Key Points
Question
Does an association exist between genetic factors and the development of persistent chemotherapy-induced alopecia?
Findings
This agnostic genome-wide association study of 215 women with breast cancer treated with docetaxel-based therapies found genetic variants in the ABCB1 gene that were significantly associated with the risk to develop persistent chemotherapy-induced alopecia. This finding was replicated in an independent cohort.
Meaning
To our knowledge, this is the first report suggesting an association between genetic variants and the risk to develop persistent chemotherapy-induced alopecia.
Abstract
Importance
Persistent chemotherapy-induced alopecia (pCIA) has been recently described in patients with breast cancer and in its most severe form occurs in up to 10% of these patients. Genetic risk factors associated with pCIA have not been adequately explored.
Objective
To identify genetic variants associated with pCIA.
Design, Setting, and Participants
In this genetic association study, 215 women with breast cancer treated with docetaxel-based chemotherapy with a follow-up of 1.5 to 10 years after the end of the treatment were recruited retrospectively through 3 hospital oncology units across Spain between 2005 and 2018. Severe pCIA was defined as lack of scalp hair recovery (Common Terminology Criteria for Adverse Events, version 3.0, grade 2) 18 months or more after the end of treatment. Patients with grade 2 pCIA were selected as cases, and those with no sign of residual alopecia 12 months after the end of docetaxel treatment were selected as controls. A genome-wide association study in a discovery phase was conducted, and logistic regression was used to identify variants associated with the risk to develop this adverse effect. The validity of the association was addressed through a replication phase.
Exposures
Docetaxel-based chemotherapy.
Main Outcomes and Measures
Genotypes of single-nucleotide variants associated with pCIA.
Results
In total, 215 women with breast cancer (median age, 51.6 years; interquartile range, 44-60 years) were recruited (173 patients for the discovery phase and 42 patients for the replication phase). In the discovery phase, ABCB1 genetic variants were associated with risk to develop pCIA. In particular, single-nucleotide variation rs1202179, a regulatory variant located in an enhancer element that interacts with the ABCB1 promoter, was associated with the occurrence of pCIA. This finding was validated in the replication cohort (combined odds ratio, 4.05; 95% CI, 2.46-6.67; P = 3.946 × 10−8). This variant is associated with ABCB1 mRNA expression, and the risk allele was associated with decreased ABCB1 expression levels (P = 1.64 × 10−20).
Conclusions and Relevance
This is the first study, to our knowledge, that identifies an association between a regulatory variant in the ABCB1 gene and the occurrence of pCIA in patients with breast cancer who were treated with docetaxel-based therapies. This finding suggests an important insight into the biological mechanisms underlying pCIA and opens the opportunity to explore personalized treatment of these patients.
This genetic association study explores whether an association exists between genetic factors and the development of persistent chemotherapy-induced alopecia in women with breast cancer who are treated with docetaxel.
Introduction
Alopecia is a common toxic effect of chemotherapy, especially among patients with breast cancer. This chemotherapy-induced alopecia (CIA) has been described as one of the main causes of distress of chemotherapy and has severe psychological consequences in cancer survivors.1 Although CIA is a transient adverse effect in the great majority of patients, treatment with taxanes, particularly docetaxel, has been recently associated with an 8 times higher risk of developing persistent CIA (pCIA).2,3,4 The lack of hair recovery seems to be definitive because it persists for at least several years after chemotherapy, with a strong association with the emotional adjustment and the quality of life of patients who survived breast cancer.2 These cases had been considered unusual, but recent epidemiological studies found that up to 50% of patients with breast cancer present minor forms of persistent alopecia, and 10% of patients with breast cancer present severe forms, with a recovery of less than 10% of the original hair.3,4,5 To date, only a few genetic studies have been conducted assessing CIA6; however, to date, no studies have focused on pCIA. Hence, our aim was to evaluate whether genetic factors were associated with developing pCIA because such genetic factors may be useful as biomarkers.
