Abstract
Objective
Targeted testing and treatment of persons with latent tuberculosis infection (LTBI) is a critical component of the US tuberculosis (TB) elimination strategy. In January 2016, the California Department of Public Health issued a tool and user guide for TB risk assessment (California tool) and guidance for LTBI testing, and in September 2016, the US Preventive Services Task Force (USPSTF) issued recommendations for LTBI testing in primary care settings. We estimated the epidemiologic effect of adherence to both recommendations in California.
Methods
We used an individual-based Markov micro-simulation model to estimate the number of cases of TB disease expected through 2026 with baseline LTBI strategies compared with implementation of the USPSTF or California tool guidance. We estimated the risk of LTBI by age and country of origin, the probability of being in a targeted population, and the probability of presenting for primary care based on available data. We assumed 100% adherence to testing guidance but imperfect adherence to treatment.
Results
Implementation of USPSTF and California tool guidance would result in nearly identical numbers of tests administered and cases of TB disease prevented. Perfect adherence to either recommendation would result in approximately 7000 cases of TB disease averted (40% reduction compared with baseline) by 2026. Almost all of this decline would be driven by a reduction in the number of cases among non–US-born persons.
Conclusions
By focusing on the non–US-born population, adherence to LTBI testing strategies recommended by the USPSTF and the California tool could substantially reduce the burden of TB disease in California in the next decade.
Keywords: tuberculosis, LTBI, guidelines, tuberculosis elimination, simulation modeling
Elimination of tuberculosis (TB) is a global public health priority.1 Strategies to control TB in the United States are shifting from a focus on identifying and treating persons with TB disease toward testing and treating persons with latent TB infection (LTBI).2,3 According to the most recent estimates from 2011-2012, approximately 5% of the US population has LTBI.4,5 An estimated 85% of US TB cases are attributable to reactivation of LTBI; approximately 70% occur among non–US-born persons.6,7 In response to these trends, focus is being placed on scaling up targeted LTBI testing and treatment among populations at high risk for progressing to active TB disease.8
In January 2016, the California Department of Public Health (CDPH) introduced the California Tuberculosis Risk Assessment Tool (hereinafter, California tool) to help primary care providers scale up LTBI testing and treatment.9 CDPH together with the California Tuberculosis Controllers Association developed the California tool by combining available evidence on the risks associated with TB exposure and the risk for progression to TB disease, ranked by relative risk, and based on values in the literature. Stakeholders estimated the epidemiology of TB risk factors among recent TB cases in California from published data or current surveillance data from California, including cohort risk estimates (personal communication, P. Barry, CDPH, January 2019). The final risk assessment prioritized 3 groups for LTBI testing and treatment who are at highest risk for either recent TB infection or progression from LTBI to active disease. The California tool recommends testing non–US-born persons from countries with a high incidence of TB (ie, 20 countries with the highest absolute number of incident cases annually),1 contacts of persons with active TB, and persons who have immunosuppressive conditions (eg, HIV infection), persons who are undergoing immunosuppressive therapies (eg, persons who use tumor necrosis factor [TNF]–α inhibitors), and organ transplant recipients. In September 2016, the US Preventive Services Task Force (USPSTF) also issued recommendations for LTBI testing and treatment. The Task Force based its findings on evidence synthesis, systematic reviews, and expert opinion; these findings are detailed elswhere.10,11 The Task Force recommended that adults born or formerly residing in countries with high TB prevalence1 and current and former residents of high-risk congregate settings (HRCSs; eg, prisons, nursing homes, homeless shelters) be tested for LTBI in primary care settings.12
Although multiple studies estimate the importance of LTBI testing and treatment in populations at high risk for TB, such as non–US-born persons and persons with medical comorbidities,13-16 no studies have assessed the effect of implementing LTBI testing and treatment guidance. We assessed the potential effect of these recommendations by modeling the implementation of the USPSTF and California tool recommendations to estimate the number of TB cases in California that are potentially preventable.
Methods
Modeling Approach
We previously developed a model of TB in California.15 For this analysis, we added detail to the model on relevant populations at high risk of TB. Our model is an individual-based 1% scale portrayal of the California population aged ≥18, implemented in Python 2.717 with demographic attributes of persons in the model obtained from local data sources. The model simulates the natural history of TB with a Markov chain. Persons entered the model by aging in (at 18 years) or by immigrating and exited via death or emigration. We portrayed transmission by using a semi-random mixing model that assumed an increased probability of interaction with persons of the same race–nativity group. Each time cycle represented 1 month. Details of the model structure, model inputs, and calibration are provided in the technical supplement available from the authors upon request.
