Abstract
A recent study found a decreased risk of Parkinson disease (PD) associated with the β2 adrenergic agonist (β2-agonist) salbutamol. However, other mechanisms might explain this apparent association. Using the UK Clinical Practice Research Datalink, we formed a cohort of 2,430,884 patients aged 50 years or older between 1995 and 2016. During follow-up, 8,604 cases of PD were identified and matched to 86,040 controls on sex, age, date of cohort entry, and duration of follow-up, after applying a 1-year latency time window. Incidence rate ratios of PD associated with use of β2-agonists were estimated using conditional logistic regression. Ever-use of β2-agonists was associated with a 17% decreased rate of PD (rate ratio = 0.83, 95% confidence interval: 0.75, 0.91) compared with no use. However, this association was limited to early short-term use and was no longer observed after more than 2 years of cumulative duration of use (rate ratio = 0.97, 95% confidence interval: 0.80, 1.17). A similar pattern was observed when stratifying by time since first β2-agonist prescription and by duration of follow-up. The apparent association of β2-agonists with a decreased risk of PD is likely the result of reverse causality rather than a biological effect of these drugs on the risk of PD.
Keywords: β-blockers, β2-agonists, Parkinson disease, reverse causality
Abbreviations
- β2-agonist
β2 adrenergic agonist
- CPRD
Clinical Practice Research Datalink
- LABA
long-acting selective β2-agonists
- PD
Parkinson disease
- SABA
short-acting β2-agonists
One of the neuropathological hallmarks of Parkinson disease (PD) is the development of abnormal aggregated α-synuclein, known as Lewy bodies, encoded by the gene SNCA (1). A recent study identified β2 adrenergic agonists (β2-agonists) as drugs capable of reducing SNCA expression, thus potentially slowing down the formation of Lewy bodies and the development of PD (2). In a cohort study using the Norwegian administrative health databases, the same authors found a 34% reduction in the incidence of PD associated with salbutamol, the most commonly used β2-agonist in Norway, compared with no use, and a markedly increased risk of PD with use of the β-blocker (β-antagonist) propranolol (2).
β2-agonists such as salbutamol are already marketed and readily available; therefore, these drugs might represent a unique opportunity for the prevention and treatment of PD. As such, the above findings generated substantial scientific interest, given the lack of neuroprotective drugs in PD. On the other hand, concern has been raised that these findings, if not reliable, might influence physicians’ and patients’ behavior, leading to unregulated off-label use of β2-agonists to treat PD (3). Indeed, alternative explanations, in particular reverse causality, might be responsible for these results. Specifically, early signs and symptoms of yet undiagnosed PD, such as tremor, might prompt initiation of some medications such as β-blockers and, conversely, deter physicians from prescribing drugs such as β2-agonists. This phenomenon might lead to spurious associations between these medications and PD. Therefore, we aimed to further explore the putative neuroprotective effect of β2-agonists on the incidence of PD in a large population-based cohort study. As a secondary objective, we also assessed the association between use of β-blockers and the incidence of PD.
METHODS
Source population
This study was conducted using the UK Clinical Practice Research Datalink (CPRD) (4–6). This database contains the complete primary-care medical record for more than 13 million people enrolled in more than 700 general practices (6). The geographic distribution of the participating practices has been shown to be representative of the UK population, and age and sex distributions of patients in the CPRD are similar to those reported by the National Population Census (4, 6). Information collected includes demographic characteristics, lifestyle factors, medical diagnoses, laboratory tests, prescriptions, and referrals to specialists and hospitals. Prescriptions written by general practitioners are automatically transcribed into computer records. Read codes are used to capture medical diagnoses and procedures (7), and a coded drug dictionary based on the British National Formulary is used for recording prescriptions. The recorded information on drug exposures and diagnoses in the CPRD has been validated and shown to be of high quality (8–10).
The study protocol was approved by the Independent Scientific Advisory Committee of the CPRD (#18_063R) and by the Research Ethics Committee of the Jewish General Hospital, Montreal, Canada.
Cohort definition
We conducted a cohort study, with a nested case-control approach to analysis, within the CPRD population. We assembled a cohort of all individuals in the CPRD aged 50 years or older between January 1, 1995, and December 31, 2016, who were members of a practice that fulfilled predefined quality criteria (“up to standard”) (Figure 1). Cohort entry was defined as the latest of the following events: January 1, 1995; calendar date of a patient’s 50th birthday; or 1 year after the patient’s registration date with an up-to-standard practice. We excluded patients with a diagnosis of PD or secondary parkinsonism, or a prescription of an antiparkinson drug any time before cohort entry, as well as patients with extrapyramidal disease not otherwise specified in the year before cohort entry. Patients treated with antipsychotic drugs or related drugs such as metoclopramide in the year before cohort entry were also excluded because these drugs might induce parkinsonism. To identify new users of the study drugs and of respiratory antimuscarinics, we also excluded patients with 1 or more prescriptions of selective and nonselective β2-agonists, β-blockers, or respiratory antimuscarinic drugs any time before cohort entry. Respiratory antimuscarinic drugs have indications of use similar to those of β2-agonists but do not activate the β2-adrenoceptor and have not been associated with PD; thus, they were used as a negative control exposure (see sensitivity analysis).