Methods
Study Population and Alopecia Assessment
Women with breast cancer who received docetaxel-based chemotherapy with a follow-up of 1.5 to 10 years after the end of the treatment were recruited retrospectively through 3 hospital oncology units across Spain between 2005 and 2018. We assessed pCIA using Common Terminology Criteria for Adverse Events, version 3.0, at least 18 months after the end of the treatment.3 Patients with severe toxic effects (pCIA grade 2) were selected as cases, and those with complete recovery of hair 12 months or more after the end of docetaxel chemotherapy were selected as controls (eAppendix 1 in the Supplement). Alopecia was assessed by a specialist, and only patients with grade 2 pCIA were included to minimize subjective evaluation. In total, 173 patients with breast cancer were included in the discovery cohort (122 controls and 51 cases), and 42 patients with breast cancer (22 controls and 20 cases) were included in the replication phase (eFigure 1 in the Supplement). Patient age, treatment regimens, cumulative docetaxel doses, and hormonal therapy regimens were recorded. This study was approved by the ethics committee of each participating hospital. Written informed consent was obtained from all participants in a manner consistent with the Common Rule requirements before we obtained samples or obtained clinical photographs. No one received compensation or was offered any incentive for participating in this study.
Genome-Wide Association Study and Statistical Analysis
A genome-wide association study was performed for DNA samples from the discovery cohort using Illumina OmniExpress BeadChips containing 713 599 single-nucleotide variations (SNVs; formerly SNPs). Logistic regression and statistical analyses were performed using PLINK, version 1.9 (Christopher Chang/Grail Inc), SPSS, version 19.0 (SPSS Inc), and R, version 3.3 (The R Foundation) software. Data were imputed using Impute2 for fine-mapping of the associated region (eAppendix 2 in the Supplement). Allelic discrimination with TaqMan technology was used for validation of candidate-imputed SNVs and for genotyping in the replication cohort (eAppendix 3 in the Supplement). A threshold of 5 × 10−6 was established in the discovery cohort to determine the associated markers for further replication, and a 2-sided P < .05 was considered statistically significant for the replication cohort.
Results
The clinical characteristics of the discovery and replication cohorts are shown in Table 1. In total, 215 patients with breast cancer (median age, 51.6 years; interquartile range, 44-60 years) were recruited (173 patients for the discovery phase and 42 patients for the replication phase). Older age and higher cumulative docetaxel dose were associated with a higher rate of pCIA (Table 1). Punch biopsy specimens from cases showed a marked reduction in large terminal hairs with a reciprocal increase in small, vellus-like hair follicles. No significant perifollicular inflammation or fibrosis was found (eFigure 2 in the Supplement).
Table 1. Summary of Patient Characteristics.
| Characteristic | Discovery (n = 173) | Replication (n = 42) | Combined cohort P value (n = 215) | ||||
|---|---|---|---|---|---|---|---|
| No. (%) | P value | No. (%) | P value | ||||
| Cases (n = 51) | Controls (n = 122) | Cases (n = 20) | Controls (n = 22) | ||||
| Age at time of treatment, median (IQR), y | 56 (44-61) | 51 (43-60) | .003 | 50 (42-54) | 46 (41-54) | .89 | .01 |
| Cumulative dose, median (range), mg/m2 | 434.2 (300-550) | 422.7 (400-600) | .002 | 420.5 (420-435) | 421.7 (420-450) | .11 | .001 |
| Hormonal therapya | |||||||
| AIs | 20 (39.2) | 43 (35.2) | .46 | 10 (50.0) | 5 (22.7) | .11 | .18 |
| Steroidal AIs | 1 (2.0) | 8 (6.6) | .45 | 2 (10.0) | 1 (4.5) | .60 | .75 |
Abbreviations: AIs, aromatase inhibitors; IQR, interquartile range.
Data available for only 186 patients, with 144 in the discovery phase.