We adapted our model to simulate implementation of USPSTF guidance by including current and former residence in HRCSs as attributes of persons. We also made HRCS residence at any point in time a risk factor for progression from LTBI to TB disease, with risk ratios estimated by expert opinion and refined by calibration. Furthermore, we assumed that infectious HRCS residents could transmit only to other residents of the same HRCS. Because no individual-level data were used and all modeling scenarios were hypothetical, institutional review board review was not sought.
Data Inputs
Population
The primary source for data on the size and demographic composition of the California population and new entrants is the American Community Survey.18 Data sources used to determine the size and composition of the populations residing in HRCSs were the Point in Time survey and the Homeless Management Information System,19 the National Corrections Reporting System,20 the California Department of Corrections and Rehabilitation 2011 Adult Institutions Outcome Evaluation Report,21 and the California Office of Statewide Planning and Development.22
Risk of reactivation and transmission
Risk of progression from LTBI to TB disease followed an exponential decay curve as a function of the number of months since infection and a baseline (first month) risk of reactivation. We included estimates of the baseline risk of reactivation, rate of exponential decay, risk ratios for progression from LTBI to TB disease, and number of persons infected by 1 infected person (details available from the authors upon request).
LTBI and TB disease prevalence
We estimated LTBI prevalence trends for US-born and non–US-born persons who had been in the United States for ≥5 years by using data from the National Health and Nutrition Examination Survey (NHANES), stratified by age, race, and sex.23 We compared LTBI prevalence for US-born persons in the 2011-2012 NHANES with LTBI prevalence for US-born persons in the 1999-2000 NHANES to establish a monthly decline in the risk of LTBI for persons entering the model (through internal migration, aging in, or in the first cycle). For entrants within the past 5 years, we estimated prevalence rates and recent infection rates from estimated prevalence in the country of origin.4 Persons with LTBI were also assigned a number of months since infection.
Primary care provider visits
We estimated the monthly probability of primary care provider visits by estimating the total number of primary care provider visits in California by age group, sex, smoking status, and diabetes status from the 2015 National Ambulatory Medical Care Survey.24 To account for differences in primary care provider use by nativity or length of residence in the United States, we adjusted the probabilities calculated from the National Ambulatory Medical Care Survey by using data on health care access from the 2015 National Health Interview Survey.25
Testing Strategies
We assumed that all testing would be conducted using the interferon gamma release assay Quantiferon-Gold (Qiagen, Hilden, Germany), which is more specific than the tuberculin skin test, particularly in the non–US-born population, because it includes many persons vaccinated with Bacillus Calmette Guerin.26 Previous modeling work has suggested that the interferon gamma release assay is more cost-effective than the tuberculin skin test for LTBI testing and treatment of non–US-born persons.14,26 We assumed 100% adherence to testing by primary care providers for all patients who met eligibility criteria and 100% agreement to testing by patients.
Treatment Strategies
We assumed that LTBI treatment would consist of 12 weeks of isoniazid and rifapentine using directly observed therapy.27 We estimated that 82% of patients had initiated treatment and that 6% of patients who began the month of treatment discontinued treatment each month.28 Persons who discontinued treatment returned to the untreated LTBI state.
Targeted Testing and Treatment Scenarios
We compared populations targeted for testing in the baseline, USPSTF, and California tool models (Table 1). We assumed that all persons tested under the baseline scenario would also be tested under the USPSTF and California tool models. We ran 500 iterations of each testing scenario.
Table 1.
Comparison of model implementation of US Preventive Services Task Force (USPSTF) recommendations and risk scenarios from the California Tuberculosis Risk Assessment Toola
| Characteristics | Baseline | USPSTF Recommendations |
California Tuberculosis Risk Assessment Tool |
|---|---|---|---|
| General population | 2% probability of testing per year | 2% probability of testing per year | 2% probability of testing per year |
| Non–US-born persons | Not applicable | Tested once | Tested once |
| Health care workers | Annual testing | Annual testing | Annual testing |
| Contacts of tuberculosis case | Tested once, at time of contact investigation | Tested once, at time of contact investigation | Tested once, at time of contact investigation |
| Immunosuppressedb | |||
| HIV positive | Tested once | Tested once | Tested once |
| TNF-α inhibitor use | 85% tested once | 85% tested once | Tested once |
| Transplant recipient | Tested once | Tested once | Tested once |
| Current residents of HRCSs | |||
| Long-term care facilities | Tested on admission | Tested annually | Tested on admission |
| Homeless shelters | Not applicable | Tested annually | Not applicable |
| Correctional facilities | Tested on admission | Tested annually | Tested on admission |
| Former residents of HRCSs | |||
| Long-term care facilities | Not testedc | Tested once | Not testedc |
| Homeless shelters | Not testedc | Tested once | Not testedc |
| Correctional facilities | Not testedc | Tested once | Not testedc |
Abbreviations: HRCS, high-risk congregate setting; TNF, tumor necrosis factor.