Figure 1.
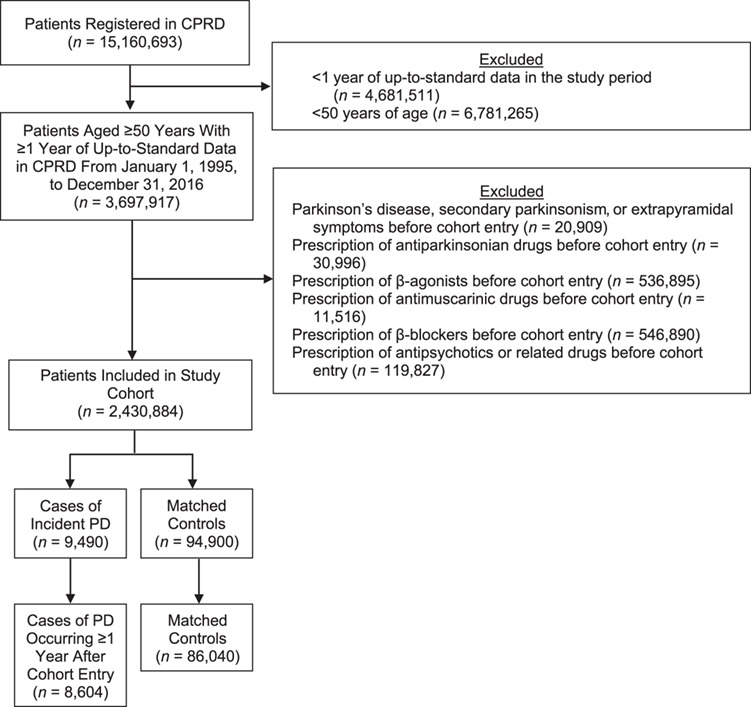
Study flow chart illustrating the cohort formation and selection of cases of Parkinson disease (PD) and matched controls in the Clinical Practice Research Datalink (CPRD), United Kingdom, between 1995 and 2016. β2-agonist, β2 adrenergic agonist.
Patients meeting these criteria were followed until a first diagnosis of PD or censored at the earliest of the following: a first diagnosis of secondary parkinsonism, death from any cause, date they transferred out of the practice, prescription for an antipsychotic drug, prescription for other specified drugs (vilanterol, aclidinium, or umeclidinium—respectively, a new β2-agonist marketed in 2013 and 2 new antimuscarinic drugs marketed in 2012 and 2014, for which the effect on PD is unknown), or end of the study (December 31, 2016).
Case and control selection
Within the cohort defined above, we used a nested case-control analysis because of the time-varying nature of exposure, the size of the cohort, and the long duration of follow-up (11–13). In this context, the nested case-control approach is computationally more efficient than a time-dependent survival analysis while producing equivalent estimates (12, 14). Thus, we identified all patients within our cohort with a first-time Read code for idiopathic PD recorded during follow-up. The first date of PD diagnosis in the study period was defined as the index date for the cases.
For each case of PD, up to 10 controls were randomly selected among the risk set defined by the case after matching on sex, age (within 1 year), date of cohort entry (within 1 year), and duration of follow-up. Because controls were selected from the risk set defined by each case, all controls were necessarily alive, active in the practice, and event-free when matched to their corresponding case. The date resulting in the same duration of follow-up for the case and controls defined the index date for the controls. We restricted all analyses to cases and matched controls with at least 1 year of follow-up between cohort entry and index date to allow for a latency time window, because cases of PD identified shortly after cohort entry are likely to be prevalent cases. Web Figure 1, available at https://academic.oup.com/aje, summarizes cohort formation and case-control selection. Baseline characteristics for cases and controls are summarized in Table 1.
Table 1.
