et al. Housing status, medical care, and health outcomes among people living with HIV/AIDS: a systematic review. Am J Public Health. 2016;106(1):e1-e23. 10.2105/AJPH.2015.302905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leaver CA., Bargh G., Dunn JR., Hwang SW. The effects of housing status on health-related outcomes in people living with HIV: a systematic review of the literature. AIDS Behav. 2007;11(6 suppl):85-100. 10.1007/s10461-007-9246-3 [DOI] [PubMed] [Google Scholar]
- 12. Pitman R., Fisman D., Zaric GS. et al. Dynamic transmission modeling: a report of the ISPOR-SMDM Modeling Good Practices Task Force Working Group-5. Med Decis Making. 2012;32(5):712-721. 10.1177/0272989X12454578 [DOI] [PubMed] [Google Scholar]
- 13. Nosyk B., Zang X., Krebs E. et al. Ending the epidemic in America will not happen if the status quo continues: modeled projections for human immunodeficiency virus incidence in 6 US cities. Clin Infect Dis. 2019;69(12):2195-2198. 10.1093/cid/ciz1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bradley H., Rosenberg ES., Holtgrave DR. Data-driven goals for curbing the U.S. HIV epidemic by 2030. AIDS Behav. 2019;23(3):557-563. 10.1007/s10461-019-02442-7 [DOI] [PubMed] [Google Scholar]
- 15. Bernard CL., Owens DK., Goldhaber-Fiebert JD., Brandeau ML. Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: a model-based analysis. PLoS Med. 2017;14(5):e1002312. 10.1371/journal.pmed.1002312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Juusola JL., Brandeau ML., Owens DK., Bendavid E. The cost-effectiveness of preexposure prophylaxis for HIV prevention in the United States in men who have sex with men. Ann Intern Med. 2012;156(8):541-550. 10.7326/0003-4819-156-8-201204170-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uzun Jacobson E., Hicks KA., Tucker EL., Farnham PG., Sansom SL. Effects of reaching national goals on HIV incidence, by race and ethnicity, in the United States. J Public Health Manag Pract. 2018;24(4):e1-e8. 10.1097/PHH.0000000000000717 [DOI] [PubMed] [Google Scholar]
- 18. Shah M., Perry A., Risher K., Kapoor S. et al. Effect of the US National HIV/AIDS Strategy targets for improved HIV care engagement: a modelling study. Lancet HIV. 2016;3(3):e140-e146. 10.1016/S2352-3018(16)00007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abuelezam NN., McCormick AW., Surface ED. et al. Modelling the epidemiologic impact of achieving UNAIDS fast-track 90-90-90 and 95-95-95 targets in South Africa. Epidemiol Infect. 2019;147:e122. 10.1017/S0950268818003497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dutta A., Barker C., Kallarakal A. The HIV treatment gap: estimates of the financial resources needed versus available for scale-up of antiretroviral therapy in 97 countries from 2015 to 2020. PLoS Med. 2015;12(11):e1001907. 10.1371/journal.pmed.1001907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stover J., Bollinger L., Izazola JA. et al. What is required to end the AIDS epidemic as a public health threat by 2030? The cost and impact of the fast-track approach. PLoS One. 2016;11(5):e0154893. 10.1371/journal.pone.0154893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stuart RM., Fraser-Hurt N., Kerr CC. et al. The City of Johannesburg can end AIDS by 2030: modelling the impact of achieving the Fast-Track targets and what it will take to get there. J Int AIDS Soc. 2018;21(1):e25068. 10.1002/jia2.25068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bansi-Matharu L., Cambiano V., Apollo T. et al. 90-90-90 by 2020? Estimation and projection of the adult HIV epidemic and ART programme in Zimbabwe—2017 to 2020. J Int AIDS Soc. 2018;21(11):e25205. 10.1002/jia2.25205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lima VD., St-Jean M., Rozada I. et al. Progress towards the United Nations 90-90-90 and 95-95-95 targets: the experience in British Columbia, Canada. J Int AIDS Soc. 2017;20(3). 10.1002/jia2.25011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khanna AS., Schneider JA., Collier N. et al. A modeling framework to inform preexposure prophylaxis initiation and retention scale-up in the context of “Getting to Zero” initiatives. AIDS. 2019;33(12):1911-1922. 10.1097/QAD.0000000000002290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Forrester JW. Industrial Dynamics. MIT Press; 1961. [Google Scholar]
- 27. Sterman JD. Business Dynamics: Systems Thinking and Modeling for a Complex World. Irwin/McGraw-Hill; 2000. [Google Scholar]
- 28. Black LJ. When visuals are boundary objects in system dynamics work. Syst Dyn Rev. 2013;29(2):70-86. [Google Scholar]
- 29. Anderson RM., May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford University Press; 1991. [Google Scholar]
- 30. Martin EG., MacDonald RH., Smith LC. et al. Policy modeling to support administrative decision making on the New York State HIV testing law. J Policy Anal Manage. 2015;34(2):403-423. 10.1002/pam.21797 [DOI] [PubMed] [Google Scholar]
- 31. Martin EG., MacDonald RH., Smith LC. et al. Mandating the offer of HIV testing in New York: simulating the epidemic impact and resource needs. J Acquir Immune Defic Syndr. 2015;68(suppl 1):S59-S67. 10.1097/QAI.0000000000000395 [DOI] [PubMed] [Google Scholar]
