Abstract
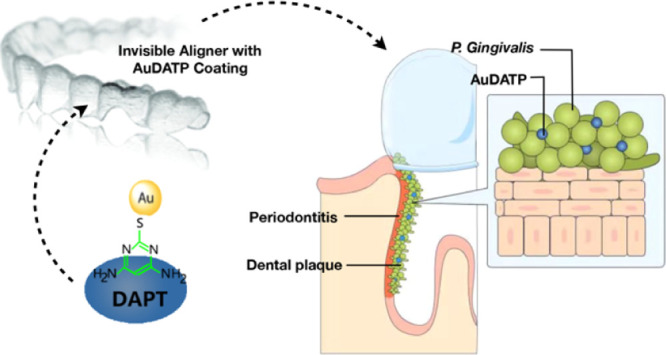
Oral microbiology could directly influence overall health. Porphyromonas gingivalis (P. gingivalis) is a highly pathogenic bacterium that causes periodontitis and other related systematic diseases, including Alzheimer’s disease. Orthodontic devices (e.g., invisalign aligner) is commonly used in populations with periodontitis who are also at a high risk of systematic diseases. In this study, newly explored antibacterial 4,6-diamino-2-pyrimidinethiol-modified gold nanoparticles (AuDAPT) were coated onto aligners. The coated aligners showed favorable antibacterial activity against P. gingivalis. In the presence of the coated aligner, the number of planktonic cells was decreased, and biofilm formation was prevented. This material also showed favorable biocompatibility in vivo and in vitro. This study reveals a new method for treating oral P. gingivalis by coating aligners with AuDAPT, which has typical advantages compared to other treatments for both periodontitis and related systematic diseases.
Introduction
Porphyromonas gingivalis (P. gingivalis) is an asaccharolytic Gram-negative oral pathogenetic anaerobe affecting both oral and general health. P. gingivalis is involved in the pathogenesis of periodontitis, an inflammatory disease that destroys the tissues supporting the tooth, which may eventually lead to tooth loss.1P. gingivalis infection is also associated with a variety of diseases such as atherosclerotic vascular disease, diabetes mellitus, and chronic nephritis.2−5 The recent identification of P. gingivalis in the brain of Alzheimer’s disease patients has received public attention.6−9
However, the clinical treatments for P. gingivalis-related systematic diseases are not satisfying. The major virulence factor produced by P. gingivalis is gingipain,10 and treatment with broad-spectrum antibiotics rarely eradicates P. gingivalis and may lead to resistance.11
P. gingivalis is mainly found in the oral cavity during gingival and periodontal infections; however, transient bacteremia of P. gingivalis can occur during common activities such as brushing, flossing, and chewing, as well as during dental procedures,12 resulting in translocation to a variety of tissues including the coronary arteries,13 placenta,14 liver,15 and brain,9 which inspires us that dental treatment device may play as a drug delivery for general P. gingivalis infection.
Clear aligners, such as Invisalign, is a dental treatment device that has been widely used in the last decade because of several obvious advantages.16,17 Additionally, during aligner treatment, both the teeth and gingiva are covered for nearly the entire day with aligners. Thus, with proper modification, the Invisalign system may be used as a long-term drug delivery system for patients with P. gingivalis infection.
Nanomaterials have been recently reported to show antimicrobial ability, including silver, gold, zinc, or metal oxide nanoparticles (NPs).18,19 However, the biological safety stays a vital problem for most metal nanomaterials, except for AuNPs, which exerts favorable biocompatibility.20−22 Besides, AuNPs could be easily modified as it is size-controllable and chemically stable. 4,6-Diamino-2-pyrimidinethiol (DAPT)-conjugated gold NPs (AuNPs) (AuDAPT) were previously reported to kill multidrug-resistant Gram-negative bacteria efficiently and to induce drug resistance to a much-smaller degree than conventional antibiotics.21 Zheng et al.23 fabricated AuDAPT on various solid surfaces by electrostatic self-assembly, and this coating method shows superiorities over some currently used methods24,25 in terms of expense, time, or morphology popularity.26
In this study, surface-modified Invisalign with a stable antimicrobial coating was developed by electrostatic self-assembly of AuDAPT on its surface. To investigate the anti-P. gingivalis properties of this system, optical antibacterial density measurement, contact assay, and scanning electron microscopy (SEM) were performed. The effect of AuDAPT on biofilm formation was also investigated. Both in vitro and in vivo assays were performed to evaluate the biocompatibility of AuDAPT-based coating, including its influence on human periodontal ligament fibroblast cell (hPDLC) viability and irritation of the rat oral mucosa. This approach may be useful for treating P. gingivalis infection. Compared with the traditional oral administration method, a removable anti-bacterial appliance that requires long-term wear maintains a continuous supply of drugs, avoiding the impairment of treatment efficiency or even the development of drug resistance resulting from poor compliance or pharmacodynamics.
Materials and Methods
Synthesis and Characterization of AuDAPT-Coated Invisalign
AuDAPT-coated Invisalign was prepared as previously reported.21,23 The aligners were first treated with oxygen plasma for 3 min (PDC-MG, Chengdu Mingheng Technology Co., Ltd., Chengdu, China) and immediately immersed in a solution of AuDAPT for 12 h. After soaking, the samples were washed with water and unbound AuDAPTs were removed by washing them with phosphate-buffered saline (PBS) with ultrasound treatment for 1 h. The AuDAPT-coated Invisalign aligners were dried and stored at around 20 °C. The incubation concentration of AuDAPT was 517 ± 12.6 μg/mL. The concentration of AuDAPT coating was 1.109 ± 0.021 μg/cm2. The concentration of AuDAPTs in solution or coated on the aligners was measured with an inductively coupled plasma optical emission spectrometer (ICP–OES, PerkinElmer Optima 5300 V, Waltham, MA, USA).