Baseline Characteristics of Study Cases of Parkinson Disease and Matched Controls at Cohort Entry, United Kingdom, 1995–2016
| Cases of PD (n = 8,604) | Controls a (n = 86,040) | |||
|---|---|---|---|---|
| Characteristic | No. | % | No. | % |
| Male sex | 5,542 | 64.4 | 55,420 | 64.4 |
| Age, yearsb | 66.3 (9.9) | 66.3 (10.0) | ||
| Years of follow-upb | 7.6 (4.7) | 7.6 (4.7) | ||
| Smoking status | ||||
| Never-smoker | 3,956 | 46.0 | 33,090 | 38.5 |
| Ever-smoker | 2,581 | 30.0 | 29,803 | 34.6 |
| Unknown | 2,067 | 24.0 | 23,147 | 26.9 |
| Alcohol abuse | 167 | 1.9 | 1,512 | 1.8 |
| Body mass indexc | ||||
| <25 | 2,455 | 28.5 | 23,672 | 27.5 |
| 25–29 | 2,533 | 29.4 | 23,624 | 27.5 |
| ≥30 | 908 | 10.6 | 8,863 | 10.3 |
| Unknown | 2,708 | 31.5 | 29,881 | 34.7 |
| Comorbidities | ||||
| Hypertension | 1,801 | 20.9 | 16,955 | 19.7 |
| Ischemic heart disease | 529 | 6.1 | 4,751 | 5.5 |
| Hyperlipidemia | 850 | 9.9 | 7,503 | 8.7 |
| Diabetes | 493 | 5.7 | 4,401 | 5.1 |
| Cerebrovascular disease | 366 | 4.3 | 2,842 | 3.3 |
| COPD | 332 | 3.9 | 3,059 | 3.6 |
| Asthma | 154 | 1.8 | 1,436 | 1.7 |
| Dementia | 63 | 0.7 | 354 | 0.4 |
| Cancer | 479 | 5.6 | 4,296 | 5.0 |
| Head injury | 153 | 1.8 | 1,234 | 1.4 |
| Depression | 896 | 10.4 | 7,086 | 8.2 |
| Medications | ||||
| Aspirin and other antiplatelets | 818 | 9.5 | 6,637 | 7.7 |
| Antidiabetic drugs | 309 | 3.6 | 2,717 | 3.2 |
| Antihypertensive drugs | 1,434 | 16.7 | 12,977 | 15.1 |
| Lipid-lowering drugs | 522 | 6.1 | 4,571 | 5.3 |
| NSAIDs | 1,443 | 16.8 | 13,699 | 15.9 |
| No. of drugs | ||||
| 0 | 2,289 | 26.6 | 28,241 | 32.8 |
| 1 | 1,487 | 17.3 | 14,539 | 16.9 |
| 2–3 | 2,136 | 24.8 | 19,968 | 23.2 |
| 4–7 | 1,897 | 22.0 | 16,979 | 19.7 |
| ≥8 | 795 | 9.2 | 6,313 | 7.3 |
| No. of physician visits | ||||
| 0 | 1,529 | 17.8 | 20,179 | 23.5 |
| 1–2 | 1,936 | 22.5 | 20,171 | 23.4 |
| 3–7 | 2,911 | 33.8 | 26,807 | 31.2 |
| 8–14 | 1,472 | 17.1 | 12,737 | 14.8 |
| ≥15 | 756 | 8.8 | 6,146 | 7.1 |
Abbreviations: COPD, chronic obstructive pulmonary disease; NSAID, nonsteroidal antiinflammatory drug; PD, Parkinson disease.
a Cases and controls were matched on sex, age, date of cohort entry, and duration of follow-up.
b Values are expressed as mean (standard deviation).
c Weight (kg)/height (m)2.
Definition of exposure
For each case and matched controls, we identified from the computerized medical records all prescriptions for short- and long-acting selective β2-agonists (SABAs and LABAs) between cohort entry and index date.
For all exposure definitions below, exposure was lagged by 1 year to account for a biologically plausible latency time window, given that treatments initiated shortly before PD diagnosis were unlikely to have influenced its occurrence. Exclusion of a time period prior to the index date (i.e., latency window) also minimizes detection bias, where initiation of a new treatment might lead to an increase in diagnostic investigations, thereby increasing the probability of identifying PD. Finally, this period minimizes reverse-causality bias, where initiation of a treatment might have been influenced by early signs or symptoms of PD (e.g., tremor). Thus, for all cases and their matched controls, prescriptions issued in the year before the index date were not considered. Because the length of the true latency window is uncertain, sensitivity analyses were conducted by varying the latency time window to 0, 2, 3, and 5 years.
First, patients were considered ever exposed to β2-agonists if they had been issued at least 1 prescription between cohort entry and the year before the index date. Second, among patients who ever used β2-agonists, cumulative duration of use was defined as the total number of years of exposure to β2-agonists, calculated by summing the durations of all prescriptions between cohort entry and the year before the index date, with a 30-day grace period added to the end of a prescription to allow for refill time. For this analysis, we assumed a duration of 2 months for SABA prescriptions written “as needed.” In sensitivity analyses, the duration for SABA prescriptions was changed to 1 month and 6 months. This was an issue only for SABAs because LABAs are rarely prescribed as needed. Cumulative duration of use was stratified according to the tertiles of the distribution of use in the controls.
We also assessed separately the association between β-blockers and the risk of PD. Thus, the same exposure definitions described above were applied to define exposure to β-blockers.
Data analysis
Given the nested case-control approach to analysis, we used conditional logistic regression to compute odds ratios, which are unbiased estimators of incidence rate ratios, with little or no loss in precision (14).
In the primary analyses, we estimated the rate ratios and 95% confidence intervals of PD associated with ever use and cumulative duration of use of β2-agonists compared with no use. By the matching process, all rate ratios were adjusted for sex, age, calendar time, and duration of follow-up. In addition, all models adjusted for the following potential confounding factors measured at cohort entry: body mass index, excessive alcohol use, smoking status, comorbidities (hypertension, hyperlipidemia, diabetes, ischemic heart disease, cerebrovascular disease, asthma, chronic obstructive pulmonary disease, dementia, depression, cancer, and history of head injury) measured at any time before cohort entry, and use of medications (aspirin and other antiplatelet drugs, statins, and nonsteroidal antiinflammatory agents) measured in the year before cohort entry. Finally, all models adjusted for the number of physician visits (as a measure of health utilization) and the number of unique medications (as a measure of overall health) in the year before cohort entry. In addition, when exploring the association between β2-agonists and PD, models also adjusted for ever use of β-blockers and antimuscarinic drugs. Conversely, when assessing the risk associated with β-blockers, models adjusted for ever use of β2-agonists and antimuscarinic drugs. We also conducted stratified analyses to assess whether the risk of PD varied by type of β2-agonist (SABA vs. LABA) and by administration (inhaled vs. oral). Finally, all analyses were repeated to estimate the risk of PD associated with β-blockers.