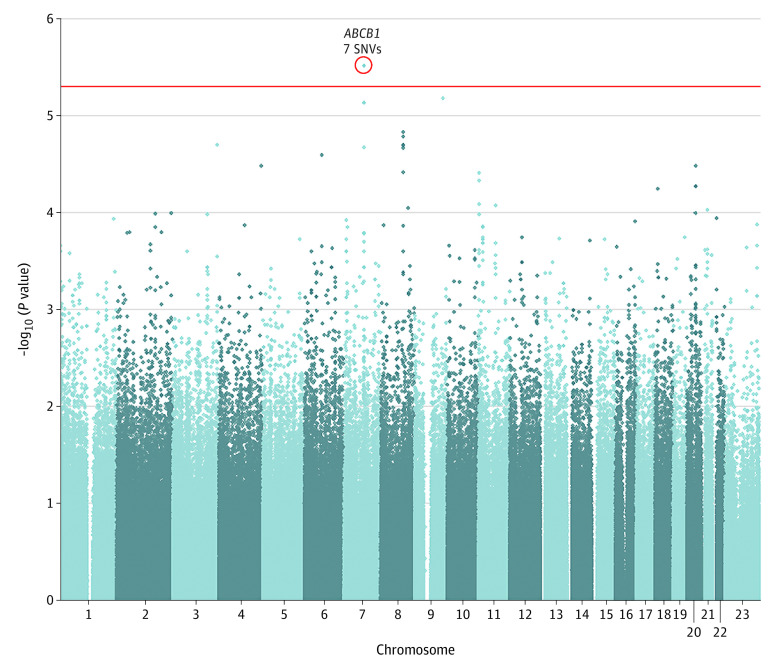
After quality control, 155 of 173 patients with breast cancer (47 cases and 108 controls) and 693 898 SNVs were included in the subsequent analysis (eTable 1 and eFigures 1, 3, and 4 in the Supplement). Single-SNV associations using logistic regression analyses were evaluated, and a cluster of 7 variants in complete linkage disequilibrium located in the ABCB1 gene were identified as the best significant signal associated with pCIA (odds ratio, 3.79; 95% CI, 2.17-6.62; P = 3.05 × 10−6) (Figure; Table 2). Similar results were found when associated clinical factors were included as covariates (odds ratio, 5.28; 95% CI, 2.65-10.54; P = 2.262 × 10−6) (eAppendix 2 in the Supplement).
Figure. Manhattan Plot of the Genome-Wide Association Analysis in the Discovery Cohort, Showing the Association Between the Genotypes of 693 898 Single-Nucleotide Variants (SNVs) and Risk of Persistent Chemotherapy-Induced Alopecia.
The P values derived by logistic regression analyses on a −log10 scale are plotted against their physical chromosomal position. A P value of 5 × 10−6 was established to determine the threshold of genome-wide significance and suggestive associations (red line).
Table 2. ABCB1 SNVs Associated With pCIA in the Discovery Cohort Using Logistic Regression Analysis.
| SNV | CHR | Base pair | A1 | OR (95% CI) | P valuea | R2b | Source |
|---|---|---|---|---|---|---|---|
| rs1202179 | 7 | 87204279 | C | 3.79 (2.17-6.62) | 3.05 × 10−6 | 0.98 | Genotyped |
| rs1989831 | 7 | 87205479 | T | 4.01 (2.24-7.17) | 2.87 × 10−6 | 0.98 | Imputed |
| rs1989830 | 7 | 87205663 | A | 3.79 (2.17-6.62) | 3.05 × 10−6 | 0.99 | Genotyped |
| rs1989829 | 7 | 87205707 | T | 3.79 (2.17-6.62) | 3.05 × 10−6 | 0.99 | Imputed |
| rs1202177 | 7 | 87207914 | C | 3.79 (2.17-6.62) | 3.05 × 10−6 | 0.99 | Imputed |
| rs1202176 | 7 | 87208987 | G | 3.79 (2.17-6.62) | 3.05 × 10−6 | 0.99 | Imputed |
| rs1202175 | 7 | 87209150 | G | 3.79 (2.17-6.62) | 3.05 × 10−6 | 0.99 | Genotyped |
| rs1202174 | 7 | 87209372 | T | 3.79 (2.17-6.62) | 3.05 × 10−6 | 0.99 | Imputed |
| rs1202173 | 7 | 87210259 | A | 3.79 (2.17-6.62) | 3.05 × 10−6 | 1 | Imputed |
| rs1202172 | 7 | 87210974 | C | 3.79 (2.17-6.62) | 3.05 × 10−6 | 1 | Imputed |
| rs1202171 | 7 | 87211045 | T | 3.79 (2.17-6.62) | 3.05 × 10−6 | 1 | Genotyped |
| rs1202186 | 7 | 87213258 | C | 3.79 (2.17-6.62) | 3.05 × 10−6 | 1 | Genotyped |
| rs1202185 | 7 | 87213384 | C | 3.79 (2.17-6.62) | 3.05 × 10−6 | 1 | Genotyped |
| rs1202182 | 7 | 87215304 | G | 3.79 (2.17-6.62) | 3.05 × 10−6 | 1 | Genotyped |
| rs1202181 | 7 | 87216150 | G | 3.79 (2.17-6.62) | 3.05 × 10−6 | 1 | Genotyped |
| rs1202180 | 7 | 87203840 | C | 3.57 (2.06-6.19) | 5.49 × 10−6 | 0.97 | Imputed |
| rs1209054 | 7 | 87208225 | A | 3.91 (2.23-6.84) | 1.86 × 10−6 | 0.96 | Imputed |
| rs1211151 | 7 | 87208255 | T | 3.79 (2.17-6.62) | 3.05 × 10−6 | 0.98 | Imputed |
| rs28381820 | 7 | 87216216 | AAG | 3.79 (2.17-6.62) | 3.05 × 10−6 | 0.94 | Imputed |
Abbreviations: A1, allele 1, effect allele; CHR, chromosome; OR, per-allele odds ratio associated with effect allele; pCIA, persistent chemotherapy-induced alopecia; SNV, single-nucleotide variation.
Obtained in logistic regression analysis.