aModeled scenarios varied by the key populations prioritized for targeted tuberculosis testing and treatment and frequency of testing. Data sources: California Department of Public Health9 and USPSTF.12
bPersons with HIV undergo testing at the time of antiretroviral therapy initiation, which is used as a proxy for time of diagnosis. Persons who use TNF-α antagonists and recipients of solid-organ or bone marrow transplants undergo testing before initiation of immunosuppressive therapy or transplantation.
cNot tested refers to persons in this population not being tested an additional time in the model based on lack of a recommendation to do so in current guidelines or practice.
Continuation of the baseline
Testing on admission is currently conducted in almost all California state and county correctional facilities and is mandated on admission to long-term care facilities.29 Therefore, the baseline testing scenario assumes 100% testing in the first month of residence in these settings. We based the baseline probability of testing among immunocompromised persons on expert opinion and estimated it to be 100% for persons with HIV infection at the time of diagnosis, 100% for organ transplant recipients immediately before transplantation, and 85% for persons initiating TNF-α inhibitor treatment. We also assumed testing of recent contacts to infectious cases, annual testing of health care workers, and 2% random testing of all other persons each year.
Implementation of USPSTF guidance
We implemented USPSTF guidance in the model from January 2017 through 2026 in primary care provider settings. Populations recommended for testing under USPSTF guidance are non–US-born persons and HRCS residents (Table 1).
Implementation of the California tool
We implemented the California tool guidance in the model from January 2017 through 2026 in primary care provider settings. Persons targeted under these recommendations are non–US-born persons and persons with certain immunosuppressive comorbidities, including TNF-α inhibitor users who are not tested at initiation of therapy (Table 1).
Targeted testing of subgroups
In addition to modeling the 2 sets of official recommendations, we estimated the number of TB disease cases and tests performed in scenarios in which only non–US-born persons and HRCS residents were targeted for testing.
Outcomes and Analysis
For each intervention strategy, we estimated the number of incident TB cases and the number of TB cases prevented. We also estimated the number of persons with untreated LTBI, the number of tests performed, and the number of transmissions. We created point estimates by taking the mean value across all simulations and 95% simulation intervals (SIs) by taking the 97.5th and 2.5th percentile of each outcome across all simulations. SIs reflect the stochastic nature of transitions between states on each of the Markov chains and stochasticity in the composition of the model population.
Sensitivity Analysis
We conducted a sensitivity analysis by varying adherence to recommendations, with perfect adherence defined as testing of all eligible patients when they present for a primary care provider visit. Adherence, in this case, does not include acceptance of treatment or level of treatment discontinuation by patients. Adherence levels modeled were 25%, 50%, and 100%.
Results
Baseline Epidemiologic Projections
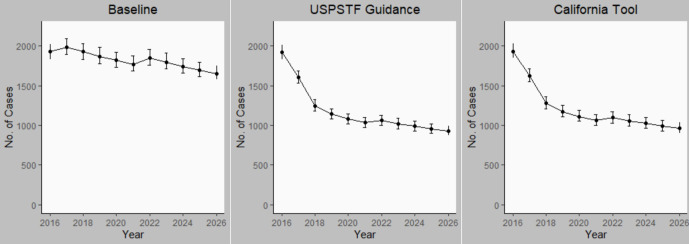
In the baseline scenario, the annual number of projected cases of TB disease declined from 1988 (95% SI, 1896-2087) in 2017 to 1655 (95% SI, 1576-1746) in 2026 (Figure 1) for a cumulative total of 18 123 cases. The projected annual number of tests performed increased from 2.78 million (95% SI, 2.75 million-2.81 million) in 2017 to 3.17 million (95% SI, 3.13 million-3.20 million) in 2026, for a 10-year total of 29.79 million. The cumulative number of TB cases was projected to be 15 274 (95% SI, 14 669-15 964) among non–US-born persons, 582 (95% SI, 332-1048) among HRCS residents, and 1309 (95% SI, 1095-1531) among persons with immunosuppressive disorders. The estimated number of persons living with untreated LTBI was projected to decrease from 2.17 million (95% SI, 2.14 million-2.20 million) in 2017 to 1.82 million (95% SI, 1.79 million-1.84 million) in 2026. The model projected 55 641 (95% SI, 52 577-58 973) TB transmissions occurring in California from 2017 to 2026.