Eight sensitivity analyses were performed to assess the robustness of our results. First, we varied the latency time window for the exposure definition to 0, 2, 3, and 5 years (i.e., 0-, 2-, 3-, and 5-year lags). Second, we repeated the primary analyses using a grace period of 60 days between prescriptions to evaluate the potential for misclassification of exposure. Third, we changed the exposure definition by requiring at least 4 prescriptions of β2-agonists within a 12-month period to be considered ever exposed. Fourth, in the analyses stratified by duration of use, we changed the duration of SABA prescriptions with unknown duration from 2 months to 1 month and 6 months. Fifth, to assess the robustness of our outcome definition, we restricted cases to those who also had at least 2 prescriptions for antiparkinson drugs (levodopa, dopamine agonists, monoamine-oxidase-b inhibitors, antimuscarinic drugs, catechol-O-methyltransferase inhibitors) along with a PD diagnosis, all within 1 year. In these analyses, the index date was the latest of the PD diagnosis or the second antiparkinson drug prescription. Sixth, we used multiple imputation for variables with missing values (i.e., body mass index, smoking) (15–17). Seventh, we assessed the risk of PD in users of salbutamol as done in the recent study by Mittal et al. (2). Finally, to assess the potential for residual confounding, we used respiratory antimuscarinic drugs as a negative control exposure, because these drugs do not activate the β2-adrenoceptor and have not been associated with PD.
To assess the potential for reverse causality (18), whereby patients with early symptoms of PD such as tremor would be less likely to be prescribed β2-agonists, we repeated the analyses stratified by time since the first β2-agonist and by duration of follow-up. Conversely, patients with tremor and not yet diagnosed with PD might be more likely to initiate β-blockers. Thus, we repeated the analysis estimating the association between β-blockers and PD with the index date defined as the earliest of the first record of tremor, first antiparkinson prescription (if any), or PD diagnosis, whichever occurred first during follow-up, with the same corresponding index date for their matched controls.
All computations were performed using SAS, version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Within a cohort of 2,430,884 patients meeting all inclusion criteria, 9,490 patients were diagnosed with PD during follow-up, and matched to 94,900 controls (Figure 1). After applying a 1-year latency time window, 8,604 cases and 86,040 matched controls were included in the analyses. Table 1 describes the characteristics of the cases of PD and their matched controls. Cases were predominantly male, had a lower prevalence of smoking, and had a similar prevalence of respiratory diseases compared with controls. As expected, cases were on average sicker than controls, with a higher prevalence of depression and antiplatelets prescriptions as well as a higher number of drugs prescribed in the year before cohort entry and a higher number of physician visits.
A total of 632 (7.3%) cases and 7,394 (8.6%) controls received at least 1 selective β2-agonist prescription during follow-up, and 98.8% of β2-agonists prescriptions in cases and 98.3% in controls were inhaled β2-agonists. Ever use of selective β2-agonists was associated with a 17% decreased rate of PD (rate ratio = 0.83, 95% confidence interval: 0.75, 0.91) compared with no use (Table 2). However, this decreased rate was no longer observed after more than 2 years of cumulative duration of use (rate ratio = 0.97, 95% confidence interval: 0.80, 1.17). When stratified by time since first β2-agonist prescription, the association was strongest with β2-agonists initiated shortly before PD diagnosis. Similarly, in analyses stratified by duration of follow-up, the decreased rate associated with β2-agonists was limited to the subgroup of patients with a short follow-up and less than 2 years of duration of use (Table 3). In analyses stratified by type of β2-agonists (SABAs, LABAs) and route of administration (inhaled, oral) the lowest rate of PD was also observed in short-term users (Web Table 1). In sensitivity analyses applying different lag times, the same pattern was observed with 2- and 3-year lags whereas there was no overall association of β2-agonists with the incidence of PD with a 5-year lag (Table 4). Results of the other sensitivity analyses were consistent with those of the primary analyses overall, with an association limited to short-term use of β2-agonists (Web Tables 2–7). Ever use of antimuscarinics (negative control exposure) was not associated with the incidence of PD (Web Table 8).
Table 2.
Crude and Adjusted Rate Ratios of Parkinson Disease Associated With Ever Use of β2 Adrenergic Agonists Compared With No Use, United Kingdom, 1995–2016
| Cases | Controls a | Adjusted RR b | |||||
|---|---|---|---|---|---|---|---|
| Exposure | No. | % | No. | % | Crude RR | RR | 95% CI |
| No use | 7,972 | 92.7 | 78,646 | 91.4 | 1.00 | 1.00 | Referent |
| β2-agonists | 632 | 7.3 | 7,394 | 8.6 | 0.84 | 0.83 | 0.75, 0.91 |
| Time since first β2-agonist prescription, years | |||||||
| 1–2 | 174 | 2.0 | 2,236 | 2.6 | 0.77 | 0.75 | 0.64, 0.88 |
| 3–5 | 195 | 2.3 | 2,353 | 2.7 | 0.81 | 0.81 | 0.69, 0.94 |
| 6–21 | 263 | 3.1 | 2,805 | 3.3 | 0.92 | 0.91 | 0.79, 1.05 |
| Cumulative duration of use, months | |||||||
| <3 | 257 | 3.0 | 2,896 | 3.4 | 0.87 | 0.83 | 0.72, 0.94 |
| 3–24 | 219 | 2.5 | 2,762 | 3.2 | 0.78 | 0.77 | 0.66, 0.88 |
| >24 | 156 | 1.8 | 1,736 | 2.0 | 0.88 | 0.97 | 0.80, 1.17 |
Abbreviations: β2-agonist, β2 adrenergic agonist; CI, confidence interval; RR, rate ratio.