Pairwise correlation between the SNV (R2) and rs1202186.
These ABCB1 SNVs were located in a linkage disequilibrium block, and after data imputation, 12 additional SNVs correlated with the genotyped variants (R2 > 0.97) were found to be associated with pCIA risk with similar P values (eFigure 5 in the Supplement; Table 2). Adjustment for 1 of the markers, rs1202186, confirmed a single association signal. Next, we combined numerous sources of in silico annotation, and rs1202179 was selected as a potential functional candidate owing to its location in an enhancer element interacting with the ABCB1 promoter and the decreased ABCB1 expression showed in the presence of the rs1202179 risk allele C (P = 1.64 × 10−20) (eAppendix 2 in the Supplement, eFigure 6 in the Supplement). The imputed rs1202179 genotype was analyzed using an allelic discrimination assay to confirm the genotypes in the discovery cohort (eAppendix 2 in the Supplement). We then analyzed the marker in our validation cohort, replicating our association with pCIA risk (validation odds ratio, 4.66; 95% CI, 1.53-14.14; P = .002; combined odds ratio, 4.05; 95% CI, 2.46-6.67; P = 3.946 × 10−8) (eAppendix 3 in the Supplement). We explored whether significant signals in the well-known ABCB1 variants7 could be found, but none of them reached our threshold of significance (eTable 2 in the Supplement).
Discussion
This genetic association study found an association between the intronic variant rs1202179 of the ABCB1 gene and risk for pCIA, and this finding was replicated in an independent cohort of patients. The ABCB1 gene encodes P-glycoprotein, an efflux pump responsible for transporting drugs (including docetaxel) out of cells. Owing to its main role in drug pharmacokinetics, P-glycoprotein has been described as a therapeutic target by enhancing or inhibiting its function;8 in addition, ABCB1 genetic variants have been associated with several toxic effects.9 Enhancing ABCB1 expression has been described as one of the main cellular defense mechanisms.10 The ABCB1 gene is expressed in the human hair follicle and, specifically, in hair follicle stem cells.11 The irreversible damage to hair follicle stem cells caused by chemotherapeutics has been proposed to be the cause of pCIA.12 The variant rs1202179 is located in an enhancer region interacting with the ABCB1 promotor, and the risk allele C is associated with decreased ABCB1 expression. This decrease may produce decreased P-glycoprotein levels in carriers of the risk allele, causing decreased docetaxel elimination and thus its intracellular accumulation. After exposure to docetaxel, the highly proliferative matrix cells of the hair follicle are very susceptible to damage, but bulge stem cells are typically left intact, enabling hair regrowth after docetaxel is removed. However, in carriers of the risk allele who experience a high drug exposure, alopecia may become permanent owing to the destruction of hair follicle stem cells, preventing the production of new hair follicles. This hypothesis would explain the different predisposition to pCIA among docetaxel-treated patients.3 Nevertheless, functional studies demonstrating the association of the risk allele with ABCB1 expression and hair follicle stem cell death are essential.