Figure 1.
Annual number of cases of tuberculosis (TB) disease in California from 2016 through 2026 under 3 scenarios for targeted TB testing: baseline (continuation of preguidance testing levels), US Preventive Services Task Force (USPSTF) recommendations, and California TB Risk Assessment Tool (California Tool) recommendations. Error bars indicate 95% simulation intervals. Data sources: California Department of Public Health9 and USPSTF.12
Modeled LTBI Testing and Treatment Scenarios Based on Guidance
Number of TB cases averted
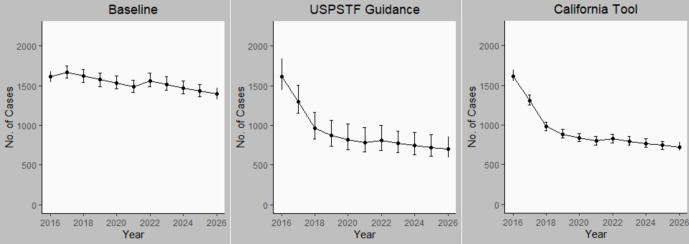
Our model projections estimated that full adherence to either the USPSTF recommendations or the California tool would prevent an additional 7000 cases of TB disease (n = 7063; 95% SI, 6139-7574 for USPSTF; n = 6726; 95% SI, 5741-7708 for the California tool) within the first 10 years after implementation or a reduction of approximately 40% of cases from the baseline scenario. The steepest decline occurred in the first 2 years after implementation (Figure 1). Most prevented TB cases (96% for USPSTF and 98% for the California tool) would occur among non–US-born persons. USPSTF guidance implementation was projected to prevent 6775 (95% SI, 5989-7574) TB cases and California tool implementation was projected to prevent 6601 (95% SI, 5798-7375) TB cases among non–US-born persons during the decade (Figure 2). USPSTF and California tool guidance implementation was projected to prevent 273 and 189 cases, respectively, among HRCS residents and 412 and 533 cases, respectively, among persons with immunosuppressive conditions. However, these numbers were too small to obtain reliable SIs. The LTBI testing and treatment scenario targeting only non–US-born persons for testing averted 6552 (95% SI, 5586-7708) cases during the 10-year period, whereas targeting only HRCS residents averted 805 cases; however, the numbers were too small to obtain reliable SIs.
Figure 2.
Annual number of cases of tuberculosis (TB) disease among non–US-born persons in California from 2016 through 2026 under 3 scenarios for targeted TB testing: baseline (continuation of pre-guidance testing levels), US Preventive Services Task Force (USPSTF) recommendations, and California TB Risk Assessment Tool (California Tool) recommendations. Error bars indicate 95% simulation intervals. Data sources: California Department of Public Health9 and USPSTF.12
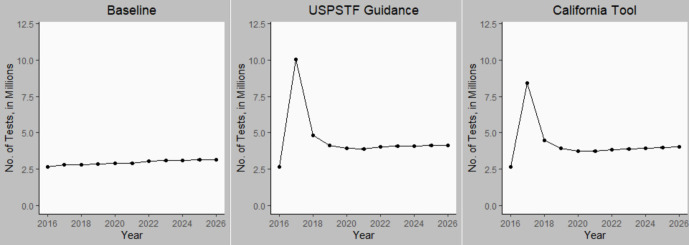
Number of tests performed
The cumulative number of additional LTBI tests performed during 2016-2026 was 16.53 million (95% SI, 16.20 million-16.86 million) for USPSTF and 13.25 million (95% SI, 12.77 million-13.71 million) for California tool recommendations (Figure 3). This change represents a 55% (USPSTF) and 44% (California tool) increase in testing. The incremental number needed to test for LTBI using interferon gamma release assay to prevent 1 case of TB disease (compared with the baseline scenario) was similar under the USPSTF (n = 2352; 95% SI, 2041-2639) and California tool (n = 1980; 95% SI, 1718-2316) guidance. The LTBI testing and treatment scenario targeting only non–US-born persons for testing led to an additional 8.90 million tests (95% SI, 8.54 million-9.24 million) and a number needed to test of 1366 (95% SI, 1167-1589) during the 10-year period. Targeting only HRCS residents resulted in 7.86 million additional tests and a point estimate of 9764 for the number needed to test; however, the SI for this number needed to test was not defined because the lower bounds of the 95% SI for cases prevented were negative.
Figure 3.