a Cases and controls were matched on age, sex, date of cohort entry, and duration of follow-up.
b Adjusted for variables listed in Table 1 and use of β-blockers and antimuscarinic drugs.
Table 3.
Crude and Adjusted Rate Ratios of Parkinson Disease Associated With Ever Use of β2 Adrenergic Agonists, Stratified by Duration of Follow-Up, United Kingdom, 1995–2016
| Cases | Controls a | Adjusted RR b | |||||
|---|---|---|---|---|---|---|---|
| Exposure | No. | % | No. | % | Crude RR | RR | 95% CI |
| No use | 7,972 | 92.7 | 78,646 | 91.4 | 1.00 | 1.00 | Referent |
| β2-agonists | 632 | 7.3 | 7,394 | 8.6 | 0.84 | 0.83 | 0.75, 0.91 |
| Follow-up, 1–5 years | |||||||
| No use | 3,677 | 97.2 | 36,374 | 96.1 | 1.00 | 1.00 | Referent |
| β2-agonists | 107 | 2.8 | 1,466 | 3.9 | 0.72 | 0.66 | 0.53, 0.81 |
| Cumulative duration of use, months | |||||||
| <3 | 52 | 1.4 | 731 | 1.9 | 0.70 | 0.63 | 0.47, 0.84 |
| 3–24 | 45 | 1.2 | 638 | 1.7 | 0.69 | 0.66 | 0.48, 0.90 |
| >24 | 10 | 0.3 | 97 | 0.3 | 1.02 | 1.05 | 0.53, 2.08 |
| Follow-up, 7–13 years | |||||||
| No use | 3,462 | 90.1 | 34,181 | 88.9 | 1.00 | 1.00 | Referent |
| β2-agonists | 382 | 9.9 | 4,259 | 11.1 | 0.88 | 0.90 | 0.80, 1.02 |
| Cumulative duration of use, months | |||||||
| <3 | 155 | 4.0 | 1,612 | 4.2 | 0.95 | 0.93 | 0.78, 1.10 |
| 3–24 | 136 | 3.5 | 1,576 | 4.1 | 0.85 | 0.86 | 0.71, 1.04 |
| >24 | 91 | 2.4 | 1,071 | 2.8 | 0.84 | 0.93 | 0.73, 1.19 |
| Follow-up, 14–22 years | |||||||
| No use | 833 | 85.3 | 8,091 | 82.9 | 1.00 | 1.00 | Referent |
| β2-agonists | 143 | 14.7 | 1,669 | 17.1 | 0.83 | 0.86 | 0.70, 1.06 |
| Cumulative duration of use, months | |||||||
| <3 | 50 | 5.1 | 553 | 5.7 | 0.88 | 0.86 | 0.63, 1.16 |
| 3–24 | 38 | 3.9 | 548 | 5.6 | 0.67 | 0.69 | 0.49, 0.97 |
| >24 | 55 | 5.6 | 568 | 5.8 | 0.94 | 1.15 | 0.81, 1.62 |
Abbreviations: β2-agonist, β2 adrenergic agonist; CI, confidence interval; RR, rate ratio.
a Cases and controls were matched on age, sex, date of cohort entry, and duration of follow-up.
b Adjusted for variables listed in Table 1, plus use of β-blockers and respiratory antimuscarinics.
Table 4.