Scalp cooling has been associated with decreased rates of acute, reversible CIA and pCIA,3 and scalp cooling is widely used nowadays. However, the effectiveness of this treatment in preventing acute CIA depends largely on the chemotherapy regimen.3,13,14 With docetaxel regimens, the success rate of scalp cooling was only 64% to 65% in 2 recent randomized clinical trials.13,14 These data are relevant because docetaxel-containing regimens are among the most frequently used adjuvant regimens for patients with breast cancer.15
The genetics variants identified in this study could be associated with the failure of scalp-cooling therapy in preventing acute docetaxel-induced alopecia, but this hypothesis needs to be established in further studies. If our hypothesis is correct, our findings could help stratify patients for more intensive preservation maneuvers. Moreover, our results point to the ABCB1 transporter as a potential therapeutic target in the development of new antialopecia therapies.16
Limitations
Although the identification of a genetic risk factor associated with pCIA is an important finding of this study, the limitations of the study should be addressed. These limitations include the retrospective case-control design and the relatively small sample size.
Conclusions
This is the first study, to our knowledge, that identifies a genetic risk factor, a genetic variant located in the ABCB1 gene, associated with severe pCIA in patients with breast cancer. This association was replicated in an independent cohort, corroborating our finding. The identification of the involvement of ABCB1 provides insight into the biological mechanisms that may underlie pCIA and provides an opportunity to explore a personalized management of therapy for these patients.
eAppendix 1. Study Population
eFigure 1. Flowchart of the Patients Included in the Study
eFigure 2. A-B. Histological Images of Punch Biopsies Obtained From Persistent Chemotherapy-Induced Alopecia (pCIA) Grade 2 Patients. C-D. Clinical Photos of Grade 2 pCIA Patients Scalp.
eAppendix 2. Genome-Wide Association Study
eFigure 3. Principal Component Analysis of Discovery Cohort
eTable 1. Summary of Main Characteristics of Patients Included in the GWAS Analysis After Quality Control Analysis
eFigure 4. Quantile-Quantile Plot of the Discovery Cohort
eFigure 5. Association and Recombination Plot of the Risk Locus and ± 50-kb Boundaries
eFigure 6. Functional Annotation of the 7q21.1 Risk Locus Using Data From ENCODE
eTable 2. Well-Known ABCB1 Haplotype Frequencies and Their Association With pCIA
eAppendix 3. Replication Analysis
eReferences
References
- 1.Trusson D, Pilnick A. The role of hair loss in cancer identity: perceptions of chemotherapy-induced alopecia among women treated for early-stage breast cancer or ductal carcinoma in situ. Cancer Nurs. 2017;40(2):E9-E16. doi: 10.1097/NCC.0000000000000373 [DOI] [PubMed] [Google Scholar]
- 2.Freites-Martinez A, Chan D, Sibaud V, et al. Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol. 2019;155(6):724-728. doi: 10.1001/jamadermatol.2018.5071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martín M, de la Torre-Montero JC, López-Tarruella S, et al. Persistent major alopecia following adjuvant docetaxel for breast cancer: incidence, characteristics, and prevention with scalp cooling. Breast Cancer Res Treat. 2018;171(3):627-634. doi: 10.1007/s10549-018-4855-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang D, Kim I-R, Choi E-K, et al. Permanent chemotherapy-induced alopecia in patients with breast cancer: a 3-year prospective cohort study. Oncologist. 2019;24(3):414-420. doi: 10.1634/theoncologist.2018-0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim GM, Kim S, Park HS, et al. Chemotherapy-induced irreversible alopecia in early breast cancer patients. Breast Cancer Res Treat. 2017;163(3):527-533. doi: 10.1007/s10549-017-4204-x [DOI] [PubMed] [Google Scholar]