Annual number of tuberculosis (TB) tests performed in California from 2016 through 2026 under 3 scenarios for targeted TB testing: baseline (continuation of preguidance testing levels), US Preventive Services Task Force (USPSTF) recommendations, and California TB Risk Assessment Tool (California Tool) recommendations. Error bars indicate 95% simulation intervals. Data sources: California Department of Public Health9 and USPSTF.12
LTBI prevalence
Under implementation of USPSTF guidance, the estimated number of prevalent cases of untreated LTBI dropped from 1.60 million (95% SI, 1.58 million-1.63 million) in 2017 to 0.99 million (95% SI, 0.97 million-1.02 million) in 2026. Similarly, implementation of California tool guidance reduced the number of prevalent untreated LTBI cases (including persons who discontinued treatment before completion) to 1.03 million (95% SI, 1.01 million-1.05 million) in 2026. The 2026 projections under the 2 LTBI testing and treatment scenarios represent a >45% decrease in the prevalence of untreated LTBI compared with the baseline projections for 2026.
Transmissions
The number of new transmissions of TB infection under the USPSTF and California tool scenarios was 41 020 (95% SI, 35 544-46 139) and 41 958 (95% SI, 37 283-47 329), respectively, which is a 26% and 25% reduction from the baseline scenario, respectively.
Sensitivity Analysis
We projected the number of cases averted and the number of additional tests performed compared with baseline during 2017 through 2026 with varying adherence to both guideline scenarios. Adherence to recommendations by primary care providers 25%, 50%, and 75% of the time resulted in fewer cases averted compared with perfect implementation simulated as 100% adherence (n = 4987 [95% SI, 4590-5333], n = 6148 [95% SI, 5842-6307], and 6364 [95% SI, 6153-6465] cases averted, respectively). Lower adherence to the guidelines also resulted in fewer tests performed (Table 2). However, >70% of the cases averted under 100% adherence were projected to be prevented even at as low as 25% adherence.
Table 2.
Sensitivity analysis describing the effect of adherence to latent tuberculosis (TB) targeted testing and treatment recommendations on TB epidemiology and testing patterns in California, 2017-2026a
| Adherence | US Preventive Services Task Forceb | California Tuberculosis Risk Assessment Toolb | ||
|---|---|---|---|---|
| No. of Cases Averted (95% SI)c | No. of Tests (95% SI)c | No. of Cases Averted (95% SI)c | No. of Tests (95% SI)c | |
| 25% | 4987 (4590-5333) | 12.48 (12.38-12.58) | 5026 (3970-5751) | 8.49 (8.36-8.52) |
| 50% | 6148 (5842-6307) | 14.66 (14.64-14.67) | 5799 (5376-6309) | 10.35 (10.09-10.63) |
| 75% | 6364 (6153-6465) | 15.88 (15.69-16.14) | 6487 (6199-6762) | 11.95 (11.83-11.98) |
Abbreviation: SI, simulation interval.
aResults in the table reflect model projections of the number of cases of TB averted and the additional number of tests (in millions) required to conduct guideline-adherent targeted testing and treatment for latent TB infection using either the US Preventive Services Task Force (USPSTF)12 or California Tuberculosis Risk Assessment Tool9 recommendations from 2017-2026 and compared with a baseline scenario. Each row represents a different level of adherence to recommendations by primary care providers; 95% SIs are included. Under the baseline scenario, a cumulative total of 18 123 cases of TB disease and 29.8 million TB tests were predicted.
bThese are in comparison with results of perfect (100%) adherence to recommendations (USPSTF: 7063 [95% SI, 6139-7574] cases of TB averted and 16.53 million [95% SI, 16.20-16.53 million] tests conducted; California Risk Assessment tool: 6726 [95% SI, 5741-708] cases of TB averted and 13.25 million [95% SI, 12.77-13.71 million] tests conducted).
cSimulation intervals describe uncertainty in point estimates due to the stochastic nature of the model. They are derived by taking the 97.5th and 2.5th percentile of each outcome across all model simulations.
Discussion
The results of this model suggest a substantial potential effect of implementation of LTBI testing and treatment on TB epidemiology in California. Hypothetical testing and treatment with 100% adherence to testing by either USPSTF or California tool testing recommendations would be expected to cause a 40% decline in the number of cases of TB in the first decade after implementation and an approximately 45% decline in untreated LTBI prevalence in California. Approximately 50% of the decline in TB incidence is projected to occur within the first 2 years of implementation. In addition, even testing of only 25% of eligible patients during primary care provider encounters is still likely to prevent a substantial proportion of TB cases compared with full implementation (approximately 30% decline from baseline). Because long-term trends in immigration are difficult to predict, and future developments may render current tests and LTBI treatment regimens obsolete, we chose to model a relatively short timeline after implementation. Even so, most of the decrease in TB incidence would occur in the first 2 years after implementation and then stabilize at approximately 55% of the projected baseline scenario incidence. Thus, barring major changes to incidence or LTBI testing and treatment technology, we project that full implementation of either LTBI testing and treatment recommendation would prevent approximately 40% to 45% of TB cases from the second decade after implementation.