Crude and Adjusted Rate Ratios of Parkinson Disease Associated With Ever Use β2 Adrenergic Agonists Compared With No Use Using Different Lag Times, United Kingdom, 1995–2016
| Cases | Controls a | Adjusted RR b | |||||
|---|---|---|---|---|---|---|---|
| Exposure | No. | % | No. | % | Crude RR | RR | 95% CI |
| With no exposure lag | |||||||
| No use | 7,953 | 92.4 | 78,529 | 91.3 | 1.00 | 1.00 | Referent |
| β2-agonists | 651 | 7.6 | 7,511 | 8.7 | 0.85 | 0.84 | 0.77, 0.92 |
| Cumulative duration of use, months | |||||||
| <3 | 260 | 3.0 | 2,888 | 3.4 | 0.89 | 0.84 | 0.74, 0.96 |
| 3–24 | 223 | 2.6 | 2,797 | 3.2 | 0.78 | 0.77 | 0.67, 0.89 |
| >24 | 168 | 2.0 | 1,826 | 2.1 | 0.90 | 1.01 | 0.84, 1.21 |
| With a 2-year exposure lag | |||||||
| No use | 7,188 | 93.0 | 71,093 | 92.0 | 1.00 | 1.00 | Referent |
| β2-agonists | 544 | 7.0 | 6,227 | 8.0 | 0.86 | 0.85 | 0.77, 0.94 |
| Cumulative duration of use, months | |||||||
| <3 | 236 | 3.0 | 2,529 | 3.4 | 0.92 | 0.88 | 0.77, 1.01 |
| 3–24 | 176 | 2.3 | 2,290 | 3.0 | 0.76 | 0.75 | 0.64, 0.88 |
| >24 | 132 | 1.7 | 1,408 | 1.8 | 0.92 | 1.01 | 0.82, 1.24 |
| With a 3-year exposure lag | |||||||
| No use | 6,470 | 93.4 | 64,122 | 92.6 | 1.00 | 1.00 | Referent |
| β2-agonists | 458 | 6.6 | 5,158 | 7.4 | 0.87 | 0.87 | 0.78, 0.97 |
| Cumulative duration of use, months | |||||||
| <3 | 187 | 2.7 | 2,119 | 3.1 | 0.87 | 0.84 | 0.72, 0.98 |
| 3–24 | 164 | 2.4 | 1,926 | 2.8 | 0.84 | 0.84 | 0.71, 0.99 |
| >24 | 107 | 1.5 | 1,113 | 1.6 | 0.95 | 1.04 | 0.83, 1.30 |
| With a 5-year exposure lag | |||||||
| No use | 5,121 | 93.9 | 51,057 | 93.6 | 1.00 | 1.00 | Referent |
| β2-agonists | 334 | 6.1 | 3,493 | 6.4 | 0.95 | 0.97 | 0.85, 1.10 |
| Cumulative duration of use, months | |||||||
| <3 | 149 | 2.7 | 1,514 | 2.3 | 0.98 | 0.96 | 0.81, 1.14 |
| 3–24 | 125 | 2.3 | 1,304 | 2.4 | 0.95 | 0.96 | 0.79, 1.16 |
| >24 | 60 | 1.1 | 675 | 1.2 | 0.88 | 1.01 | 0.75, 1.35 |
Abbreviations: β2-agonist, β2 adrenergic agonist; CI, confidence interval; RR, rate ratio.
a Cases and controls were matched on age, sex, date of cohort entry, and duration of follow-up.
b Adjusted for variables listed in Table 1, and use of β-blockers and respiratory antimuscarinic drugs.
Conversely, ever use of β-blockers was associated with a 45% increased risk of PD (rate ratio = 1.45, 95% confidence interval: 1.37, 1.54) compared with no use (Table 5). The rate was highest with less than 1 year of cumulative duration of use and decreased thereafter. In sensitivity analyses, applying 2-, 3-, and 5-year latency windows yielded a similar pattern with no increased rate of PD with more than 5 years of use of β-blockers (Web Table 9). Defining the index date as the earliest of first tremor symptoms, first antiparkinson drug prescription, or first PD diagnostic resulted in lower point estimates overall and no increased rate of PD with more than 5 years of use of β-blockers (Table 6).
Table 5.
Crude and Adjusted Rate Ratios of Parkinson Disease Associated With Ever Use of β-Blockers Compared With No Use, United Kingdom, 1995–2016
| Cases | Controls a | Adjusted RR b | |||||
|---|---|---|---|---|---|---|---|
| Exposure | No. | % | No. | % | Crude RR | 95% | CI |
| No use | 6,786 | 78.9 | 72,552 | 84.3 | 1.00 | 1.00 | Referent |
| β-blockers | 1,818 | 21.1 | 13,488 | 15.7 | 1.49 | 1.45 | 1.37, 1.54 |
| Cumulative duration of use, years | |||||||
| <1 | 780 | 9.1 | 4,830 | 5.6 | 1.76 | 1.70 | 1.57, 1.85 |
| 1–5 | 696 | 8.1 | 5,339 | 6.2 | 1.43 | 1.39 | 1.28, 1.52 |
| >5 | 342 | 4.0 | 3,319 | 3.9 | 1.15 | 1.13 | 1.00, 1.27 |
Abbreviations: CI, confidence interval; RR, rate ratio.
a Cases and controls were matched on age, sex, date of cohort entry, and duration of follow-up.
b Adjusted for variables listed in Table 1 and use of β-blockers and antimuscarinic drugs.
Table 6.
Crude and Adjusted Rate Ratios of Parkinson Disease Associated With Ever Use of β-Blockers Relative to No Use, Considering First Symptoms of Tremor, First Diagnostic of Parkinson Disease, or First Antiparkinson Drug Prescription, Whichever Came First, as Index Date, United Kingdom, 1995–2016
| Cases | Controls a | Adjusted RR b | |||||
|---|---|---|---|---|---|---|---|
| Effect | No. | % | No. | % | Crude RR | RR | 95% CI |
| No use | 6,801 | 82.7 | 69,187 | 85.5 | 1.00 | 1.00 | Referent |
| β-blockers | 1,425 | 17.3 | 11,818 | 14.6 | 1.24 | 1.20 | 1.13, 1.28 |
| Cumulative duration of use, years | |||||||
| <1 | 571 | 6.9 | 4,327 | 5.3 | 1.35 | 1.30 | 1.19, 1.43 |
| 1–5 | 565 | 6.9 | 4,716 | 5.8 | 1.23 | 1.19 | 1.09, 1.31 |
| >5 | 289 | 3.5 | 2,775 | 3.4 | 1.07 | 1.04 | 0.92, 1.19 |
Abbreviations: CI, confidence interval; RR, rate ratio.
a Cases and controls were matched on age, date of cohort entry, and duration of follow-up.
b Adjusted for variables listed in Table 1, and use of β-agonists, and respiratory antimuscarinic drugs.