Bacterial Culture and Cell Growth Assay
P. gingivalis (W83) was obtained from the Center laboratory, Peking University Hospital of Stomatology. The bacterial cells were grown on blood–brain heart infusion plates (2% agar, 10% vitamin K, 5% sheep blood, and 1% heme chloride). A single colony was inoculated in a blood–brain heart infusion medium (10% vitamin K, 1% heme chloride) and grown in an anaerobic bag at 37 °C. After incubation for 48 h, P. gingivalis cells were harvested in the mid-exponential growth phase and diluted to 104, 105, and 108 colony-forming units (CFU)/mL for further bacterial analysis.
The Invisalign aligners were cut into circles (diameter of 6 mm) and sterilized with glutaraldehyde, rinsed with sterilized water three times, and dried in clean air. The circles were placed in centrifuge tubes with 2.5 mL of bacterial suspension at a concentration of 104 or 105 CFU/mL. The cells were grown statically at 37 °C in anaerobic bags. After 20, 24, and 32 h, cell growth was measured by recording the optical density at 600 nm (OD600) using a microplate reader (Bio-Rad 680, Hercules, CA, USA). The cell growth of bacterial suspension at a concentration of 104 CFU/mL was observed until 48 h27. At least three samples were evaluated for each group.
Biofilm Formation and Crystal Violet Staining Assay (CV Staining)
Crystal violet (CV) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Saliva was collected from healthy volunteers, and informed consent was obtained. The study and the associated research protocols were approved by the ethics committees of the Peking University Hospital of Stomatology (PKUSSIRB-201520012). The saliva was filtered with the Sterile Syringe Filter (0.22 μm, SLGP033RB, Millipore) to get the sterile saliva. Sterilized Invisalign fragments and circles were placed in sterilized saliva for 24 h in anaerobic bags. The samples were removed from the bags and placed in centrifuge tubes with 2.5 mL of bacterial suspension at a concentration of 107 CFU/mL. After 48 h of incubation, the biofilm colonized on the samples was gently rinsed three times with 1 mL of PBS and air-dried for several minutes. The biofilm on the fragments was stained with 3 mL of 0.1% (w/v) CV at room temperature for 15 min. After removing the excess stain, the samples were gently washed three times with PBS.27 A digital camera (Canon EOS 60D, Tokyo, Japan) was used to record the stained biofilm. The biomass was quantified by measuring the OD570 using a microtiter plate reader (Bio-Rad 680) after dissolving the biofilms in 5 mL of 95% ethanol. All measurements were carried out at least three times.
Biofilms on aligner circles were scraped off with a knife blade and dissolved in 1 mL of PBS. Next, 100 μL of the bacterial suspension was spirally inoculated on the plate. After 7 days of anaerobic culture, the colony number was determined. At least three samples were evaluated for each group.
Contact Antibacterial Assay
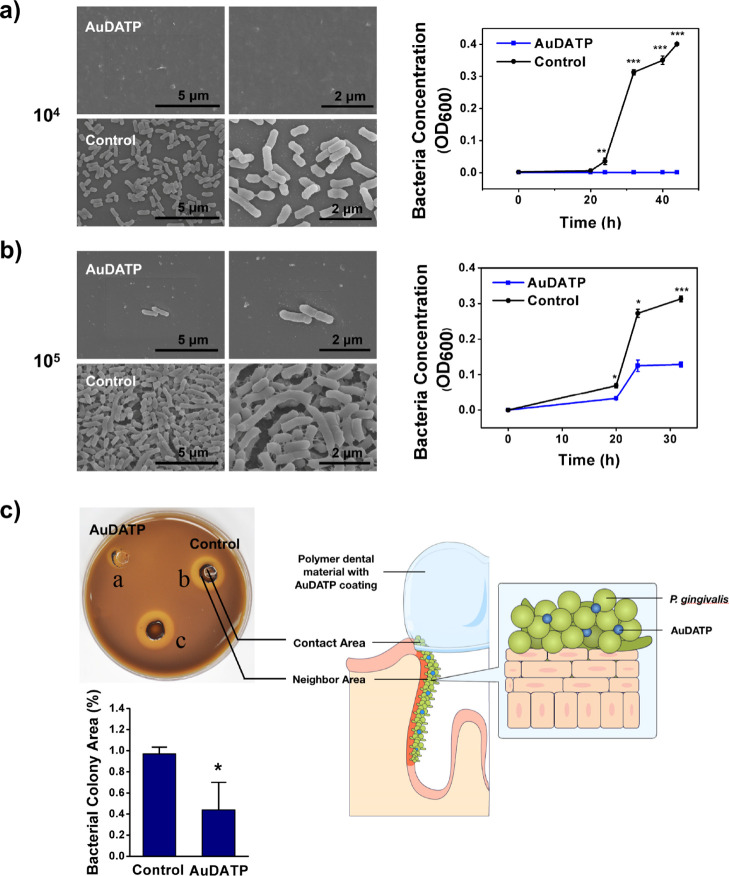
We dropped a drop of 20 μL of bacterial suspension on the agar plate. The circular samples (diameter of 6 mm) were placed on top of the drop for the edge of the drops to overflow. The plates were placed in the anaerobic bag at 37 °C. After 7 days of culture, the bacterial colony area was measured. The ratio of the colony area with the samples to that without the samples was calculated. The bacterial colony without the sample was used as a blank control. The ratio reflects the ability that the sample inhibits the bacterial growth. In order to explain the method more clearly, we marked the bacterium area in Figure 2 with a, b, and c and compared the ratio “a/c” with “b/c”. The ratio of the AuDAPT samples was compared to that of the untreated group. Three samples were evaluated for each group.