Although both recommendations target some US-born persons with special risk factors, almost all (96% and 98%, respectively, for USPSTF and California tool implementation) of the prevented cases are expected to occur among non–US-born persons. Non–US-born persons have the largest burden of both LTBI and TB incidence in California30 and are the largest group targeted under both USPSTF and California tool recommendations. Our results also demonstrate a decline in the number of TB cases among immunosuppressed persons and HRCS residents. Although these declines do not reflect a high number of cases averted or a proportional reduction in population-level TB incidence or LTBI prevalence, implementation of these recommendations may reduce the incidence rate substantially within these groups.
Strengths and Limitations
This analysis had several strengths. First, we structured our model to illuminate TB epidemiology in populations at high risk for TB through an individual approach that integrates population dynamics and demography with health indicators and outcomes. Second, our approach included parameters that reflect adherence to LTBI testing and treatment recommendations as a way to describe the potential effect of variable implementation. Our simulation was limited to California, which has a very large non–US-born population (27% in 2016)31 and already tests persons incarcerated in state and local correctional facilities and long-term care facility residents. Although our results may not be generalizable to other contexts in which demographic characteristics differ, our methods could be replicated by using local data and a baseline that reflects local practices. Third, our model reflects the effect of engaging primary care providers to expand the reach and coverage of LTBI testing and treatment interventions. An estimated 80% of LTBI are conducted in public health clinics in the United States.32 Public health clinics often lack resources to scale up LTBI testing and treatment to levels required to reach TB elimination goals.33 Policy shifts such as the Patient Protection and Affordable Care Act have increased the number of persons with access to preventive care services. We assessed this development, which provides an opportunity to massively scale up LTBI testing and treatment in community clinics.34
Both sets of recommendations provide guidance on core elements of an LTBI testing and treatment implementation strategy, specifically a focus on non–US-born persons and primary care settings, but there are differences. In addition to geographic reach (USPSTF guidance applies to the entire United States), the HRCSs of interest also differ. Although it is possible that the national standards will supersede the guidance issued by CDPH in primary care provider settings, the question of which standard is more widely adopted makes little practical difference, because the performance of the 2 sets of recommendations is similar. A more important consideration is how to optimize adherence to either guidance. Future research should focus on understanding gaps in the current cascade of care for LTBI testing of groups at high risk of TB and using results to develop and test interventions that enhance implementation of these recommendations across the continuum of screening, diagnosis, and treatment of LTBI. Examples of such strategies include increasing access to primary care for non–US-born persons or strengthening the ability of community providers to deliver LTBI care.
Fourth, as with all simulation models, our model is a highly simplified attempt to re-create a complex natural process. We made certain assumptions about reactivation risk by age, multiplicity of risk factors, and population dynamics because of limited data or to simplify the model. These assumptions may not be realistic given the known uncertainty in estimates of these related parameters. We also used fixed estimates for certain parameters, although these estimates are uncertain. Future research could focus on conducting sensitivity analyses by varying assumptions for some of the parameters with the lowest quality evidence to inform estimates. Potential parameters of interest include probabilities of transitioning into and out of HRCS residence, LTBI and TB disease prevalence of new entrants to California, and probability of migration out of California for US-born and non–US-born residents. Another possible area for sensitivity analysis is the assumption of multiplicativity for risk factors for progression to TB disease.
Finally, there is a lack of data on the epidemiologic parameters of TB in HRCSs. Therefore, we resorted to using estimates determined by expert opinion and refined by manual calibration. Although baseline case counts and infections prevented in HRCS populations and users of TNF-α inhibitors were modeled, group-specific numbers varied widely across simulations because of the small numbers of projected cases and stochastic nature of the model. We did not use empirical evidence on the uptake of evidence-based guidelines on LTBI testing and treatment among patients with immunosuppressive comorbidities and relied on expert opinion to inform those input parameters. Use of empirical estimates may have improved the accuracy of forecasts of testing in the baseline scenario. In addition, all models included testing of HIV-positive persons only at the time of infection, whereas other guidance recommends annual testing of HIV-positive persons “who are at high risk for repeated or ongoing exposure to persons with active TB.”35 However, we do not expect these uncertainties to have a substantial effect on our estimates of the overall number of tests performed and TB cases averted, because only 5% of TB cases in 2017 occurred among persons experiencing homelessness within the past year, and 5% of cases occurred among persons with HIV infection, organ transplants, or TNF-α inhibitor use combined.31 The small numbers of persons in some groups at high risk of TB limited the utility of a stochastic scale model for forecasting cases in risk groups because of wide SIs.