DISCUSSION
In this large population-based study of over 2.4 million people, including more than 8,500 cases of PD, we found that ever use of β2-agonists was associated with a decreased rate of PD, in accordance with the results from a large cohort study in Norway (2). However, this association was limited to recent and short-term cumulative duration of use, suggesting that reverse causality is a plausible explanation for these findings. Conversely, use of β-blockers was associated with an increased risk of PD that was highest with short duration of use and decreased thereafter. Thus, a similar mechanism of reverse causality could, at least in part, be responsible for the observed association.
Evaluating the long-term effect of medications on the incidence of PD can be challenging. PD is a slowly developing neurodegenerative disease with an uncertain date of onset. Consequently, first symptoms might not be readily attributed to PD, or the diagnosis might be suspected early but only recorded in the medical file at a more advanced or clinically overt stage of the disease. Meanwhile, the changing health status of a patient will trigger changes in prescribing, with physicians initiating or stopping medications in response to these first, yet undiagnosed, manifestations of PD. Consequently, some drugs initiated (e.g., β-blockers) or stopped or not prescribed (e.g., β2-agonists) because of these early manifestations of PD might appear to be associated with an increase or decrease incidence of PD, respectively. This phenomenon, referred to as reverse causality or protopathic bias (18), is a likely explanation for the previously reported association of β2-agonists with the incidence of PD; we showed that this association was limited to short-term recent use of these medications. Indeed, given the nature of PD, an association is not expected with a drug given shortly before PD diagnosis and only for a short time but rather with a drug given earlier and with a more pronounced decreased risk associated with longer exposure. Our results were robust, as shown in several sensitivity analyses. Also, in keeping with these results, changing the exposure definition from ever use to at least 4 prescriptions of β2-agonists within 1 year, to capture more regular users, showed no association of β2-agonists with PD. The same phenomenon of reverse causality likely explained the increased rate of PD associated with use of β-blockers. Indeed, the observed increased rate was highest with short-term use of β-blockers and decreased with longer exposure. Moreover, there was no association with longer duration of cumulative use in sensitivity analyses applying 2- to 5-year exposure lags. Similarly, moving the index date to the earliest recorded manifestations of PD weakened the overall association, with no association of long cumulative use of β-blockers with the incidence of PD.
In a recent study, β2-agonists were shown to reduce α-synuclein gene expression in neuronal cells and mouse models of PD, suggesting a potential protective role of β2-agonists (2). To corroborate their experimental findings, the authors examined the association between salbutamol and the incidence of PD in a population-based cohort in Norway (2). They reported a 34% lower rate of PD associated with ever use of salbutamol compared with no use. However, in a subsequent analysis stratified by cumulative dose, a strong (40%) decrease in rate of PD was already present with doses as small as defined daily doses of 60–180 micrograms, an illogical finding that raises concerns about this particular analysis. Moreover, analyses were adjusted only for age, sex, and education level, and no latency time windows were used to account for the insidious and progressive nature of PD—methodological omissions whose effects could partly explain the strong association observed. In a recent nested case-control study based on an Israeli electronic medical records database, the authors reported a decreased risk of PD with β2-agonists overall (19). The association varied with duration of use, but the definition of exposure was unclear in this latter analysis. Finally, a case control study using a US health administrative database found inconsistent results, from an increased risk of PD with salbutamol in analyses adjusted for demographic factors to an overall null association after adjustment for use of care and history of smoking and a decreased risk with inhaled salbutamol (20). Aside from applying various lag times (up to 18 months only in one study (20)), no further analyses were conducted to explore in detail the potential for reverse causality for the association between β2-agonists and PD (19, 20). The same studies also examined the risk of PD associated with β-blockers with inconsistent findings (2, 19, 20). We investigated the potential for reverse causality in several analyses. The present analyses all showed that the associations for both β2-agonists and β-blockers were limited to short-term use, findings that consistently support attributing the apparent associations to reverse causality.
Our study has a number of strengths, including the population-based nature of the cohort and the large number of cases of PD identified in a database of electronic medical records that has been extensively used for pharmacoepidemiologic studies, including in PD (21, 22). Also, the CPRD contains information on lifestyle risk factors, such as smoking, that are not available in administrative health databases. Finally, prescriptions issued are automatically recorded in the database, mitigating the potential for misclassification of exposure. However, some limitations also need to be considered. Definition of exposure was based solely on prescriptions issued by general practitioners (prescriptions issued by specialists are not recorded in CPRD). However, general practitioners play a central role in the UK health-care system and are responsible for regular follow-up and renewal of prescriptions issued by specialists, so exposure misclassification is likely to be small. Moreover, the sensitivity analysis extending the grace period yielded consistent results. Finally, some misclassification of PD diagnosis is possible. To increase the specificity of our case definition, we performed a sensitivity analysis restricting our case definition to patients having a PD diagnosis and at least 2 antiparkinson drugs prescriptions, with results consistent with those of the primary analysis.