Figure 2.
(a,b) SEM images of bacterial cells accumulated on the sample surface when immersed into bacterial suspension and curves of optical density at 600 nm of bacterial suspension with the tested sample [(a) density of bacterial suspension: 104 CFU/mL, (b) density of bacterial suspension: 105 CFU/mL, n = 3 in each group, data in graphs is the average of multiple experiments; magnification: 10.0k× and 20.0k×; voltage: 5.0 kV]. (c) Contact assay: comparison of bacteria growth area ratio of the AuDAPT-coated samples and untreated samples [(a) AuDAPT-coated sample contacting area; (b) untreated sample contacting area; (c) blank control; control: (b/c); AuDAPT: (a/c), n = 3, data in graphs is the average of multiple experiments] (P < 0.05).
Scanning Electron Microscopy
The morphologies of the bacterial cells and biofilm were observed by SEM. The bacterial cells were incubated for 24 h, biofilm was incubated for 48 h, and then, the samples were washed gently three times with PBS and fixed with 2% glutaraldehyde for 2 h at 4 °C. The fixed samples were dehydrated through a gradient ethanol series. The samples were coated with gold and observed by SEM (FEI, Magellan 400, Hillsboro, OR, USA) at a magnification of 10 × 103 and 20 × 103 and an accelerating voltage of 5.0 kV. At least three samples were evaluated for each group.
Confocal Laser Scanning Microscopy
The biofilm samples were rinsed gently three times with PBS and stained with 500 μL of concanavalin A-Alexa Fluor 647 conjugate solution [Invitrogen, Carlsbad, CA, USA, 50 μg/mL, red fluorescence, labeled extracellular polymeric substances (EPS)] for 15 min. EPS production was observed by confocal laser scanning microscopy.6 The excitation/emission for Alexa Fluor 647 was 650/668 nm. Stack images were obtained by scanning the biofilm along the Z-axis at 1.8 μm intervals. For each image stack, EPS biomass is indicated as the area occupied by EPS labeled by concanavalin A-Alexa Fluor 647 (red fluorescence). At least three samples were evaluated for each group.
Cell Culture and Toxicity Assays
The behavior of hPDLCs (provided by Peking University Hospital of Stomatology, approved by the ethics committees of the Peking University Hospital of Stomatology, LA2018008) was evaluated in terms of cell proliferation in the medium after soaking the samples. 6 mm diameter circles of AuDAPT-coated aligner or untreated aligner were soaked in 100 mL of medium in a 12-well plate. Three samples were evaluated for each group. The cells were cultured at a density of 2 × 105 in each well. Cell proliferation was recorded with an automated microscope system (IncuCyte; Essen Bioscience, Ann Arbor, MI, USA) for up to 144 h.
Hemolytic Test
We then do the hemolytic test with the fresh mice blood. This animal study was approved by the Institutional Animal Care and Use Committee, Institute of Process Engineering, Chinese Academy of Sciences (IACUC, IPE–CAS, IRB number: SYXK 2019-0189). We first collect blood from the mice eye socket, dilute the fresh blood with 0.9% sodium chloride solution to 4% mixed suspension, and then treat the 4% blood suspension with different concentrations of AuDAPT (2.5, 5, 10, 20, 40, and 80 μg/mL), using the sodium chloride solution and 0.2% Triton X-100 as negative and positive controls, respectively. Next, we place them in the constant temperature box of 37 °C s for 1 h. Finally, we record the pictures and UV–vis spectrophotometer testing at 540 nm absorbance values after 1200 r/min, centrifugal 15 min. At least three samples were evaluated for each group.
Mucosal Contact Test
The golden hamster (Syrian Golden Hamster, supplied by Dental Medical Devices Testing Center of Peking University School of Stomatology) is used for mucosa irritation tests. This study and the associated research protocols were approved by the ethics committees of the Peking University Hospital of Stomatology (LA2018008). This test was conducted based on the Chinese medical industry standard YY/T 0127.13-2009. Animals were housed in groups in plastic cages. Conditions conformed to standard operational procedures; certified commercial diet was provided daily. The room temperature and room humidity were monitored daily, and animal welfare was ensured. The test and control materials were cut into circles with a diameter of approximately 6 mm and used directly. Next, 20% of ethyl carbamate was intraperitoneally administered to the hamsters at a dosage of 0.6 mL/100 g body weight. The cheek pouches were everted and washed with 0.9% sodium chloride solution after anesthetization. After confirming the absence of abnormalities, the test sample was placed in the right cheek pouch, while the control sample was placed in the left cheek pouch. In each group, three hamsters were tested. The animals were examined clinically every day during the test period (7 days). On day 8 of the test, excess 20% ethyl carbamate was administered for sacrifice by intraperitoneal injection.
Changes in the position of the test article placed in the pouch mucosa were examined after removing the test article. Congestion, swell, erosion, ulceration, or other changes were investigated and recorded. Differences in the pouch between the test side and control side were compared. The graded table for erythema reactions of each pouch surface is given in Table S1. The grades for each animal were added, and the sum was divided by the number of animals to determine the average grade per animal.
Histological observation was also conducted. Tissue samples from representative areas of the pouches were removed and fixed in 10% formalin. The tissue samples were embedded in paraffin, cut into sections, and subjected to hematoxylin and eosin staining. Each tissue sample was graded according to the system shown in Table S2.