- 32. New York State Department of Health, Bureau of HIV/AIDS Epidemiology, AIDS Institute New York State HIV/AIDS surveillance annual report for persons diagnosed through December 2016. 2017. Accessed March 17, 2019 https://www.health.ny.gov/diseases/aids/general/statistics/annual
- 33. Centers for Disease Control and Prevention Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years—United States, 2008. MMWR Recomm Rep. 2008;57(RR-10):1-12. [PubMed] [Google Scholar]
- 34. Martin EG., Paltiel AD., Walensky RP., Schackman BR. Expanded HIV screening in the United States: what will it cost government discretionary and entitlement programs? A budget impact analysis. Value Health. 2010;13(8):893-902. 10.1111/j.1524-4733.2010.00763.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mindel A., Tenant-Flowers M. ABC of AIDS: natural history and management of early HIV infection. BMJ. 2001;322(7297):1290-1293. 10.1136/bmj.322.7297.1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shrestha RK., Gardner L., Marks G. et al. Estimating the cost of increasing retention in care for HIV-infected patients: results of the CDC/HRSA Retention in Care Trial. J Acquir Immune Defic Syndr. 2015;68(3):345-350. 10.1097/QAI.0000000000000462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grant RM., Lama JR., Anderson PL. et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587-2599. 10.1056/NEJMoa1011205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tesoriero JM., Johnson BL., Hart-Malloy R. et al. Improving retention in HIV care through New York’s expanded partner services data-to-care pilot. J Public Health Manag Pract. 2017;23(3):255-263. 10.1097/PHH.0000000000000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jenness SM., Goodreau SM., Rosenberg E. et al. Impact of the Centers for Disease Control’s HIV preexposure prophylaxis guidelines for men who have sex with men in the United States. J Infect Dis. 2016;214(12):1800-1807. 10.1093/infdis/jiw223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abbas UL., Glaubius R., Mubayi A., Hood G., Mellors JW. Antiretroviral therapy and pre-exposure prophylaxis: combined impact on HIV transmission and drug resistance in South Africa. J Infect Dis. 2013;208(2):224-234. 10.1093/infdis/jit150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baeten JM., Donnell D., Ndase P. et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399-410. 10.1056/NEJMoa1108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grohskopf LA., Chillag KL., Gvetadze R. et al. Randomized trial of clinical safety of daily oral tenofovir disoproxil fumarate among HIV-uninfected men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2013;64(1):79-86. 10.1097/QAI.0b013e31828ece33 [DOI] [PubMed] [Google Scholar]
- 43. Governor Cuomo announces all HIV-positive individuals in New York City to become eligible for housing, transportation and nutritional support [news release] New York State; June 23, 2016. https://www.governor.ny.gov/news/governor-cuomo-announces-all-hiv-positive-individuals-new-york-city-become-eligible-housing
- 44. Buchbinder SP., Havlir DV. Getting to Zero San Francisco: a collective impact approach. J Acquir Immune Defic Syndr. 2019;82(suppl 3):S176-S182. 10.1097/QAI.0000000000002200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scheer S., Hsu L., Schwarcz S. et al. Trends in the San Francisco human immunodeficiency virus epidemic in the “Getting to Zero” era. Clin Infect Dis. 2018;66(7):1027-1034. 10.1093/cid/cix940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Escudero DJ., Bennett B., Suarez S., Darrow WW., Mayer KH., Seage GR. Progress and challenges in “Getting to Zero” new HIV infections in Miami, Florida. J Int Assoc Provid AIDS Care. 2019;18:2325958219852122. 10.1177/2325958219852122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Institute of Medicine Monitoring HIV Care in the United States. National Academies Press; 2012. [PubMed] [Google Scholar]
- 48. New York State Department of Health HIV-positive adults in care in New York State, Medical Monitoring Project, 2009-2014. 2017. Accessed March 17, 2019 https://www.health.ny.gov/diseases/aids/general/statistics/docs/medical_monitoring_project.pdf
- 49. Carlile PR. A pragmatic view of knowledge and boundaries: boundary objects in new product development. Organ Sci. 2002;13(4):442-455. 10.1287/orsc.13.4.442.2953 [DOI] [Google Scholar]
- 50. Carlile PR. Transferring, translating, and transforming: an integrative framework for managing knowledge across boundaries. Organ Sci. 2004;15(5):555-568. 10.1287/orsc.1040.0094 [DOI] [Google Scholar]
- 51. New York State Department of Health, AIDS Institute HIV care in New York State, 2016: linkage to care and viral suppression among persons residing in New York State. Updated 2017. Accessed March 17, 2019 https://www.health.ny.gov/diseases/aids/general/statistics/cascade_reports/docs/linkage_retention_2016.pdf
- 52. Rahmandad H., Sterman J. Heterogeneity and network structure in the dynamics of diffusion: comparing agent-based and differential equation model. Manag Sci. 2008;54(5):998-1014. [Google Scholar]
- 53. Centers for Disease Control and Prevention Diagnoses of HIV infection in the United States and dependent areas, 2018 (preliminary). HIV Surveill Rep. 2019;30:1-129. [Google Scholar]