In summary, our findings suggest that the apparent decreased risk of PD associated with β2-agonists previously reported is likely the result of reverse causality rather than a biological effect of these drugs. Consideration of this potential but important bias is warranted to avoid prematurely concluding that there is a potential new indication for this class of drugs.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Center for Clinical Epidemiology, Lady Davis Research Institute, Jewish General Hospital, McGill University, Montreal, Quebec, Canada (Francesco Giorgianni, Pierre Ernst, Sophie Dell’Aniello, Samy Suissa, Christel Renoux); Department of Epidemiology, Biostatistics, and Occupational Health, McGill University, Montreal, Quebec, Canada (Pierre Ernst, Samy Suissa, Christel Renoux); and Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, Canada (Christel Renoux).
This study was supported by a grant from Parkinson Canada and by infrastructure funding from the Canadian Institutes of Health Research and the Canadian Foundation for Innovation. Christel Renoux holds a Chercheur-Boursier Junior 2 Award from the Fonds de recherche du Québec – Santé. Samy Suissa is the recipient of the James McGill Chair.
We are indebted to Prof. J.-L. Montastruc for his comments on an earlier version of the manuscript.
The funders had no role in study design; data collection, data analysis, or data interpretation; writing of the report; or the decision to submit the paper for publication.
Dr. Suissa has received research grants from Boehringer Ingelheim and has participated in advisory board meetings or as speaker for AstraZeneca, Boehringer-Ingelheim, Novartis, and Pfizer. All other authors have no conflicts of interest to disclose.
REFERENCES
- 1. Shults CW. Lewy bodies. Proc Natl Acad Sci USA. 2006;103(6):1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mittal S, Bjornevik K, Im DS, et al. . β2-adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease. Science. 2017;357(6354):891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Snyder EY. Finding a new purpose for old drugs. Science. 2017;357(6354):869–870. [DOI] [PubMed] [Google Scholar]
- 4. García Rodríguez LA, Pérez Gutthann S. Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol. 1998;45(5):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walley T, Mantgani A. The UK General Practice Research Database. Lancet. 1997;350(9084):1097–1099. [DOI] [PubMed] [Google Scholar]
- 6. Herrett E, Gallagher AM, Bhaskaran K, et al. . Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chisholm J. The Read clinical classification. BMJ. 1990;300(6732):1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991;302(6779):766–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jick SS, Kaye JA, Vasilakis-Scaramozza C, et al. . Validity of the General Practice Research Database. Pharmacotherapy. 2003;23(5):686–689. [DOI] [PubMed] [Google Scholar]
- 10. Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract. 2010;60(572):e128–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breslow NE, Day NE. Fitting models to continuous data In: Statistical Methods in Cancer Research Volume II: The Design and Analysis of Cohorts Studies. Lyon, France: International Agency for Research on Cancer; 1987:178–229. [Google Scholar]
- 12. Essebag V, Genest J Jr, Suissa S, et al. . The nested case-control study in cardiology. Am Heart J. 2003;146(4):581–590. [DOI] [PubMed] [Google Scholar]
- 13. Suissa S. Novel approaches to pharmacoepidemiology study design and statistical analysis In: Strome BL, ed. Pharmacoepidemiology. New York, NY: John Wiley & Sons, Ltd; 2000:785–805. [Google Scholar]
- 14. Essebag V, Platt RW, Abrahamowicz M, et al. . Comparison of nested case-control and survival analysis methodologies for analysis of time-dependent exposure. BMC Med Res Methodol. 2005;5(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schafer JL. Analysis of Incomplete Multivariate Data. Boca Raton, Florida: Chapman & Hall/CRC; 1997. [Google Scholar]
- 16. Rubin DB, Wiley I. Multiple Imputation for Nonresponse in Surveys. Hoboken, New Jersey: John Wiley & Sons; 1987. [Google Scholar]
- 17. Sterne JA, White IR, Carlin JB, et al. . Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horwitz RI, Feinstein AR. The problem of "protopathic bias" in case-control studies. Am J Med. 1980;68(2):255–258. [DOI] [PubMed] [Google Scholar]
- 19. Gronich N, Abernethy DR, Auriel E, et al. . β2-adrenoceptor agonists and antagonists and risk of Parkinson's disease. Mov Disord. 2018;33(9):1465–1471. [DOI] [PubMed] [Google Scholar]
- 20. Searles Nielsen S, Gross A, Camacho-Soto A, et al. . β2-adrenoreceptor medications and risk of Parkinson disease. Ann Neurol. 2018;84(5):683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Becker C, Jick SS, Meier CR. Use of statins and the risk of Parkinson's disease: a retrospective case-control study in the UK. Drug Saf. 2008;31(5):399–407. [DOI] [PubMed] [Google Scholar]
- 22. Hernán MA, Logroscino G, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and the incidence of Parkinson disease. Neurology. 2006;66(7):1097–1099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