The irritation index was obtained by subtracting the control group average from the test group average. The average grade was determined as the irritation index. Combined with the results of macroscopic observation, the final result was obtained.
Statistical Analysis
Student’s t-test was applied to compare data. A P-value of less than 0.5 was considered significant.
Results and Discussion
Characterization of AuDAPT-Coated Invisalign Aligners
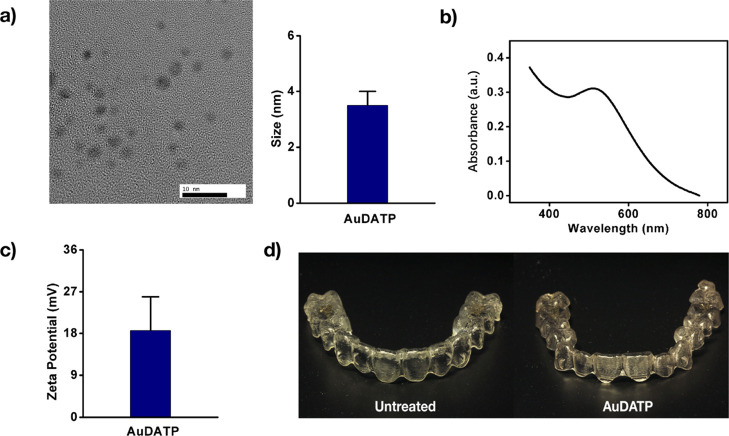
We synthesize the antibiotic AuDAPTs according to our previous work.8 The transmission electron microscopy (TEM) image indicates that the diameter of AuDAPTs is less than 4 nm (Figure 1a). AuDAPTs exhibit a maximum absorbance at 0.3 with a wavelength at 510 nm (Figure 1b). They show a positive charge of 18.6 mV, which is easy for them to be modified by negatively charged agents (Figure 1c).
Figure 1.
Characterizations of AuDAPTs. (a) TEM image and size of AuDAPTs. (b) Absorption spectrum of AuDAPTs is analyzed. (c) Zeta potential of AuDAPTs. (d) Photographs of AuDAPTs-coated aligners.
To obtain the AuDAPT-coated Invisalign aligners, we treat the aligners with oxygen plasma, then incubate them with AuDAPTs, and treat them in ultrasound. These AuDAPT-modified aligners are transparent because the solution of AuDAPTs is nearly colorless (Figure 1d). We use the concentration of the decorated AuDAPTs, which is obtained by ICP–OES, as the main index to characterize the density of the modified particles on the aligners. The concentration of AuDAPT coating was 1.109 ± 0.021 μg/cm2.
Antibacterial Behavior of AuDAPT-Coated Aligners
To investigate the antibacterial activities of AuDAPT-coated substrates, the sample circles (diameter 6 mm) were placed in bacterial suspensions of P. gingivalis. The optical density values indicated that the AuDAPT-coated aligner efficiently inhibited the growth of P. gingivalis. At a concentration of 104 CFU/mL, P. gingivalis stopped growing (Figure 2a). At a concentration of 105 CFU/mL, P. gingivalis showed slow growth (Figure 2b). The lag, log, and stationary phases were delayed. The AuDAPT-coated Invisalign showed bactericidal properties.
The number of viable P. gingivalis cells was determined after 40 h of incubation using an easy spiral inoculator (Interscience, France). No colonies formed from the suspension (undiluted) on AuDAPT-coated Invisalign at a concentration of 104 CFU/mL, whereas a large number of colonies formed from the 104 and 105 CFU/mL suspensions on an untreated Invisalign (Figure S1).
The SEM images confirmed that fewer P. gingivalis were live on the AuDAPT-coated Invisalign (Figure 2a,b), and biofilm did not form compared to that on untreated substrates. On the AuDAPT-coated Invisalign immersed in 104 CFU/mL bacteria, no live bacterial cell was observed. As a highly pathogenic but antibiotic-resistant bacteria, P. gingivalis could be efficiently treated by AuDAPT, which provides a promising way to treat related diseases. Previous reports revealed that AuDAPT could eradicate bacteria by disrupting their membranes owing to its antibacterial activity.21,23 Thus, in bacterial suspension, only the bacteria contacting AuDAPT were to be killed. Moreover, AuDAPT existed both as a stabilized molecule and in suspension that is released from samples.
The contact assay showed that bacteria did not grow in the areas contacting and neighboring the AuDAPT-coated Invisalign circles (Figure 2c). The proportion of colonies on the untreated aligner and those on AuDAPT-coated aligner was significantly different.
The pathogenic biofilm typically extends from the supragingival area to the subgingival area. However, the aligner can only cover the supragingival tooth area. Therefore, how to effectively deliver the drug into the supragingival area and periodontal pocket is still a problem to be solved. Zheng et al. calculated the Au content on the cell culture plates before and after bacterial incubation based on ICP–OES measurement and detected no measurable decrease in Au on the coating after incubation, demonstrating that electrostatic self-assembly can stabilize AuNPs during cell culture.23,28 However, the current results indicate that maybe a small amount of AuDAPT released from the substrates can have an antibacterial effect on P. gingivalis. During clinical treatment, it is suggested that aligners should be changed every 7–14 days, which would provide a sustainable AuDAPT level with effects exerted by both immobilized and released AuDAPT. However, the animal model or other quantified evidences should be provided in the future, including the effectiveness on organism infection. Although our results indicated that the released AuDAPT may be effective to the subgingival bacteria area, and further exploration is needed to investigate the efficient way to treat dental pockets plaques.