Despite this dramatic projected progress, adherence to either set of LTBI testing and treatment recommendations is unlikely to result in achievement of the World Health Organization or US TB elimination goals.36,37 Progress in the near term, however, is within reach; by increasing testing and treatment by just 4- to 6-fold, the number of TB cases can be reduced substantially, saving many lives and reducing costs resulting from TB disease.
Conclusion
Adherence to the USPSTF and California tool recommendations is likely to substantially reduce TB disease incidence and untreated LTBI prevalence in California, primarily because of testing and treatment of persons born outside the United States. We estimate that approximately 40% of new cases are preventable with full adherence to testing guidance, with steep declines in the first few years. Future studies may wish to focus on the cost-effectiveness of LTBI testing and treatment in groups targeted under the USPSTF and California tool recommendations and, in particular, on the incremental cost-effectiveness of testing persons in HRCSs or persons with immunosuppression in addition to non–US-born persons.15
Footnotes
Authors’ Note: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or the authors’ affiliated institutions. References in this article to any commercial products, process, service, manufacturer, or company do not constitute their endorsement or recommendation by the US government or CDC.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the CDC, National Center for HIV, Viral Hepatitis, STD, and TB Prevention Epidemiologic and Economic Modeling Agreement #5U38PS004649.
ORCID iDs
Andrea Parriott https://orcid.org/0000-0002-9457-1319
Priya B. Shete https://orcid.org/0000-0002-0279-8170
References
- 1. World Health Organization Global Tuberculosis Programme Global Tuberculosis Report 2018. World Health Organization; 2018. [Google Scholar]
- 2. Lönnroth K., Mor Z., Erkens C. et al. Tuberculosis in migrants in low-incidence countries: epidemiology and intervention entry points. Int J Tuberc Lung Dis. 2017;21(6):624-637. 10.5588/ijtld.16.0845 [DOI] [PubMed] [Google Scholar]
- 3. Dye C., Glaziou P., Floyd K., Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013;34:271-286. 10.1146/annurev-publhealth-031912-114431 [DOI] [PubMed] [Google Scholar]
- 4. Houben RM., Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. 10.1371/journal.pmed.1002152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miramontes R., Hill AN., Yelk Woodruff RS. et al. Tuberculosis infection in the United States: prevalence estimates from the National Health and Nutrition Examination Survey, 2011-2012. PLoS One. 2015;10(11):e0140881. 10.1371/journal.pone.0140881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmit KM., Wansaula Z., Pratt R., Price SF., Langer AJ. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(11):289-294. 10.15585/mmwr.mm6611a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuen CM., Kammerer JS., Marks K., Navin TR., France AM. Recent transmission of tuberculosis—United States, 2011-2014. PLoS One. 2016;11(4):e0153728. 10.1371/journal.pone.0153728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. United States Agency for International Development (USAID) United States Government Global Tuberculosis Strategy 2015-2019 . USAID; 2015. [Google Scholar]
- 9. California Department of Public Health California Adult Tuberculosis Risk Asessment and User Guide. California Department of Public Health; 2017. Accessed November 20, 2019 https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/TB-Risk-Assessment.aspx
- 10. California Department of Public Health Report on Tuberculosis in California, 2017 . California Department of Public Health; 2018. [Google Scholar]
- 11. Kahwati LC., Feltner C., Halpern M. et al. Primary care screening and treatment for latent tuberculosis infection in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316(9):970-983. 10.1001/jama.2016.10357 [DOI] [PubMed] [Google Scholar]
- 12. US Preventive Services Task Force. Bibbins-Domingo K, Grossman DC. et al. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316(9):962-969. 10.1001/jama.2016.11046 [DOI] [PubMed] [Google Scholar]
- 13. Menzies NA., Cohen T., Hill AN. et al. Prospects for tuberculosis elimination in the United States: results of a transmission dynamic model. Am J Epidemiol. 2018;187(9):2011-2020. 10.1093/aje/kwy094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shrestha S., Hill AN., Marks SM., Dowdy DW. Comparing drivers and dynamics of tuberculosis in California, Florida, New York, and Texas. Am J Respir Crit Care Med. 2017;196(8):1050-1059. 10.1164/rccm.201702-0377OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goodell AJ., Shete PB., Vreman R. et al. Outlook for tuberculosis elimination in California: an individual-based stochastic model. PLoS One. 2019;14(4):e0214532. 10.1371/journal.pone.0214532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsang CA., Langer AJ., Navin TR., Armstrong LR. Tuberculosis among foreign-born persons diagnosed ≥10 years after arrival in the United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2017;66(11):295-298. 10.15585/mmwr.mm6611a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Rossum G., Drake FL Jr. Python Reference Manual . Centrum voor Wiskunde en Informatica; 1995. [Google Scholar]