- 6.Chung S, Low S-K, Zembutsu H, et al. A genome-wide association study of chemotherapy-induced alopecia in breast cancer patients. Breast Cancer Res. 2013;15(5):R81. doi: 10.1186/bcr3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolking S, Schaeffeler E, Lerche H, Schwab M, Nies AT. Impact of genetic polymorphisms of ABCB1 (MDR1, P-glycoprotein) on drug disposition and potential clinical implications: update of the literature. Clin Pharmacokinet. 2015;54(7):709-735. doi: 10.1007/s40262-015-0267-1 [DOI] [PubMed] [Google Scholar]
- 8.Silva R, Vilas-Boas V, Carmo H, et al. Modulation of P-glycoprotein efflux pump: induction and activation as a therapeutic strategy. Pharmacol Ther. 2015;149:1-123. doi: 10.1016/j.pharmthera.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 9.PharmGKB. ABCB1. Accessed June 26, 2020. https://www.pharmgkb.org/gene/PA267/
- 10.Callaghan R, Crowley E, Potter S, Kerr ID. P-glycoprotein: so many ways to turn it on. J Clin Pharmacol. 2008;48(3):365-378. doi: 10.1177/0091270007311568 [DOI] [PubMed] [Google Scholar]
- 11.Haslam IS, El-Chami C, Faruqi H, Shahmalak A, O’Neill CA, Paus R. Differential expression and functionality of ATP-binding cassette transporters in the human hair follicle. Br J Dermatol. 2015;172(6):1562-1572. doi: 10.1111/bjd.13549 [DOI] [PubMed] [Google Scholar]
- 12.Paus R, Haslam IS, Sharov AA, Botchkarev VA. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14(2):e50-e59. doi: 10.1016/S1470-2045(12)70553-3 [DOI] [PubMed] [Google Scholar]
- 13.Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. 2017;317(6):596-605. doi: 10.1001/jama.2016.20939 [DOI] [PubMed] [Google Scholar]
- 14.Rugo HS, Klein P, Melin SA, et al. Association between use of a scalp cooling device and alopecia after chemotherapy for breast cancer. JAMA. 2017;317(6):606-614. doi: 10.1001/jama.2016.21038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparano JA, Gray RJ, Makower DF, et al. Clinical outcomes in early breast cancer with a high 21-gene recurrence score of 26 to 100 assigned to adjuvant chemotherapy plus endocrine therapy: a secondary analysis of the TAILORx randomized clinical trial. JAMA Oncol. 2019;10461. doi: 10.1001/jamaoncol.2019.4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haslam IS, Pitre A, Schuetz JD, Paus R. Protection against chemotherapy-induced alopecia: targeting ATP-binding cassette transporters in the hair follicle? Trends Pharmacol Sci. 2013;34(11):599-604. doi: 10.1016/j.tips.2013.09.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Study Population
eFigure 1. Flowchart of the Patients Included in the Study
eFigure 2. A-B. Histological Images of Punch Biopsies Obtained From Persistent Chemotherapy-Induced Alopecia (pCIA) Grade 2 Patients. C-D. Clinical Photos of Grade 2 pCIA Patients Scalp.
eAppendix 2. Genome-Wide Association Study
eFigure 3. Principal Component Analysis of Discovery Cohort
eTable 1. Summary of Main Characteristics of Patients Included in the GWAS Analysis After Quality Control Analysis
eFigure 4. Quantile-Quantile Plot of the Discovery Cohort
eFigure 5. Association and Recombination Plot of the Risk Locus and ± 50-kb Boundaries
eFigure 6. Functional Annotation of the 7q21.1 Risk Locus Using Data From ENCODE
eTable 2. Well-Known ABCB1 Haplotype Frequencies and Their Association With pCIA
eAppendix 3. Replication Analysis
eReferences