Inhibition of Biofilm Formation by AuDAPT-Coated Aligners
Bacteria inhabit natural and artificial systems in the form of homogeneous or heterogeneous biofilms rather than as planktonic individuals, with bacterial communities embedded in self-produced three-dimensional EPS. Gingivitis and chronic periodontitis are initiated and sustained by microbial biofilm formed by dental plaque.29 Therefore, effectively inhibiting biofilm formation is very important in clinical treatment.
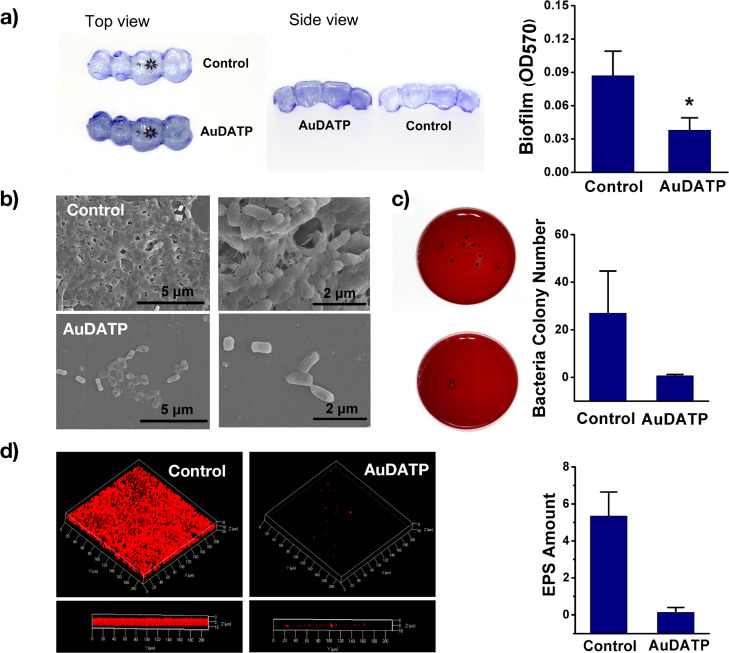
Biofilms consist of the EPS and the wrapped planktonic cells.30 To evaluate the effect of the modified aligner on biofilm formation, the total biomass of the biofilm, EPS, and wrapped living bacteria were measured. The optical density (OD570) based on the CV staining indicated that the total biomass of the biofilm was decreased in the coating group from 0.09 ± 0.02 to 0.04 ± 0.01 (Figure 3a), which was consistent with the SEM observation (Figure 3b). The living bacteria wrapped in the biofilm was calculated from the number of colonies formed from the biofilm. A volume of 100 μL from 1 mL of PBS containing suspended bacterial wrapped in the biofilm formed approximately 27 colonies compared to 0.6 colonies in the coating group (Figure 3c). Confocal microscopy observation was performed to measure and compare the amount of biofilm EPS stained by a concanavalin A-Alexa Fluor 647 conjugate, revealing a difference (Figure 3d). These results indicate that AuDAPT decreased the number of living bacteria, the amount of EPS, and total biofilm biomass. Planktonic bacteria drive the beginning of biofilm formation. AuDAPT reduced the number of planktonic cells, thus delaying the biofilm formation and maturation.
Figure 3.
(a) CV staining images of biofilms and comparison of OD 570 value from CV staining (P < 0.05) (n = 3). (b) SEM images of bacteria biofilms formed on the sample surface when immersed into bacterial suspension (density of bacterial suspension: 107 CFU/mL, magnification: 10.0k× and 20.0k×; voltage: 5.0 kV). (c) Comparison of the colony number formed by live bacteria in biofilm (n = 3). (d) Confocal images of biofilm EPS, stained with Con-A Alexa Fluor (red) (n = 3). (All graphs represent the average data of multiple experiments).
However, there are still limitations in our exploration. First, dental plaque in organisms does not consist of a single species. Oral bacterial species identified using DNA probes from gingival and subgingival samples were divided into microbial complexes labeled by different colors.31 A climax community of periodontal anaerobes, including P. gingivalis, was present in the red complex,32 and successful treatments were associated with a reduction in these complexes and increase in host-compatible microorganisms.33 Therefore, the present study could not simulate the actual environment in which a biofilm is formed in periodontal tissues. More kinds of microorganisms included in complexes and their coordination should be further investigated. However, the powerful influence of AuDAPT coating on P. gingivalis, a highly pathogenic and drug-resistant Gram-negative bacterium, was promising for disease treatment.
The treatment for biofilms involves a variety of materials and mechanism, and gold NPs have their own advantages. Biofilms enhance bacterial drug-resistance in complex manners. Several strategies for targeting biofilms, including silver coating,34 antibiotics,35 and enzymes,36 have been reported for certain groups of microorganisms and life cycle stages of biofilm; however, comprehensive methods for targeting microbial biofilms have not been developed. Nanomaterials including single-wall carbon nanotubes,37 vanadium pentoxide NPs,38 zinc oxide NPs,39 and graphene oxide–silver nanocomposites30 have also been reported to not only kill planktonic bacteria but also inhibit biofilm formation and even eliminate mature biofilms. However, their broad applications in the clinic remain controversial because their stabilities and potential toxicities to humans and the environment are unclear.
Effective anti-biofilm strategies cannot be confined to a single methodology that can disrupt one pathway but should simultaneously target various routes adopted by microorganisms for survival within their ecosystem.34 As a promising antibacterial nanomaterial, the antibacterial mechanism of gold NPs is currently an active field of research. Evidence indicates that gold NPs exert their antibacterial activities in complicated manners such as by decreasing membrane potential, inhibiting ATPase activities from lowering ATP levels, and inhibiting ribosome subunits from binding to tRNA. They also enhance chemotaxis in the early-phase reaction. The action of gold NPs does not involve reactive oxygen species-related mechanisms.40 These multiple mechanisms make resistance development more difficult and enhance antibiofilm effects.