- 18. US Census Bureau American Community Survey (ACS): PUMS data. 2018. Accessed March 25, 2018 https://www.census.gov/programs-surveys/acs/data/pums.html
- 19. Solari CD., Morris S., Shivji A., de Souza T. 2015 Annual Homeless Assessment Report (AHAR) to Congress . US Department of Housing and Urban Development; 2016. [Google Scholar]
- 20. National Archive of Criminal Justice Data National Corrections Reporting Program resource guide. Accessed March 25, 2018 https://www.icpsr.umich.edu/icpsrweb/content/NACJD/guides/ncrp.html
- 21. California Department of Corrections and Rehabilitation, Office of Research . 2011 Adult Institutions Outcome Evaluation Report . California Department of Corrections and Rehabilitation; 2016. [Google Scholar]
- 22. California Office of Statewide Health Planning and Development Long-term care facility utilization. Accessed March 25, 2018 https://oshpd.ca.gov/data-and-reports/healthcare-utilization/long-term-care-utilization
- 23. Centers for Disease Control and Prevention, National Center for Health Statistics National Health and Nutrition Examination Survey 2011-2012. Accessed March 25, 2018 https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2011
- 24. Centers for Disease Control and Prevention, National Center for Health Statistics National Ambulatory Medical Care Survey: 2015 state and national summary tables. Accessed March 25, 2018 https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2015_namcs_web_tables.pdf
- 25. Centers for Disease Control and Prevention, National Center for Health Statistics National Health Interview Survey. 2015. Accessed March 25, 2018 https://www.cdc.gov/nchs/nhis/index.htm
- 26. Mazurek GH., Jereb J., Vernon A. et al. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25. [PubMed] [Google Scholar]
- 27. Tasillo A., Salomon JA., Trikalinos TA., Horsburgh CR., Marks SM., Linas BP. Cost-effectiveness of testing and treatment for latent tuberculosis infection in residents born outside the United States with and without medical comorbidities in a simulation model. JAMA Intern Med. 2017;177(12):1755-1764. 10.1001/jamainternmed.2017.3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sterling TR., Villarino ME., Borisov AS. et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155-2166. 10.1056/NEJMoa1104875 [DOI] [PubMed] [Google Scholar]
- 29.Patient Care Policies and Procedures Barclays California Code of Regulations Title 22 §72523(c)(2)(C) (2020).
- 30. California Department of Public Health TB performance trends for national and California objectives. 2018. Accessed March 25, 2018 https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/TBCB-Performance-Trends-for-US-CA-Objectives.pdf
- 31. California Department of Public Health Report on tuberculosis in California, 2015. 2016. Accessed March 25, 2018 https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/TBCB_Report_2015.pdf
- 32. Marks SM., Hill AN., Yelk Woodruff R., Asay G., Owusu-Edusei K. Estimations of testing for latent TB infection in the United States in 2013. Poster presented at: National TB Controllers Association Annual Conference; May 21-24, 2018; Palm Springs, CA. [Google Scholar]
- 33. Ehman M., Flood J., Barry PM. Tuberculosis treatment managed by providers outside the public health department: lessons for the Affordable Care Act. PLoS One. 2014;9(10):e110645. 10.1371/journal.pone.0110645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Balaban V., Marks SM., Etkind SC. et al. Tuberculosis elimination efforts in the United States in the era of insurance expansion and the Affordable Care Act. Public Health Rep. 2015;130(4):349-354. 10.1177/003335491513000413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Panel on Opportunistic Infections in HIV-infected Adults and Adolescents Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health and the HIV Medicine Association of the Infectious Diseases Society of America. Accessed March 25, 2018 https://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
- 36. World Health Organization The End TB Strategy: Global Strategy and Targets for Tuberculosis Prevention, Care and Control After 2015. World Health Organization; 2014. Accessed March 25, 2018 https://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1
- 37. Centers for Disease Control and Prevention Strategic planning for tuberculosis (TB) elimination in the United States and prevention and control of TB globally. Accessed February 8, 2020 https://www.cdc.gov/tb/about/strategicplan.pdf