Biological Safety of AuDAPT-Coated Surfaces
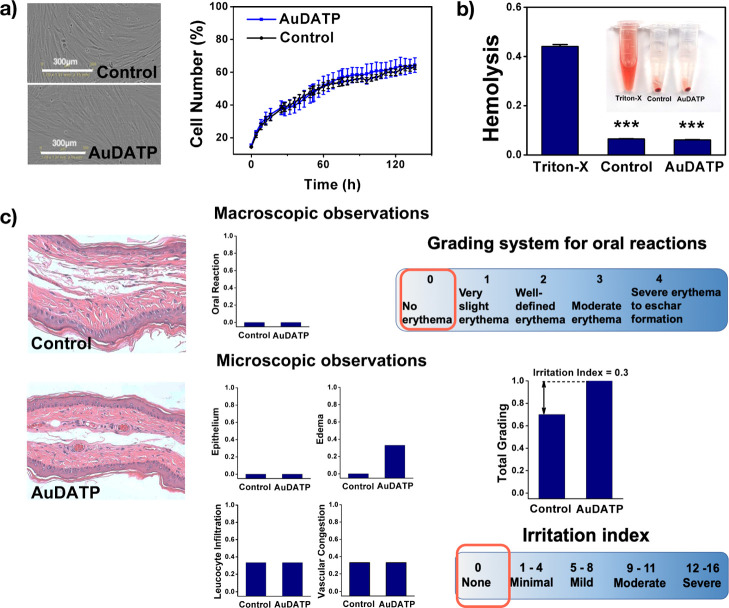
During a 6 day culture, hPDLCs grew favorably in both groups, and the cell numbers were not significantly different. Image analysis of the two groups revealed a similar density and morphology in the same phase (Figure 4a).
Figure 4.
Biocompatibility of AuDAPT-coated aligners. (a) Images and growth curve of cells in culture medium with immersed test samples (n = 3). (b) Hemolysis ratio of AuDAPT-coated aligners compared with the untreated group. (c) Histological images of contact pouch areas (n = 3). The grading system includes macroscopic examination: erythema and eschar formation and microscopic examination: the reaction of epithelium, leucocyte infiltration, vascular congestion, and edema. Irritation index is the average of microscopic grading results (all graphs represent the average data of multiple experiments).
It was reported that AuDAPT does not affect the viability of human umbilical vein endothelial cells at concentrations below 100 μg/mL.23 A similar phenomenon was also observed in cells subjected to orthodontic treatment in the current study.
To investigate the biocompatibility of AuDAPT, we test the hemolytic property on mice erythrocytes (Figure 4b). Hemolysis must be avoided when we use materials in blood-contacting applications (gingival bleeding is a representative symptom of periodontitis). After measuring the absorbance at 545 nm, we find that the AuDAPT-coated samples will not induce any hemolysis.
In mucosal contact test, no erythema or eschar formation, cell degeneration or flatting, or other abnormal manifestation was observed (Figure 4c) in the AuDAPT group. The mucosal irritation was evaluated from both the macroscopic and microscopic level. In the macroscope evaluation, no erythema was observed in both control and AuDAPT group. Therefore, their grades are both zero. In the microscopic evaluation, reactions of epithelium, leucocyte infiltration, vascular congestion, and edema were all considered for an irritation index. The average irritation index, according to microscopic evaluation, was among none to minimal value (Figure 4c).
Under the conditions of this study, the AuDAPT-coated aligner did not irritate the oral mucosa. In addition to hPDLCs, the oral mucosa is a site at which long-term contact and rubbing by aligners during orthodontic treatment can easily lead to irritation effects. In the mucosa irritation experiment, no abnormalities were found for the contacted mucosa tissues of all test and control sides. Thus, AuDAPT can be applied for oral applications.
Conclusions
In conclusion, AuDAPT-coated aligners have antibacterial effects on suspension of P. gingivalis, a drug-resistant pathogenic oral bacterium. The strength of these effects depends on the amount of AuDAPT and bacterial concentration. The neighboring area of the material was also affected. AuDAPT-coated aligners slowed biofilm formation and showed favorable biocompatibility. This method could be used to treat periodontal-related bacterial and systemic infections, but further explorations are needed. Microorganisms in the oral cavity are much more complicated than a single kind of bacterium. More in-depth exploration should be conducted in the future to simulate the biological environment and develop proper ways to treat oral bacterial-related diseases through dental devices.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 81400561, 21761142006) and the Tribology Science Fund of State Key Laboratory of Tribology (SKLTKF19B11). We thank the Dental Medical Devices Testing Center of Peking University School of Stomatology for assistance in animal test.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01532.
Grading system for oral reactions, grading system for microscopic examination for oral tissue reaction, and bacterial suspension with untreated and AuDAPT-coated samples (cultured for 48 h) and spirally inoculation colonies (PDF)
Author Contributions
⊥ M.Z., X.L. and Y.X. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Mysak J.; Podzimek S.; Sommerova P.; Lyuya-Mi Y.; Bartova J.; Janatova T.; Prochazkova J.; Duskova J. Porphyromonas gingivalis: major periodontopathic pathogen overview. J. Immunol. Res. 2014, 2014, 476068. 10.1155/2014/476068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teratani G.; Awano S.; Soh I.; Yoshida A.; Kinoshita N.; Hamasaki T.; Takata Y.; Sonoki K.; Nakamura H.; Ansai T. Oral health in patients on haemodialysis for diabetic nephropathy and chronic. Clin. Oral Invest. 2013, 17, 483–489. 10.1007/s00784-012-0741-1. [DOI] [PubMed] [Google Scholar]
- Holmlund A.; Lampa E.; Lind L. Oral health and cardiovascular disease risk in a cohort of periodontitis patients. Atherosclerosis 2017, 262, 101–106. 10.1016/j.atherosclerosis.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Graziani F.; Gennai S.; Solini A.; Petrini M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes An update of the EFP-AAP review. J. Clin. Periodontol. 2018, 45, 167–187. 10.1111/jcpe.12837. [DOI] [PubMed] [Google Scholar]
- Liljestrand J. M.; Paju S.; Pietiäinen M.; Buhlin K.; Persson G. R.; Nieminen M. S.; Sinisalo J.; Mäntylä P.; Pussinen P. J. Immunologic burden links periodontitis to acute coronary syndrome. Atherosclerosis 2018, 268, 177–184. 10.1016/j.atherosclerosis.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Kaye E. K.; Valencia A.; Baba N.; Spiro A. III; Dietrich T.; Garcia R. I. Tooth loss and periodontal disease predict poor cognitive function in older men. J. Am. Geriatr. Soc. 2010, 58, 713–718. 10.1111/j.1532-5415.2010.02788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz M.; Mortimer J. A.; Fratiglioni L.; Johansson B.; Berg S.; Reynolds C. A.; Pedersen N. L. Potentially modifiable risk factors for dementia in identical twins. Alzheimer’s Dementia 2006, 2, 110–117. 10.1016/j.jalz.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Kamer A. R.; Pirraglia E.; Tsui W.; Rusinek H.; Vallabhajosula S.; Mosconi L.; Yi L.; McHugh P.; Craig R. G.; Svetcov S.; Linker R.; Shi C.; Glodzik L.; Williams S.; Corby P.; Saxena D.; de Leon M. J. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol. Aging 2015, 36, 627–633. 10.1016/j.neurobiolaging.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy S. S.; Lynch C.; Ermini F.; Benedyk M.; Marczyk A.; Konradi A.; Nguyen M.; Haditsch U.; Raha D.; Griffin C.; Holsinger L. J.; Arastu-Kapur S.; Kaba S.; Lee A.; Ryder M. I.; Potempa B.; Mydel P.; Hellvard A.; Adamowicz K.; Hasturk H.; Walker G. D.; Reynolds E. C.; Faull R. L. M.; Curtis M. A.; Dragunow M.; Potempa J. Porphyromonas gingivalisin Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulou P. G.; Galicia J. C.; Benakanakere M. R.; Garcia C. A.; Potempa J.; Kinane D. F. Porphyromonas gingivalis induce apoptosis in human gingival epithelial cells through a gingipain-dependent mechanism. BMC Microbiol. 2009, 9, 107. 10.1186/1471-2180-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemmig T. F.; Milián E.; Karch H.; Klaiber B. Differential clinical treatment outcome after systemic metronidazole and amoxicillin in patients harboring Actinobacillus actinomycetemcomitans and/or Porphyromonas gingivalis. J. Clin. Periodontol. 1998, 25, 380–387. 10.1111/j.1600-051x.1998.tb02459.x. [DOI] [PubMed] [Google Scholar]
- Forner L.; Larsen T.; Kilian M.; Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J. Clin. Periodontol. 2006, 33, 401–407. 10.1111/j.1600-051x.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- Mahendra J.; Mahendra L.; Nagarajan A.; Mathew K. Prevalence of eight putative periodontal pathogens in atherosclerotic plaque of coronary artery disease patients and comparing them with noncardiac subjects: A case-control study. Indian J. Dent. Res. 2015, 26, 189–195. 10.4103/0970-9290.159164. [DOI] [PubMed] [Google Scholar]
- Katz J.; Chegini N.; Shiverick K. T.; Lamont R. J. Localization of P. gingivalisin Preterm Delivery Placenta. J. Dent. Res. 2009, 88, 575–578. 10.1177/0022034509338032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M.; Yoshida K.; Okamura H.; Ochiai K.; Takamura H.; Fujiwara N.; Ozaki K. Oral Porphyromonas gingivalis translocates to the liver and regulates hepatic glycogen synthesis through the Akt/GSK-3β signaling pathway. Biochim. Biophys. Acta 2013, 1832, 2035–2043. 10.1016/j.bbadis.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Azaripour A.; Weusmann J.; Mahmoodi B.; Peppas D.; Gerhold-Ay A.; Noorden C. J. V.; Willershausen B. Braces versus Invisalign: gingival parameters and patients’ satisfaction during treatment: a cross-sectional study. BMC Oral Health 2015, 15, 69. 10.1186/s12903-015-0060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miethke R.-R.; Brauner K. A Comparison of the periodontal health of patients during treatment with the Invisalign system and with fixed lingual appliances. J. Orofac. Orthop. 2007, 68, 223–231. 10.1007/s00056-007-0655-8. [DOI] [PubMed] [Google Scholar]
- Huh A. J.; Kwon Y. J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Controlled Release 2011, 156, 128–145. 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Fernandez-Moure J. S.; Evangelopoulos M.; Colvill K.; Van Eps J. L.; Tasciotti E. Nanoantibiotics: a new paradigm for the treatment of surgical infection. Nanomedicine 2017, 12, 1319–1334. 10.2217/nnm-2017-0401. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Chen Z.; Chen Y.; Xu J.; Li J.; Jiang X. Synergy of Non-antibiotic Drugs and Pyrimidinethiol on Gold Nanoparticles against Superbugs. J. Am. Chem. Soc. 2013, 135, 12940–12943. 10.1021/ja4058635. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Tian Y.; Cui Y.; Liu W.; Ma W.; Jiang X. Small Molecule-Capped Gold Nanoparticles as Potent Antibacterial Agents That Target Gram-Negative Bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. 10.1021/ja1028843. [DOI] [PubMed] [Google Scholar]
- Xie Y.; Xianyu Y.; Wang N.; Yan Z.; Liu Y.; Zhu K.; Hatzakis N. S.; Jiang X. Functionalized Gold Nanoclusters Identify Highly Reactive Oxygen Species in Living Organisms. Adv. Funct. Mater. 2018, 28, 1702026. 10.1002/adfm.201702026. [DOI] [Google Scholar]
- Zheng W.; Jia Y.; Chen W.; Wang G.; Guo X.; Jiang X. Universal coating from electrostatic self-Assembly to prevent multidrug-resistant bacterial colonization on medical devices and solid surfaces. ACS Appl. Mater. Interfaces 2017, 9, 21181–21189. 10.1021/acsami.7b05230. [DOI] [PubMed] [Google Scholar]
- Lee D.; Cohen R. E.; Rubner M. F. Antibacterial properties of Ag nanoparticle loaded multilayers and formation of magnetically directed antibacterial microparticles. Langmuir 2005, 21, 9651–9659. 10.1021/la0513306. [DOI] [PubMed] [Google Scholar]
- Jain P.; Pradeep T. Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng. 2005, 90, 59–63. 10.1002/bit.20368. [DOI] [PubMed] [Google Scholar]
- Haynes C. L.; Van Duyne R. P. Nanosphere lithography: A versatile nanofabrication tool for studies of size-dependent nanoparticle optics. J. Phys. Chem. B 2001, 105, 5599–5611. 10.1021/jp010657m. [DOI] [Google Scholar]
- Xie Y.; Zhang M.; Zhang W.; Liu X.; Zheng W.; Jiang X. Gold Nanoclusters-Coated Orthodontic Devices Can Inhibit the Formation of Streptococcus mutans Biofilm. ACS Biomater. Sci. Eng. 2020, 6, 1239–1246. 10.1021/acsbiomaterials.9b01647. [DOI] [PubMed] [Google Scholar]
- Tang R.; Moyano D. F.; Subramani C.; Yan B.; Jeoung E.; Tonga G. Y.; Duncan B.; Yeh Y.-C.; Jiang Z.; Kim C.; Rotello V. M. Rapid coating of surfaces with functionalized nanoparticles for regulation of cell behavior. Adv. Mater. 2014, 26, 3310–3314. 10.1002/adma.201306030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane D. F.; Stathopoulou P. G.; Papapanou P. N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- Liu S.; Cao S.; Guo J.; Luo L.; Zhou Y.; Lin C.; Shi J.; Fan C.; Lv M.; Wang L. Graphene oxide-silver nanocomposites modulate biofilm formation and extracellular polymeric substance (EPS) production. Nanoscale 2018, 10, 19603–19611. 10.1039/c8nr04064h. [DOI] [PubMed] [Google Scholar]
- Socransky S. S.; Haffajee A. D.; Cugini M. A.; Smith C.; Kent R. L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S.; Haffajee A. D. Periodontal microbial ecology. Periodontol 2000 2005, 38, 135–187. 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- Colombo A. P. V.; Tanner A. C. R. The role of bacterial biofilms in dental caries and periodontal and peri-implant diseases: A Historical Perspective. J. Dent. Res. 2019, 98, 373–385. 10.1177/0022034519830686. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Alam A.; Rani M.; Ehtesham N. Z.; Hasnain S. E. Biofilms: Survival and defense strategy for pathogens. Int. J. Med. Microbiol. 2017, 307, 481–489. 10.1016/j.ijmm.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Yasuda H.; Ajiki Y.; Koga T.; Kawada H.; Yokota T. Interaction between biofilms formed by Pseudomonas aeruginosa and clarithromycin. Antimicrob. Agents Chemother. 1993, 37, 1749. 10.1128/aac.37.9.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto K.; Hirota K.; Ono T.; Murakami K.; Murakami K.; Nagao D.; Miyake Y. Effect of Varidase (streptokinase) on biofilm formed by Staphylococcus aureus. Chemotherapy 2000, 46, 111–115. 10.1159/000007264. [DOI] [PubMed] [Google Scholar]
- Rodrigues D. F.; Elimelech M. Toxic effects of single-walled carbon nanotubes in the development of E. coli biofilm. Environ. Sci. Technol. 2010, 44, 4583–4589. 10.1021/es1005785. [DOI] [PubMed] [Google Scholar]
- Natalio F.; André R.; Hartog A. F.; Stoll B.; Jochum K. P.; Wever R.; Tremel W. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat. Nanotechnol. 2012, 7, 530–535. 10.1038/nnano.2012.91. [DOI] [PubMed] [Google Scholar]
- Applerot G.; Lellouche J.; Perkas N.; Nitzan Y.; Gedanken A.; Banin E. ZnO nanoparticle-coated surfaces inhibit bacterial biofilm formation and increase antibiotic susceptibility. RSC Adv. 2012, 2, 2314–2321. 10.1039/c2ra00602b. [DOI] [Google Scholar]
- Cui Y.; Zhao Y.; Tian Y.; Zhang W.; Lü X.; Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. 10.1016/j.biomaterials.2011.11.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.